Abstract
We investigated associations between retrospectively assessed timing of pubertal development, interpersonal interactions, and hypothalamic-pituitary-adrenal (HPA) axis reactivity to an interpersonal stress task in 110 young adult women. Participants provided salivary cortisol samples at points prior and subsequent to a video-taped conflict discussion with their romantic partner. Participants also provided subjective global ratings of their discussion on dimensions of conflict and support. For earlier developing girls, higher levels of interpersonal conflict were associated with greater physiological stress in anticipation of the discussion task and less physiological recovery following the discussion. In contrast, for later developing girls, low levels of conflict were associated with greater anticipatory stress and less physiological recovery. These findings have implications for understanding the influence of off-time pubertal development on the life time development of young women.
The influence of early timing of puberty on the well-being of adolescent girls has been repeatedly established (e.g., Ge, Conger & Elder, 2001a; Graber, et al, 1997). For example, associated with earlier onset of pubertal development are increased conflictual interactions with parents (Steinberg, 1987), greater involvement in delinquent activities and deviant behaviors (Wiesner & Ittel, 2002; Wichstrom, 2001; Lanza & Collins, 2002; Caspi et al., 1993), greater amounts of sexual activity (Stattin & Magnusson, 1990), an increase in eating problems (Swarr & Richards, 1996), and an increased prevalence and intensity of psychological distress (Ge, Conger, & Elder, 1996). Early pubertal timing also increases the influence of other risk factors on the well-being of adolescent girls, such as heightened risk for violent behavior when living in a disadvantaged neighborhood (Obeidallah et al, 2004), delinquent behaviors when enrolled in mixed-sex schools (Caspi et al, 1993), exacerbation of behavioral problems among girls who were predisposed to behavioral problems earlier in childhood (Caspi & Moffitt, 1991), and increased levels of depressive symptoms in the presence of stressful life events (Ge et al., 2001).
More recently, research has indicated the impact of early timing on the lifetime occurrence of mental disorders and a lasting influence on other psychosocial outcomes into early adulthood. Earlier maturation has been associated with lower life satisfaction, smaller social networks, and poorer relationship quality in young adulthood (Graber, Seeley, Brooks-Gunn, & Lewinsohn, 2004). There is evidence that earlier pubertal timing increases a girl’s risk for depressive symptoms later in adolescence (Ge, Conger, & Elder, 2001a; Petersen, Sarigiani, & Kennedy, 1991), and although during puberty late-maturing girls tend to resemble their on-time developing peers on most psychological outcomes (e.g. Ge, Conger, & Elder, 2001a) , a consistent negative effect of later maturation for girls appears to be higher rates of lifetime depression evidenced in late adolescence and early adulthood (Graber, Brooks-Gunn, &Archibald, 2005; Herva et al, 2004).
Life time risk as a result of off-time development may be due to the social and developmental sequelae associated with the problems inherent in off-time development. For example, pubertal timing influences association with particular social contexts which themselves present amplified risk for earlier maturing girls (Stattin & Magnusson, 1990; Caspi et al, 1993; Ge, Conger, & Elder, 1996; Ge et al, 2003; Ge et al, 2006). Girls who develop physically before their same-age peers and begin to associate with chronologically older peers may find themselves facing challenges and difficulties in navigating more developmentally advanced social situations (Stattin & Magnusson, 1990; Caspi et al, 1993; Ge et al., 1996). In addition, early maturing girls may lack the opportunities to develop as effective social skills as their on-time or later maturing peers (Graber, Brooks-Gunn, & Archibald, 2005). Similarly, Masten and colleagues (2005) determined that externalizing problems in childhood tended to undermine academic achievement in adolescence, which in turn resulted in increased internalizing problems in young adulthood. A child at higher risk for engaging in delinquent behaviors due to her pubertal timing is also at greater risk for school failure or drop-out, which subsequently increases her risks for other negative future outcomes. The impact of these social sequelae may be further amplified by patterns of physiological reactivity formed by earlier childhood experiences (Boyce & Ellis, 2005), which having possibly influenced the timing of puberty (Ellis, 2004), may continue to describe patterns of reactivity in adulthood.
Interpersonal contexts such as new school environments for children (Bruce, Davis, & Gunnar, 2002) and interpersonal conflict negotiation tasks for adults (Kiecolt-Glaser et al., 1996) are activating stimuli for the hypothalamic-pituitary-adrenal (HPA) axis. Cortisol is one of the hormonal responses to stress produced by the HPA-axis, and has been shown to be responsive to varying interpersonal stressors in adults and children (Klimes-Dugan, Hastings, Granger, Usher, & Zahn-Waxler, 2001). Beyond the potential contributions to pubertal onset (Ellis, 2004), the HPA-axis is one of the hormonal systems that changes at puberty, including changes in response to stressors (Stroud, Papandonatos, Williamson, & Dahl, 2004). The off-time maturing girl, whether early or late, may have physiological reactivity that is particularly sensitized to contexts (Boyce & Ellis, 2005; Ellis, Essex, & Boyce, 2005). This reactivity sensitivity may be further challenged by a potential lack in the skills and resources to effectively manage the social environments in which a girl may find herself as a function of her pubertal timing. Thus, the increase in interpersonal conflict and risk behaviors associated with puberty may reinforce, in additive and interactive ways, a biological pathway already sensitized by pubertal onset or family environment. It is important to emphasize that we were interested not in the relative timing of the attainment of stages of pubertal development (i.e. status of pubertal development) but rather in the women’s experiences of their overall development compared with their same-aged peers concurrent with their development. Particularly as a retrospective measure, the self-report of pubertal timing is described as a stable and reliable estimate of development, as it is able to take into account one’s development across adolescence and categorize it in a personally meaningful context (Dubas, Graber, & Petersen, 1991). Self-perception of pubertal development may have particular relevance to interpersonal interactions, as visible signs of development (i.e. development of secondary sex characteristics) may elicit behaviors and beliefs about coinciding cognitive or emotional development. In this way, off-time pubertal timing may create a social and psychological context which interacts with physiological physiological reactivity and continues to have an impact throughout the lifespan.
The present study investigated whether pubertal timing could predict unique patterns of salivary cortisol reactivity and recovery in response to an interpersonal stressor experienced post-puberty. Specifically, we hypothesized that off-time maturing girls would evidence greater variation in reactivity to an interpersonal stressor than on-time maturing girls. We hypothesized that this would be particularly true for those off-time girls who engaged in greater conflict or less support in their interactions.
Method
Participants
The sample consisted of 110 young women, age 18–21 years (M=19.2, SD=1.8) drawn from study of young adult heterosexual couples (Powers et al., 2006). Participants were recruited by means of fliers distributed in the community and throughout 5 college campuses. Eighty-five percent of the sample was European-American (Caucasian), 6% Asian American or Native American, 6% Hispanic, and 2% African American. 42% of participants’ mothers worked full time outside of the home, and 30% were stay-at-home parents. 5.8% of participants’ fathers were unemployed or retired. To be eligible for participation, participants were required to be in a romantic relationship of at least 2 months at the time of the initial contact with the study.
Procedure
Participants attended a 3-hour session with their romantic partners that took place in our university laboratory offices. In addition to individually completing a series of self-report measures, the couple completed a video-taped discussion about an unresolved issue in their relationship, provided ratings of their behaviors during the discussion, and provided seven saliva samples. Each of these procedures is further described below.
In order to ensure that the discussion topic was of particular salience to each couple, participants were each asked to identify a topic of conflict that they felt was currently an issue in their relationship. Research assistants then randomly selected one of the topics for the video-taped discussion. In a private room with a couch and three small, but visible video cameras, participants were instructed to spend 15 minutes discussing the selected topic with a goal of resolving the conflict. Immediately following the discussion each participant indicated her overall perceptions of her behavior and her partner’s behavior during the discussion (this rating form is described below).
Participants’ HPA reactivity to the interpersonal task was assessed at seven time points by means of a non-intrusive saliva sampling. In response to stress, the hypothalamus releases corticotrophin releasing hormone (CRH), which stimulates the secretion of adrenocorticotropin hormone (ACTH) by the pituitary. This activation leads to the release of cortisol by the adrenal cortex. Cortisol secretions are discernable in saliva in about 15 minutes after a stress event. An initial saliva sample was collected at the start of the session. An anticipatory cortisol sample was collected 15-minutes following an explicit and vivid description of the upcoming discussion task. Additional samples were attained at 10, 20, 30, 45, and 60 minutes following the discussion. In order to maximize the effect of the interpersonal stressor on cortisol levels, all data collection sessions began at 4pm as cortisol levels are most stable at that time of day (Kirschbaum & Hellhammer, 1989). Saliva samples were collected according to procedures suggested by Salimetrics, LLC. Participants were instructed to “passively drool down a straw and into a small plastic vial” with their heads tilted forward until the required amount of saliva was collected. The vial was then sealed and immediately placed in frozen storage (−20 degrees C) until shipped on dry ice to Salimetrics, LLC for analysis of cortisol levels. All samples were assayed for salivary cortisol in duplicate using a highly-sensitive enzyme immunoassay (Salimetrics, PA). The test used 25 ul of saliva (for singlet determinations), has a lower limit of sensitivity of .003 ug/dl, range of sensitivity from .003 to 1.2 ug/dl, and average intra-and inter-assay coefficients of variation 4.13% and 8.89% respectively. Method accuracy, determine by spike recovery, and linearity, determined by serial dilution are 105% and 95 %. Values from matched serum and saliva samples show the expected strong linear relationship, r (17) = .94, p < .0001.
Measures
Pubertal Timing
Timing of puberty was assessed by retrospective self-report. In this study, young women were asked to assess their timing relative to those of their same age peers at the time of puberty, from 1 (“a lot before my peers”) to 5 (a lot later than my peers”). This assessment was used as a continuous measure of pubertal timing from earlier to later maturation. However, descriptively, a frequency distribution of the current sample indicates 24% of participants experienced timing that was a little or a lot before most of their peers, 41% had timing that was the same as their peers, and 34% experienced timing that was a little or a lot later than their peers. This distribution is consistent with other work that has assigned categories of early/ontime/late to their samples at 30/40/30 (Ge, Conger, & Elder, 2001a) or 20/60/20 (Brooks-Gunn & Warren, 1989).
Age at menarche provides assessment of a particular event in pubertal development, and in this study can provide a more objective assessment of timing relative to the present sample. The measurement of contextual pubertal timing and the age of menarche was strongly related within this sample (r = .567, p <.01). This is comparable with a reported correlation between self-report of timing and an objective measure of pubertal timing (age at peak height velocity) of .56 among 12th grade girls (Dubas, Graber, Petersen, 1991). To increase the reliability of the reported findings in regards to the influence of contextual timing, we ran matching analyses using a continuous variable of age at menarche. It should be noted that this too is a retrospective report provided by the participants.
Interpersonal Interaction
The Global Ratings Scale (GRS) was designed by the researchers to assess the couples’ impressions of their behaviors during the conflict discussion task. The discussions were characterized as high or low in conflict and support by self-report ratings provided by the participants immediately following the discussion. Subjective ratings are able to incorporate information that cannot be observed by independent raters (Powers, Welsh, & Wright, 1994) and provide descriptions of the interaction that are potentially more salient assessments of the individual’s experience of the interaction. An individual’s reactions and responses to the interaction – both interpersonally and physiologically – are grounded in her relationship with her partner and in her interpersonal history; as Klimes-Dugan and colleagues (2001) point out the “rise and fall of circulating cortisol levels may vary depending on the nature of the stressor and the subjective experience of the challenging event” (p. 696–697). As a result, it is her characterization of the behaviors as supportive or conflictual that is most likely to be related to her physiological reactivity.
Ratings described the participant’s perception of her conflictual or supportive behavior, and that of her partner’s, on a scale from “not at all [conflictual]” (0) to “very [conflictual]” (4). A total score assessing the extent to which the interaction was rated as conflictual or supportive was calculated by adding a participant’s ratings of her behavior and her ratings of her partner’s behaviors. For example, the combination of a participant’s perception of herself as highly conflictual (rating of 4) and her perception of her partner as moderately conflictual (rating of 3), resulted in a total conflict score of 7 on an 8-point scale.
HPA Axis Reactivity
HPA axis reactivity was measured by two different indices: anticipatory reaction and extent of recovery. Participants’ reactivity to the stress of anticipating the upcoming discussion task was computed by subtracting the cortisol level obtained from the first sample from the second cortisol sample, which was collected after an explicit description of the impending discussion task. The extent of recovery is indicated by decline in cortisol levels as represented in lower final sample levels and was computed by subtracting the level of the final cortisol sample from the level of the first sample; thus, a positive level on the extent of recovery variable is indicative of a final sample lower than the initial sample and greater recovery whereas a negative level is indicative of a higher final sample compared with the initial sample and less recovery.
Results
Means and standard deviations and zero-order correlations between all predictor and outcome variables are presented in Table 1. Descriptive data describing the cortisol samples is presented in Table 2.
Table 1.
Descriptives and Correlations Between All Variables
| Pubertal timing |
Age at Menarche |
Anticipatory Reactivity |
Extent of Recovery |
Conflict | Support | ||
|---|---|---|---|---|---|---|---|
| Pubertal timing | - | ||||||
| Age at Menarche | .567** | - | |||||
| Anticipatory reactivity | .133 | .116 | - | ||||
| Extent of recovery | −.036 | −.087 | −.309** | - | |||
| Conflict | .109 | .175 | .094 | −.052 | - | ||
| Support | .078 | −.057 | .075 | .096 | −.460*** | - | |
| M | 3.06 | 13.12 | .030 | .016 | 4.12 | 5.59 | |
| SD | 1.01 | 1.21 | .098 | .108 | 1.42 | 1.64 | |
| Min – Max | 1 – 5 | 9 – 16 | −.51 – .31 | −.23 – .54 | 2 – 8 | 2 – 8 | |
Table 2.
Cortisol Samples
| Time point relative to beginning of interaction |
Description of Cortisol Sample | Min | Max | Mean | SD | |
|---|---|---|---|---|---|---|
| Initial Sample | −20 minutes | Level prior to arrival at lab. | .050 | .798 | .199 | .116 |
| Pre-task Sample | − 5 minutes | Level in response to description of upcoming task | .061 | 1.031 | .232 | .144 |
| Post-task Sample 1 | + 10 minutes | Level during interaction task | .044 | 1.212 | .243 | .181 |
| Post-task Sample 2 | + 20 minutes | Level at completion of task (completion) | .027 | .747 | .219 | .138 |
| Post-task Sample 3 | + 30 minutes | Level 15 minutes after end of task (recovery) | .024 | .735 | .208 | .131 |
| Post-task Sample 4 | + 45 minutes | Level 30 minutes after end of task (recovery) | .023 | .628 | .198 | .118 |
| Post-task Sample 5 | + 60 minutes | Level 45 minutes after end of task (recovery) | .040 | .508 | .184 | .096 |
Two different sets of hierarchical multiple regression analyses were conducted predicting each of the outcome variables (anticipatory reactivity and extent of recovery) by pubertal timing and age at menarche. Each equation tested for main effects of pubertal timing or age at menarche and ratings of conflict or support, and in a second step, tested for interaction effects between pubertal timing or age at menarche and conflict or support. In addition, all equations included the first cortisol sample to control for initial levels. All variables were centered prior to inclusion in the regression analyses as recommended by Aiken and West (1991), by subtracting the mean value of the variable from each individual’s score. As a result, each main effect coefficient estimates the size of the effect of that predictor when the other predictor from the interaction is equal to the average value of that predictor, rather than equal to 0. i
Pubertal Timing, Ratings of Conflict, and HPA-axis Reactivity
Anticipatory Reactivity
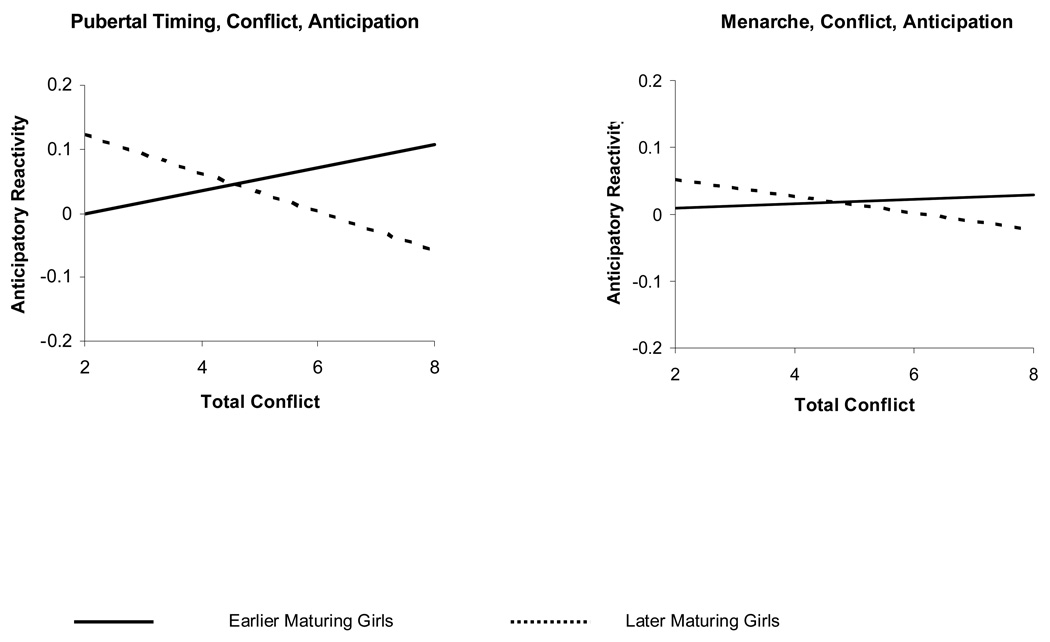
As seen in Table 3 and Figure 1, conflict behavior moderates the relation between pubertal timing and anticipatory physiological reactions, after accounting for the relative influence of initial cortisol levels. For earlier maturing young women, higher levels of conflict were associated with higher levels of anticipatory reactivity. For later maturing young women, lower levels of conflict were associated with greater anticipatory reactivity. These results were replicated using age at menarche as the variable of pubertal timing. Again, only the interaction between age at menarche and ratings of conflict predicted anticipatory reactivity. For young women who experienced menarche at 11 years of age or earlier, higher levels of conflict were associated with higher levels of anticipatory reactivity. In contrast, those who experienced menarche at age 14 or older, lower levels conflict of were associated with higher levels of anticipatory reactivity.
Table 3.
Summary of Hierarchical Regression Analyses Predicting Anticipatory Reactivity by Pubertal Timing and Conflict
| Anticipatory Reactivity by Pubertal Timing and Conflict | ||||||
|---|---|---|---|---|---|---|
| Step 1 | Step 2 | |||||
| Variable | B | SE B | β | B | SE B | β |
| Initial Cortisol | −.099 | .085 | −.117 | −.059 | .084 | −.070 |
| Pubertal Timing | .013 | .010 | .138 | .012 | .009 | .124 |
| Total Conflict | .006 | .007 | .087 | .002 | .007 | .025 |
| Timing by Conflict | −.019 | .007 | −.267** | |||
| R2 | .038 | .103 | ||||
| F for change in R2 | 1.273 | 7.023** | ||||
| F | 1.273 | 2.769** | ||||
| Anticipatory Reactivity by Age at Menarche and Conflict | ||||||
| Step 1 | Step 2 | |||||
| Variable | B | SE B | β | B | SE B | β |
| Initial Cortisol | −.084 | .085 | −.100 | −.063 | .084 | −.074 |
| Menarche | .008 | .008 | .101 | .007 | .007 | .097 |
| Total Conflict | .005 | .007 | .068 | .005 | .007 | .068 |
| Menarche by Conflict | −.010 | .005 | −.197* | |||
| R2 | .029 | .067 | ||||
| F for change in R2 | .969 | 3.886* | ||||
| F | .969 | 1.720 | ||||
Note: p < .05.
p < .01.
Figure 1.
Anticipatory reactivity at different levels of conflict, for groups of early and late maturing girls determined by perceived timing or age at menarche. Pubertal Timing categories of early and late represent the 24% of participants with reported timing that was either “a little before” or “a lot before” most of their peers (“Early”) and the 34% of participants with reported timing that was “a little later” or “a lot later” than their peers (“Late”). Age at Menarche groups represent the 28% of participants with reported age of menarche at least one standard deviation less than the average reported age (“Early”) and the 17% of participants with reported age of menarche at least one standard deviation greater than the average reported age (“Late”). Note that higher values indicate greater physiological recovery.
Extent of Recovery
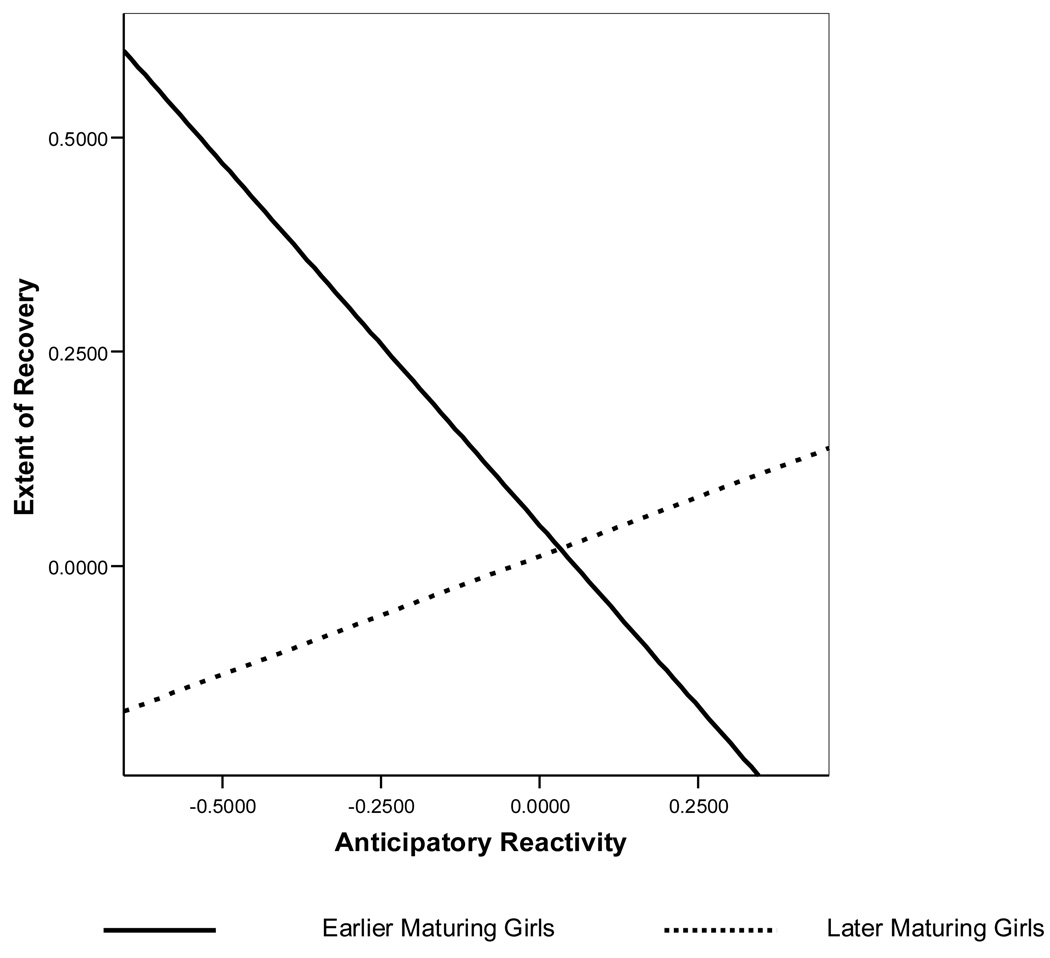
Because anticipatory reactivity and extent of recovery were significantly correlated, we included anticipatory reactivity as a control variable and as a moderator of pubertal timing and conflict behavior. As presented in Table 4 and Figure 2, after accounting for the influence of anticipatory reactivity (and that of initial levels of cortisol), the interaction between pubertal timing and anticipatory reactivity significantly predicted the extent of recovery. For young women reporting earlier maturation, high anticipatory reactivity was associated with less recovery. In contrast, for those reporting later maturation, high anticipatory reactivity was associated with greater recovery. A three-way interaction between pubertal timing, level of conflict, and anticipatory reactivity, did not significantly predict the extent of recovery. These results were replicated using age at menarche as the timing variable presented in Table 5. Again, the interaction between menarche and anticipatory reactivity alone predicted the extent of recovery; however, this relationship was rendered non-significant with the addition of the also non-significant three-way interaction between age at menarche, level of conflict, and anticipatory reactivity.
Table 4.
Summary of Hierarchical Regression Analyses Predicting Extent of Recovery by Anticipatory Reactivity, Pubertal Timing and Conflict
| Extent of Recovery by Anticipatory Reactivity, Pubertal Timing, and Conflict. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | |||||||
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Initial Cortisol | .571 | .071 | .612*** | .430 | .080 | .461*** | .430 | .080 | .461*** |
| Anticipatory. Reactivity | −.259 | .084 | −.235*** | −.141 | .090 | −.128 | −.138 | .091 | −.125 |
| Pubertal Timing | −.007 | .008 | −.065 | −.005 | .008 | −.048 | −.005 | .008 | −.050 |
| Conflict | −.004 | .006 | −.053 | .004 | .006 | .051 | .003 | .006 | .045 |
| Conflict by Pubertal Timing | .009 | .006 | .119 | .009 | .007 | .115 | |||
| Anticipatory Reactivity by Timing | .307 | .087 | .305*** | .299 | .090 | .296*** | |||
| Conflict by Anticipatory Reactivity | .025 | .051 | .041 | .017 | .056 | .027 | |||
| Conflict by Timing By Anticipatory Reactivity | −.019 | .052 | −.032 | ||||||
| R2 | .461 | .530 | .531 | ||||||
| F for change in R2 | 20.513*** | 4.595** | .132 | ||||||
| F | 20.513*** | 15.008*** | 13.025*** | ||||||
Note: p < .05.
p < .01.
p < .005.
Figure 2.
Extent of cortisol recovery at different levels of anticipatory reactivity for early and late maturing girls determined by perceived timing. Pubertal Timing categories of early and late represent the 24% of participants with reported timing that was either “a little before” or “a lot before” most of their peers (“Early”) and the 34% of participants with reported timing that was “a little later” or “a lot later” than their peers (“Late”). Note that higher values indicate greater physiological recovery.
Table 5.
Summary of Hierarchical Regression Analyses Predicting Extent of Recovery by Anticipatory Reactivity, Age at Menarche Conflict.
| Extent of Recovery by Anticipatory Reactivity, Age at Menarche, and Conflict. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | |||||||
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Initial Cortisol | .564 | .071 | .604*** | .521 | .074 | .558*** | .498 | .077 | .534*** |
| Anticipatory. Reactivity | −.265 | .084 | −.240*** | −.207 | .089 | −.188* | −.153 | .101 | −.139 |
| Menarche | −.003 | .006 | −.038 | −.002 | .006 | −.020 | −.002 | .006 | −.026 |
| Conflict | −.004 | .006 | −.051 | −.000 | .006 | −.001 | −.001 | .006 | −.008 |
| Conflict by Menarche | .002 | .004 | .034 | .000 | .004 | .008 | |||
| Anticipatory Reactivity by Menarche | .204 | .088 | .205* | .176 | .091 | .176 | |||
| Conflict by Anticipatory Reactivity | −.010 | .054 | −.017 | −.009 | .054 | −.014 | |||
| Conflict by Menarche By Anticipatory Reactivity | −.097 | .086 | −.115 | ||||||
| R2 | .458 | .490 | .497 | ||||||
| F for change in R2 | 20.291*** | 1.952 | 1.279 | ||||||
| F | 20.291*** | 12.776*** | 11.372*** | ||||||
Note: p < .05.
p < .01.
p < .005.
Pubertal Timing, Ratings of Support, and HPA-axis Reactivity
There were no observed main effects or interaction effects for pubertal timing (either perceived or age at menarche) and ratings of support in the prediction of any of the reactivity variables.
Discussion
Timing of puberty in girls that is off-time with that of peers is linked with a number of negative outcomes that occur in adolescence and may have far reaching developmental implications. To our knowledge, this study is the first to demonstrate a process level connection between pubertal timing and later HPA reactivity to interpersonal interaction. We proposed that off-time pubertal timing (early or late) may have a lasting influence on hormonal reactivity to interpersonal stress.
Young women who reported earlier or later maturation compared with their peers, were found to have distinct patterns of reactivity that were moderated by their experiences during the conflict discussion. Specifically, those who were highly reactive when they were anticipating the upcoming stressor subsequently experienced different interpersonal contexts during the stress task depending on their pubertal timing. Earlier maturing young women, who showed a strong physiological response during anticipation of the discussion task, experienced their discussions as high in conflict. In contrast, later maturing young women, who experienced a strong physiological reaction during anticipation of the task, experienced less conflictual interactions. Furthermore, for those who had a strong physiological anticipatory response, pubertal timing predicted differing rates of physiological recovery following the stressor. Ultimately, those with high anticipatory reactivity who reported earlier maturation experienced less recovery following the interaction than those who reported later maturation.
The longitudinal impact of puberty on emotional well-being may be due to the establishment of a vulnerable developmental trajectory that is a combination of contextual and biological forces. The data presented here support the contention that during early adolescence family and friends may mistakenly perceive early developing girls to be socially and cognitively more mature than their peers. As a result, these girls may be drawn into socially challenging environments, which demand that they develop more assertive styles of coping and possibly greater proclivity for conflict in solving interpersonal problems. Evidence from prior studies that is consistent with this hypothesis includes early maturing girls’ increased interpersonal conflict (Steinberg, 1987), greater involvement with older peers and delinquent activities (Caspi et al., 1993; Lanza & Collins, 2002; Sattin & Magnusson, 1990; Wiesner & Ittel, 2002, Wichstrom, 2001), heightened risk for violence (Obeidallah et al., 2004). It is has been unclear to what extent effects of this developmental trajectory (i.e. a greater tendency for interpersonal conflict in early and mid adolescence) extend beyond early adolescence into the transition to adulthood, although one study showing that early developing girls have poorer social networks and relationship quality in young adulthood and suggests that effects could be long-lasting (Graber et al., 2004). This hypothesized developmental trajectory fits our data well. That is, in the present study, after early developing girls have matured into young women, the greater their stress reactions in anticipation of an upcoming discussion of an interpersonal problem, the more they perceive the behaviors of themselves and their partners as high in conflict. They also struggle more to recover physiological equilibrium, which could underlie the psychological disorders, such as depression and anxiety, observed among young adult women who experienced earlier maturation (Graber et al, 2004).
A different picture emerges for young women who experienced the pubertal development later than their peer group. The hypothesized developmental trajectory for this group is that later physical development may result in family and friends perceiving these girls as less mature than their peers, thus providing less challenging social environments for these girls to practice interpersonal coping skills. Evidence from other studies suggests that later maturation may be somewhat protective for the adolescent girl in that later maturing girls frequently resemble their “on-time” peers in terms of psychological outcomes during adolescence (e.g. Ge, Conger, & Elder, 2001a). Our data indicate that when young women are highly reactive in anticipation of interpersonal conflict, those reporting later maturation experience greater physiological recovery than do their earlier maturing peers. This may be a good example of a protective factor associated with later maturation, suggesting adaptive psychological and/or physiological coping to manage the physiological stress. Young women who reported later maturation in adolescence have been observed to have a greater incidence of major life experiences yet also fewer psychological difficulties or psychosocial issues than their earlier maturing peers (Graber et al, 2004). Our findings may indicate a preliminary explanation for this resiliency.
Thus, our data are consistent with the assertion that the social environment created by an off-time transition through puberty may result in emotional and interpersonal experiences that have ongoing influences on their experiences of interpersonal conflict. When off-time puberty combines with particular interpersonal contexts– such as heightened conflict or avoided conflict – it is associated with heightened physiological responses to interpersonal stressors, which may result in chronic individual challenges. Although these data do not address gender differences, this heightened response to interpersonal stress may be particularly salient to the well-being of young women who biologically may be more predisposed than young men to reduce conflict and increase affiliative behaviors (Taylor et al, 2000).
Strengths and Limitations
A particular strength of this work is in the replication of findings from two different – though related – retrospective measurements of pubertal timing. The use of menarche as a marker of pubertal timing replicated the associations between perceived pubertal timing and physiological reactivity, though associations were generally weaker. This may be because menarche is an event that is marked by the adolescent herself and used in determining her sense of timing relative to her peers. Thus, menarche is subsumed under the subjective perception of timing provided by the girls themselves. As such, early menarche is likely to be associated with the same risks as early timing (such as increased conflictual behaviors, for example) but does not assess the same contextual information implied by the subjective comparative rating of timing. Furthermore, categorization of early and late based on menarche was based on comparison with this sample and not with the girls’ peer group at the time of development, which may account for the overall weaker associations.
The demographic distribution of our sample made it unfortunately untenable to conduct comparisons by racial/ethnic groups. Much of research on pubertal timing to date has been conducted on Caucasian middle class girls and the proposed conceptual model may not be relevant to other racial, ethnic, or socioeconomic groups. While there is evidence to suggest that off-time development is associated with similar behavioral risks in girls of other racial backgrounds, for example, pubertal timing was found to exacerbate the risk of peer context on early substance use in African American girls (Ge et al, 2006), this risk may be the result of different mediating or moderating processes. Indeed, White, African American and Latina girls have been shown to be differentially influenced by the interplay of pubertal timing and friendship groups in regards to early sexual behaviors (Cavanagh, 2004). Certainly, the variations in timing of normative development associated with race may have direct implications for the contextual risk of timing proposed in this paper; for example, after controlling for socioeconomic status, Latina girls reach menarche earlier than African American girls, however no differences persist between White girls and Latina girls or White girls and African American girls (Obeidallah, Brennan, Brooks-Gunn, Kindlon, & Earls, 2000). Furthermore, as racial background has been associated with differing family interaction styles, in addition to different timetables for pubertal development, investigation of pubertal timing, interpersonal interactions and the impact on physiological reactivity to stress is highly warranted. Similarly, this study did not investigate the proposed associations in young men. There is evidence that pubertal timing has equally significant effects on the psychological well being and behavioral outcomes of boys as girls (e.g. Susman, Dorn, & Chrousos, 1991; Graber et al, 1997; Ge, Conger, & Elder, 2001b), and such processes as described here, may or may not be similar for young men and have equally important implications for their development. However, we feel the data presented here still reveal important aspects of development for young women regardless of how they may be similar (or not) to the experiences of young men. Future investigations into these processes for young men and how they compare with young women would certainly further add to our understanding of pubertal timing, interpersonal interactions, and stress reactivity.
A second limitation of this study is that all data were collected at one time point, using retrospective assessments of pubertal timing, and requiring some theoretical assumptions to remain untested. Specifically, we proposed that greater conflict interactions experienced by early maturing adolescent girls, possibly at a developmental point when they are less able to effectively handle such conflict, reinforce a physiological reaction to conflict that is maintained in their later intimate relationships. The findings of this study emphasize that further exploration of the questions posed in this study would be well served by a longitudinal study, which could incorporate both family interactions and physiological reactivity during pubertal timing and subsequently in early adulthood. In addition, future investigations could consider alternate methods of assessing interaction characteristics and collect information on other coping behaviors, using observer ratings or video recall methods (Powers, Welsh, & Wright, 1994).
There is an additional notable limitation of this study: the majority of participants were students enrolled in undergraduate college. Despite community recruitment efforts, only a few couples were not attending college. As a result those early maturing girls who had particular academic or behavioral difficulties in high-school may be unintentionally excluded from the sample, and therefore our “earlier maturing” group may not be typical of those girls who experienced the most difficulty associated with their developmental timing.
Finally, it should be noted that the physiological anticipation of an upcoming stressful interaction may be appropriately considered a marker of psychological anticipation, but these were not differentiated in the current study, thereby limiting the interpretation of the association between physiological anticipation and levels of conflict. The degree of conscious awareness of the physiological anticipatory stress – in the form of anxiety regarding the upcoming task - is unknown and could provide valuable contextual information to the reported findings.
Conclusions
This study contributes to identifying processes by which pubertal development has long term significance, including subjective perception of pubertal timing, interpersonal conflict, and physiological reactivity to an interpersonal stressor. The lasting influence of pubertal timing may lie in the interaction between social context and biological reactivity. Future investigations into specific interaction behaviors, coping styles, and particular psychosocial outcomes could further clarify the risk to girls and women of off-time pubertal development.
Acknowledgments
This study was supported in part by Grant R01 MH60228-01A1 from the National Institute of Mental Health to Sally I. Powers.
Footnotes
In addition to ratings of conflict and support, participants provided ratings of the intensity of the discussion, the degree of stressfulness of the interaction, and the degree of resolution attained by the end of the discussion. Pearson correlations indicated significant moderate associations between these perceptions and levels of conflict (intensity: r = .432, p<.01, stress: r =.492, p<.01, resolution: r =−.335, p<.01) and support (intensity: r =−.250p<.05, stress: r = − .434, p<.01, resolution: r = .483, p<.01). In order to highlight the impact of conflict and support behaviors on physiological reactivity, ratings of discussion intensity, stressfulness, and resolution were included in analyses as control variables. However, as these variables were found to not contribute to the models in significant ways they were removed from the final equations presented here.
Contributor Information
Anne Emilie Smith, Yale University School of Medicine, Department of Psychiatry, 1 Long Wharf, Suite 102, New Haven, CT 06520.
Sally I. Powers, University of Massachusetts, Department of Psychology, 134 Hicks Way, Amherst, MA 01002
References
- Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Thousand Oaks: Sage; 1991. [Google Scholar]
- Brooks-Gunn J, Graber JA, Paikoff RL. Studying links between hormones and negative affect: Models and measures. Journal of Research on Adolescence. 1994;4:469–486. [Google Scholar]
- Brooks-Gunn J, Warren MP. Biological and social contributions to negative affect in young adolescent girls. Child Development. 1989;60:40–55. doi: 10.1111/j.1467-8624.1989.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Bruce J, Davis EP, Gunnar MR. Individual differences in children's cortisol response to the beginning of a new school year. Psychoneuroendocrinology. 2002;27:635–650. doi: 10.1016/s0306-4530(01)00031-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Lynam D, Moffitt TE, Silva PA. Unraveling girls' delinquency: Biological, dispositional, and contextual contributions to adolescent misbehavior. Developmental Psychology. 1993;29:19–30. [Google Scholar]
- Caspi A, Moffitt TE. Individual differences are accentuated during periods of social change. The sample case of girls at puberty. Journal of Personality and Social Psychology. 1991;61:157–168. doi: 10.1037//0022-3514.61.1.157. [DOI] [PubMed] [Google Scholar]
- Cavanagh SE. The sexual debut of girls in early adolescence: The intersection of race, pubertal timing, and friendship group characteristics. Journal of Research on Adolescence. 2004;14:285–312. [Google Scholar]
- Dorn LD, Crockett LJ, Petersen AC. The relations of pubertal status to intrapersonal changes in young adolescents. Journal of Early Adolescence. 1988;8:405–419. [Google Scholar]
- Dubas JS, Graber JA, Petersen AC. A longitudinal investigation of adolescents' changing perceptions of pubertal timing. Developmental Psychology. 1991;27:580–586. [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Coming of age to early: Pubertal influences on girls' vulnerability to psychological distress. Child Development. 1996;67:3386–3400. [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001a;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. The relation between puberty and psychological distress in adolescent boys. Journal of Research on Adolescence. 2001b;11:49–70. [Google Scholar]
- Ge X, Jin R, Natsuaki M, Gibbons FX, Brody GH, Cutrona CE, Simons RL. Pubertal maturation and early substance use risks among African American children. Psychology of Addictive Behaviors. 2006;20:404–414. doi: 10.1037/0893-164X.20.4.404. [DOI] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J, Archibald AB. Links between girls’ puberty and externalizing and internalizing behaviors: Moving from demonstrating effects to identifying pathways. In: Stoff DM, Susman EJ, editors. Developmental psychobiology of aggression. New York, NY: Cambridge University Press; 2005. pp. 87–113. [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber JA, Petersen AC, Brooks-Gunn J. Pubertal processes: Methods, measures, and models. In: Graber JA, Brooks-Gunn J, Petersen AC, editors. Transitions through adolescence: Interpersonal domains and context. Mahwah, NJ: Lawrence Erlbaum; 1996. pp. 23–52. [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Granger D, Stansbury K, Henker B. Preschooler's behavioral and neuroendocrine responses to social challenge. Merrill-Palmer Quarterly. 1994;40:190–211. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Herva A, Jokelainen J, Pouta A, Veijola J, Timonen M, Karvonen JT, Joukama M. Age at menarche and depression at the age of 31 years. Findings from the Northern Finland 1966 Birth Cohort Study. Journal of Psychosomatic Research. 2004;57:359–362. doi: 10.1016/j.jpsychores.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. A model of family relational transformations during the transition to adolescence: Parent-adolescent conflict and adaptation. In: Graber JA, Brooks-Gunn J, Petersen AC, editors. Transitions through adolescence: Interpersonal domains and context. Mahwah, NJ: Lawrence Erlbaum; 1996. pp. 167–199. [Google Scholar]
- Holmbeck GN, Hill JP. Conflictive engagement, positive affect, and menarche in families with seventh-grade girls. Child Development. 1991;62:1030–1048. [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral Reviews. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo J, MacCallum R, Glaser R, Malarkey WB. Marital conflict and endocrine functioning: Are men really more physiologically affected than women? Journal of Consulting and Clinical Psychology. 1996;64:324–332. doi: 10.1037//0022-006x.64.2.324. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Laitinen-Krispijn S, Van der Ende J, Hazebroke-Kampshreur A, Verhulst F. Pubertal maturation and the development of behavioral and emotional problems in early adolescence. Acta Psychiatrica Scandinavica. 1999;99:16–25. doi: 10.1111/j.1600-0447.1999.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Lanza S, Collins LM. Pubertal timing and the onset of substance use in females during early adolescence. Prevention Science. 2002;3:69–82. doi: 10.1023/a:1014675410947. [DOI] [PubMed] [Google Scholar]
- Malina RM, Bouchard C. Growth, maturation, and physical activity. Champaign, IL: Human Kinetics; 1991. [Google Scholar]
- Masten AS, Roisman GI, Long JF, Burt KB, Obradovic J, Riley JR, et al. Developmental cascades: Linking academic achievement and externalizing and internalizing symptoms over 20 years. Developmental Psychology. 2005;41:733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- Obeidallah DA, Brennan RT, Brooks-Gunn J, Earls F. Links between pubertal timing and neighborhood contexts: Implications for girls’ violent behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;43:1460–1468. doi: 10.1097/01.chi.0000142667.52062.1e. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Sarigiani PA, Kennedy RE. Adolescent depression: Why more girls? Journal of Youth and Adolescence. 1991;20:247–271. doi: 10.1007/BF01537611. [DOI] [PubMed] [Google Scholar]
- Powers SI, Pietromonaco P, Gunlicks M, Sayer A. Dating couples’ attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. Journal of Personality and Social Psychology. 2006;90(4):613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- Powers SI, Welsh DP, Wright V. Adolescents' affective experiences of family behaviors: The role of subjective understanding. Journal of Research on Adolescence. 1994;4:585–600. [Google Scholar]
- Stattin H, Magnusson D. Paths Through Life. Vol. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1990. [Google Scholar]
- Steinberg L. Impact of puberty on family relations: Effects of pubertal status and pubertal timing. Developmental Psychology. 1987;23:451–460. [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in the effects of pubertal development on responses to a corticotropin-releasing hormone challenge: The Pittsburgh Psychobiologic Studies. Annals of the New York Academy of Sciences. 2004;1021:348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Swarr AE, Richards MH. Longitudinal effects of adolescent girls’ pubertal development, perceptions of pubertal timing, and parental relations of eating problems. Developmental Psychology. 1996;32:636–646. [Google Scholar]
- Warren MP, Brooks-Gunn J. Mood and behavior at adolescence: evidence for hormonal factors. Journal of Clinical Endocrinology and Metabolism. 1989;69:77–83. doi: 10.1210/jcem-69-1-77. [DOI] [PubMed] [Google Scholar]
- Wichstrom L. The impact of pubertal timing on adolescents' alcohol use. Journal of Research on Adolescence. 2001;11:131–150. [Google Scholar]
- Wiesner M, Ittel A. Relations of pubertal timing and depressive symptoms to substance use in early adolescence. Journal of Early Adolescence. 2002;22:5–23. [Google Scholar]
- Yehuda R, Boisoneau D, Mason JW, Giller EL. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biological Psychiatry. 1993;34:18–25. doi: 10.1016/0006-3223(93)90252-9. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Resnick H, Kahan B, Giller EL. Long lasting hormonal alterations to extreme stress in humans: Normative or maladaptive? Psychosomatic Medicine. 1993;55:287–297. doi: 10.1097/00006842-199305000-00006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, et al. Low urinary cortisol excretion in patients with post traumatic stress disorder. Journal of Nervous and Mental Disease. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]