Abstract
Bz-423 is a pro-apoptotic 1,4-benzodiazepine with therapeutic properties in murine models of lupus demonstrating selectivity for autoreactive lymphocytes. Bz-423 modulates the F1F0-ATPase, inducing the formation of superoxide within the mitochondrial respiratory chain, which then functions as a second messenger initiating apoptosis. In order to understand some of the features that contribute to the increased sensitivity of lymphocytes, we report the signaling pathway engaged by Bz-423 in a Burkitt lymphoma cell line (Ramos). Following the generation of superoxide, Bz-423-induced apoptosis requires the activation of Bax and Bak to induce mitochondrial outer membrane permeabilization and cytochrome c release. Knockdown of the BH3-only proteins Bad, Bim, Bik, and Puma inhibits Bz-423 apoptosis, suggesting that these proteins serve as upstream sensors of the oxidant stress induced by Bz-423. Treatment with Bz-423 results in superoxide-dependent Mcl-1 degradation, implicating this protein as the link between Bz-423-induced superoxide and Bax and Bak activation. In contrast to fibroblasts, B cell death induced by Bz-423 is independent of c-Jun N-terminal kinase. These results demonstrate that superoxide generated from the mitochondrial respiratory chain as a consequence of a respiratory transition can signal a specific apoptotic response that differs across cell types.
Keywords: Mcl-1, Bax, Bak, Benzodiazepine, Apoptosis, Superoxide
1. Introduction
Bz-423 is a pro-apoptotic 1,4-benzodiazepine with potent therapeutic properties in murine lupus linked to specific deletion of autoreactive lymphocytes [1, 2]. Bz-423 binds to the oligomycin-sensitivity conferring protein (OSCP), a component of the mitochondrial F1F0-ATPase, and induces the formation of superoxide via a state 3 to state 4 respiratory transition [3]. The reactive oxygen species (ROS) generated by Bz-423 induces lymphocyte apoptosis both in vivo and in vitro [2]. The absence of either general toxicities or significant effects on normal immune responses in treated mice indicates that Bz-423 has selective effects on cells that are pathogenic in autoimmune disease.
We previously characterized the apoptosis induced by Bz-423 in mouse embryonic fibroblasts (MEFs) containing knockouts of key apoptotic proteins [4]. In this cell type, Bz-423-induced superoxide is followed by caspase activation, mitochondrial electrochemical gradient (ΔΨm) collapse, and the release of cytochrome c into the cytoplasm, consistent with mitochondrial outer membrane permeabilization (MOMP) and the release of cytochrome c from the mitochondrial inter-membrane space [5]. Following these events, morphological and biochemical evidence of apoptosis is detected. In isolated mitochondria, Bz-423 induces ROS, but does not cause gradient collapse or swelling. These data show that Bz-423-induced superoxide does not directly trigger opening of the permeability transition pore, and implicates extra-mitochondrial factors in the mechanism coupling Bz-423-induced ROS to apoptosis.
In MEFs, apoptosis signal-regulating kinase 1 (ASK1) was found to be a critical upstream cellular redox sensor linking mitochondrial superoxide generation to apoptosis [4]. Activation of ASK1 initiates a mitogen activated protein (MAP) kinase cascade culminating in the activation of c-Jun N-terminal kinase (JNK). Activated JNK is then necessary for activation of pro-apoptotic Bax and Bak resulting in MOMP and a commitment to apoptotic cell death, as a small molecule JNK inhibitor prevents all of these steps.
Because MAP kinases are differentially regulated across cell types [6], we sought to determine if Bz-423 activates this pathway in lymphocytes, a cell type which is more sensitive to Bz-423. In particular, we sought to identify the extra-mitochondrial factors that link Bz-423-induced superoxide to apoptosis in Ramos B cells, in order to understand the differences in the response between lymphocytes and fibroblasts. In contrast to fibroblasts, Bz-423 does not activate MAP kinases in B cells, but apoptosis nonetheless still requires activation of Bax and Bak. We identify a superoxide-dependent decrease in Mcl-1 levels, and find that Bz-423 activates multiple BH3-only proteins in order to activate Bax and Bak and induce MOMP. These results demonstrate that superoxide generated from the mitochondrial respiratory chain as a consequence of a respiratory transition can signal specific apoptotic responses that differ across cell types. Our data suggest that differences in the levels of antioxidants and expression of Bcl-2 proteins help to explain the increased sensitivity of lymphocytes to Bz-423.
2. Materials and Methods
2.1. Reagents
Bz-423 was synthesized as previously described [7]. Dihydroethidium (DHE) and 3,3’-dihexyloxacarbocyanine iodide (DIOC6(3)) were obtained from Invitrogen Corp. (Carlsbad, CA, USA). Manganese (III) tetrakis (4-benzoic acid)porphyrin (MnTBAP) was purchased from Alexis Biochemicals (Lausen, Switzerland). Unless otherwise specified, all additional reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Cell lines and culture
Ramos cells were obtained from the American Type Culture Collection and maintained in RPMI 1640 media (Mediatech, Manassas, VA, USA) supplemented with heat-inactivated fetal bovine serum (FBS, 10%), penicillin (100 U/mL), streptomycin (100 µg/mL), and L-glutamine (290 µg/mL). In vitro experiments were conducted in media containing 2% FBS unless otherwise noted. Organic compounds were dissolved in media containing 0.5% DMSO.
2.3. Transient transfections
Small interfering RNA molecules (siRNAs) were purchased from Dharmacon (Lafayette, CO, USA). The sense strand sequences of the RNA duplexes used were as follows: Bim, ACC GAG AAG GUA GAC AAU U dtdt; Bax, GAA CUG AUC AGA ACC AUC AUU; Bik, Bak, Bad, Bid, and Puma were purchased as siGENOME SMARTpool reagents. Cells were washed once with ice-cold phosphate-buffered saline, suspended in Amaxa electroporation buffer T (Lonza Walkersville Inc, Walkersville, MD, USA) at a density of 7.5 × 106 cells/mL, and subjected to electroporation with desired siRNA (2 µg) with an Amaxa Nucleofector apparatus and a 2.0-mm electroporation cuvette using program N-16. Cells were transfected once daily for three days. Four days after the initial transfection, they were subjected to immunoblot analysis or incubated with Bz-423.
2.4. Immunofluorescence
Ramos cells were cultured on glass chamber slides (Nalge Nunc International, Rochester, NY, USA). Cells were fixed (0.25 h, RT) with PBS containing paraformaldehyde (2%). To remove the fixative, cells were washed five times with PBS containing saponin (10% w/v) and heat inactivated FBS (5%). Cells were incubated (overnight, 4 °C, 1 µg/mL) with antibodies for detection of activated Bax (catalog # 06-499, Millipore, Charlottesville, VA, USA) and Bak (06-536, Millipore). Following six washes, the cells were incubated (0.5 h, RT, 5 µg/mL) with biotinylated goat anti-rabbit IgG (BA-1000, Vector Laboratories, Burlingame, CA, USA). Following six washes, the cells were incubated (0.5 h, RT, 5 µg/mL) with fluorescein-conjugated avidin D (A-2001, Vector Laboratories). Samples were examined by microscopy using a Leica DM-LB microscope. Images (630X) were captured using a SPOT RS slider digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA) interfaced to a Macintosh PC.
2.5. Detection of intracellular superoxide, ΔΨm, cell death, and hypodiploid DNA
Detection of intracellular superoxide formation was performed by monitoring the oxidation of DHE to oxyethidium by flow cytometry using the FL2 channel (585 nm) of a FACSCalibur flow cytometer (BD Biosciences Inc., Franklin Lakes, NJ, USA). DHE (4 µM) was added to cells 30 min prior to flow cytometric analysis. For measurement of ΔΨm, cells were labeled with DIOC6(3) (37 °C, 15 min, 20 nM) prior to detection by flow cytometry using the FL1 channel (530 nm). Cell viability was assessed by staining with propidium iodide (PI; 1 µg/mL) prior to detection by flow cytometry using the FL3 channel (>650 nm). Measurement of hypodiploid DNA was conducted after incubating cells (4°C, overnight) in labeling solution (50 µg/ml of PI in PBS containing 0.2% Triton and 10 µg/ml RNase A). Total cellular DNA content was measured by flow cytometry in the FL2 channel after excluding aggregates.
2.6. Preparation of cellular extracts
For the preparation of whole cell extracts, 20 ×106 cells were pelleted and washed with PBS prior to lysis with WCE lysis buffer (25 mM Hepes pH 7.7, 150 mM NaCl, 2.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 20 mM β-glycerophosphate, 0.5 mM DTT containing 1 mM phenylmethylsulfonyl fluoride (PMSF), complete protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN, USA), 3.3 mM NaF, and 0.1 mM sodium orthovanadate). Following incubation on ice (30 min), the lysate was centrifuged (16,000 g, 0.5 h, 4 °C) to pellet insoluble cellular debris. Total protein content in the supernatant was quantified by the Bradford protein assay (Bio-rad Laboratories, Hercules, CA, USA).
For mitochondrial extracts, 107 cells were harvested and washed with ice cold PBS, followed by resuspension in ice-cold buffer A (200 µL, 20 mM Hepes-KOH, pH 7.5, 10 mM KCl, 10 mM β-glycerophosphate, 5 mM NaF, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM DTT, 1 mM sodium orthovanadate, 250 mM sucrose, complete protease inhibitor cocktail tablet and 0.1 mM PMSF). The cell suspension was allowed to sit on ice (20 min) and then was disrupted by 10 strokes through a 28.5 G needle. The homogenate was centrifuged (1000 g, 10 min, 4 °C) to pellet nuclei. The resulting supernatant was centrifuged (10,000 g, 30 min, 4 °C) to obtain the mitochondrial fraction. The supernatant from this centrifugation was harvested as the cytosolic fraction. The purity of fractions was tested by immunoblotting with antibodies specific for the cytosolic proteins β-tubulin (T4026, Sigma-Aldrich) and/or glyceraldehyde-3-phosphate dehydrogenase (GAPDH, MAB374, Millipore), or the mitochondrial protein β-subunit of complex V (V-β, A21351, Invitrogen). Total glutathione (GSH) concentrations of mitochondrial and cytosolic extracts were determined by an enzymatic GSH recycling assay [8].
2.7. Immunoblot Analysis and Immunoprecipitations
Cell lysates were separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes as previously described [2]. The membranes were incubated with primary antibodies for proteins of interest, including: cytochrome c (556433, BD Biosciences), Bax (06-499, Millipore), Bak (06-536, Millipore), Bcl-2 (M0887, Dako, Carpenteria, CA, USA), Bcl-xL (556361, BD Biosciences), Mcl-1 (sc-819, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bad (610392, BD Biosciences), phospho-Bad Ser112 (06-853, Millipore), phospho-Bad Ser136 (06-846, Millipore), Bim (559685, BD Biosciences), Bid (2002, Cell Signaling Technology, Danvers, MA, USA), Bik (sc-10770, Santa Cruz Biotechnology), Bmf (ab9655, Abcam, Cambridge, MA, USA), Puma (14–6041, eBioscience, San Diego, CA, USA), Noxa (OP180, EMD Biosciences, Gibbstown, NJ, USA), ASK1 (sc-7931, Santa Cruz Biotechnology), thioredoxin (sc-20146, Santa Cruz Biotechnology), JNK (9252, Cell Signaling Technology), phospho-JNK Thr183/Tyr185 (9251, Cell Signaling Technology), p38 (9212, Cell Signaling Technology), phospho-p38 Thr180/Tyr182 (9215, Cell Signaling Technology), ERK (9102, Cell Signaling Technology), phospho-ERK Thr202/Tyr204 (9101, Cell Signaling Technology). Blots were then incubated with horseradish peroxidase conjugated secondary antibodies (NA931 or NA934, GE Healthcare Bio-sciences, Piscataway, NJ, USA) and reacted with chemiluminescence reagents (GE Healthcare Biosciences). Immunoprecipations for ASK1 and thioredoxin were performed as previously described [4]. For JNK blots, lysates obtained from 293 cells treated with UV light were used as a positive control (9253, Cell Signaling Technology).
2.8. ASK1 In Vitro Kinase Assay
To detect ASK1 activity, inactive GST-MKK6 (0.1 mg, Millipore) was incubated (15 min, 25 °C) with immunoprecipitated ASK1 in kinase assay buffer (Millipore). Subsequently, GST-p38α (0.5 µg, Millipore) was added to this reaction and incubated (15 min, 25 °C) followed by detection of phospho-GST-p38 via immunoblot as described above.
2.9. Statistical analysis
Where indicated, statistical significance was assessed by a Student’s t test. P values are two-tailed, and all data are presented as mean ± one standard deviation, unless otherwise noted. Figures contain representative data of experiments performed in triplicate.
3. Results
3.1. Lymphocytes display increased sensitivity to Bz-423
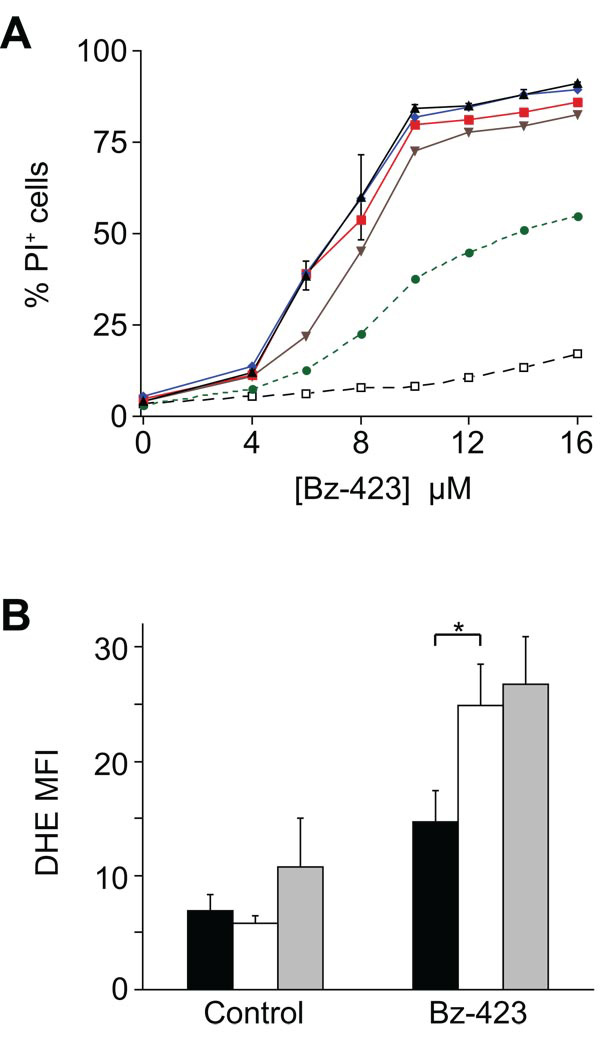
The cell death pathway activated by Bz-423 in fibroblasts in vitro requires >10 h exposure to [Bz-423] > 10 µM [4]. In contrast, apoptosis of Ramos cells begins in 3–4 h at lower [Bz-423] (Figure 1A). As previously reported, cell death in both cell types depends on Bz-423-induced superoxide [2, 4]. Indeed, consistent with the increased sensitivity of Ramos cells to this agent, Bz-423 induces a larger superoxide response in Ramos cells at lower [Bz-423] (Figure 1B). The apoptotic response is specific to superoxide as pre-treatment with a cell permeant form of catalase conjugated to polyethylene glycol (PEG-CAT, [9]) does not alter Bz-423-induced superoxide (Figure 1B) or inhibit Bz-423-induced cell death (Table 1). To help explain the difference in sensitivity between fibroblasts and lymphocytes, we measured the levels of glutathione, the major cellular antioxidant, in both cell types [10]. As can be seen in Table 2, Ramos cells have lower levels of reduced glutathione in both the mitochondrial (49% lower) and cytosolic (63% lower) compartments compared to fibroblasts, consistent with the increased sensitivity of this cell type.
Fig. 1. Bz-423 causes rapid death of Ramos B cells.
(A) Ramos cells were incubated with the indicated concentrations of Bz-423 and then Bz-423 was washed out of the media at various time points (1 h: black open squares, 2 h: green circles, 3 h: brown inverted triangles, 4 h: red squares, 5 h: blue diamonds, no wash: black triangles). Cell viability was determined by propidium iodide (PI) exclusion after 24 h of total culture. (B) Superoxide production measured by the oxidation of DHE (1 h) in response to Bz-423 and reported as median fluorescent intensity (MFI) values. Black bars = MEFs (no inhibitor, [Bz-423] =12 µM); White bars = Ramos cells (no inhibitor, [Bz-423] = 8 µM); Grey bars = Ramos cells pre-treated with PEG-CAT (200 U/mL, overnight) followed by Bz-423 (8 µM). Control MEF vs. Ramos: p >0.05; * = Bz-423 MEF vs. Ramos: p = 0.004; Control Ramos no inhibitor vs. PEG-CAT: p >0.05; Bz-423 Ramos no inhibitor vs. PEG-CAT: p >0.05.
Table 1.
Catalase does not inhibit Bz-423.
Cells were pre-treated with PEG-CAT (overnight, 200 U/mL).
Values reported are % PI positive cells (24 h).
P > 0.05 for no inhibitor vs. PEG-CAT
[Bz-423] = 8 µM.
Table 2.
Intracellular glutathione concentrations
mGSH = mitochondrial GSH
cGSH = cytosolic GSH
nmol/mg protein
P < 0.001 for Ramos vs. MEF
3.2. Effect on MAP Kinases
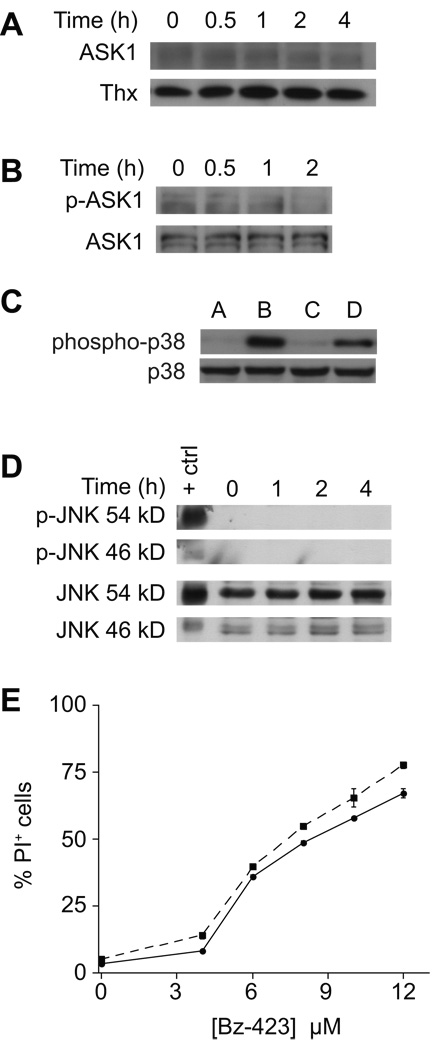
In MEFs, activation of ASK1 is the most proximal superoxide-dependent signal detected following treatment with Bz-423 [4]. Activated ASK1 triggers a MAP kinase cascade that results in phosphorylation and activation of JNK [4]. To determine if this response occurs in other cell types, ASK1•thioredoxin (Thx) complexes were immunoprecipitated from lysates of Ramos B cells treated with Bz-423. In contrast to what is observed in MEFs, treatment of Ramos cells with Bz-423 does not cause release of ASK1 from its complex with thioredoxin (Figure 2A), nor does it cause ASK1 phosphorylation (Figure 2B). To confirm that ASK1 is functional in these cells, immunoprecipitated ASK1 from cells treated with hydrogen peroxide was found to activate MKK6 detected by phosphorylation of p38 in an in vitro kinase assay (Figure 2C). Consistent with the lack of ASK1 activation, treatment with Bz-423 does not result in phosphorylation of JNK, and inhibition of JNK with SP600125 does not protect Ramos cells from Bz-423-induced apoptosis (Figure 2D–E). To determine if the oxidative stress induced by Bz-423 activates other MAP kinases, the effect of Bz-423 treatment on p38 and ERK phosphorylation was determined. Similar to the results with JNK, Bz-423 does not induce phosphorylation of these MAP kinases (data not shown). These results suggest that different mechanisms couple Bz-423-induced superoxide to apoptosis in Ramos B cells and fibroblasts.
Fig. 2. Bz-423 does not activate ASK1/JNK in Ramos cells.
(A) After treatment with Bz-423 (10 µM) for the indicated times, Thx was immunoprecipitated from cellular lysates, and the immune complexes blotted for ASK1 and Thx. (B) Lysates prepared from cells treated with Bz-423 (10 µM) were immunoprecipitated for ASK1 and then blotted to detect total ASK1 and phospho-ASK1. (C) ASK1 was immunoprecipitated from Ramos cell lysates treated with H2O2 (1 mM, 1 h) and reacted with MKK6 and p38 as described in the Methods section. Lane A: no lysate + inactive MKK6 + inactive p38; lane B: no lysate + active MKK6 + inactive p38; lane C: control lysate + inactive MKK6 + inactive p38; lane D: H2O2-treated lysate + inactive MKK6 + inactive p38. (D) Lysates prepared from Ramos cells treated with Bz-423 (10 µM) were immunoblotted to detect total and phosphorylated JNK. (E) Following pre-treatment with SP600125 (1 µM, dashed line) or vehicle (solid line), Ramos cells were incubated with Bz-423 and viability (24 h) was determined by PI exclusion.
3.3. Bz-423-induced apoptosis depends upon Bcl-2 proteins
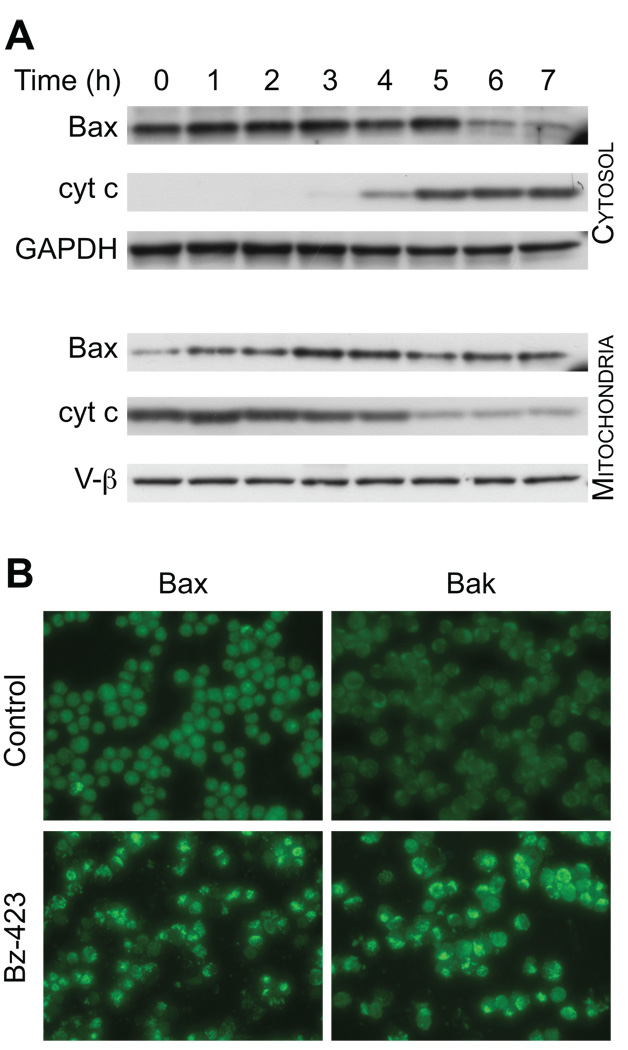
Since Bz-423 does not activate ASK1-JNK in Ramos cells, we determined if elements of the apoptotic mechanism downstream of JNK in fibroblasts are part of the Bz-423 response in these lymphoid cells. In fibroblasts, activation of Bax and/or Bak is critical for Bz-423 to induce apoptosis, as knockout of these proteins inhibits cell killing [4]. Treatment with Bz-423 increases the amount of Bax in the mitochondrial fraction and decreases its cytoplasmic concentration starting at 3 h (Figure 3A). These findings are consistent with Bz-423 causing Bax activation and translocation prior to the release of cytochrome c which is observed starting at 4 h (Figure 3A). To confirm that Bax undergoes activation and assess whether Bak is also activated in response to Bz-423, Ramos cells were incubated with antibodies to Bax and Bak that recognize a cryptic N-terminal epitope that is only accessible after activation. Cells treated with Bz-423 display a bright, punctate staining pattern with each antibody by immunofluorescence microscopy (Figure 3B). This pattern is not seen in control cells, and these results are consistent with activation and mitochondrial localization of these proteins in response to Bz-423. These results indicate that in both lymphoid cells and fibroblasts, apoptosis induced by Bz-423 involves some common elements of the cells apoptotic machinery – activation of Bax and Bak.
Fig. 3. Bz-423 activates Bax and Bak.
(A) Ramos cells were treated with Bz-423 (10 µM) for the indicated times followed by cell fractionation. Cytosolic and mitochondrial Bax and cytochrome c was detected by immunoblot. (B) Ramos cells were treated with vehicle or Bz-423 (10 µM, 12 h) followed by detection of activated N-terminal Bax or Bak by immunofluorescence microscopy.
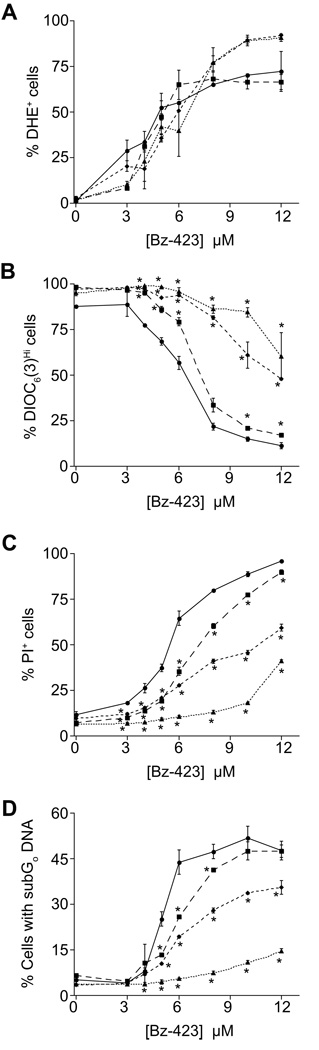
To determine if Bax and/or Bak is required for the Bz-423 death response, Ramos cells were transfected with siRNAs to reduce the expression of both proteins [11]. Transfection with siRNAs specific for Bax, Bak, or a combination of the two reduced expression by greater than 90% relative to control cells transfected with a siRNA control sequence that is not present in the human genome (data not shown). Knockdown of Bax, Bak, or both proteins does not inhibit Bz-423-induced superoxide formation, as detected by DHE (Figure 4A). This result is consistent with Bz-423 binding to the mitochondrial F1F0-ATPase to induce superoxide formation directly as a consequence of target binding [12]. In contrast, knockdown of each protein inhibits Bz-423-triggered ΔΨm collapse and cell death (Figure 4B–D). Interestingly, knockdown of Bak alone is more inhibitory than knockdown of Bax alone, while the combination almost completely inhibits Bz-423-induced apoptosis. These results indicate that activation of Bax and Bak is central to the Bz-423 death mechanism in Ramos cells.
Fig. 4. Knockdown of Bax and Bak inhibit Bz-423-induced apoptosis.
Ramos cells were transfected with siRNA for control (circles, solid line), Bax (squares, long dashes), Bak (diamonds, short dashes), or both Bax and Bak (triangles, dotted line) followed by treatment with Bz-423. (A) Superoxide production (1 h) was measured using DHE. (B) ΔΨm (6 h) was measured using DIOC6(3). * = P < 0.05 for Control vs. Bax or Bak or Bax/Bak; P < 0.05 also for Bax vs. Bak at [Bz-423] ≥ 5 µM. (C) Cell viability (24 h) was measured by PI exclusion. * = P < 0.05 for Control vs. Bax or Bak or Bax/Bak; P < 0.05 also for Bax vs. Bak at [Bz-423] ≥ 6 µM. (D) Apoptosis (24 h) was measured by identifying cells with subG0-DNA content. * = P < 0.05 for Control vs. Bax or Bak or Bax/Bak; P < 0.05 also for Bax vs. Bak at [Bz-423] ≥ 5 µM.
3.4. How does Bz-423 activate Bax and Bak in Ramos B cells?
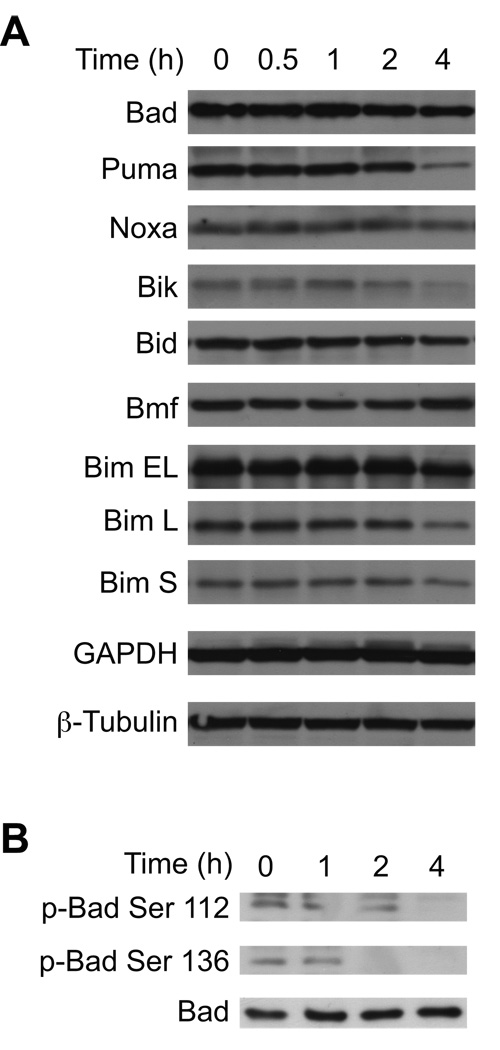
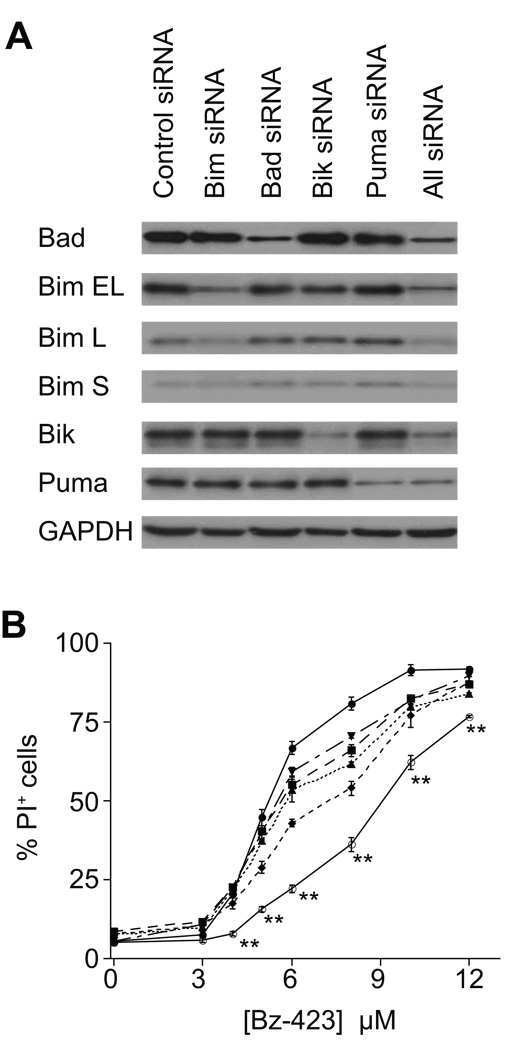
In MEFs Bax and Bak activation is dependent on JNK and protein synthesis, neither of which are required in Ramos cells (vide supra and [13]). Therefore, we examined the response of upstream Bcl-2 proteins to define the mechanism that couples Bz-423-induced superoxide to Bax/Bak activation in the lymphoid cells. Increased expression or post-translational modifications of BH3-only proteins activate Bax and Bak in response to a variety of cellular stresses [14, 15]. Cellular lysates were therefore screened with antibodies against Bad, Puma, Noxa, Bik, Bid, Bmf, and Bim. As can be seen in Figure 5A, Bz-423 does not increase the expression of any of these pro-apoptotic proteins, consistent with the inability of cycloheximide to prevent Bz-423-induced cell death [13]. In addition to increased expression, the BH3-only protein Bad can be regulated by post-translational phosphorylation of Ser-112 and Ser-136 whereby dephosphorylation triggers its activation and release from 14-3-3 proteins [14]. Treatment with Bz-423 induces dephosphorylation of Bad at both serine residues within 2 h (Figure 5B) indicating that in Ramos cells, Bz-423 is activating this BH3-only protein via post-translational mechanisms. To determine the functional significance of Bad and evaluate the roles of other selected BH3-only proteins in the Bz-423 response mechanism, Ramos cells were transfected with siRNAs specific for Puma, Bad, Bik, and Bim. The transfections decreased expression of the target proteins by 60–90% relative to control siRNA (Figure 6A and Table 3). Decreasing the expression of each of these BH3-only proteins generates partial resistance to Bz-423-induced cell death (Figure 6B). Knockdown of Bik provides the most protection followed by knockdown of Bim, Bad, and Puma. Knockdown of all four BH3 proteins together provides greater protection than any individual BH3-only protein. This result suggests that superoxide induced by Bz-423 is translated into an array of upstream BH3-only protein responses that lead to Bax and Bak activation.
Fig. 5. Changes in BH3-only proteins following treatment with Bz-423.
(A) Following treatment with Bz-423 (10 µM) for the indicated times, total cell lysates were analyzed by immunoblot for the indicated BH3-only proteins. (B) Following treatment with Bz-423 (10 µM) for the indicated times, total cell lysates were analyzed by immunoblot for phospho-Bad.
Fig. 6. Knockdown of BH3-only proteins inhibits Bz-423-induced cell death.
(A) Ramos cells were transfected with siRNA for the indicated BH3-only proteins followed by preparation of total cell lysates and determination of Bad, Bim, Bik, and Puma by immunoblot. All represents lysates from cells transfected with siRNA for Bad, Bim, Bik, and Puma. (B) Ramos cells were transfected with siRNA for control (circles, solid line), Bad (squares, long dashes), Bik (diamonds, short dashes), Bim (triangles, dotted line), Puma (inverted triangles, alternating long and short dashes), or All (open circles, solid line) followed by treatment with Bz-423. Cell viability (24 h) was determined by PI exclusion. ** = P < 0.001 for Control vs. All. In addition, P < 0.01 for Control vs. Bad at [Bz-423] ≥ 6 µM; P < 0.01 for Control vs. Bik at [Bz-423] ≥ 5 µM; P < 0.02 for Control vs. Bim at [Bz-423] ≥ 5 µM; P < 0.02 for Control vs. Puma at [Bz-423] ≥ 6 µM; P < 0.05 for All vs. Bad or Bik or Bim or Puma at all [Bz-423] ≥ 3 µM.
Table 3.
Quantitative % decrease in BH3-only protein expression
| Single siRNA | Combined siRNA | |
|---|---|---|
| Bad | 80 | 83 |
| Bim EL | 85 | 79 |
| Bim L | 64 | 70 |
| Bim S | 59 | 60 |
| Bik | 91 | 83 |
| Puma | 88 | 86 |
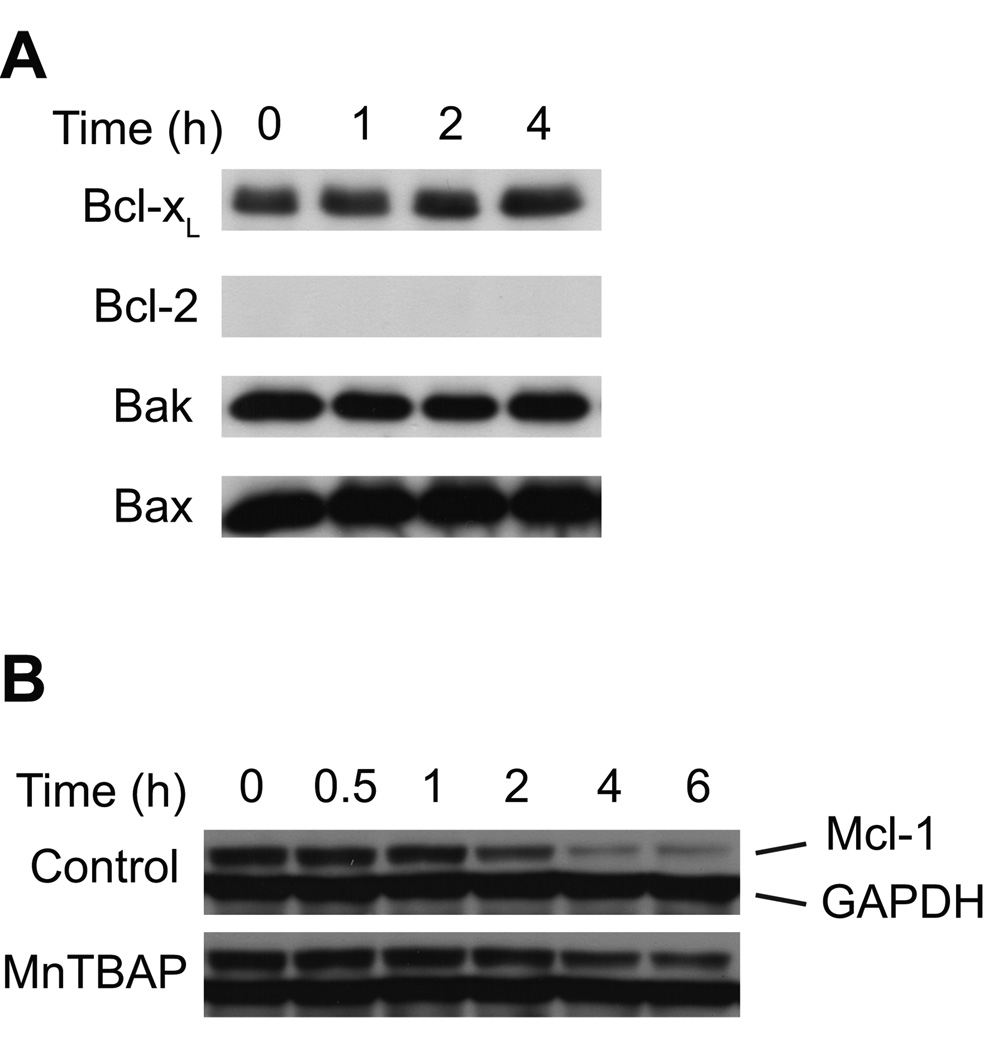
The balance between the levels of pro- and anti-apoptotic Bcl-2 proteins regulates activation of Bax and Bak [16]. Given the generalized BH3-only response of Ramos cells to Bz-423, we measured the levels of anti-apoptotic Bcl-2 proteins (e.g., Bcl-2, Bcl-xL, Mcl-1) because a decrease in their expression can lead to an imbalance with pro-apoptotic BH3-only proteins that favors cell death. In order to detect specific early responses to Bz-423-induced superoxide, we screened cellular lysates to detect changes in these proteins at time points preceding MOMP, as monitored by cytochrome c release and ΔΨm collapse. Bz-423 does not affect levels of Bax, Bak, Bcl-2 or Bcl-xL (Figure 7A) but it decreases the level of anti-apoptotic Mcl-1 starting at 2 h (Figure 7B). The decrease in Mcl-1 expression is prevented by pretreatment with MnTBAP. Thus, Mcl-1 is downstream of the superoxide response and temporally positioned to potentially account for Bax and Bak activation which occur at later times. These results suggest that in Ramos cells Bz-423-induced superoxide leads to Bax and Bak activation via changes in Mcl-1 expression and functional activation of BH3-only proteins.
Fig. 7. Levels of multi-domain Bcl-2 proteins.
(A) Ramos cells were treated with Bz-423 (10 µM) for the indicated times followed by detection of total cellular Bcl-xL, Bcl-2, Bax and Bak by immunoblot. (B) Ramos cells were pretreated with MnTBAP (100 µM, 0.5 h) or vehicle prior to treatment with Bz-423 (10 µM) for the indicated times followed by detection of total cellular Mcl-1 by immunoblot.
4. Discussion
While modulation of the F1F0-ATPase in lymphocytes and fibroblasts results in the formation of superoxide, this signal is propagated differently in each cell type, resulting in different cell death pathways. Lymphocytes are more sensitive to Bz-423 than fibroblasts in vitro and in vivo, suggesting that the different responses result from differences in factors that limit superoxide signaling, such as cellular antioxidant defenses which include antioxidant enzymes (e.g. superoxide dismutases, catalases, peroxidases) and low molecular weight antioxidants (e.g. glutathione) [17, 18]. Indeed, lymphocyte-rich splenic tissue has low levels of dismutase and peroxidase activities [19]. We have demonstrated that compared to fibroblasts, Ramos B cells have low levels of glutathione, which as the major cellular redox buffer is a representative indicator of the overall redox environment of the cell [18]. Decreased antioxidant stores is likely one critical factor contributing to increased sensitivity of B cells to Bz-423.
In all cell types studied, apoptosis induced by Bz-423 critically depends upon the activation of pro-apoptotic Bcl-2 proteins Bax and Bak. In fibroblasts, Bax and Bak can substitute for one another, while in Ramos cells, knockdown of Bak protects more than knockdown of Bax [4]. It is well accepted that activation of Bax and Bak depends upon on the balance between anti-apoptotic Bcl-2 proteins such as Bcl-2, Bcl-xL, and Mcl-1, and pro-apoptotic BH3-only proteins, however the specific steps that mediate this activation remain controversial [20]. Indeed, recent reports have demonstrated that not all anti-apoptotic Bcl-2 proteins are functionally equivalent [21]. For example, Bak preferentially binds to Mcl-1 and Bcl-xL, and forced overexpression of Bcl-2 is unable to prevent Bak activation [21]. To determine if changes in anti-apoptotic Bcl-2 protein expression activates Bax and Bak, we examined the levels of Bcl-2, Bcl-xL, and Mcl-1. In contrast to fibroblasts which express constant levels of all three proteins (unpublished observations), Ramos cells do not express Bcl-2, and they have stable levels of Bcl-xL. Interestingly, treating Ramos cells with Bz-423 decreases levels of Mcl-1. Although it is possible for Bcl-xL to be functionally inactivated by BH3-only proteins in the absence of changes in protein level [22], the decrease in Mcl-1 levels following Bz-423 treatment suggests that the anti-apoptotic threshold in these cells depends primarily upon Mcl-1. Given the preferential association between Mcl-1 and Bak, the early decrease in Mcl-1 induced by Bz-423 supports the prominent role for Bak in Bz-423-induced apoptosis.
The decrease in Mcl-1 is likely a proximal step in the signal transduction pathway activated by Bz-423, as it is first detected at 2 h, prior to Bax translocation (~3 h), cytochrome c release (4–5 h), caspase activation (3-4 h), and ΔΨm collapse (5–6 h) (vide supra and [2]). In addition, Mcl-1 levels are preserved when cells are pre-treated with MnTBAP, making the decrease dependent upon and downstream of superoxide formation. Mcl-1 is a labile protein subject to rapid degradation by multiple pathways. In response to some stimuli, Mcl-1 is specifically cleaved by caspase 3 after Asp-127 and Asp-157 to yield 27 and 19 kD fragments [23, 24]. It is unlikely that this pathway is active in Ramos cells following Bz-423 treatment, as Mcl-1 levels start to decrease before caspase activation or MOMP is observed and because we do not observe the formation of the Mcl-1 fragments by western blot (unpublished observation). Binding of BH3-only proteins to Mcl-1 can also trigger a decrease in this protein via proteasomal degradation, as has been demonstrated with overexpression of Noxa [21]. However, in our system, we do not detect increased levels of Noxa or other BH3-only proteins following treatment with Bz-423. We have previously demonstrated in Ramos B cells that Bz-423, via superoxide-dependent signaling, increases the efficiency of targeting short-lived proteins such as c-myc for proteasomal degradation [25]. In a manner similar to c-myc, basal levels of Mcl-1 are tightly controlled by the E3 ubiquitin ligase Mule, such that a superoxide-dependent increase in proteasomal degradation may contribute to the ability of Bz-423 to decrease Mcl-1 levels [26].
The steps intervening between Bz-423-induced superoxide, Mcl-1 degradation, and Bax/Bak activation are currently unclear. Activation of BH3-only proteins is one well-established mechanism by which Bax and Bak are activated following an apoptotic stimulus [20]. These proteins are regulated by changes in expression as well as post-translational modifications including phosphorylation and changes in cellular localization [14, 15]. As noted above, and consistent with the lack of protection from cycloheximide, Bz-423 does not induce increased levels of expression of the seven BH3-only proteins examined. Knockdown of several BH3-only proteins does however inhibit Bz-423-induced apoptosis, suggesting that post-translational regulation of these pro-apoptotic proteins may be more relevant. Indeed, we observe Bad dephosphorylation following Bz-423 treatment. Among its many targets, activated Akt suppresses apoptosis by phosphorylating Bad to inhibit its pro-apoptotic actions [27]. The treatment of U937 leukemia cells with 2-methoxyestradiol to induce superoxide formation can trigger apoptosis via inhibition of Akt [28]. In a parallel manner, Bz-423-induced superoxide may inhibit Akt to trigger Bad activation and Mcl-1 degradation.
The absence of Bz-423-mediated activation of ASK1 and MAP kinases in Ramos cells is surprising given their importance in stress responses and the role they play in fibroblast apoptosis induced by this compound. Since ASK1 is functional in Ramos cells (e.g., see Figure 2C), activation of this protein must be regulated differently in these cells. Indeed, MAP kinase activation is tightly regulated across cell types and stages of development and involves the balance between pro- and anti-survival signals [6]. MAPK activation is affected by the basal activity of dual specificity of phosphatases such as MKP-1, and the presence of scaffolding proteins that are necessary to coordinate phosphorylation cascades [6, 29, 30]. There is also crosstalk between the Bcl-2 proteins and MAP kinases as high levels of anti-apoptotic Bcl-2 proteins inhibit MAP kinase activation [31]. The higher levels of Bcl-xL expressed in Ramos cells compared to fibroblasts (unpublished observations) may limit the ability of Bz-423 to activate JNK in lymphocytes.
In summary, we found that the signal transduction pathways that link Bz-423-induced superoxide to apoptosis in lymphocytes and fibroblasts are distinct. Although both cell types couple superoxide to the activation of Bax and Bak, the upstream apoptotic response to this signal is propagated differently in B cells and fibroblasts. In particular, Mcl-1 expression is down-regulated by Bz-423 in B cells. Low levels of intracellular antioxidants and the potential for superoxide to induce Mcl-1 degradation have been identified as characteristics to explain differential sensitivity to Bz-423 between B cells and fibroblasts. Such differences are likely key elements of the mechanism that underlies the selectivity observed in vivo, favoring apoptotic effects on disease-causing lymphocytes.
Acknowledgments
This work was supported by grants from the NIH (R01-AI 47450 to G.D.G, R01-CA 10456 to A.W.O.). N.B.B. is supported by a training grant from the NIH (T32 DK065517).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Neal B. Blatt, Email: nblatt@umich.edu.
Anthony E. Boitano, Email: aboitano@umich.edu.
Costas A. Lyssiotis, Email: clyssiot@umich.edu.
Anthony W. Opipari, Jr., Email: aopipari@umich.edu.
Gary D. Glick, Email: gglick@umich.edu.
References
- 1.Bednarski JJ, Warner RE, Rao T, Leonetti F, Yung R, Richardson BC, et al. Attenuation of autoimmune disease in Fas-deficient mice by treatment with a cytotoxic benzodiazepine. Arthritis Rheum. 2003;48:757–766. doi: 10.1002/art.10968. [DOI] [PubMed] [Google Scholar]
- 2.Blatt NB, Bednarski JJ, Warner RE, Leonetti F, Johnson KM, Boitano A, et al. Benzodiazepine-induced superoxide signals B cell apoptosis: mechanistic insight and potential therapeutic utility. J Clin Invest. 2002;110:1123–1132. doi: 10.1172/JCI16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW, Glick GD. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem Biol. 2005;12:485–496. doi: 10.1016/j.chembiol.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Blatt NB, Boitano AE, Lyssiotis CA, Opipari AW, Glick GD. Bz-423 superoxide signals apoptosis via selective activation of JNK, Bak, and Bax. Free Radic Biol Med. 2008;45:1232–1242. doi: 10.1016/j.freeradbiomed.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Bunin BA, Plunkett MJ, Ellman JA. The combinatorial synthesis and chemical and biological evaluation of a 1,4-benzodiazepine library. Proc Natl Acad Sci USA. 1994;91:4708–4712. doi: 10.1073/pnas.91.11.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3169. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JS, Minor RL, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- 10.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 11.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 12.Johnson KM, Cleary J, Fierke CA, Opipari AW, Glick GD. Mechanistic basis for therapeutic targeting of the mitochondrial F1F0-ATPase. ACS Chem Biol. 2006;1:304–308. doi: 10.1021/cb600143j. [DOI] [PubMed] [Google Scholar]
- 13.Bednarski JJ, Lyssiotis CA, Roush R, Boitano A, Glick GD, Opipari AW. A novel benzodiazepine increases the sensitivity of B cells to receptor stimulation with synergistic effects on calcium signaling and apoptosis. J Biol Chem. 2004;279:29615–29621. doi: 10.1074/jbc.M403507200. [DOI] [PubMed] [Google Scholar]
- 14.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcription and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 15.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2006;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–1402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 17.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 18.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363:91–97. doi: 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher JI, Huang DCS. Controlling the cell death mediators Bax and Bak. Puzzles and conundrums. Cell Cycle. 2008;7:39–44. doi: 10.4161/cc.7.1.5178. [DOI] [PubMed] [Google Scholar]
- 21.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng C, Li Y, Xu D, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491–10500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- 24.Lin K-R, Lee S-F, Hung C-M, Li C-L, Yang-Yen H-F, Yen JJY. Survival factor withdrawal-induced apoptosis of TF-1 cells involves a TRB2-Mcl-1 axis-dependent pathway. J Biol Chem. 2007;282:21962–21972. doi: 10.1074/jbc.M701663200. [DOI] [PubMed] [Google Scholar]
- 25.Sundberg TB, Ney GM, Subramanian C, Opipari AW, Glick GD. The immunomodulatory benzodiazepine Bz-423 inhibits B-cell proliferation by targeting c-myc protein for rapid and specific degradation. Cancer Res. 2006;66:1775–1782. doi: 10.1158/0008-5472.CAN-05-3476. [DOI] [PubMed] [Google Scholar]
- 26.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Gao N, Rahmani M, Dent P, Grant S. 2-Methoxyestradiol-induced apoptosis in human leukemia cells proceeds through a reactive oxygen and Akt-dependent process. Oncogene. 2005;24:3797–3809. doi: 10.1038/sj.onc.1208530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuura H, Nishitoh H, Takeda K, Matsuzawa A, Amagasa T, Ito M, et al. Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the ASK1-JNK signaling pathway. J Biol Chem. 2002;277:40703–40709. doi: 10.1074/jbc.M202004200. [DOI] [PubMed] [Google Scholar]
- 30.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 31.Jeong H-S, Choi H-Y, Choi T-W, Kim B-W, Kim J-H, Lee E-R, et al. Differential regulation of the antiapoptotic action of B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma extra long (Bcl-xL) by c-Jun N-Terminal protein kinase (JNK) 1-involved pathway in neuroglioma cells. Biol Pharm Bull. 2008;31:1686–1690. doi: 10.1248/bpb.31.1686. [DOI] [PubMed] [Google Scholar]