Abstract
The insulin-like growth factors (IGFs) signaling system has been shown to play important roles in neoplasia. The IGF receptor type 1 (IGF-IR) is overexpressed in many types of solid and hematopoietic malignancies, and there is substantial experimental and clinical evidence that targeting IGF-IR is a promising therapeutic strategy against cancer. It has been previously reported that a mouse monoclonal antibody (mAb), 4G11, blocked IGF-I binding to IGF-IR and downregulated the IGF-IR in MCF-7 cells. We cloned this antibody, constructed a human-mouse chimeric antibody, designated m590, and characterized it. The chimeric IgG1 m590 bound to cell-associated IGF-IR on NWT c43 stably transfected cells and MCF-7 breast cancer cells as efficiently as the parental murine antibody. Using purified IGF-IR extracellular domains, we found that both the chimeric m590 and the parental 4G11 antibodies bind to conformational epitopes on IGF-IR. Neither of these antibodies bound to the insulin receptor (IR) ectodomain. Furthermore, IgG1 m590 blocked the binding of IGF-I and IGF-II to IGF-IR, and inhibited both IGF-I and IGF-II induced phosphorylation of IGF-IR in MCF-7 cells. These results suggest that m590 could be an useful antibody in diagnosis and treatment of cancer, as well as a research tool.
Keywords: antibody, IGF-IR, phosphorylation, signal transduction
Introduction
Insulin-like growth factors (IGF) I and II are overexpressed by many tumors, resulting in increased proliferation, motility and survival. They bind to the type I insulin-like growth factor receptor (IGF-IR), which is also involved in cell transformation induced by tumor virus proteins and oncogene products. Tumor growth and metastasis can be blocked by agents that inhibit IGF-IR expression or function, suggesting that IGF-IR is a promising cancer treatment target. Strategies that involve IGF signaling system targeting include reduction of ligand levels or bioactivity, and inhibition of receptor function using receptor-specific antibodies or small-molecule tyrosine kinase inhibitors.1–3
Many IGF-IR-specific antibodies have undergone preclinical study, and several are being evaluated in clinical trials.3 The most advanced of these are human monoclonal antibody (mAb) CP751,871 (Pfizer) and humanized mAb MK-0646 (Pierre-Fabre/Merck),3 which are in Phase III clinical studies.4,5 Other anti-IGF-IR antibodies include fully human mAbs AmG479 (Amgen),7 IMC-A12 (ImClone),8 R1507 (Hoffmann LaRoche),3 and robatumumab (Schering-Plough). Various combinations of IGF-IR-specific antibodies in combination with marketed agents are also being evaluated as treatments for medical needs.9 Results from these clinical trials are promising. Antibodies to IGF-IR have shown additive effects with traditional chemotherapy drugs,9–11 and anti-Her2 mAb trastuzumab in cancer therapy.12,13
We previously reported the development of three novel anti-IGF-II fully human mAbs.14 They bound with high (subnanomolar) affinity to IGF-II, did not cross-react with IGF-I and insulin, and potently inhibited signal transduction mediated by the IGF-IR interaction with IGF-II. The most potent neutralizer, IgG1 m610, inhibited phosphorylation of the IGF-IR and the IR, as well as phosphorylation of the downstream kinases Akt and mitogen-activated protein kinase with an IC50 of the order of 1 nmol/L at IGF-II concentration of 10 nmol/L. m610 also inhibited growth of the prostate cancer cell line DU145, and migration of the breast cancer line cells MCF-7.
While we are testing the immunotherapeutic potential of IgG1 m610 in preclinical study, we intend to develop mAbs to IGF-IR to be used in combination with m610 and other antibodies or agents targeting the IGF system. 4G11 is a mouse IgG2b kappa mAb developed against IGF-IR by immunizing mice with mouse embryo fibroblasts overexpressing the human IGF-IR.15 In addition to inhibiting the binding of IGF-I to the fibroblast receptor, 4G11 also potently downregulates the IGF-IR in MCF-7 cells resulting in inhibition of Akt and MAPK activation by IGF-I.
Here, we report further characterization of 4G11, as well as characterization of the chimeric antibody m590, which was derived by cloning of the antibody gene from the 4G11 hybridoma and construction of a human-mouse chimeric version. We found that both 4G11 and m590 bind to cell-associated IGF-IR and recombinant IGF-IR extracellular ectodomain, but not to the IR ectodomain. We further found that both murine and chimeric antibodies inhibited not only IGF-I induced, but also IGF-II induced phosphorylation of IGF-IR in MCF-7 cells, suggesting that they have potential use as cancer therapeutics.
Results
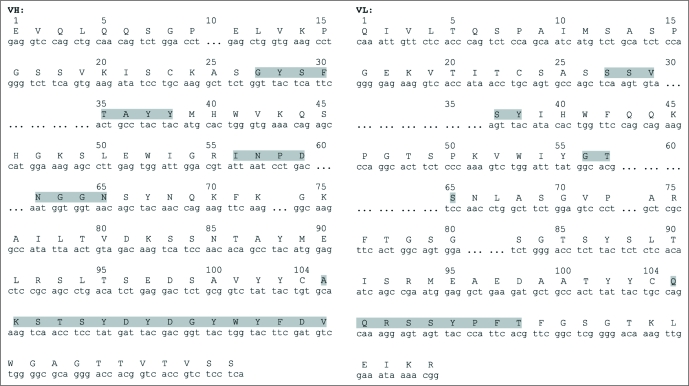
Molecule cloning of the 4G11 antibody gene heavy and light chain variable regions and construction of human-mouse chimeric antibody IgG1 m590. Murine 4G11 antibody heavy and light chain variable regions (VH and VL) were PCR amplified using a set of primers specific for different families of mouse antibody framework 1 and J chains. Amplified VH and VL were assembled with human antibody heavy chain first constant region (CH1) and light chain constant region (CL), respectively, and cloned to pComb3X for expression as Fab fragment. The murine VH and chimeric light chain (LC) were then subcloned to pDR12 containing genomic sequence of human heavy chain constant region for expression as IgG1. The final human-mouse chimeric IgG1 was designated m590. Both murine and chimeric antibody genes were sequenced and confirmed in frame (Fig. 1). The sequence analysis using IMGT database showed that m590 VH belongs to VH1 family and is most likely derived from VH1-34*01, DH2–4*01 and JH1*01 recombination. m590 VL belongs to VK4 family and is most likely derived from VK4–57*01 and JK4*01 recombination.
Figure 1.
Chimeric m590 antibody VH and VL DNA and deduced amino acid sequences. Complementarity determining regions in both VH and VL are highlighted in grey.
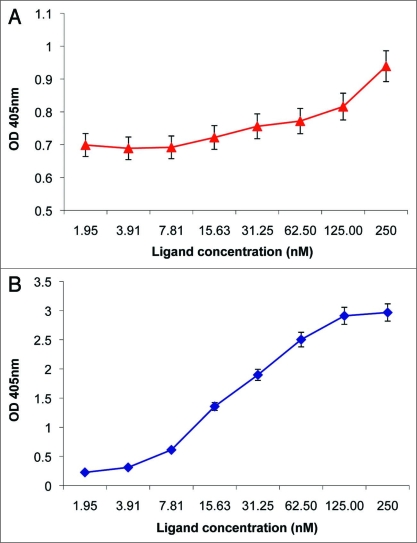
Production and characterization of IGF-IR ectodomain. IGF-IR extracellular domain gene was PCR amplified from pBluescript-IGF-IR construct and cloned to pSectag 2 plasmid via Eco RI and Not I sites. Recombinant IGF-IR ectodomain containing c-myc and his 6 tags was affinity purified by IMAC using the supernatant from transient transfection of free-style 293 cells. Purified IGF-IR ectodomain showed binding activity to IGF-I (Fig. 2A) and premature form IGF-II in ELISA (Fig. 2B).
Figure 2.
Binding of IGF-I and IGF-II to recombinant IGF-IR ectodomain. Two micrograms per milliliter recombinant IGF-IR ectodomain were coated on 96-well Maxisorp plate. After blocking the plates with 3% BSA in PBS, two-fold serially diluted IGF-I and IGF-II premature form with a starting concentration of 5 µg/ml were added to the plates. Bound IGF-I were detected with goat anti-IGF-I pAb (1:1,000) and HRP conjugates to anti-goat pAb (1:1,000). Bound IGF-II premature form were detected with HRP conjugates to penta-his tag antibody (1:1,000). Optical density at 405 nm was measured 20 min after adding ABTS substrate.
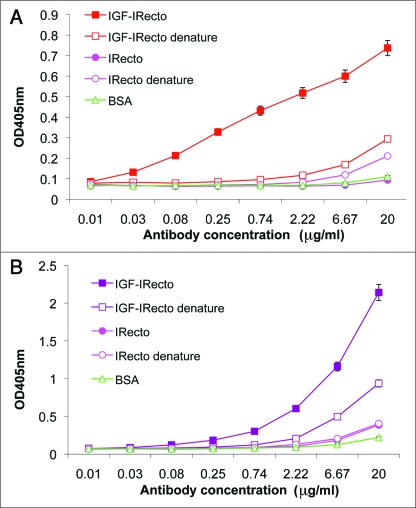
Binding of murine 4G11 and chimeric m590 antibodies to the IGF-IR ectodomain, but not to the IR ectodomain. Purified IGF-IR ectodomain was used to characterize murine 4G11 antibody and chimeric m590 antibody. ELISA assay using non-denatured and denatured IGF-IR and IR ectodomains demonstrated that both 4G11 (Fig. 3A) and m590 (Fig. 3B) bound to non-denatured IGF-IR ectodomain, but not to denatured IGF-IR ectodomian and IR ectodomain regardless of its conformation. This result indicates that 4G11 and m590 bind to conformational epitopes on IGF-IR.
Figure 3.
Binding of mouse IgG2b 4G11 and chimeric IgG1 m590 to non-denatured and denatured IGF-IR and IR ectodomains. Both ectodomains were coated at 200 ng/well on Maxisorp plates. Denatured ectodomains were prepared as described in Materials and Methods. After blocking the plates with 3% BSA in PBS, three-fold serially diluted antibodies with a starting concentration of 20 µg/ml were added to the plates and bound antibodies were detected with anti-mouse IgG conjugated to HRP for 4G11 and anti-human Fc conjugated to HRP for m590.
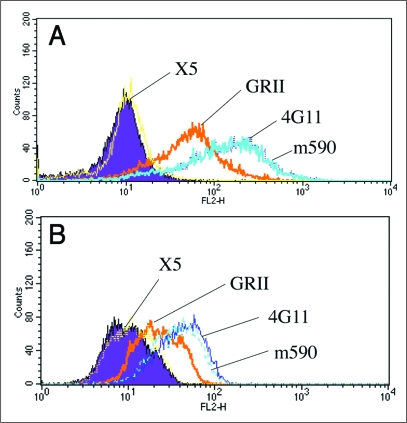
Binding of 4G11 and m590 to cell-associated IGF-IR. We measured binding of the two antibodies to cell-associated IGF-IR by fluorescence-activated cell sorting (FACS). Both 4G11 and m590 bind well to IGF-IR stably transfected cell line NWT c43 (Fig. 4A) and breast cancer cell line MCF-7 (Fig. 4B). The binding of these antibodies was stronger than that of commercially available anti-IGF-IR b subunit mouse mAb GRII. The binding was also specific because the control human mAb IgG1 X5 to HIV-1 did not show binding to these two cell lines.
Figure 4.
Binding of human-mouse chimeric IgG1 m590, mouse IgG2b 4G11, anti-IGF-IR b subunit antibody GRII and control antibody anti-HIV-1 IgG1 X5 to NWT C43 cells and MCF-7 cells. Ten 10 mg/ml of each antibody were incubated with stably transfected NWT C43 cells or breast cancer cell line MCF-7. Bound chimeric IgG1 m590 and control antibody IgG1 X5 were revealed by PE labeled anti-human IgG, F(ab')2, mouse IgG2b 4G11 and GRII by PE labeled anti-mouse polyclonal antibody.
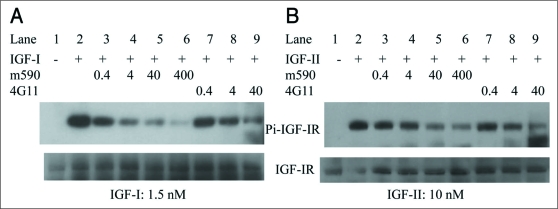
Inhibition of IGF-I and IGF-II induced phosphorylation of IGF-IR by chimeric m590 antibody and parental murine 4G11 antibody in MCF-7 cells. 4G11 blocks IGF-I binding to IGF-IR and downregulates IGF-IR in NWT c43, MCF-7 breast cancer cells, a panel of colon cancer cell lines and MG-63 human osteosarcoma cells.15 In addition, downregulation of IGF-I receptor by 4G11 antibody results in inhibition of IGF-I-stimulated activation of Akt and MAPK. We therefore examined the effect of 4G11 and m590 on binding of IGF-II to IGF-IR and IGF-II induced phosphorylation of IGF-IR (Fig. 5B). IGF-I binding and IGF-I induced phosphorylation of IGF-IR were also included in the experiment (Fig. 5A). The result indicated that both m590 and 4G11 antibodies inhibited IGF-I and IGF-II induced phosphorylation of IGF-IR in MCF-7 cells. For this experiment, we incubated cells with the antibodies at 37°C for 30 min prior to addition of IGF-I and IGF-II to cells, so the inhibitory effect on receptor phosphorylation attributes to blocking of the binding of IGF-I and IGF-II to IGF-IR, rather than to downregulation of IGF-IR. Combined with the previous observation that 4G11 blocks IGF-I binding to IGF-IR and inhibits IGF-I stimulated activation of Akt and MAPK, we conclude that both m590 and 4G11 antibodies block the binding of IGF-I and IGF-II to IGF-IR, and inhibit IGF-I and IGF-II induced phosphorylation of IGF-IR.
Figure 5.
Inhibition of IGF-I and IGF-II induced phosphorylation of IGF-IR by anti-IGF-IR human-mouse chimeric antibody IgG1 m590 and parental murine antibody IgG2b 4G11 in MCF-7 cells. MCF-7 cells starved in serum-free medium were pre-incubated with indicated concentrations (in nM) of IgG1 m590 (Lanes 3 to 6) and IgG2b 4G11 (Lanes 7 to 9) for 30 min followed by addition of 1.5 nM IGF-I (left) or 10 nM IGF-II (right) for 20 min. Total IGF-IR was immunoprecipitated with rabbit anti-IGF-IR beta subunit pAb. Phosphorylated IGF-IR was detected with mAb 4G10 specific to phosphotyrosine (top gels). The blots were re-probed with rabbit anti-IGF-IR beta subunit pAb (bottom gels) to show the total IGF-IR protein among the samples. Cells treated with IGF-I or IGF-II alone (Lane 2 in both panels) were included as positive control.
Discussion
We report here the construction and characterization of a human-mouse chimeric antibody, m590 that is specific for the IGF-IR. The parental murine 4G11 antibody was also further characterized in this study. We demonstrated that the chimeric m590 antibody retained characteristics of the parental murine 4G11 antibody. The combined results from a previous study of the murine 4G11 antibody14 and the current study on both chimeric m590 antibody and murine 4G11 antibody, suggest that m590 has potential as a therapeutic agent against cancer. Downregulation of IGF-IR and blocking of the IGF-IR mediated signaling are both important in the development of IGF-IR targeting therapeutics against cancer. This antibody demonstrated both properties. We plan to humanize this antibody to avoid potential immune responses generated by administration of a chimeric antibody in vivo. The humanized version of m590 might potentially be used alone or in combination with other mAbs targeting either the IGF-II ligand (such as m610) or IGF-IR, or with other anti-cancer agents targeting the IGF signaling system or other targets. Further experiments in animal models and human clinical trials will be necessary to evaluate this possibility.
Our experimental data showed that both m590 and 4G11 antibodies bind to the IGF-IR ectodomain, but not to the IR ectodomain. We do not have experimental data to exclude the possibility of their binding to heterodimers of IGF-IR and IR. We observed an increase of FL2-H signal of 4G11 and m590 in FACS analyses compared with the control mouse mAb GRII which binds to the IGF-IR β subunit (Fig. 3), but it does not suggest cross-reactivity of 4G11 and m590 with hybrid-receptor. GRII and 4G10 or m590 bind to different epitopes on IGF-IR and they have different affinity for IGF-IR. The differences of the FL2-H signal may reflect the difference in the antibody affinity. Lack of binding of 4G11 and m590 to denatured IGF-IR ectodomain suggests that this antibody may not bind to heterodimers of IGF1R and IR. If this is the case, then this antibody may have a major advantage in clinical use over existing mAbs targeting the IGF-IR. Recent study has shown that anti-IGF-IR antibodies also downregulate IR,15 which could lead to side effects. We plan to further characterize this antibody and engineer it if necessary. In summary, this antibody, or its humanized version may have potential as an IGF-IR targeted cancer therapy, as well as a diagnostic and research tool.
Materials and Methods
Cells, hybridoma, constructs, proteins and antibodies. NWT c43 stably transfected cells expressing IGF-IR, 4G11 hybridoma and pBlue-Script-IGF-IR construct were kindly provided by Derek LeRoith at the National Institutes of Health (NIH). Free-style 293 cells were purchased from Invitrogen. Recombinant human IGF-I, IGF-II and IR ectodomain were purchased from Xpress-Bio life science. Purified 4G11 antibodies were kindly provided by Cheryl Thomas and Peter Nissley at NIH. Premature IGF-II was produced in our laboratory as described.14 Penta-His antibody was purchased from Qiagen. Anti-human IGF-I or IGF-II polyclonal antibodies (pAb) were purchased from R&D systems and anti-IGF-IR β subunit mAb GRII were purchased from Millpore (MAB1123). Anti-IGF-IR beta subunit pAb were purchased from Santa Cruz Biotechnologies. The second antibodies including HRP conjugated to anti-human or anti-mouse pAbs used in ELISA, and PE conjugated to anti-human or mouse pAbs used in FACS analyses were purchased from Jackson Immuno laboratory.
Cloning of 4G11 VH and VL genes and construction of chimeric IgG1 m590. Total RNA was isolated from 4G11 hybridoma growing in serum-free medium. Message RNA was then isolated from total RNA and used in RT-PCR to amplify VH and VL using a set of primers specific for mouse antibody genes. Amplified VH and VL genes were assembled with human CH1 and CL by SOE-PCR and cloned to pComb3X. Fab m590 in pComb3X was then converted to IgG1 m590 in pDR12 containing genomic DNA sequence of human heavy chain constant region. All the constructs were sequenced after each subcloning.
Expression of chimeric IgG1 m590 and recombinant human IGF-IR ectodomain. IgG1 m590 was expressed by transient transfection of free-style 293 cells. 293Fectin was used to transfect free-style 293 cells according to the instructions of the manufacturer (Invitrogen). Four days post-transfection, the culture supernatant was harvested and IgG1 m590 purified on protein A affinity column.
Recombinant human IGF-IR ectodomain gene was PCR-amplified from pBlueScript-IGF-IR construct and subcloned to pSecTag 2C vector. C-myc and his 6 tagged IGF-IR ectodomain was expressed by transient transfection of adherent 293 cells, followed by booster with vTF7.3 vaccinia virus expressing T7 RNA polymerase. Two days post-transfection, the culture supernatant was harvested and IGF-IR ectodomain purified by IMAC.
Enzyme-linked immunosorbent assays (ELISAs). ELISAs were performed on Maxisorp 96-well plates. Binding of IGF-I and IGF-II to IGF-IR ectodomain was carried out by coating 200 ng/well IG-IR ectodomain in sodium carbonate coating buffer (pH 8.3). The plates were blocked with 3% BSA in PBS, pH 7.4 by incubation at 37°C for 1 h. Two-fold serially diluted IGF-I and premature IGF-II protein with a starting concentration of 5 µg/ml was then added to the wells. Bound IGF-I was detected with goat anti-IGF-I pAb (1:1,000) followed by addition of HRP-anti-goat pAb (1:1,000) as second antibody and ABTS as substrate. Bound premature IGF-II was detected with HRP-Penta-His antibody (1:1,000) as second antibody and ABTS as substrate. Optical density at 405 nm was measured after addition of the substrate and incubation at room temperature for 20 min.
Binding of mouse IgG2b 4G11 and chimeric IgG1 m590 to IGF-IR and IR ectodomains were carried out by coating the ectodomains at 200 ng/well in coating buffer. Denatured ectodomains were prepared as follow: ectodomains were diluted to 20 µg/ml in denaturing buffer containing 1% SDS and 50 mM DTT. After incubation at room temperature for 30 min, ectodomains were diluted 1:10 in coating buffer and coated onto the plates. Three-fold serially diluted antibodies with a starting concentration of 20 µg/ml were added to the wells and bound antibodies were detected with anti-mouse IgG conjugated to HRP for 4G11 and anti-human Fc conjugated to HRP for m590. The rest steps were the same as described above.
FACS analysis. NWT c43 or MCF-7 cells (1 × 106) were suspended in 10 µg/ml mAbs (murine mAb 4G11, or chimeric IgG1 m590, or control mouse mAb GRII, or control human mAb IgG1 X5) for 45 min at 4°C to avoid receptor internalization. After washing, the cells were incubated with PE-labeled anti-mouse IgG pAb for detection of bound 4G11 and GRII or PE-labeled anti-human IgG, F(ab')2 for detection of bound chimeric m590 and X5 for 30 min at 4°C in the dark. The cells were washed again and kept on ice. FACS analysis was then performed on FACSort, Becton Dickinson.
Phosphorylation assay. Phosphorylation assay was performed as described previously.14 Briefly, MCF-7 cells starved in serum-free medium were pre-incubated with chimeric IgG1 m590 and murine IgG2b 4G11 antibodies for 30 min followed by addition of 1.5 nM IGF-I or 10 nM IGF-II for 20 min. Total IGF-IR was immunoprecipitated with rabbit anti-IGF-IR beta subunit pAb. Phosphorylated IGF-IR were detected with mAb 4G10 specific to phosphotyrosine. The blots were re-probed with rabbit anti-IGF-IR beta subunit pAb to show the total IGF-IR protein among the samples. Cells treated with IGF-I or IGF-II alone were included as positive control.
Acknowledgements
We thank Derek LeRoith (Diabetes Branch, NIDDK, NIH, Bethesda, MD), Cheryl Thomas (Metabolism Branch, CCR, NCI, NIH, Bethesda, MD), and Peter Nissley (National Cancer Institute, NIH, Bethesda, MD) for providing reagents and helpful discussions.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, by federal funds from the NIH, National Cancer Institute, under contract no. NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- IGF
insulin-like growth factors
- IGF-IR
IGF receptor type 1
- IR
insulin receptor
- mAb
monoclonal antibody
- IMAC
immobilized metal ion affinity chromatography
- VH and VL
antibody heavy and light chain variable regions
- FACS
fluorescence-activated cell sorting
- DTT
dithiothreitol
- BSA
bovine serum albumin
- PBS
phosphate-buffered saline
- ABTS
2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)
- PE
phycoerythrin
- HRP
horse radish peroxidase
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/9580
References
- 1.Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov. 2009;4:54–72. doi: 10.2174/157489209787002515. [DOI] [PubMed] [Google Scholar]
- 2.Pollak M. Targeting insulin and insulin-like growth factor signalling in oncology. Curr Opin Pharmacol. 2008;8:384–392. doi: 10.1016/j.coph.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 4.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 5.Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulin-like growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 6.Descamps G, Gomez-Bougie P, Venot C, Moreau P, Bataille R, Amiot M. A humanised anti-IGF-1R monoclonal antibody (AVE1642) enhances Bortezomib-induced apoptosis in myeloma cells lacking CD45. Br J Cancer. 2009;100:366–369. doi: 10.1038/sj.bjc.6604839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 8.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549–5555. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 10.Maloney EK, McLaughlin JL, Dagdigian NE, Garrett LM, Connors KM, Zhou XM, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073–5083. [PubMed] [Google Scholar]
- 11.Goetsch L, Gonzalez A, Leger O, Beck A, Pauwels PJ, Haeuw JF, et al. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113:316–328. doi: 10.1002/ijc.20543. [DOI] [PubMed] [Google Scholar]
- 12.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 13.Bender LM, Nahta R. Her2 cross talk and therapeutic resistance in breast cancer. Front Biosci. 2008;13:3906–3912. doi: 10.2741/2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Zhu Z, Xiao X, Choudhry V, Barrett JC, Dimitrov DS. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol Cancer Ther. 2006;5:114–120. doi: 10.1158/1535-7163.MCT-05-0252. [DOI] [PubMed] [Google Scholar]
- 15.Jackson-Booth PG, Terry C, Lackey B, Lopaczynska M, Nissley P. Inhibition of the biologic response to insulin-like growth factor I in MCF-7 breast cancer cells by a new monoclonal antibody to the insulin-like growth factor-I receptor. The importance of receptor downregulation. Horm Metab Res. 2003;35:850–856. doi: 10.1055/s-2004-814144. [DOI] [PubMed] [Google Scholar]