Abstract
Glucocorticoids (GCS) are capable of stimulating the secretion of interleukin (IL)-10 by leucocytes; however, the potential of GCS to modulate leucocyte susceptibility to IL-10-mediated actions has not yet been studied. In the current paper, we performed a detailed cross-sectional analysis of IL-10 receptor (IL-10R) expression by CD4+ and CD8+ T cells, natural killer (NK) cells, monocytes and neutrophils. Next, we analysed the effects of short-term oral GCS treatment on surface IL-10R expression by various leucocyte subpopulations in asthmatic patients. All leucocyte subsets studied presented with substantial levels of surface IL-10R. The highest levels of IL-10R were found on monocytes, predominantly with CD142+CD16+ and CD14+CD16+ phenotypes, and on CD4+CD25high T cells. In contrast, levels of IL-10R on CD8+ T cells, NK cells and neutrophils were significantly lower and similar to each other in intensity. GCS treatment resulted in a significant decrease of IL-10R expression on all analysed peripheral blood leucocyte subsets. Our data suggest that down-regulation of IL-10R could counterbalance the otherwise suppressive action of GCS.
Keywords: asthma, cell surface molecules, cytokine receptors
Introduction
Interleukin (IL)-10 is an immunosuppressive anti-inflammatory cytokine secreted by a number of cells, including monocytes, macrophages, lymphocytes T and B, eosinophils and mast cells. IL-10 down-regulates T helper type 1 (Th1) and Th2 responses by attenuating the function of T cells, dendritic cells and monocytes and macrophages. In addition, IL-10 decreases production and secretion of proinflammatory mediators such as tumour necrosis factor (TNF)-α, granulocyte–macrophage colony-stimulating factor, IL-4 and IL-5 (summarized in [1,2]). Allergic diseases, including asthma, have been associated with decreased IL-10 production [3]. Severely asthmatic patients are reported to have decreased levels of IL-10-producing monocytes and T cells [4,5]. In contrast, administration of IL-10 has been associated with suppression of allergic inflammation in sensitized animals [6]. In human subjects, clinically effective allergen-specific immunotherapy results in induction of tolerance/anergy, dependent on IL-10-mediated mechanisms [7–10].
It has been reported that secretion of endogenous IL-10 might be augmented by glucocorticoids (GCS) [11–14]. GCS are potent anti-inflammatory drugs that are used widely in asthma and other inflammatory conditions. Surprisingly, despite well-documented interplay between GCS and IL-10, there are no data describing the effects of GCS treatment on IL-10 receptor (IL-10R) expression on peripheral blood leucocytes. Moreover, the pattern of IL-10R expression on different peripheral blood cells has not yet been specified. In this study we performed for the first time an extensive time–course analysis of IL-10R expression on different peripheral blood leucocyte subsets in asthmatic patients who underwent transient oral GCS treatment. We demonstrate that 7-day administration of 30 mg of methylprednisolone results in down-regulation of IL-10R on different monocyte subsets, CD4+ and CD8+ lymphocytes, natural killer (NK) cells and neutrophils.
Material and methods
Patients
We recruited 11 moderately asthmatic patients aged 39–54 years (median 45·2 years) treated with moderate doses of inhaled GCS and long-acting beta-agonists, classified according to Global Initiative for Asthma criteria, with exacerbation requiring rescue oral GCS. Exacerbation was defined by an increase in dyspnoea and the use of rescue beta-agonist, a fall of forced expiratory volume in 1 s by at least 20% and an increase in exhaled nitric oxide level. These patients had normal white blood cell counts, normal C-reactive protein and sedimentation rate levels and no evidence of respiratory infection. Patients were hospitalized in the Department of Allergology and Internal Medicine and were administered 30 mg methylprednisolone for 7 days in addition to their current treatment. The control group consisted of eight patients with asthma exacerbation, aged 31–44 years (median 37·4 years) who were observed for 7 days in the out-patient allergy clinics with no oral GCS added to their current treatment. All patients were atopic as defined by at least two positive skin prick tests to common allergens. Current smokers, ex-smokers and subjects with a history of respiratory infection during the previous 4 weeks were excluded from the study.
Cell culture
Whole blood samples were cultured with either medium plus methylprednisolone at a concentration of 3 or 0·3 µg/ml, or medium alone as a negative control. The cultures were incubated at 37°C in a humidified 5% CO2 atmosphere for 6 h. After incubation, cells were washed in Dulbecco's phosphate-buffered saline (PBS) with 2% fetal bovine serum, harvested and processed immediately for surface staining. In four individuals remaining cells (precultured with either methylprednisolone or medium alone) were incubated with phorbol myristate acetate at 50 ng/ml and ionomycin at 0·5 µg/ml in the presence of 1 µg/ml brefeldin A (GolgiPlug, BD PharMingen, Erembodegen, Belgium). After 6 h cells were harvested for intracellular cytokine analysis.
Immunostaining and flow cytometric analysis
Fresh and cultured whole blood samples were incubated for 30 min at room temperature with 10 µl of monoclonal antibodies: anti-CD210 (IL-10R), anti-CD14, anti-CD16, anti-CD3, anti-CD4, anti-CD8, anti-CD25 and anti-CD56 (all from BD PharMingen). The red blood cells were lysed; the remaining cells were washed twice with PBS and fixed with 2% paraformaldehyde. Portions of cells prepared for intracellular cytokine analysis were permeabilized and incubated for 30 min at room temperature with 10 µl of anti-TNF-α and anti-IL-4 monoclonal antibodies (from BD PharMingen). Flow cytometry analysis was performed using a fluorescence activated cell sorter Calibur cytometer (BD Immunocytometry Systems, San Jose, CA, USA), as described previously [15,16]. Flow cytometry data were collected in list mode and analysed using CellQuest software (BD Immunocytometry Systems). First, lymphocytes, monocytes and granulocytes were gated by a combination of forward and side scatter (FSC/SSC). The gating of different monocyte subsets was performed using anti-CD14 and anti-CD16 monoclonal antibodies and appropriate isotype-matched controls, as described elsewhere [17]. The gating of different lymphocyte subsets was performed using anti-CD3, anti-CD4, anti-CD8 and anti-CD25 monoclonal antibodies and appropriate isotype-matched controls. NK cells were identified based on FSC/SSC gating and subsequent staining with anti-CD3, anti-CD16 and anti-CD56 monoclonal antibodies (CD3−CD16+CD56+) and appropriate isotype-matched controls. Neutrophils were identified by FSC/SSC gating and staining with anti-CD16.
To assess the expression of CD210 (IL-10R), TNF-α and IL-4, the gate was set initially with isotype-matched control antibody. As CD210 labelling displays with monophasic distribution, the data are presented as the mean fluorescence intensity (MFI) of leucocytes expressing CD210 within each considered cell subset.
Statistical analysis
We used Kruskal–Wallis anova and Mann–Whitney non-parametric tests to analyse statistically significant differences among groups. The non-parametric Wilcoxon test was used to analyse the pre- and post-treatment values. In all tests, statistically significant results were identified by a P value of <0·05.
Results
Expression of IL-10R on peripheral blood leucocyte subsets
We found substantial levels of IL-10R on all studied peripheral blood leucocytes; however, the intensity of expression varied among different subsets (Fig. 1). Interestingly, the highest levels of IL-10R were found consistently on monocytes [median MFI = 9·26, interquartile range (IQR) = 2·10, P < 0·05, in comparison with CD4+ and CD8+ T cells and NK cells]. With regard to monocytes, the largest expression of IL-10R was observed in subsets bearing CD16 antigen, namely CD14+CD16+ and CD142+CD16+[12·7 (IQR = 3·70) and 10·25 (IQR = 2·52) respectively]. IL-10R levels on both CD16+ subsets were significantly higher than on CD142+CD16− monocytes (P < 0·05). With regard to T cells, populations of CD4+ and CD8+ T cells presented with comparable intensity of IL-10R staining: 5·37 (IQR = 3·65) for CD4+ T cells and 5·30 (IQR = 2·03) for CD8+ T cells. Notably, CD4+CD25high T cells presented with levels of IL-10R expression that were significantly higher compared with CD4+CD25− T cells [12·0 (IQR = 2·90) versus 5·05 (IQR = 3·80); P < 0·001] and to CD4+CD25intermediate T cells [12·0 (IQR = 2·90) versus 7·10 (IQR = 2·70), P < 0·05]. In contrast, there was no statistically significant difference in IL-10R expression between CD8+CD25− T cells and CD8+CD25+ T cells [5·50 (IQR = 2·60) versus 4·70 (IQR = 2·20), P > 0·05]. IL-10R levels, similar to those seen in bulk populations of CD4+ and CD8+ T-cells, were also found on NK cells and neutrophils [5·00 (IQR = 2·40) and 6·80 (IQR = 11·52) respectively]. A concordant pattern of IL-10R expression by different peripheral blood leucocyte subsets was found in healthy controls and asthmatic patients at stable stages of disease (data not shown).
Fig. 1.
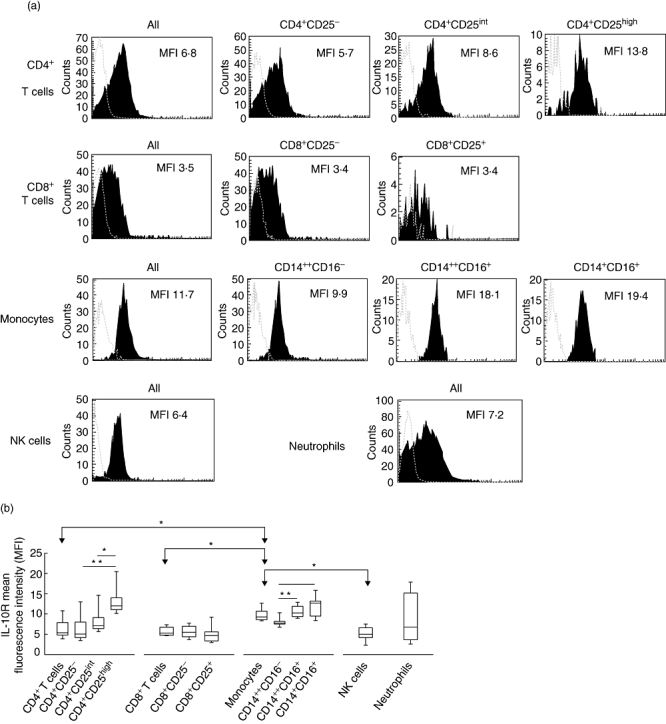
Interleukin (IL)-10R expression by peripheral blood leucocyte subsets. (a) Flow cytometric histograms representing the levels of IL-10R expression (black areas) in different leucocyte subsets in a representative sample of asthmatic patients. Values refer to mean fluorescence intensity (MFI) of IL-10R expression by the leucocyte subsets indicated. Grey broken lines represent isotype controls. (b) Summary of analyses of IL-10R expression by leucocyte subsets in asthmatic patients. Results are for the MFI of the IL-10R+ subsets. Data are depicted as box-plots. Medians are noted by inside bars and the boxes span the 25th and 75th percentiles, while whiskers represent the 5th and 95th percentiles. *P < 0·05; **P < 0·001: statistically significant differences between leucocyte subsets.
Effects of treatment with GCS on IL-10R expression on peripheral blood leucocytes
Seven-day oral administration of 30 mg methylprednisolone resulted in a significant decrease of IL-10R expression on CD4+ and CD8+ T cells, monocytes, NK cells and neutrophils (Fig. 2a–d). The following down-regulation of IL-10R expression was observed (in decreasing sequence): in CD4+ T-cells: from 5·37 (IQR = 3·65) to 4·35 (IQR = 3·05), P = 0·003 (compared with baseline values); in monocytes: from 9·26 (IQR = 2·10) to 8·30 (IQR = 2·50), P = 0·010; in neutrophils: from 6·80 (IQR = 11·52) to 4·00 (IQR = 7·82), P = 0·016; in CD8+ T cells: from 5·30 (IQR = 2·03) to 4·60 (IQR = 2·26), P = 0·042; and in NK cells: from 5·00 (IQR = 2·40) to 3·75 (IQR = 1·50), P = 0·049. A significant decrease of IL-10R expression after GCS treatment was seen in all CD4+ subsets (regardless of CD25 expression) but only in CD8+CD25− T cells. With regard to monocytes, a decrease of IL-10R levels was found in all subsets, but reached statistical significance only for CD142+CD16+ and CD14+CD16+ monocytes. In contrast, we did not observe any significant changes in IL-10R expression in the control group of eight asthmatic patients who were observed for 7 days (without addition of oral GCS to their current treatment) (Fig. 2e).
Fig. 2.
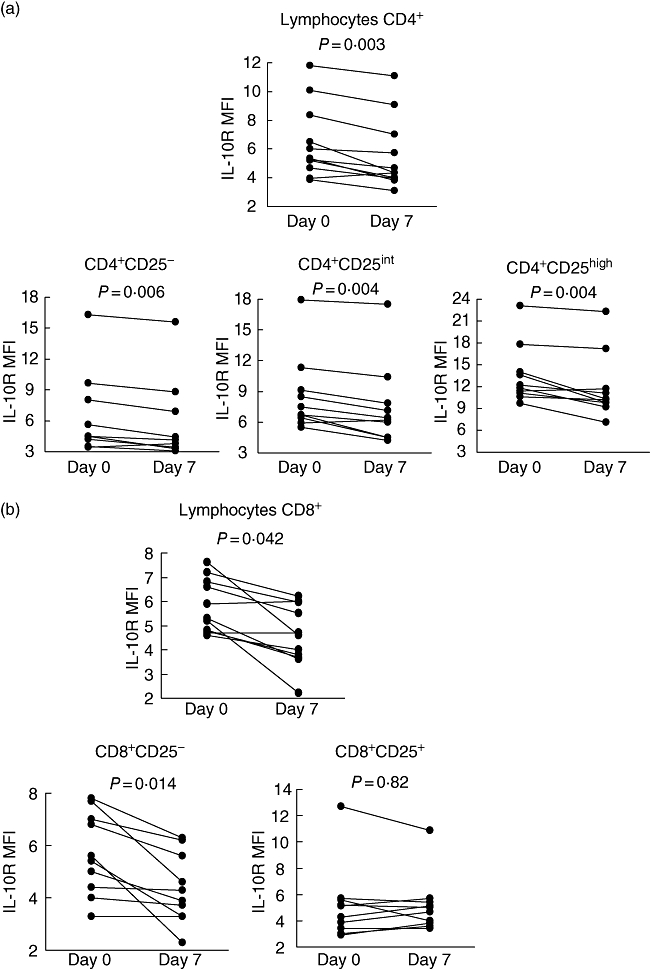
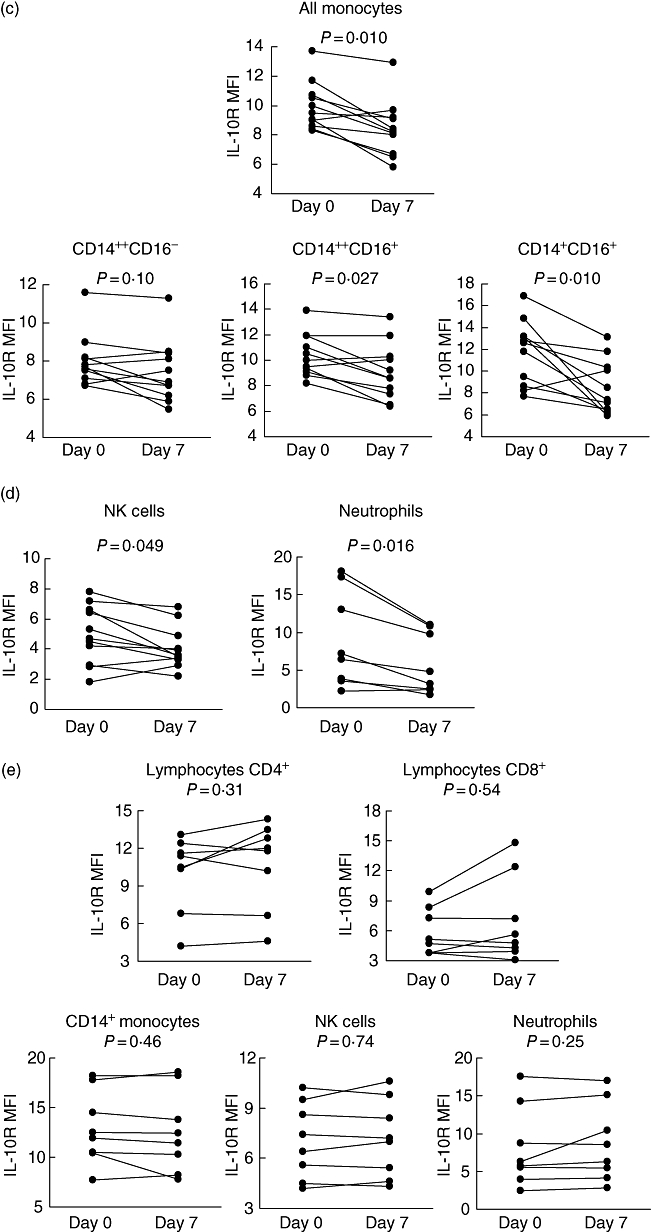
Detailed time–course analysis of frequencies of interleukin (IL)-10R+ leucocytes: (a) CD4+ T cells; (b) CD8+ T cells; (c) monocytes; and (d) natural killer cells and neutrophils, before and after administration of methylprednisolone. Statistically significant differences between pre- and post-treatment values are indicated. (e) Combined time–course analysis of frequencies of IL-10R+ leucocytes in the control group (with no oral glucocorticoids).
Modulation of IL-10R expression by culture with GCS
Having found the decreased frequencies of IL-10R+ peripheral blood leucocytes, we wished to explore whether this effect is due to direct action of GCS on IL-10R surface expression.
Whole blood samples were cultured for 6 h in the presence of different concentrations of methyprednisolone. In general, the staining for IL-10R in cultured samples was less intense compared with freshly processed samples. As expected, incubation with GCS led to significant down-regulation of IL-10R expression on both whole blood and isolated monocytes, CD4+ and CD8+ T cells, NK cells and neutrophils (Fig. 3a and data not shown). Interestingly, however, despite the decrease in IL-10R expression, subsequent stimulation of GCS preincubated cells with mitogen did not result in significant change of cytokine production by CD3+ T cells (Fig. 3b).
Fig. 3.
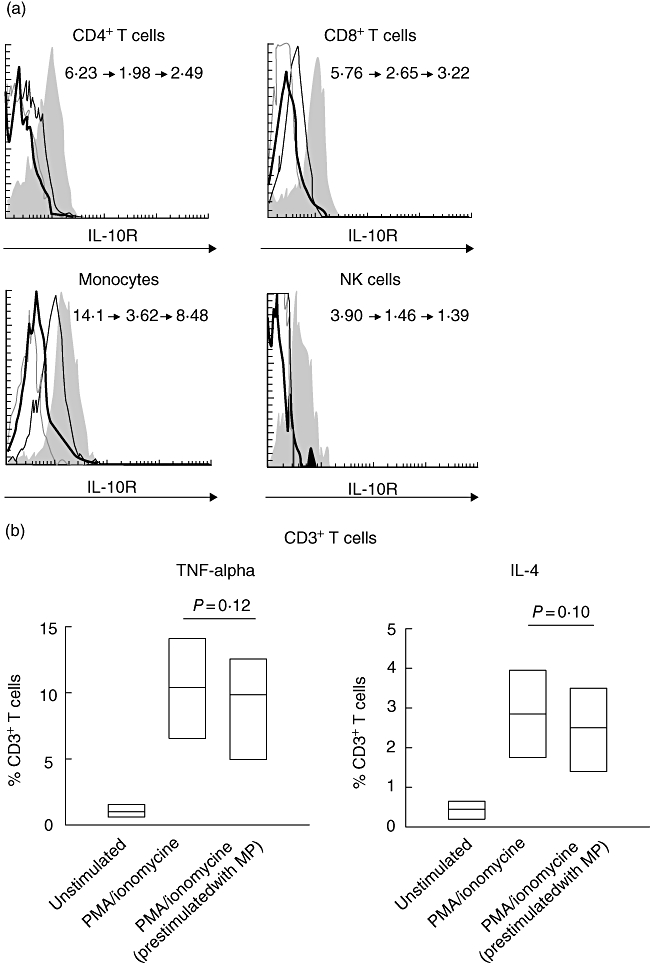
(a) Flow cytometric histograms representing levels of interleukin (IL)-10R expression in different leucocyte subsets in whole blood cultures with medium alone (grey areas), 3 µg/ml methylprednisolone (solid lines) or 0·3 µg/ml methylprednisolone (thin lines). Grey broken lines represent isotype controls. The values in histograms are for mean fluorescence intensity (MFI) of IL-10R expression by indicated leucocyte subsets in the following conditions: unstimulated (left), stimulated with methylprednisolone at 3 µg/ml concentration (middle) and stimulated with methylprednisolone at 0·3 µg/ml concentration (right). (b) The summary of analyses of tumour necrosis factor (TNF)-α and IL-4 secretion by glucocorticoids preincubated CD3+ T cells stimulated with phorbol myristate acetate and ionomycin. Results are for the percentage of TNF-α-positive and IL-4-positive CD3+ T cells. Data are depicted as box-plots. Medians are noted by inside bars and the boxes span the 25th and 75th percentiles (n = 4). MP, methylprednisolone.
Discussion
In the current paper we performed a parallel analysis of IL-10R expression on main leucocyte subsets in asthmatic patients subject to short-term administration of oral GCS. Notably, we did not limit our analysis to only a single subset but, rather, explored the effects of GC treatment on different cell types in parallel. We noted that GCS induced a decrease in IL-10R expression on all leucocyte subsets studied. That GC-induced down-regulation of IL-10R is not restricted to only one subset indicates the existence of a general pattern of responsiveness to IL-10-mediated signals.
To date there are only anecdotal reports analysing quantitatively IL-10R expression either on bulk peripheral blood mononuclear cells or on NK cells and neutrophils [18–20]. Here we demonstrate that IL-10R is expressed substantially by all leucocyte subsets studied. The highest levels of IL-10R+ cells were found on monocytes, which indicates that those cells could be an important target for IL-10-mediated signals. Our analysis revealed that IL-10R expression is expressed most highly by CD16+ monocytes. CD16+ monocytes have been considered more mature cells capable of becoming tissue macrophages, and IL-10 treatment was reported to accelerate this process [21]. Our current notion of high levels of IL-10R on those cells is also in agreement with our previous study, wherein substantial levels of IL-10R were found on airway macrophages of asthmatic patients [22].
With regard to T lymphocytes, higher levels of IL-10R (compared with other subsets) were found on CD4+CD25high T cells and, to a lesser extent, on CD4+CD25intermediate T cells, but not on CD8+CD25+ T cells. In general, the fact that GCS treatment exerted stronger effects on CD4+ than on CD8+ T cells or NK cells is surprising in light of recent studies, indicating lower levels of the glucocorticoid receptor on CD4+ T cells compared with other lymphocyte subsets [16,23]. On the other hand, our finding that CD4+CD25high lymphocytes bear the highest levels of IL-10R among CD4+ lymphocytes is interesting, considering that the CD4+CD25high phenotype is considered specific for natural regulatory T cells. Our current data suggest that CD4+CD25high T cells are significantly more susceptible to IL-10-mediated signals than other CD4+ T cells with non-regulatory phenotypes.
It could be hypothesized that some of the effects of IL-10R down-regulation by GCS might be associated with a treatment-induced decrease of frequency of subsets with high levels of IL-10R. This could be possible in monocytes, wherein GCS induces depletion of CD16+ subsets. However, CD16+ monocytes, although bearing the highest levels of IL-10R on their surface, constitute only a minority of the entire monocyte population. On the other hand, we also observed a trend of IL-10R down-regulation in CD142+CD16− monocytes that are not decreased by GCS treatment [17,24]. Similarly, oral GCS treatment does not lead to significant changes in frequencies of CD4+CD25high bearing the highest levels of IL-10R among all CD4+ T cells (Moniuszko et al., submitted).
It also remains unclear whether the decrease of IL-10R expression observed in our study was caused by the direct action of GCS exerted on surface molecules. Our data obtained from both in vivo and in vitro experiments seem to support that hypothesis; however, the possibility that IL-10R+ cells migrated to other compartments or were subject to GCS-induced programmed cell death was not excluded in the current paper and warrants further study.
Our demonstration of GCS-induced decrease in IL-10R expression is particularly interesting in light of widely described GCS-induced enhancement of IL-10 production [11–14,25]. We hypothesize that such seemingly opposite actions of GCS could, possibly, represent mechanisms of mutual counter-regulation of IL-10-mediated effects. Similarly, a pattern of interplay between IL-7 and IL-7R has been described [26–28]. Regardless of the mechanism, the clinical implications need to be elucidated in more detailed studies.
In summary, in the present report we demonstrate that IL-10R is expressed by main peripheral blood leucocyte subsets and is decreased by oral GCS treatment. We believe that our findings could contribute to a better understanding of the immunomodulating actions of GCS.
Acknowledgments
We thank Professor Loems Ziegler-Heitbrock for helpful comments, Dr Danuta Lenczewska for clinical support, Dr Krzysztof Kowal for discussions, Mrs Teresa Michno for laboratory assistance and Steven Snodgrass for editorial assistance. The study was supported by a grant from the Medical University of Bialystok.
Disclosure
The authors declare no conflict of interest.
References
- 1.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 2.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 3.Tournoy KG, Kips JC, Pauwels RA. Endogenous interleukin-10 suppresses allergen-induced airway inflammation and nonspecific airway responsiveness. Clin Exp Allergy. 2000;30:775–83. doi: 10.1046/j.1365-2222.2000.00838.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K, Inoue H, Fukuyama S, et al. Decrease of interleukin-10-producing T cells in the peripheral blood of severe unstable atopic asthmatics. Int Arch Allergy Immunol. 2004;134:295–302. doi: 10.1159/000079167. [DOI] [PubMed] [Google Scholar]
- 5.Tomita K, Lim S, Hanazawa T, et al. Attenuated production of intracellular IL-10 and IL-12 in monocytes from patients with severe asthma. Clin Immunol. 2002;102:258–66. doi: 10.1006/clim.2001.5176. [DOI] [PubMed] [Google Scholar]
- 6.Fu CL, Chuang YH, Chau LY, Chiang BL. Effects of adenovirus-expressing IL-10 in alleviating airway inflammation in asthma. J Gene Med. 2006;8:1393–9. doi: 10.1002/jgm.974. [DOI] [PubMed] [Google Scholar]
- 7.Akdis CA, Blaser K. Role of IL-10 in allergen-specific immunotherapy and normal response to allergens. Microbes Infect. 2001;3:891–8. doi: 10.1016/s1286-4579(01)01449-6. [DOI] [PubMed] [Google Scholar]
- 8.Nouri-Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 9.Vissers JL, van Esch BC, Hofman GA, et al. Allergen immunotherapy induces a suppressive memory response mediated by IL-10 in a mouse asthma model. J Allergy Clin Immunol. 2004;113:1204–10. doi: 10.1016/j.jaci.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 11.John M, Lim S, Seybold J, et al. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1α, granulocyte-macrophage colony stimulating factor and interferon-γ release from alveolar macrophages in asthma. Am J Respir Crit Care Med. 1998;157:256–62. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- 12.Richards DF, Fernandez M, Caulfield J, Hawrylowicz CM. Glucocorticoids drive human CD8(+) T cell differentiation towards a phenotype with high IL-10 and reduced IL-4, IL-5 and IL-13 production. Eur J Immunol. 2000;30:2344–54. doi: 10.1002/1521-4141(2000)30:8<2344::AID-IMMU2344>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Stelmach I, Jerzynska J, Kuna P. A randomized, double-blind trial of the effect of glucocorticoid, antileukotriene and beta-agonist treatment on IL-10 serum levels in children with asthma. Clin Exp Allergy. 2002;32:264–9. doi: 10.1046/j.1365-2222.2002.01286.x. [DOI] [PubMed] [Google Scholar]
- 14.Peek EJ, Richards DF, Faith A, et al. Interleukin-10-secreting ‘regulatory’ T cells induced by glucocorticoids and beta2-agonists. Am J Respir Cell Mol Biol. 2005;33:105–11. doi: 10.1165/rcmb.2005-0100OC. [DOI] [PubMed] [Google Scholar]
- 15.Moniuszko M, Kowal K, Zukowski S, et al. Frequencies of circulating CD4+CD25+CD127low cells in atopics are altered by bronchial allergen challenge. Eur J Clin Invest. 2008;38:201–4. doi: 10.1111/j.1365-2362.2007.01920.x. [DOI] [PubMed] [Google Scholar]
- 16.Gotovac K, Sabioncello A, Rabatic S, et al. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: lower quantity of GCR in patients with post-traumatic stress disorder (PTSD) Clin Exp Immunol. 2003;131:335–9. doi: 10.1046/j.1365-2249.2003.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol. 2009;130:338–46. doi: 10.1016/j.clim.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Valencia-Pacheco G, Layseca-Espinosa E, Niño-Moreno P, et al. Expression and function of IL-10R in mononuclear cells from patients with systemic lupus erythematosus. Scand J Rheumatol. 2006;35:368–78. doi: 10.1080/03009740600709840. [DOI] [PubMed] [Google Scholar]
- 19.Carson WE, Lindemann MJ, Baiocchi R, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85:3577–85. [PubMed] [Google Scholar]
- 20.Tamassia N, Calzetti F, Menestrina N, et al. Circulating neutrophils of septic patients constitutively express IL-10R1 and are promptly responsive to IL-10. Int Immunol. 2008;20:535–41. doi: 10.1093/intimm/dxn015. [DOI] [PubMed] [Google Scholar]
- 21.Calzada-Wack JC, Frankenberger M, Ziegler-Heitbrock HW. Interleukin-10 drives human monocytes to CD16 positive macrophages. J Inflamm. 1996;46:78–85. [PubMed] [Google Scholar]
- 22.Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Dabrowska M. Bronchial macrophages in asthmatics reveal decreased CD16 expression and substantial levels of receptors for IL-10, but not IL-4 and IL-7. Folia Histochem Cytobiol. 2007;45:181–9. [PubMed] [Google Scholar]
- 23.Berki T, Tavakoli A, Nagy KK, et al. Alterations of glucocorticoid receptor expression during glucocorticoid hormone therapy in renal transplant patients. Transplant Int. 2002;15:132–8. doi: 10.1007/s00147-002-0397-x. [DOI] [PubMed] [Google Scholar]
- 24.Dayyani F, Belge KU, Frankenberger M, et al. Mechanism of glucocorticoid-induced depletion of human CD14+CD16+ monocytes. J Leukoc Biol. 2003;74:33–9. doi: 10.1189/jlb.1202612. [DOI] [PubMed] [Google Scholar]
- 25.Unterberger C, Staples KJ, Smallie T, et al. Role of STAT3 in glucocorticoid-induced expression of the human IL-10 gene. Mol Immunol. 2008;45:3230–7. doi: 10.1016/j.molimm.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Moniuszko M, Edghill-Smith Y, Venzon D, et al. Decreased number of CD4+ and CD8+ T cells that express the interleukin-7 receptor in blood and tissues of SIV-infected macaques. Virology. 2006;356:188–97. doi: 10.1016/j.virol.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Munitic I, Williams JA, Yang Y, et al. Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood. 2004;104:4165–72. doi: 10.1182/blood-2004-06-2484. [DOI] [PubMed] [Google Scholar]
- 28.Fry TJ, Moniuszko M, Creekmore S, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–9. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]


