Abstract
Objective
Determine the effect of 15-lipoxygenase-1 (15-LO-1) on cholesterol mobilization from macrophages.
Methods and Results
Overexpression of human 15-LO-1 in RAW mouse macrophages led to enhanced cholesterol efflux, increased cholesteryl ester (CE) hydrolysis, and increased reverse cholesterol transport (RCT). Efflux studies comparing 15-LO-1 overexpressing cells to mock-transfected RAW macrophages resulted in a 3 to 7-fold increase in cholesterol efflux to apolipoprotein A-I and a modest increase in efflux to HDL. Additional experiments revealed an increase in mRNA and protein levels of ABCA1 and ABCG1 in the RAW expressing 15-LO-1 compared to controls. Efforts to examine whether the arachidonic acid metabolite of 15-LO-1, (15S)-hydroxyeicosatetraenoic acid (HETE), was responsible for the enhanced efflux revealed this eicosanoid metabolite did not play a role. Enhanced steryl ester hydrolysis was observed in 15-LO-1 overexpressing cells suggesting that the CE produced in the 15-LO-1 expressing cells was readily mobilized. To measure RCT, RAW macrophages overexpressing 15-LO-1 or mock-transfected cells were cholesterol enriched by exposure to acetylated low-density lipoprotein and [3H]-cholesterol. These macrophages were injected into wild type animals and RCT was measured as a percent of injected dose of 3H appearing in the feces at 48 h. We found 7% of the injected 3H in the feces of mice that received macrophages overexpressing 15-LO-1 and 4% in the feces of mice that received mock-transfected cells.
Conclusions
These data are consistent with a model in which overexpression of human 15-LO-1 in RAW macrophages promotes RCT through increased CE hydrolysis and ABCA1-mediated cholesterol efflux.
INTRODUCTION
Lipoxygenases are classified based on the position of polyunsaturated fatty acid oxygenation. The major product of human 15-LO-1 is (15S)-HETE, which is produced from the oxidation of arachidonic acid at C-15. 15-LO-1 also produces lesser amounts of (12S)-HETE from arachidonic acid. In contrast, the major metabolite from leukocyte-type 12/15-LO (found in mouse, pig and rabbit) is (12S)-HETE. 15-LO-1 also has activity against the fatty acyl component of cholesteryl esters (CE) and phospholipids 1.
The role of 15-LO-1 in atherosclerosis has been controversial. Fibroblasts and J774 macrophages, overexpressing 15-LO-1 and 12/15-LO respectively, are capable of oxidizing LDL, a process leading to foam cell formation, as well as enhanced monocyte chemoattractant activity, which strongly suggests a pro-atherogenic role 2,3. Published studies show mice deficient in 12/15-LO are protected from atherosclerosis in apolipoprotein E (apoE) deficient mice4,5. However, transgenic rabbits overexpressing 15-LO-1 exhibited a 45% attenuation in aortic lesion area, clearly demonstrating an anti-atherogenic role 6. Furthermore Merched et al 7 demonstrated an atheroprotective role for 12/15-LO through the local production of potent anti-inflammatory lipid mediators (lipoxin A4, resolvin D1 and protectin D1) in apoE deficient mice. Recent epidemiological studies provide strong evidence for an anti-atherogenic role of 15-LO-1. Wittwer et al 8 provides evidence of a polymorphism that occurs in 15-LO-1 resulting in higher 15-LO-1 activity in humans and is atheroprotective. Assimes et al 9 reports on a variant of 12/15-LO occurring in humans that results in decreased expression of 12/15-LO but does not offer atheroprotection. Elucidating the mechanisms underlying these conflicting data will make it possible to more rigorously assess the role of 15-LO-1 to atherosclerotic disease progression in humans.
Here we focus on the effects of one member of the LO family, human 15-LO-1 and its role in macrophage foam cells. 15-LO-1 is present in human macrophage cells 10 and is associated with neutral lipid bodies in various cell types 11,12. The absence of the reticulocyte-type 15-LO-1 in mice provides a clean model to specifically study the effects of human 15-LO-1. Therefore we chose this model to further define the role of macrophage 15-LO-1 in atherosclerosis.
METHODS
A detailed methods section can be found with the Data Supplement.
Cell Culture
RAW 267.4 (American Type Tissue Culture Collection, Manassas, VA ) murine macrophages stably transfected to overexpress human 15-LO-1 (RAW-15-LO) 4 and mock-transfected (RAW-mock) cells were routinely grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% FBS, 50 μg/ml gentamicin and 500 μg/ml geneticin.
Cholesterol Efflux
After [3H]cholesterol labeling the cells, media containing HDL (20μg/ml) or apoA-I (25μg/ml) was added for up to 4 h. In some experiments the ACAT inhibitor CP113818 (2 μg/ml) was added to the media to prevent cholesterol esterification. To determine cholesterol efflux, media were sampled at indicated times, filtered and counted by liquid scintillation counting to determine [3H] released. [3H]-Sterols in the media were compared to total [3H] at time zero to determine the percent release of [3H]cholesterol.
RESULTS
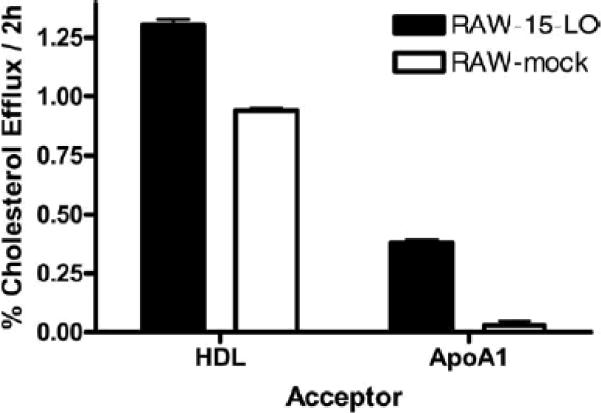
RAW murine macrophages stably transfected with human 15-LO-1 were used in these studies 13. Our initial observations indicated that cholesterol efflux to serum was enhanced when 15-LO-1 was overexpressed (data not shown). To further study this observation, macrophages were radiolabeled for 24 h in the presence of the ACAT inhibitor CP113818, to prevent CE from forming. Cells were exposed to media containing apoA-I or HDL. Efflux to HDL and apoA-I was higher in the cells expressing 15-LO-1 (Figure 1), indicating that expression of 15-LO-1 increases cholesterol efflux.
Figure 1. Cholesterol Efflux to HDL and apoA-I is Increased with Expression of 15-LO.
Macrophages were radiolabeled with [3H]-FC. Efflux to 20μg/ml apo-AI or 25μg/ml HDL was determined. Aliquots of media was removed from all wells, filtered and counted by liquid scintillation counting to determine [3H]-cholesterol released in the media. [3H]-Cholesterol counts in the media were then compared to the counts in the cells before the efflux period to determine percent release.
Since there was a dramatic increase in efflux to apoA-I (100-fold increase), we chose to use apoA-I as the extracellular acceptor in subsequent experiments. Additionally, because one of our goals was to determine the impact of 15-LO-1 expression on cellular cholesterol mobilization, we used macrophages enriched in CE in some of the experiments.
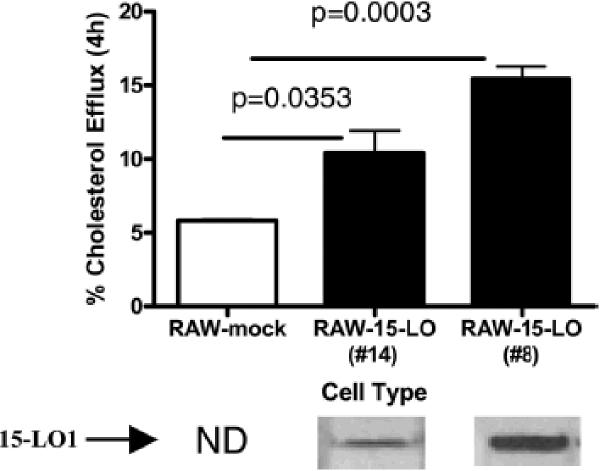
Cholesterol efflux to apoA-I was evaluated in two clones (Figure 2) and in mock-transfected cells. Figure 2 shows that 15-LO-1 expression in RAW macrophages resulted in a 2 to 3-fold increase in fractional cholesterol efflux over 4h. The increase in cholesterol efflux was related to the level of 15-LO-1 protein expression (measured by immunoblot), although not linearly (Figure 2). 15-LO-1 is not present in murine macrophages and, was not detected in the mock-transfected control (Figure 2). Over-expression of human 15-LO-1 did not affect levels of endogenous murine 5-LO levels (data not shown).
Figure 2. Effect of the Level of 15-LO Expression on Cholesterol Efflux to Apo-AI.
Cholesterol efflux was measured in two RAW clones (#8 and #14) expressing 15-LO and mock-transfected controls. Macrophages were FC-enriched and radiolabeled by incubation with [3H]-cholesterol and CP113818. Efflux to 20 μg/ml apo-AI was determined as described in Figure 1. Total cell lysates were analyzed by immunoblotting for 15-LO-1 protein.
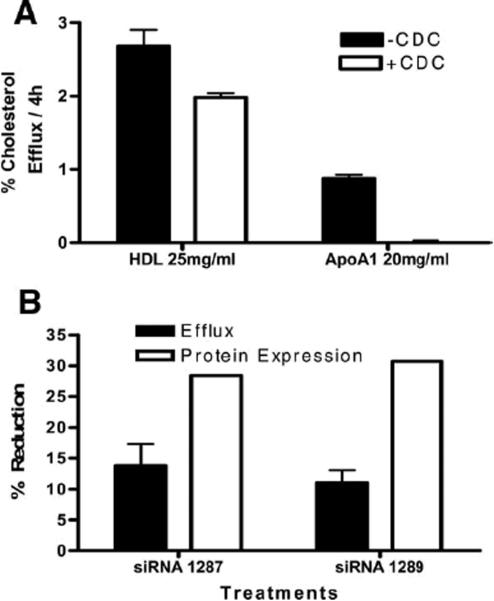
To confirm that the increase in cholesterol efflux was due to 15-LO-1 expression, we used the 15-LO-1 inhibitor, cinnamyl-3,4-dihydroxy-a-cyanocinnamate (CDC)14. When CDC was added to RAW-15-LO-1 cells, cholesterol efflux to HDL was reduced and efflux to apoA-I was eliminated (Figure 3A). CDC did not affect efflux in the mock-transfected cells (data not shown). We extended these studies by using small interfering RNA (siRNA) to knockdown expression of 15-LO-1 and assess the effect on cholesterol efflux. Figure 3B indicates that a 28–30% reduction in 15-LO-1 expression was achieved using 2 unique siRNA. This reduction in 15-LO-1 protein resulted in an 11–13% reduction in cholesterol efflux (Figure 3B). A scrambled siRNA (negative control) had no effect on 15-LO-1 expression or cholesterol efflux.
Figure 3. Inhibition of 15-LO reduces cholesterol efflux to apoA-I.
(3A) Macrophages were radiolabeled with [3H]-FC. After an 18h equilibration period in 0.2% BSA +/− CDC (5μg/ml), cells were exposed to media containing 25 μg/ml HDL or 20μg/ml apo-AI for 4h. (3B) 15-LO-1 siRNA or their scrambled control were transfected into 15-LO-1 or mock-transfected RAW cells as described in Methods. Protein levels of 15-LO-1 were determined by immunoblot. Percent efflux was measured as described in Figure 1. Data is expressed as percent reduction compared to an untreated control.
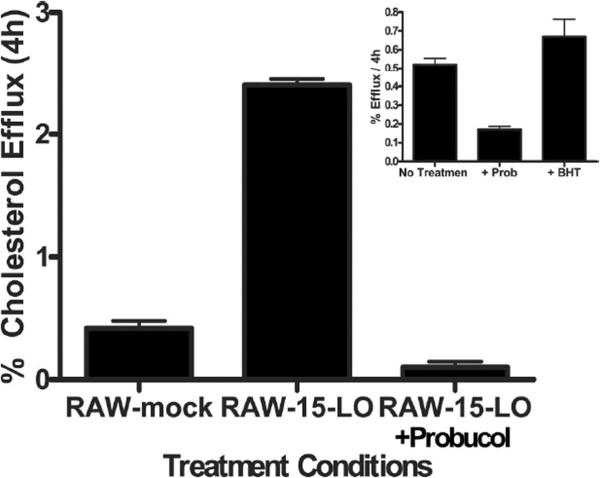
Because of the increase in efflux to apoA-I observed when 15-LO-1 was expressed, we investigated efflux via ABCA1, which is a major route of cholesterol efflux from macrophages 15. Probucol, an inhibitor of ABCA1, attenuated cholesterol efflux from RAW-15-LO-1 cells (Figure 4). Efflux experiments conducted in the presence of the antioxidant butylated hydroxytoluene (BHT) 16 demonstrated that the reduction in efflux seen with Probucol was not due to the antioxidant properties of Probucol (Figure 4 inset).
Figure 4. Probucol Effect on Cholesterol Efflux to apoA-I.
Macrophages were radiolabeled with [3H]-FC. After an equilibration in 0.2% BSA, cells were treated with either BSA alone or 20mM Probucol for 2 hours. Efflux to 20μg/ml apoA-I was measured after 4 hours. Percent efflux was determined as described in Figure 1. Inset RAW cells were treated as described above but 0.5mM BHT was substituted for Probucol.
Over-expression of 15-LO-1 may result in greater ABCA1 activity. To address this, we measured cholesterol transporter message and protein levels in the RAW-15-LO-1 and RAW-mock cells. Protein and mRNA levels of ABCA1 and ABCG1 were significantly increased (10-fold and 9-fold respectively) in RAW-15-LO-1 cells (Figure 1 in data supplement). The RNA and protein data were in agreement with the efflux data (Figure 4). We do not have a valid method for assessing ABCG1 mediated efflux in the RAW cells, but based on the data presented in Figure 5, we hypothesize that ABCG1 might also be contributing to the increase in cholesterol efflux and is responsible for the increase in efflux to HDL (Figure 1).
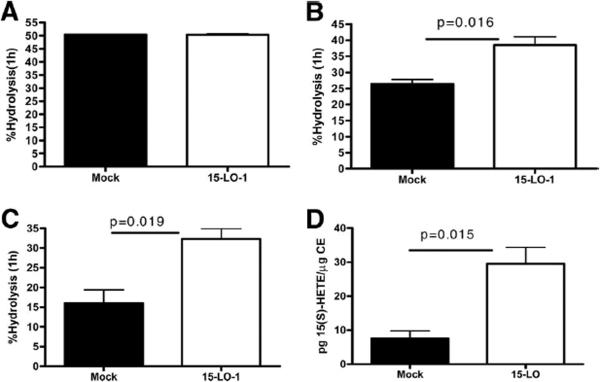
Figure 5. Steryl Ester isolated from 15-LO expressing RAW are a better substrate for neutral cholesteryl ester hydrolase.
Panel A. Commercially available [3H]-cholesteryl oleate (3μCi/ml) was added to a cell homogenate (as a source of nCEH) prepared from 15-LO-1 or mock-transfected control cells and incubated for 1h. Hydrolysis was measured as described in Methods. Panels B and C. RAW macrophages expressing human 15-LO or mock-transfected controls were cholesterol enriched and labeled as described in Methods. Cellular lipids were extracted using isopropanol. The lipid isolates were then added back, at constant CE mass, to a commercially available bovine pancreatic nCEH (Fig. 5B) or a RAW cell homogenate (as a source of nCEH, Fig 5C). Hydrolysis was allowed to occur for 1h. Percent hydrolysis was determined as described in Methods. Panel D. CE was isolated from 15-LO-1 expressing or mock-transfected cells by thin layer chromatography. (15S)-HETE in the isolated CE lipid fraction was measured using an EIA assay as described in Methods.
To address the possibility that a metabolite of 15-LO-1 was responsible for the observed changes in efflux and transporter expression, we incubated (15S)- or (12S)-HETE with mock-transfected RAW cells. Control experiments were conducted to ensure the eicosanoids were incorporated into the cells (Figure 2C in data supplement). (15S)-HETE, and to a lesser extent (12S)-HETE are products of 15-LO-1 actions on arachidonic acid. Incubation of (15S)-HETE with parent or mock-transfected RAW cells did not alter cholesterol efflux to apoA-I (Figures 2A and 2B in data supplement) or cholesterol transporter expression (Figure 3 in data supplement). Similar results were obtained when (12S)-HETE was used (Figures 2A and 2B of the data supplement). These results indicate that (15S)- or (12S)-HETE was not responsible for the cellular changes seen in the RAW-15-LO-1 macrophages.
CE hydrolysis is another aspect of cholesterol metabolism that was influenced by the over-expression of 15-LO-1. Hydrolysis was significantly increased in macrophages expressing 15-LO-1 (49±0.5%/24h in mock transfected cells; 75±0.1%/24h in 15-LO-1 cells). To determine if the observed increase in CE hydrolysis was due to an increase nCEH activity, homogenates of RAW-15-LO-1 and RAW-Mock cells were added to a commercially available [3H]-CE. Hydrolysis was calculated by comparing total cpm in CE before and after the hydrolysis period. There was not a significant difference in the amount of CE hydrolysis between the two cell types, indicating that over-expression of 15-LO-1 did not increase nCEH activity (Figure 5A). We next investigated the possibility that steryl esters produced in the RAW-15-LO-1 cells are better substrates for nCEH than that present in RAW-mock cells. To test this possibility the cellular lipids from the two cell lines were extracted and incubated with a commercially available nCEH (Figure 5B) or a RAW mock-transfected cell homogenate (Figure 5C) or 15-LO-1 cell homogenate (data not shown) as source of nCEH. The lipid extract was added back to the nCEH at constant CE mass. We measured an increase in CE hydrolysis when the lipid extracted from the RAW-15-LO-1 cells was used as substrate compared to the lipid from the RAW-mock cells (Figures 5B and 5C), no matter what the source of nCEH. Thus, the steryl esters produced in 15-LO-1 expressing cells are a more suitable substrate for nCEH and therefore are more rapidly hydrolyzed than the steryl esters isolated from mock-transfected RAW cells. We next measured the amount of the oxidized fatty acid, (15S)-HETE in the CE lipid fraction isolated from mock-transfected or 15-LO-1 expressing cells. Figure 5D indicates a 3-fold increase in the amount of (15S)-HETE in the CE produced in 15-LO-1 expressing cells compared to the CE produced in mock-transfected cells.
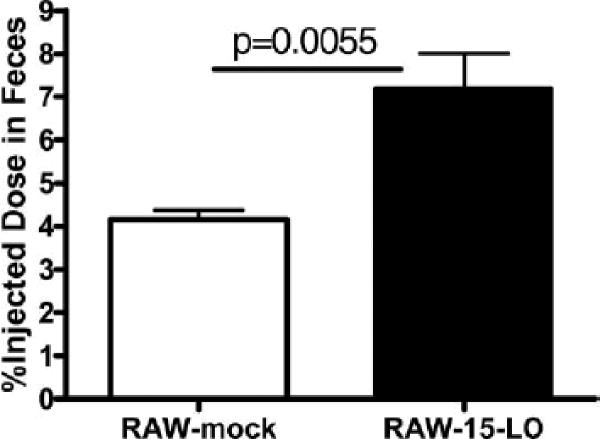
Since our data demonstrated increased cholesterol mobilization in macrophages is due to over-expression of human 15-LO-1, we next evaluated the effect over-expression of 15-LO-1 has on RCT in vivo. The model of RCT used in these studies is discussed in detail elsewhere 17. Briefly, donor macrophages are cholesterol-enriched and labeled ex vivo and then injected into the peritoneum of host animals. [3H]Cholesterol moves from the macrophages in the peritoneum (periphery) through the plasma compartment to the liver where the [3H]cholesterol is eliminated in the feces. In the present studies, cholesterol enriched RAW macrophages overexpressing 15-LO-1 or mock-transfected cells were used as the donor macrophages. The loading efficiency was similar in both cell types (83.77±1.79μg total cholesterol/mg protein (73.7% FC, 26.3% CE) in the 15-LO-1 cells and 80.01±0.60μg total cholesterol/mg protein (69.1% FC, 30.9% CE) in the mock-transfected cells). The cells were injected ip into wild type mice and plasma (retro-orbital bleed) and feces (cumulative) were collected over 48 h. After this period liver and bile samples were collected. All samples were analyzed for 3H content as described in Methods. There were no significant differences in plasma, liver and bile 3H content (data not shown) for the experimental period. However, there were significantly more fecal 3H counts in the animals injected with RAW-15-LO-1 macrophages compared to the animals receiving RAW-mock-controls (Figure 6). Since the movement of radiolabeled sterol from the injected macrophages to the feces reflects the efficiency of RCT, the increased 3H in the feces demonstrates that 15-LO-1 expression in macrophages increases RCT.
Figure 6. Expression of 15-LO in macrophages results in greater RCT.
RAW macrophages overexpressing 15-LO or mock-transfected cells were cholesterol enriched with 50μg/ml acLDL and 5μCi/ml [3H]-cholesterol in DMEM + 1% FBS for 48 hours and then injection into wild type mice as described in Methods. Feces was collected over 48h and analyzed for 3H content as described in Methods.
DISCUSSION
The initiation and progression of atherosclerosis is a complex process involving multiple pathways and risk factors. Many studies have investigated cellular and serum pro- or anti-atherogenic factors and often find that the line between a certain factor promoting or reversing atherosclerosis is blurred. Such is the case with 15-LO-1. Some of the conflicting results in the literature might be due to species differences, isoenzyme positional specificity for oxidation or expression pattern 1,7. (15S)-HETE is the major product of human reticulocyte 15-LO-1. It arises from the oxidation of arachidonic acid at C-15 to yield (15S)-hydroperoxyeicosatetraenoic acid (HPETE), which then undergoes a 2-electron reduction to (15S)-HETE. 15-LO-1 also produces smaller amounts of (12S)-HETE through the intermediate formation of (12S)-HPETE 18. Products of LO have been shown to be both pro- and anti- atherogenic 1. This study focuses on one particular component of the complex disease of atherosclerosis, the macrophage.
Foam cells of both macrophage and smooth muscle origins are responsible for a large portion of the lipid volume in atherosclerotic plaques 19. Mobilization of foam cell lipid is key to plaque regression. 15-LO-1 has been localized to macrophages in early and advanced lesions 20,21, although this too is subject to debate 22. The current studies focus on the effect of human reticulocyte-type 15-LO-1 has on cholesterol mobilization from macrophages, specifically; human 15-LO-1 was overexpressed in murine RAW macrophages 13 and were used for these studies.
Cholesterol Efflux
Our investigations revealed that cholesterol efflux to HDL and apoA-I was enhanced with the expression of human 15-LO-1. The idea that 15-LO-1 expression is responsible for increased cholesterol efflux is supported by our data demonstrating that the increase can be inhibited with CDC, a 15-LO-1 inhibitor (Figure 3), and cholesterol efflux to apoA-I is increased with expression of 15-LO-1 (Figure 2). We determined that metabolites resulting from 15-LO-1 action on arachidonic acid, (15S)-HETE and (12S)-HETE are not responsible for the increase in efflux. Recently, Nagelin et al. reported that (12S)-HETE but not (15S)-HETE reduced cholesterol efflux from J774 macrophages stably transfected with porcine 12/15-LO to HDL. The decrease in efflux is presumably due to increased degradation of ABCG1 expression 23. Interestingly, this group also found that ABCG1 expression levels returned to control levels after 24 h, suggesting that the decrease in ABCG1 expression is only temporary. The conflicting data in these reports may be due to time of incubation, cell type used for transfection, or LO species differences (porcine versus human).
Cholesterol Transporter Expression
Polyunsaturated fatty acids (PUFAs) have been shown to decrease expression of ABCA1 and ABCG1 in macrophages through destabilization of the ABCA1 protein 24,25 and inhibition of ABCA1 and ABCG1 transcription 26. In the current studies, where 15-LO-1 is overexpressed, ABCA1 and ABCG1 expression is increased in the presence or absence of exogenous PUFAs. It is possible that overexpression of 15-LO-1 results in a rapid metabolism of PUFAs, thereby eliminating any effect the PUFAs may have on cholesterol transporter expression.
Expression of 12/15-LO and its linoleic acid metabolite 13-S-HODE have been shown to activate peroxisome proliferator-activated receptors (PPARs) 27. Activation of PPARγ and PPARα affects cholesterol efflux through upregulation of cholesterol transporters ABCG1 and ABCA1 28. We report an increase in ABCG1 as well as ABCA1 protein and mRNA levels in RAW macrophages overexpressing 15-LO-1 under the experimental conditions presented in these studies. However, we did not detect 13-S-HODE in the 15-LO-1 RAW cells (data not shown) and the eicosanoid products of 15-LO-1, (12S)-HETE or (15S)-HETE, did not affect cholesterol efflux or transporter expression in our experiments (Figures 2 and 3 of the data supplement).
If oxidized metabolites of arachidonic acid were not responsible for the increase in efflux and transporter expression the question of what is causing the increase remains. It is known that LOs can oxidize the fatty acyl component of CE contained in LDL 29. Additionally, Lund et al. 30 reported that soybean LO oxidizes the B-ring of cholesterol at C-7. The hypothesis that an oxidized sterol may be responsible for the increase in cholesterol transporter expression is reasonable considering oxysterols can modulate ABCA1, ABCG1 and nCEH expression 31–33. Efforts to determine if the human 15-LO-1 has similar activity on the B-ring of cholesterol are currently underway.
ABCA1-mediated Efflux
Cholesterol efflux via ABCA1 is inhibited by Probucol34. We found that Probucol abolished the increase in cholesterol efflux to apoA-I in the 15-LO-1 expressing cells. To rule out the possibility that Probucol's antioxidant properties might also play a role in efflux reduction, we tested another antioxidant, BHT, and found no reduction to efflux to apoA-I (Figure 4 inset).
Steryl Ester Hydrolysis
We found that CE hydrolysis was substantially increased when 15-LO-1 was overexpressed. The increase in hydrolysis could be due to an increase in nCEH activity, the production of a more suitable nCEH substrate, or the formation of eicosanoid metabolites that increased CE hydrolase activity 35. Our data indicate that the increase in CE hydrolysis seen with overexpression of human 15-LO-1 is not due to an increase in nCEH activity in RAW macrophage cells (Figure 5A). Additionally, we have found that the steryl esters extracted from 15-LO-1 overexpressing foam cells are a better substrate for nCEH compared to the steryl esters extracted from mock-transfected cells. Our data is consistent with expression of 15-LO-1 leading to the oxidation of cytoplasmic CE, an idea suggested by Belkner et al. 36. (15S)-HETE was shown to be readily incorporated as the fatty acyl chain of CE 37. Our studies indicated that there is an increase in the amount of (15S)-HETE in the CE fraction isolated from 15-LO-1 expressing cells compared to CE isolated from mock-transfected cells. This data coupled with previous studies demonstrating that oxidized CE are preferentially hydrolyzed compared to non-oxidized CE 38, suggests that (15S)-HETE is incorporated into CE creating a better substrate for nCEH.
Reverse Cholesterol Transport
Expression of human 15-LO-1 in macrophages results in an increased rate of cellular CE hydrolysis and greater cholesterol efflux, which favors cholesterol mobilization. We therefore evaluated the effect expression of human 15-LO-1 in macrophages has on RCT. The model of RCT we used in this study has been validated and discussed in detail elsewhere 17. There was no difference in the two groups with regard to [3H]sterol in the blood, bile or liver. However, when human 15-LO-1 is expressed in the donor macrophages, fecal excretion is significantly higher when compared to that of mock-transfected donor macrophages. Our studies are in agreement with Merched et al who developed a macrophage-specific 12/15-LO overexpressing mouse on an apoE deficient background7. They found a significant decrease in aortic atherosclerotic lesion area with macrophage specific 12/15-LO overexpression along with a concurrent increase in lipoxin A4 production. Lipoxins are potent anti-inflammatory mediators that trigger the resolution phase of atherosclerosis. Such lipid mediators may be playing a role in the RCT system and efflux studies described in the present work.
Summary
Considering the complexity of atherosclerosis, and that the expression pattern of 15-LO-1 is not limited to peripheral foam cells, our findings do not preclude pro-atherogenic properties of 15-LO-1 such as oxidation of LDL. However, we propose that expression of human 15-LO-1 in macrophage cells results in increased cellular cholesterol mobilization through increased CE hydrolysis, ABCA1 mediated efflux and ultimately increased RCT. These observations suggest that 15-LO-1 expression in peripheral foam cells is anti-atherogenic.
Supplementary Material
AKNOWLEDGEMENTS
The authors would like to acknowledge Christine Hinkle of the University of Pennsylvania for technical assistance. We thank Dr. Colin D. Funk (Queen's University, Kingston, ON) for the pcDNA3 plasmid containing the human 15-LO-1 gene.
SOURCES OF FUNDING This project was supported by NIH PPG grant HL-22633, and NIH grants RO1CA091016, and P30ES013508.
Footnotes
DISCLOSURES None
References
- 1.Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukotrienes and Essential Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Benz DJ, Mol M, Ezaki M, Mori-Ito N, Zelán I, Miyanohara A, Friedmann T, Parthasarathy S, Steinberg D, Witztum JL. Enhanced levels of lipoperoxides in low density lipoprotein incubated with murine fibroblast expressing high levels of human 15-lipoxygenase. J Biol Chem. 1995;270:5191–5197. doi: 10.1074/jbc.270.10.5191. [DOI] [PubMed] [Google Scholar]
- 3.Scheidegger KJ, Butler S, Witztum JL. Angiotensin II increases macrophage-mediated modification of low-density lipoprotein via a lipoxygenase-dependent pathway. J Biol Chem. 1997;272:21609–21615. doi: 10.1074/jbc.272.34.21609. [DOI] [PubMed] [Google Scholar]
- 4.Cyrus T, Pratico D, Zhao L, Witztum JL, Rader DJ, Rokach J, Fitzgerald GA, Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 5.Bolick DT, Srinivasan S, Whetzel A, Fuller LC, Hedrick CC. 12/15-Lipoxygenase mediates monocyte adhesion to aortic endothelium in apolipoprotein E-deficient mice through activation of RhoA and NF-kappa B. Arterioscler Thromb and Vascular Biol. 2006;26:1260–1266. doi: 10.1161/01.ATV.0000217909.09198.d6. [DOI] [PubMed] [Google Scholar]
- 6.Shen J, Hederick E, Cornhill JF, Zsigmond E, Kim HS, Kühn H, Guevara NV, Chan L. Macrophage mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittwer J, Bayer M, Mosandl A, Muntwyler J, Hersberger M. The c.-292C> T promoter polymorphism increases reticulocyte-type 15-lipoxygenase-1 activity and could be atheroprotective. Clin Chem Lab Med. 2007;45:487–492. doi: 10.1515/CCLM.2007.103. [DOI] [PubMed] [Google Scholar]
- 9.Assimes TL, Knowles JW, Priest JR, Basu A, Borchert A, Volcik KA, Grove ML, Tabor HK, Southwick A, Tabibiazar R, Sidney S, Beorwinkle E, Iribarren A, Hlatky C, Fortmann SP, Myers RM, Kuhn H, Risch N, Quartermous T. A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis. 2008;198:136–144. doi: 10.1016/j.atherosclerosis.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stachowska E, Dziedziejko V, Safranow K, Jakubowska K, Olszewska M, Machalinski B, Chlubek D. Effect of conjugated linoleic acids on the activity of mRNA expression of 5- and 15-lipoxygenases in human macrophages. J Agric Food Chem. 2007;55:5335–5342. doi: 10.1021/jf0701077. [DOI] [PubMed] [Google Scholar]
- 11.Melo RCN, Sabban A, Weller PF. Leukocyte lipid bodies: inflammation-related organelles are rapidly detected by wet scanning electron microscopy. J Lipid Res. 2006;47:2589–2594. doi: 10.1194/jlr.D600028-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Bozza PT, Banderia-Melo C. Mechanisms of leukocyte lipid body formation and function in inflammation. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2005;100:113–120. doi: 10.1590/s0074-02762005000900020. [DOI] [PubMed] [Google Scholar]
- 13.Zhu P, Oe T, Blair IA. Determination of cellular redox status by stable isotope dilution liquid chromatograph of glutathione and glutathione disulfide. Rapid Commun Mass Spectrom. 2008;22:432–440. doi: 10.1002/rcm.3380. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Ueda M, Tamaoka M, Hamaguchi M, Aisaka K, Kiso Y, Inoue T, Ogino R, Tatsuoka T, Ishihara T, Noguchi T, Morita I, Murota S. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J Med Chem. 1991;34:1505–1508. doi: 10.1021/jm00108a039. [DOI] [PubMed] [Google Scholar]
- 15.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The role of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Marcil V, Delvin E, Sane AT, Tremblay A, Levy E. Oxidative stress influences cholesterol efflux in THP-1 macrophages: role of ATP-binding cassette A1 and nuclear factors. Cardiovascular Res. 2006;72:473–482. doi: 10.1016/j.cardiores.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Collins HL, Ramalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall A, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn H, Thiele BJ, Ostareck-Ledeerer A, Stender H, Suzuki H, Yoshimoto T, Yamamoto S. Bacterial expression, purification and partial characterization of recombinant rabbit reticulocyte 15-lipoxygenase. Biochim Biophys Acta. 1993;1168:73–78. doi: 10.1016/0005-2760(93)90268-e. [DOI] [PubMed] [Google Scholar]
- 19.Small DM. Progression and regression of atherosclerotic lesions. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- 20.Makheja AN, Bloom S, Muesing R, Simon T, Bailey JM. Anti-inflammatory drugs in experimental atherosclerosis. 7 spontaneous atherosclerosis in WHHL rabbits and inhibition by cortisone acetate. Atherosclerosis. 1989;76:155–161. doi: 10.1016/0021-9150(89)90099-3. [DOI] [PubMed] [Google Scholar]
- 21.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low-density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991;87:1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MPW, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht A. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Nat Acad Sci USA. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagelin MH, Srinivasan S, Lee J, Nadler JL, Hedrick CC. 12/15-lipoxygenase activity increases the degradation of macrophage ATP-binding cassette transporter G1. Aterioscler Throm Vasc Biol. 2008;28:1811–1819. doi: 10.1161/ATVBAHA.108.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase δ pathway. J Lipid Res. 2007;48:1062–1068. doi: 10.1194/jlr.M600437-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Kurdi-Haidar B, Oram JF. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J Lipid Res. 2004;45:972–980. doi: 10.1194/jlr.M400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Uehara Y, Miura S, von Eckardstein A, Abe S, Fujii A, Matsuo Y, Rust S, Lorowski S, Assman G, Yamada T, Saku K. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2007;191:11–21. doi: 10.1016/j.atherosclerosis.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 28.Li AC, Glass CK. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J Lipid Res. 2004;45:2161–2173. doi: 10.1194/jlr.R400010-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn H, Chan L. The role of 15-lipoxygenase in atherogenesis: pro- and antiatherogenic actions. Curr Opin Lipidol. 1997;8:111–117. doi: 10.1097/00041433-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Lund E, Diczfalusy U, Bjorkem I. On the mechanism of oxidation of cholesterol at C-7 in a lipoxygenase system. J Biol Chem. 1992;267:12462–12467. [PubMed] [Google Scholar]
- 31.Bowden K, Ridgeway ND. Oxysterol binding protein (OSBP) negatively regulates ATP-binding cassette transporter A1 (ABCA1) protein stability. J Biol Chem. 2008;283:18210–18217. doi: 10.1074/jbc.M800918200. [DOI] [PubMed] [Google Scholar]
- 32.Venkateswaran A, Repa J, Lobaccaro JA, Bronson A, Manglesdorf DJ, Edwards PA. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. J Biol Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 33.Ouimet M, Wang M, Cadotte N, Ho K, Marcel Y. Epoxycholesterol impairs cholesteryl ester hydrolysis in macrophage foam cells, resulting in decreased cholesterol efflux. Arterioscler Thromb and Vascular Biol. 2008;28:1144–1150. doi: 10.1161/ATVBAHA.107.157115. [DOI] [PubMed] [Google Scholar]
- 34.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb and Vascular Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 35.Pomerantz KB, Hajjar DP. High-density-lipoprotein-induced cholesterol efflux from atrerial smooth muscle cell derived foam cells: functional relationship of the cholesteryl ester cycle and eicosanoid biosynthesis. Biochem. 1990;29:1892–1899. doi: 10.1021/bi00459a033. [DOI] [PubMed] [Google Scholar]
- 36.Belkner J, Chaitidis P, Stender H, Gerth C, Kuban RJ, Yoshimoto T, Kuhn H. Expression of 12/15-lipoxygenase attenuates intracellular lipid deposition during in vitro foam cell formation. Arterioscler Thromb Vasc Biol. 2005;25:797–802. doi: 10.1161/01.ATV.0000157580.26858.2d. [DOI] [PubMed] [Google Scholar]
- 37.Mathur SN, Albright E, Field FJ. Incorporation of lipoxygenase products into cholesteryl esters by acyl-CoA:cholesterol acyltransferase in cholesterol-rich macrophages. Biochem J. 1998;256:807–814. doi: 10.1042/bj2560807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belkner J, Stender H, Holzhutter H, Holm C, Kuhn H. Macrophage cholesteryl ester hydrolases and hormone-sensitive lipase prefer specifically oxidized cholesteryl esters as a substrate over their non-oxidized counterparts. Biochem J. 2000;352:125–133. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.