Abstract
Currently, an effective gene therapy strategy, which not only retains cancer-specific expression but also limits toxicity, has yet to be developed for ovarian cancer. Mounting reports over the years have shown that human telomerase activity is significantly elevated in cancer cells compared with normal cells. In this study, we evaluated the hTERT promoter and showed that it can direct target gene expression preferentially in ovarian cancer cells. However, its promoter (hTERT) activity is much lower than that of CMV, a commonly used non-specific promoter. To overcome this problem, we have integrated the hTERT promoter into our recently developed VISA system (VP16-Gal4-WPRE integrated systemic amplifier) and dramatically enhanced transgene expression. In addition, to further develop this cancer-specific promoter gene expression system into an applicable therapeutic vector, we expressed E1A (an adenoviral type 5 transcription factor which possesses anti-cancer properties) through this novel VISA platform. We demonstrated that the hTERT-VISA system specifically targeted E1A’s expression to ovarian cancer cells at a level greater than or comparable to the commonly used CMV promoter, yet remained nearly silent in normal cells, and thus making this a suitable gene therapy construct. By using this cancer-specific promoter, which limits target gene expression in normal cells/tissues, potential toxicity induced by the CMV promoter would be prevented. More importantly, we showed significant antitumor activity with much less toxicity in animal models via intravenous delivery of hTERT-VISA-E1A:liposomal nanoparticles, suggesting a promising role of hTERT-VISA-E1A for ovarian cancer treatment under a gene therapy setting.
Keywords: Human telomerase, gene therapy, non-viral vector, liposome, E1A
Introduction
As the fifth leading cause of cancer deaths among women, ovarian cancer has the highest mortality rate of all gynecological cancers, with approximately 15,520 deaths and 21,650 new cases in the United States estimated in 2008 (1). Unfortunately, in spite of the improvements in cytoreductive surgery and combination chemotherapy, advanced staged-patients who do not respond to therapy or those who recurred have limited options to effectively treat this disease. Moreover, prognosis remains unfavorable especially when patients are in the advanced stages (III/IV) at the time of diagnosis with the 5-year survival rate for late-staged patients at below 25 percent (2, 3). Thus, it is crucial that more effective and/or alternative therapeutic strategies are developed to treat ovarian cancer.
One of the ongoing, but less developed areas of cancer treatment is gene therapy, which has the potential to significantly improve therapeutic outcomes (4–7). Similar to many existing anti-cancer therapies, however, gene therapy lacks specificity and the ability to specifically target transgene expression within ovarian cancer cells, attaining maximal anti-tumor activity with limited toxicity to surrounding normal cells. To address this issue, a system might be devised that utilizes an ovarian cancer-specific promoter (OCSP) to drive expression of the target gene only in cancer cells. In fact, some studies have explored this possibility (8–12) although an ideal promoter that is highly active and specific for ovarian cancer cells has yet been identified.
To find a such a promoter, we searched the literature as well as the Serial Analysis of Gene Expression database and identified three potential promoter candidates: (1) human telomerase reverse transcriptase (hTERT) (12); (2) EphA2 (13), and (3) ceruloplasmin (10). Of the three promoters, we showed that hTERT has the potential to be used for transcriptionally targeted gene therapy for ovarian cancer herein. The hTERT promoter is ideal because its activity has been linked to cancer and has been detected in many invasive cancers, but repressed in normal somatic tissues or benign tumors (14, 15). While telomerase activity is absent in normal somatic cells, greater than 85% of human cancers demonstrated high telomerase activity (14, 16, 17). A number of studies have shown that the majority of ovarian carcinoma specimens exhibit detectable telomerase activity, but little activity has been found in benign tumors or normal ovary (14, 18–20). Interestingly, analysis of the human telomerase reverse transcriptase (hTERT) promoter showed that its activity correlated with the telomerase activity (21). Therefore, the hTERT promoter might be used to drive a therapeutic gene that kills ovarian cancer cells.
Although the hTERT promoter is active in ovarian cancer cells, its activity is generally less than 5% of that of the CMV promoter, which would not be suitable for gene therapy (7, 22–25). To enhance hTERT promoter activity, we linked it to a VISA (VP16-GAL4-WPRE integrated systemic amplifier) expression vector, which was recently developed to amplify activity of a cancer-specific promoter (26). In particular, when a pancreatic-specific promoter was incorporated into the VISA vector carrying a therapeutic gene, it effectively induced cancer-cell specific killing in a pancreatic cancer model without significant toxicity compared with its CMV-driven counterpart (26). Furthermore, therapeutic gene expression under the VISA system resulted in a significant increase in expression compared with the non-specific and ubiquitous CMV promoter, making this hTERT-linked VISA vector a powerful tool for efficient production of a suitable target gene that is specifically expressed in cancer cells.
In this study, we have chosen E1A as our therapeutic target gene for ovarian cancer treatment. Briefly, the adenovirus 5 E1A gene is the first viral gene expressed in cells after adenovirus infection and is a well-known transcription factor (27, 28). Although adenoviral type 5 E1A has been classified as an immortilization oncogene due to its ability to immortalize cells, it is incapable of transforming cells by itself. Instead, numerous reports have demonstrated that E1A associates with various anti-cancer activities (29–31) prompting its evaluation in multiple clinical trials (4, 32–34). Moreover, E1A expression inhibits angiogenesis, induces apoptosis via a bystander effect (35) and activates a protein phosphatase 2a tumor suppressor that triggers a feed-forward mechanism which enhances chemotherapy-induced cancer cell death (36–38). By driving expression of E1A via systemic gene delivery under an ovarian cancer-specific promoter both primary and metastatic tumors could be targeted with minimal toxicity for normal cells. Here, we have shown that T-VISA-E1A specifically targeted ovarian cancer cells and significantly reduced tumor growth in the SKOV3.ip1 mouse model suggesting that this gene should be evaluated in clinical trials.
Materials and Methods
Constructs
The hTERT promoter (−380 to +60, relative to the start site (39)) was amplified by polymerase chain reaction (PCR) using genomic DNA extracted from LNCaP cells as template. The PCR fragment containing the hTERT promoter was subcloned into pCRII-TOPO (Invitrogen, Carlsbad, CA) to generate pCRII-TOPO-hTERT. After verification by DNA sequencing, the fragment containing the hTERT promoter was digested with Kpn I and Xho I and subsequently inserted into the corresponding sites in pGL-3 basic (Promega, Madison, WI) to obtain plasmid, pGL3-hTERT-Luc. Plasmids pGL3-EphA2-Luc, pGL3-Cerul-Luc and pGL3-CMV-Luc containing the EphA2, ceruloplasmin and CMV promoter, respectively, driving expression of the firefly luciferase gene were described previously (10, 26). Incorporation of the two-step transcriptional amplification (TSTA) system was done by subcloning the Hind III/Not I-blunted hTERT fragment of pCRII-TOPO-hTERT into the Msc I/Nhe I-blunted site of pGL3-TSTA-Luc (provided by Dr. M. Carey, UCLA School of Medicine, Los Angeles, CA) (40). Finally, construction of pGL3-hTERT-TSTA-Luc-WPRE or pGL3-T-VISA-Luc was carried out by insertion of the Not I/Bgl II fragment containing the WPRE sequence of pGL3-Luc-WPRE into the same site of pGL3-hTERT-TSTA-Luc.
A 13S E1A fragment was amplified by PCR from pE1A-Neo plasmid using primers (P1.E1A.BII, 5’-TCGAGATCTGCGCCGCCATGGGACATATTATCTGCCACGGAGGTG-3’, and P2.E1A.Nh, 5’-GCGTGCTAGCCCTCGGTTTACACCTTATGGCCTGGGGCG-3’) and substituted for the luciferase coding sequence in pGL3-T-VISA-Luc to produce pGL3-T-VISA-EIA. Construction of pUK21-T-VISA-E1A was performed by subcloning the T-VISA-E1A fragment of pGL3-T-VISA-EIA into Not I and Sal I sites of pUK21 (41). A control plasmid pUK21-T-VISA was generated by deletion of the Nhe I/Bgl II fragment from pUK21-T-VISA-E1A. To obtain pUK21-CMV-E1A, the 13S E1A fragment was amplified by PCR from pE1A-Neo plasmid using primers, E1A.P3: 5’-TCGAATTCGAGCTCTGTTCCAGCAAGTT-3’, E1A.P4: 5’-GAAAGCTTACTCTTGAGTGCCAGCGAG-3’, and subsequently inserted into the Hind III/EcoR I sites of pUK21-CMV-BikDD by ligation (42).
Cell lines and cell culture
The human ovarian cancer cell lines OVCA420, OVCA432, SKOV3.ip1, MDAH2774.c10, and normal lung fibroblasts WI-38 were grown in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 supplemented with 10% fetal bovine serum (Invitrogen). Other human ovarian cancer cell lines, including OVCAR3 and HeyA8, were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. SKOV3.ip1 was established in our laboratory as previously described (43). NOE99, NOE115, and NOE119 were primarily cultured normal ovarian epithelium cells at the time of surgery and were maintained in 1:1 of Medium 119 and MCDB 105 (Sigma-Aldrich, St. Louis, MO) with 10% fetal bovine serum and 10 ng/ml EGF (Sigma). All patients provided informed consent with institutional review board approval of all protocols.
Luciferase-expressing stable cell line
SKOV3.ip1 cells were co-transfected with 4 µg of pGL3-beta-actin promoter-luciferase plasmid and 1 µg pBabe empty vector containing a puromycin-resistance gene using electroporation. The stable clone, SKOV3.ip1-Luc, was generated by selection with 0.5 mg/ml puromycin.
Luciferase reporter assay
To determine promoter activities, cells were transiently co-transfected by DOTAP:cholesterol liposomes (42, 44, 45) complexed with 1 µg plasmid and 0.1 µg pRL-TK (internal control) in 12-well plate as previously described (26). After incubation at 37°C with 5% CO2 for 48 hours, cells were lysed using passive lysis buffer, and luciferase expression was detected by the Dual-Luciferase® Reporter Assay kit according to the commercial protocol (Promega, Madison, WI).
Plasmid preparation for animal injections
Endotoxin free plasmids were purified by Qiagen EndoFree Mega Plasmid kit (Qiagen, Valencia, CA) according to a commercial protocol. Using a chromogenic Limulus amoebocyte clotting assay kit (QCL-1000, BioWhittaker, Walkersville, MD), the endotoxin level was determined to be <10 endotoxin units/mg of DNA. The DNA/DOTAP:cholesterol complexes were prepared as previously described (26) and administered in mice through tail-vein injections.
Ovarian cancer animal model
To determine the anti-tumor effect of CMV-E1A and TV-E1A in vivo, 40 athymic female BALB/c nu/nu mice, 4–6 weeks of age per group were hosts for human orthotopic xenograft tumors. All mice were housed in a specific pathogen-free environment in compliance with institutional policy, and all animal procedures were have been approved by the appropriate institutional review boards. Luciferase-expressing SKOV3.ip1 cells (3 X 106 cells) were injected into peritoneum of the mice. Seven days after inoculation, mice were non-invasively imaged by In Vivo Imaging System (IVIS; Xenogen, Alameda, CA) to assess tumor growth and randomly assigned to one of three groups. Each group (n=12) of mice were treated with 100 µl of DNA:liposome complexes that contained 15 µg of pUK21-T-VISA-E1A, pUK21-CMV-E1A or pUK21-T-VISA (control) administered through tail-vein injection twice per week for 3 consecutive weeks and imaged once per week for a total of 6 weeks prior to each treatment. Tissues were obtained and TUNEL (terminal deoxynucleotidyl transferase [TdT]-mediated dUTP end nick labeling) assays were carried out as previously described to determine the percentage of apoptotic cells (26).
Tissue distribution of luciferase expression
To collect tumors and organs, mice were killed by cervical dislocation. Tissue specimens from tumors and heart and lungs were harvested and homogenized (46). A luminometer (Lumat LB9507; Berthold Technologies, Bad Wildbad, Germany) was used to measure the luciferase activity from each of the cell lysate, and the protein concentration was determined as described previously (46). The luminescence results were reported as relative light units per milligram of protein (RLU/mg protein).
Statistical Analyses
Survival rates were analyzed by log-rank test (Mantel-Cox test). All other data were analyzed using Student’s T-test.
Results
The hTERT promoter is specifically expressed in ovarian cancer cells
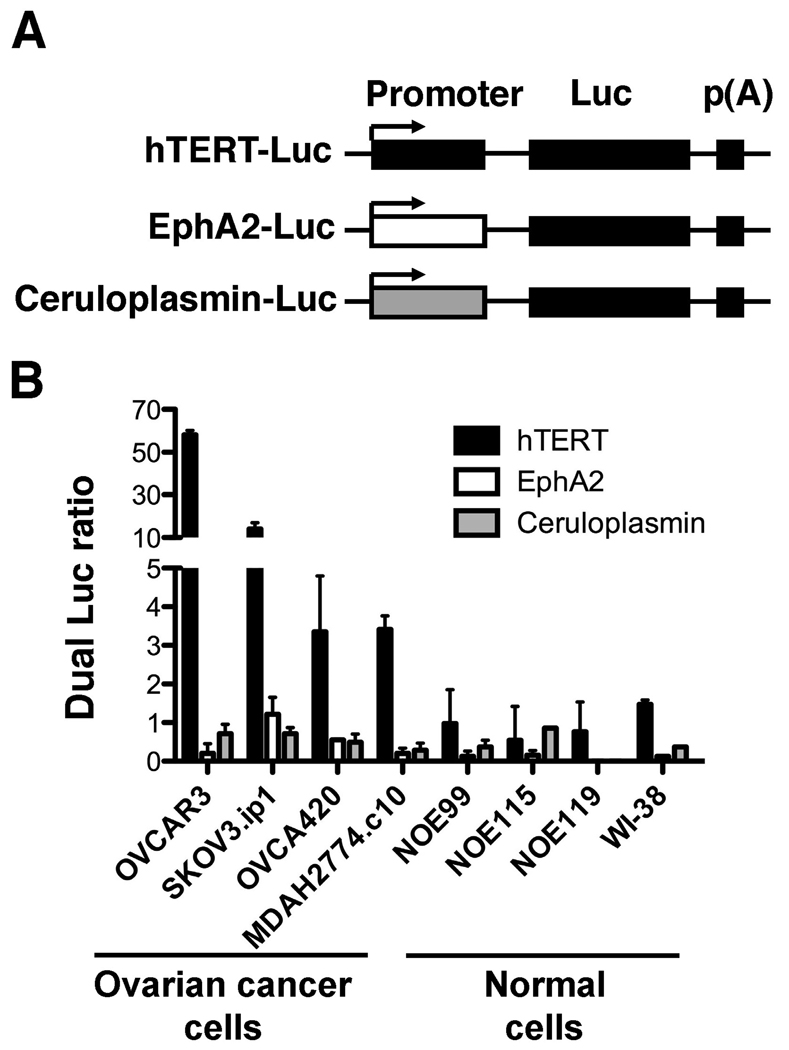
To identify an ovarian cancer-specific promoter, we searched for genes that are overexpressed in ovarian cancer due to upregulation of gene transcription such that their promoter activity would be higher in ovarian cancer cells than in normal cells. Using the Serial Analysis of Gene Expression Database and searching the literature, we found three potential candidates: human telomerase reverse transcriptase (hTERT) (12), EphA2 (13) and ceruloplasmin (10) (Figure 1A). We tested the activities of these three promoters in a panel of ovarian cancer cell lines (OVCAR3, SKOV3.ip1, OVCA420, and MDAH2774.c10) and normal cells (normal ovarian epithelial cells NOE99, NOE115, NOE119 and lung fibroblasts WI-38) using the Dual-luciferase® assay, and found that hTERT had the highest promoter activity in the ovarian cancer cell lines. Most importantly, the promoter remained inactive in normal cells (Figure 1B), suggesting that the hTERT promoter would be an excellent choice as an ovarian cancer-specific promoter to express target gene that can be applied to gene therapy.
Figure 1.
hTERT, EphA2 and ceruloplasmin promoters are active in ovarian cancer. (A) Schematic of the promoter-driven luciferase report plasmids. (B) A panel of ovarian cancer cell lines, normal ovarian epithelial cells (NOE99, NOE115, and NOE119) and fibroblasts (WI-38) were transiently cotransfected with plasmid DNA indicated and pRL-TK, the internal control. At 48h following transfectioin, dual luciferase ratio was measured as the ratio of luciferase normalized to the Renilla luciferase internal control. The data represent the mean of four independent experiments. Bar, SD.
VISA enhances the activity of the hTERT promoter while retaining its specificity
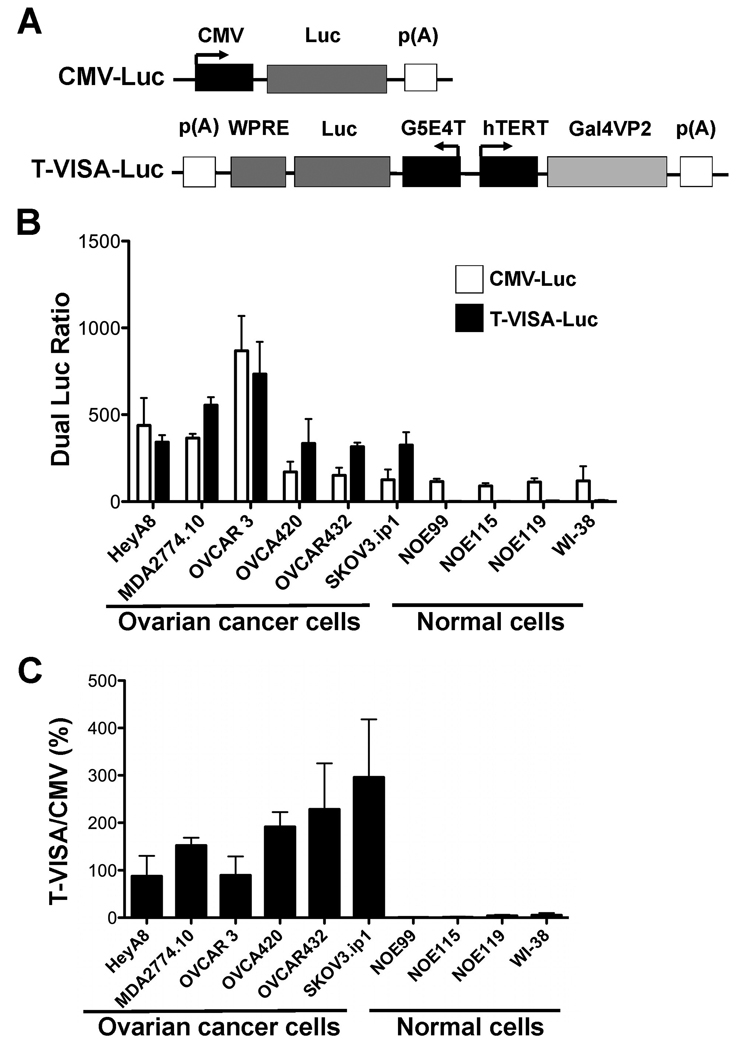
While hTERT was significantly more active in ovarian cancer cells compared with normal cells, its activity was in general less than 5% of the commonly used CMV promoter. Therefore, to enhance the efficiency of the promoter, we incorporated the hTERT promoter into a powerful VISA system that we had previously developed (26) and created the hTERT-VISA (T-VISA) expression vector (Figure 2A). Under the VISA expression platform, the hTERT promoter activity in ovarian cancer cells was dramatically increased to a level comparable or even higher than that of the CMV promoter. However, in normal cells, the activity of T-VISA remained virtually silent compared with that of the CMV promoter (Figure 2B and C). Thus, by controlling target gene expression under the hTERT promoter linked to the VISA system, we not only maintained cancer-specificity, but also achieved superb expression that exceeded the non-specific CMV promoter.
Figure 2.
hTERT-VISA is robust in ovarian cancer cell lines. (A) Schematic diagram of engineered hTERT-VISA (T-VISA) constructs in the pGL3 backbone. (B) Using a panel of ovarian cancer cell lines, normal ovarian epithelial cells (NOE99, NOE115, and NOE119) and normal lung fibroblasts (WI-38), plasmid DNA and pRL-TK were transiently cotransfected with the indicated. Forty-eight hours later, dual luciferase ratio was measured and shown as RLU (ratio) normalized to the Renilla luciferase control. The data represent the mean of four independent experiments. Bar, SD. (C) The percentage of hTERT promoter activity normalized to that of the CMV promoter.
T-VISA is specifically targeted to ovarian tumors in vivo
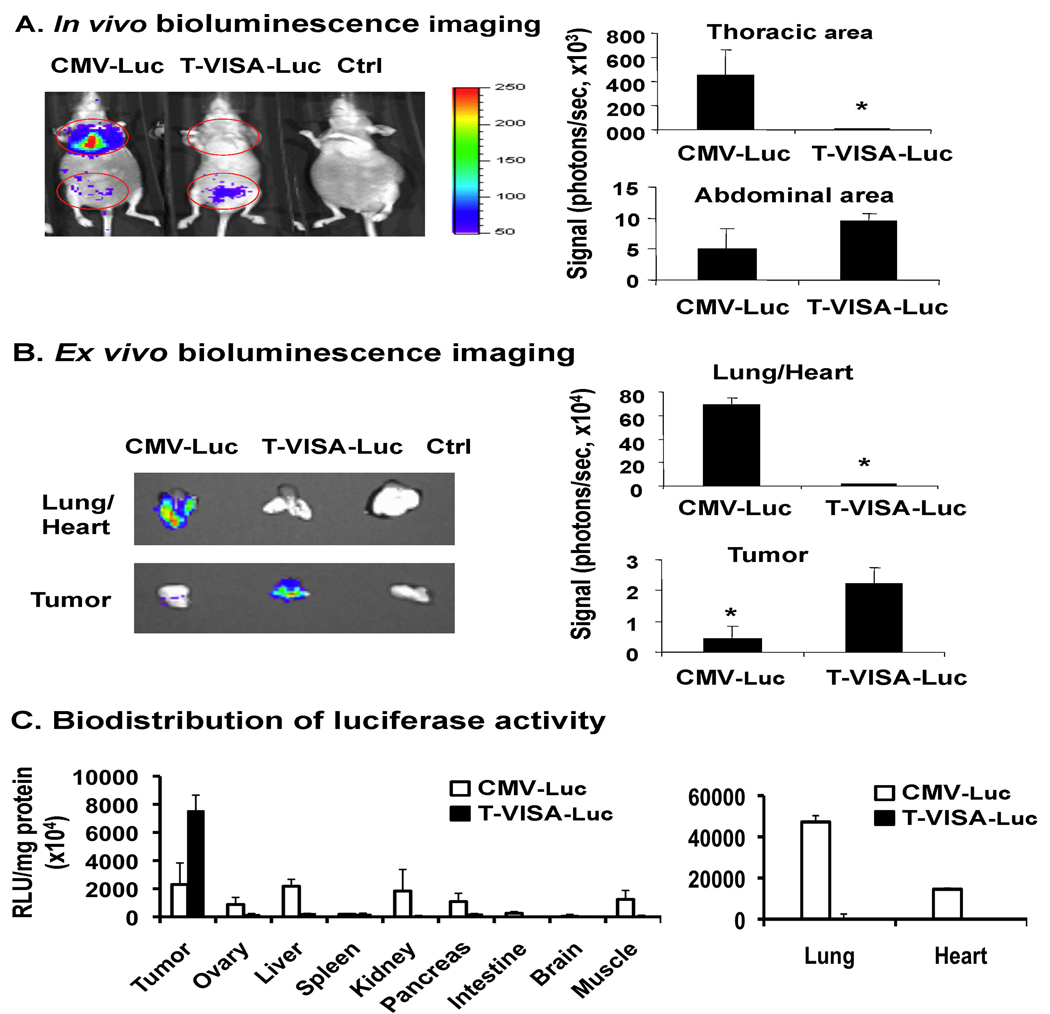
Since we determined that T-VISA was highly specific in ovarian cancer cells in vitro, we asked next whether this specificity also occurs in vivo by using an ovarian cancer orthotopic mouse model. We evaluated the promoter activity by detecting luciferase reporter gene expression after intraperitoneal inoculation of SKOV3.ip1 ovarian cancer cells and tail-vein intravenous injection of the plasmid DNA:liposome complexes carrying either CMV-Luc, T-VISA-Luc or a control vector. As shown in Figure 3A, mice that received CMV-Luc exhibited significant luciferase activity in the lungs, which is the first organ it reaches following tail-vein injection, whereas T-VISA-Luc:liposome complexes expressed luciferase primarily at the site of tumor inoculation and not in the lungs. To further confirm targeted-expression of T-VISA-Luc, we sacrificed mice, harvested the corresponding tissues/organs and imaged them for luciferase expression (Figure 3B). Contrary to the CMV-Luc vector, T-VISA-Luc exhibited high expression in the abdominal area and in tumor, but not in the lungs or heart. In addition, when luciferase expression of T-VISA-Luc was measured in a panel of normal tissues, we found that their luciferase activities were significantly lower than in the tumor (Figure 3C). In another animal model using the HeyA8 cell line, we also demonstrated the specificity of T-VISA-Luc showing a significant amount of luciferase signals in the abdominal, but not in the thoracic area, which contrasts with CMV-Luc (Panel A, Figure S1; Supplemental Data). In addition, T-VISA-Luc targeted not only the primary tumor transplant, but also metastases within the liver. As shown in Panel B of Figure S1, when we harvested the liver, we detected luciferase signals of tumor metastasized to the liver only in mice that were treated with T-VISA-Luc, but not those which received CMV-Luc. Thus, consistent with our in vitro data as well as the SKOV3.ip1 animal model described above, we showed the superiority of T-VISA-Luc over CMV-Luc in cancer-specific target gene expression.
Figure 3.
T-VISA transcriptionally targets transgene expression to SKOV3.ip1 tumors in vivo. (A) Female nude mice bearing orthotopic SKOV3.ip1 tumors were given DNA:liposome complexes containing 50 µg of plasmid DNA via tail vein injection. Two days later, mice were anesthetized and subjected to in vivo imaging 10 min after intraperitoneal injection of D-luciferin for 2 min. (B) SKOV3.ip1 tumors of mice as well as each of their heart/lungs from (A) were subjected to ex vivo imaging. The photon signals were quantified by Xenogen’s Living Imaging software (shown on the right). (C) Tissue specimens from tumor as well as other organs harvested from mice in (A) were measured for luciferase activity expressed as relative light units (RLU) per milligram of total protein. Bars, SD; n=3 per group. The plasmids indicated are as follows: CMV-Luc, pGL3-CMV-Luc; T-VISA-Luc, pGL3-hTERT-VISA-Luc; Ctrl, pGL3-T-VISA. An asterisk indicates a P-value of less than 0.05.
E1A expression under T-VISA inhibited ovarian cancer cell growth
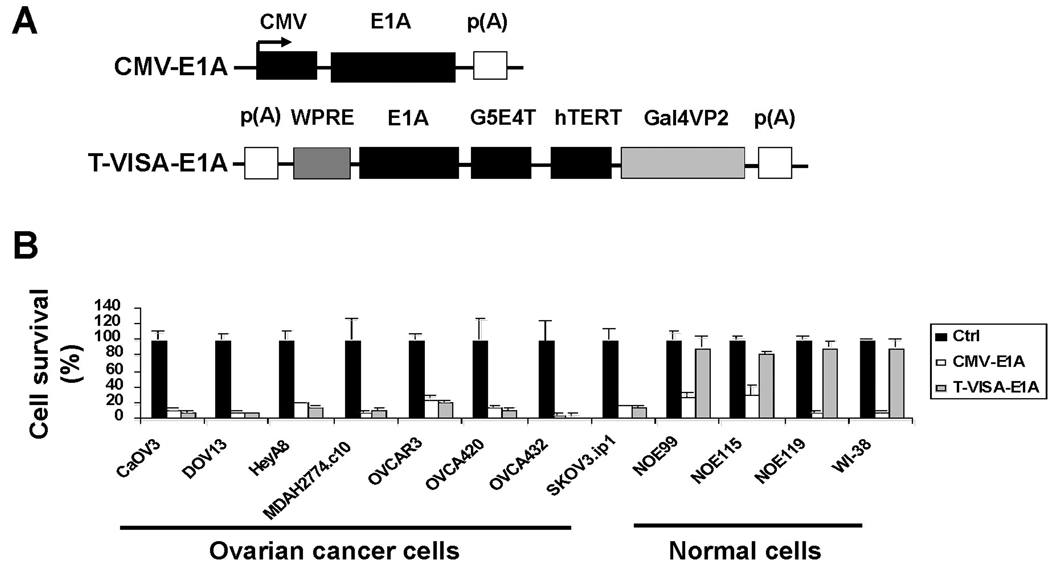
Since the T-VISA expression vector can selectively express target genes in cancer cells but not in normal cells, we engineered a therapeutic T-VISA system that drives expression of E1A (T-VISA-E1A; Figure 4A). Among the eight ovarian cancer cells lines, we showed that T-VISA-E1A inhibited cell growth in vitro at least as effectively as CMV-E1A. However, under the CMV promoter, expression of E1A resulted in a significant decrease in cell survival of normal cells that were otherwise unaffected by T-VISA-E1A (Figure 4B). Therefore, by expressing E1A under the hTERT promoter, we demonstrated that only cell growth of cancer cells, but not normal cells, is inhibited, which is crucial to establish T-VISA-E1A as a potential therapeutic agent for treating ovarian cancer.
Figure 4.
Cell killing activities of CMV-E1A and T-VISA-E1A in ovarian cancer cell lines and normal cells. A panel of ovarian cancer cell lines, normal ovarian epithelial cells, and normal fibroblasts were cotransfected with pUK21-T-VISA-E1A, pUK21-CMV-EIA, or a negative control pUK21-TV plus 100 ng of pGL3-CMV-Luc. The signals were imaged with the IVIS system two days post transfection. The percentages of cell killing are indicated by the loss of luciferase reporter signals with the negative control set at 100%. The data represent the mean of three independent experiments. Bars, SD.
T-VISA-E1A has significant anti-tumor effect against ovarian cancer in human orthotopic xenograft mouse model
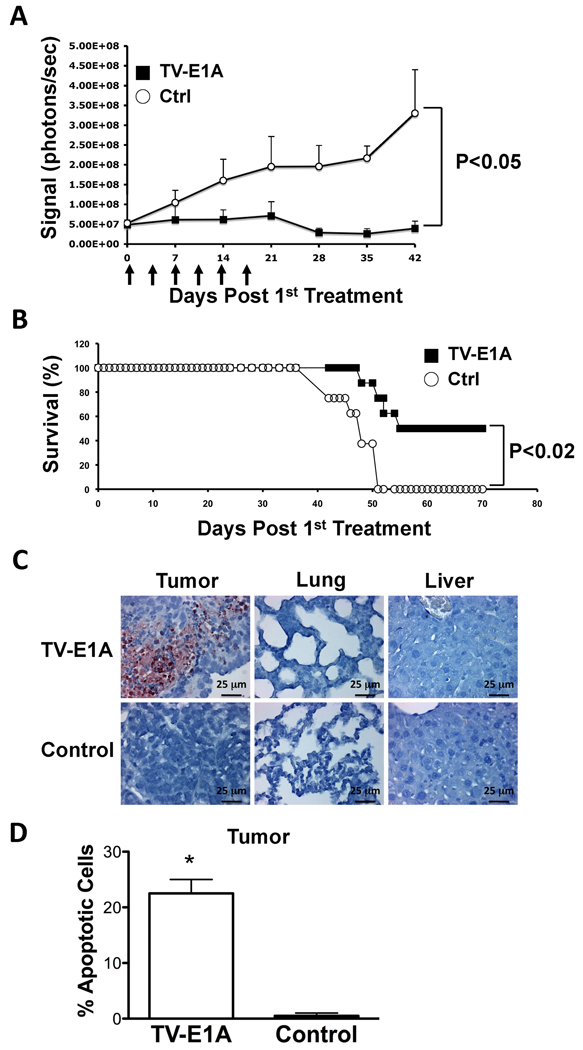
To test potential anti-tumor effect of T-VISA-E1A, we inoculated ovarian cancer cells, SKOV3.ip1-Luc, in nude mice and treated them with either T-VISA-E1A or the control vector. We found that just after two treatments, mice that received T-VISA-E1A inhibited tumor growth as indicated by relatively low luciferase signals, whereas those treated with control vector continued to show increase in tumor growth (Figure 5A). While T-VISA-E1A continued to inhibit tumor growth after six injections, mice that were treated with control vector continued to develop tumor. In addition, T-VISA-E1A treatment significantly prolonged survival in mice compared with those that were treated with the control vector (Figure 5B). We further isolated tissue samples from tumor, lung and liver of the treated mice and demonstrated by TUNEL assay (terminal deoxynucleotidyl transferase [TdT]-mediated dUTP nick end labeling) that only in the tumor, but not the lungs or the liver, did we observe apoptosis (Figure 5C). Thus, this further supports the tumor-specificity of the T-VISA-E1A vector in vivo. In addition, mice that were administered with the T-VISA-E1A vector showed more potent apoptosis compared with the control vector as indicated by the percentages of apoptosis shown in Figure 5D.
Figure 5.
T-VISA-E1A inhibited tumor growth in orthotopic xenograft animal model. (A) Nude mice bearing SKOV3.ip1-Luc tumors were i.v. injected with DNA:liposome complexes containing 15 µg of plasmid TV-E1A (■) or control (○) at time points indicated by the arrows. The photon signals were quantified by Xenogen’s living imaging software. n=12 mice/group. (B) Survival rates of mice observed for 70 days after first treatment. (C) Analysis of in vivo apoptosis tissue samples of tumor, lung and liver harvested from mice treated with either TV-E1A or control DNA-liposome complexes in panel A. (D) The percentages of apoptotic cells from two fields of the indicated tumor specimens in panel B. An asterisk indicates a P-value of less than 0.02.
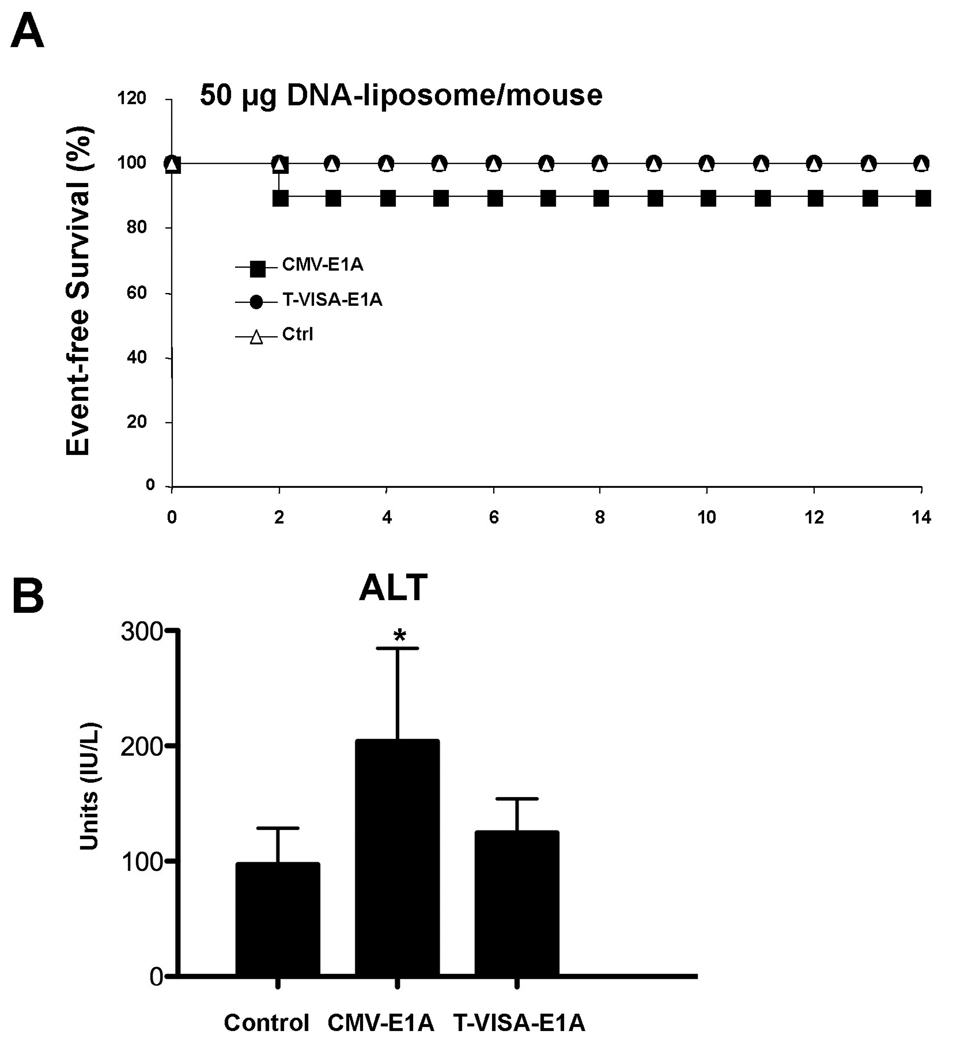
As T-VISA produces little gene expression in normal cells/tissues, we expected that T-VISA would produce fewer side effects and less toxicity than a CMV promoter-based vector. To document the safety of injected T-VISA DNA:liposome complexes we measured systemic toxicity at a dosage of 50 µg per mouse. Indeed, we showed that all mice survived following injection of T-VISA-E1A, whereas 10% of mice died after receiving CMV-E1A (Figure 6A). Moreover, when we measured the alanine aminotransferase (ALT) level to evaluate the presence of liver toxicity, we showed that the ALT level was significantly increased in mice treated with CMV-E1A compared with those injected with control vector, but not in mice that were treated with T-VISA-E1A (Figure 6B).
Figure 6.
Acute toxicity study of T-VISA-E1A versus CMV-E1A. Female CD-1 mice were given single doses of DNA:liposome complexes containig 50 µg plasmid DNA via the tail vein. Kaplan-Meier survival analysis (A) and serum levels of alanine aminotransferase (ALT) 2 days after injection (B) were determined. An asterisk indicates a P-value of less than 0.02.
Discussion
Telomerase (hTERT) has become an interesting and important target for cancer therapeutics as positive telomerase expression, which allows tumors to maintain their telomeres leading to long-term survival, is present in a majority of cancer types (47). In addition, as little telomerase is expressed in normal cells, use of its promoter to drive a target gene could provide cancer-specificity and thus minimize expression in normal cells. Several reports have explored this possibility using viral vectors to express target gene under the hTERT promoter (48). Use of non-viral vectors would, however, be ideal, since viral vector-mediated gene therapy can produce serious complications (49, 50). In this study, we developed an innovative vector that combined the hTERT promoter with a powerful non-viral expression vector called VISA (26). The VISA system can 1) selectively express target gene in ovarian cancer cells under the hTERT promoter; 2) amplify target gene without the use of viral promoter such as CMV; and 3) eliminate the risks that are associated with using viral-mediated gene delivery.
In addition, we employed the T-VISA vector in attempt to develop a novel treatment for ovarian cancer and demonstrated that hTERT maintained cancer-specificity while VISA boosted expression of the target gene in both in vitro and in vivo models. In vitro in normal ovarian epithelial cells, hTERT-driven transgene expression was notably absent. In vivo in human orthotopic xenograft mouse models, we showed that the T-VISA vector targeted primary tumor (SKOV3.ip1) as well as metastatic sites (HeyA8) without any expression in the lungs/heart. When we applied this hTERT-VISA system for expression of E1A, which has anti-tumor activity, it successfully inhibited tumor growth in mouse model. Thus far, we have demonstrated that the use of T-VISA-E1A vector as a potential therapeutic option to combat ovarian cancer.
We selected E1A as the therapeutic gene, which has been known to induce bystander effect (35) as well as sensitize cancer cells to chemotherapeutic agent (38), and was developed into a Phase I/II trial entitled “A Phase I/II Randomized Study of Intraperitoneal tgDCC-E1A and Intravenous Paclitaxel in Women with Platinum-Resistant Ovarian Cancer”. Now that we are able to overcome the limitation of efficient gene expression through the use of non-viral vector, T-VISA-E1A would undoubtedly be an excellent candidate for future development into clinical trial, and could have potential impact on ovarian cancer treatment pending additional tests to validate this approach. Since human telomerase is known to be active in other cancer types, it is possible to expand our T-VISA system to additional cancer models such as breast and prostate by expressing the appropriate therapeutic gene to target cancer cells without risking surrounding normal cells/tissues.
Supplementary Material
Acknowledgements
We are grateful to Dr. Anil K. Sood for providing ovarian cancer cell lines. We thank Dr. Xinbin Chen (Department of Cell Biology, University of Alabama at Birmingham) for providing the plasmid containing the EphA2 promoter, and also Dr. Zhenbo Han for preparation of the DOTAP:cholesterol liposomes. The work is partially supported by the Sister Institution Fund of M.D. Anderson Cancer Center and China Medical University, Ovarian Cancer SPORE (MDACC, P50 CA83639), Pancreatic Cancer SPORE (MDACC, P20 CA101936), Breast Cancer SPORE (MDACC, P50 CA116199), and the Marcus Foundation.
Nonstandard abbreviations used
- VISA
VP-16-GAL4-WPRE integrated systemic amplifier
- WPRE
woodchuck hepatitis virus post-translational regulatory element
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Matei D. Novel agents in ovarian cancer. Expert Opin Investig Drugs. 2007;16:1227–1239. doi: 10.1517/13543784.16.8.1227. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi GN, Ueno NT, Xia W, et al. Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biologic effects: a phase I clinical trial. J Clin Oncol. 2001;19:3422–3433. doi: 10.1200/JCO.2001.19.14.3422. [DOI] [PubMed] [Google Scholar]
- 5.Ueno NT, Yu D, Hung MC. E1A: tumor suppressor or oncogene? Preclinical and clinical investigations of E1A gene therapy. Breast Cancer. 2001;8:285–293. doi: 10.1007/BF02967526. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Hung MC, Wagner E, editors. Non-Viral Vectors for Gene Therapy. Second Edition: Part II. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- 7.Xie X, Zhao X, Liu Y, et al. Robust prostate-specific expression for targeted gene therapy based on the human kallikrein 2 promoter. Hum Gene Ther. 2001;12:549–561. doi: 10.1089/104303401300042483. [DOI] [PubMed] [Google Scholar]
- 8.Bao R, Connolly DC, Murphy M, et al. Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst. 2002;94:522–528. doi: 10.1093/jnci/94.7.522. [DOI] [PubMed] [Google Scholar]
- 9.Barker SD, Dmitriev IP, Nettelbeck DM, et al. Combined transcriptional and transductional targeting improves the specificity and efficacy of adenoviral gene delivery to ovarian carcinoma. Gene Ther. 2003;10:1198–1204. doi: 10.1038/sj.gt.3301974. [DOI] [PubMed] [Google Scholar]
- 10.Lee CM, Lo HW, Shao RP, et al. Selective activation of ceruloplasmin promoter in ovarian tumors: potential use for gene therapy. Cancer Res. 2004;64:1788–1793. doi: 10.1158/0008-5472.can-03-2551. [DOI] [PubMed] [Google Scholar]
- 11.Peng XY, Won JH, Rutherford T, et al. The use of the L-plastin promoter for adenoviral-mediated, tumor-specific gene expression in ovarian and bladder cancer cell lines. Cancer Res. 2001;61:4405–4413. [PubMed] [Google Scholar]
- 12.Tanyi JL, Lapushin R, Eder A, et al. Identification of tissue- and cancer-selective promoters for the introduction of genes into human ovarian cancer cells. Gynecol Oncol. 2002;85:451–458. doi: 10.1006/gyno.2002.6644. [DOI] [PubMed] [Google Scholar]
- 13.Thaker PH, Deavers M, Celestino J, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–5150. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 14.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 15.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 16.Hiyama E, Hiyama K. Telomerase as tumor marker. Cancer Lett. 2003;194:221–233. doi: 10.1016/s0304-3835(02)00709-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim NW. Clinical implications of telomerase in cancer. Eur J Cancer. 1997;33:781–786. doi: 10.1016/S0959-8049(97)00057-9. [DOI] [PubMed] [Google Scholar]
- 18.Counter CM, Hirte HW, Bacchetti S, Harley CB. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994;91:2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyo S, Kanaya T, Takakura M, et al. Expression of human telomerase subunits in ovarian malignant, borderline and benign tumors. Int J Cancer. 1999;80:804–809. doi: 10.1002/(sici)1097-0215(19990315)80:6<804::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Murakami J, Nagai N, Ohama K, Tahara H, Ide T. Telomerase activity in ovarian tumors. Cancer. 1997;80:1085–1092. [PubMed] [Google Scholar]
- 21.Braunstein I, Cohen-Barak O, Shachaf C, et al. Human telomerase reverse transcriptase promoter regulation in normal and malignant human ovarian epithelial cells. Cancer Res. 2001;61:5529–5536. [PubMed] [Google Scholar]
- 22.Pang S, Dannull J, Kaboo R, et al. Identification of a positive regulatory element responsible for tissue-specific expression of prostate-specific antigen. Cancer Res. 1997;57:495–499. [PubMed] [Google Scholar]
- 23.Qiao J, Doubrovin M, Sauter BV, et al. Tumor-specific transcriptional targeting of suicide gene therapy. Gene Ther. 2002;9:168–175. doi: 10.1038/sj.gt.3301618. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds PN, Nicklin SA, Kaliberova L, et al. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat Biotechnol. 2001;19:838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- 25.Schuur ER, Henderson GA, Kmetec LA, Miller JD, Lamparski HG, Henderson DR. Prostate-specific antigen expression is regulated by an upstream enhancer. J Biol Chem. 1996;271:7043–7051. doi: 10.1074/jbc.271.12.7043. [DOI] [PubMed] [Google Scholar]
- 26.Xie X, Xia W, Li Z, et al. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Nevins JR. Adenovirus E1A-dependent trans-activation of transcription. Semin Cancer Biol. 1990;1:59–68. [PubMed] [Google Scholar]
- 28.Nevins JR, Raychaudhuri P, Yee AS, Rooney RJ, Kovesdi I, Reichel R. Transactivation by the adenovirus E1A gene. Biochem Cell Biol. 1988;66:578–583. doi: 10.1139/o88-068. [DOI] [PubMed] [Google Scholar]
- 29.Yu D, Hung MC. The erbB2 gene as a cancer therapeutic target and the tumor- and metastasis-suppressing function of E1A. Cancer Metastasis Rev. 1998;17:195–202. doi: 10.1023/a:1006054421970. [DOI] [PubMed] [Google Scholar]
- 30.Chang JY, Xia W, Shao R, et al. The tumor suppression activity of E1A in HER-2/neu-overexpressing breast cancer. Oncogene. 1997;14:561–568. doi: 10.1038/sj.onc.1200861. [DOI] [PubMed] [Google Scholar]
- 31.Ueno NT, Yu D, Hung MC. Chemosensitization of HER-2/neu-overexpressing human breast cancer cells to paclitaxel (Taxol) by adenovirus type 5 E1A. Oncogene. 1997;15:953–960. doi: 10.1038/sj.onc.1201250. [DOI] [PubMed] [Google Scholar]
- 32.Madhusudan S, Tamir A, Bates N, et al. A multicenter Phase I gene therapy clinical trial involving intraperitoneal administration of E1A-lipid complex in patients with recurrent epithelial ovarian cancer overexpressing HER-2/neu oncogene. Clin Cancer Res. 2004;10:2986–2996. doi: 10.1158/1078-0432.ccr-03-0291. [DOI] [PubMed] [Google Scholar]
- 33.Villaret D, Glisson B, Kenady D, et al. A multicenter phase II study of tgDCC-E1A for the intratumoral treatment of patients with recurrent head and neck squamous cell carcinoma. Head Neck. 2002;24:661–669. doi: 10.1002/hed.10107. [DOI] [PubMed] [Google Scholar]
- 34.Yoo GH, Hung MC, Lopez-Berestein G, et al. Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin Cancer Res. 2001;7:1237–1245. [PubMed] [Google Scholar]
- 35.Shao R, Xia W, Hung MC. Inhibition of angiogenesis and induction of apoptosis are involved in E1A-mediated bystander effect and tumor suppression. Cancer Res. 2000;60:3123–3126. [PubMed] [Google Scholar]
- 36.Liao Y, Hung MC. Regulation of the activity of p38 mitogen-activated protein kinase by Akt in cancer and adenoviral protein E1A-mediated sensitization to apoptosis. Mol Cell Biol. 2003;23:6836–6848. doi: 10.1128/MCB.23.19.6836-6848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y, Hung MC. A new role of protein phosphatase 2a in adenoviral E1A protein-mediated sensitization to anticancer drug-induced apoptosis in human breast cancer cells. Cancer Res. 2004;64:5938–5942. doi: 10.1158/0008-5472.CAN-04-1533. [DOI] [PubMed] [Google Scholar]
- 38.Liao Y, Zou YY, Xia WY, Hung MC. Enhanced paclitaxel cytotoxicity and prolonged animal survival rate by a nonviral-mediated systemic delivery of E1A gene in orthotopic xenograft human breast cancer. Cancer Gene Ther. 2004;11:594–602. doi: 10.1038/sj.cgt.7700743. [DOI] [PubMed] [Google Scholar]
- 39.Takakura M, Kyo S, Kanaya T, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 40.Zhang L, Johnson M, Le KH, et al. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. 2003;63:4552–4560. [PubMed] [Google Scholar]
- 41.Vieira J, Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- 42.Chen JS, Liu JC, Shen L, et al. Cancer-specific activation of the survivin promoter and its potential use in gene therapy. Cancer Gene Ther. 2004 doi: 10.1038/sj.cgt.7700752. [DOI] [PubMed] [Google Scholar]
- 43.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–898. [PubMed] [Google Scholar]
- 44.Day CP, Rau KM, Qiu L, et al. Mutant Bik expression mediated by the enhanced minimal topoisomerase IIalpha promoter selectively suppressed breast tumors in an animal model. Cancer Gene Ther. 2006;13:706–719. doi: 10.1038/sj.cgt.7700945. [DOI] [PubMed] [Google Scholar]
- 45.Templeton NS, Lasic DD, Frederik PM, Strey HH, Roberts DD, Pavlakis GN. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol. 1997;15:647–652. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 46.Xie X, Luo Z, Slawin KM, Spencer DM. The EZC-prostate model: noninvasive prostate imaging in living mice. Mol Endocrinol. 2004;18:722–732. doi: 10.1210/me.2003-0316. [DOI] [PubMed] [Google Scholar]
- 47.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 48.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- 50.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.