Abstract
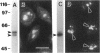
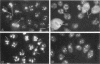
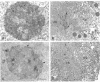
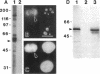
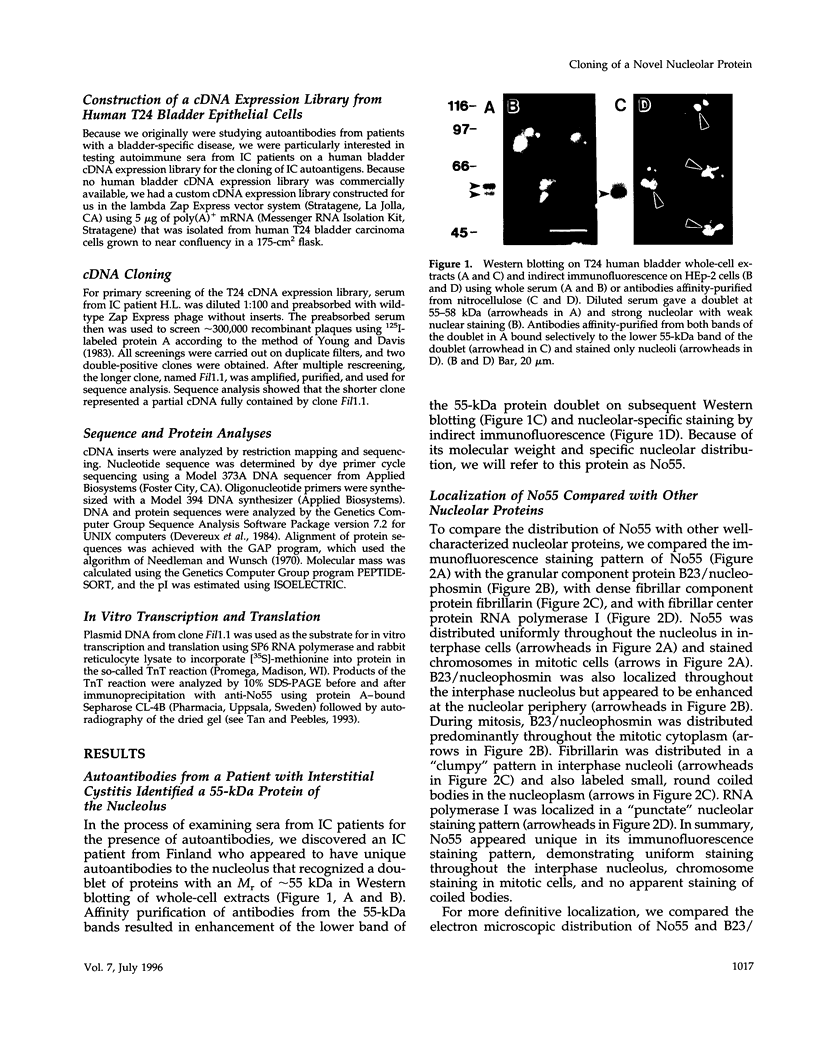
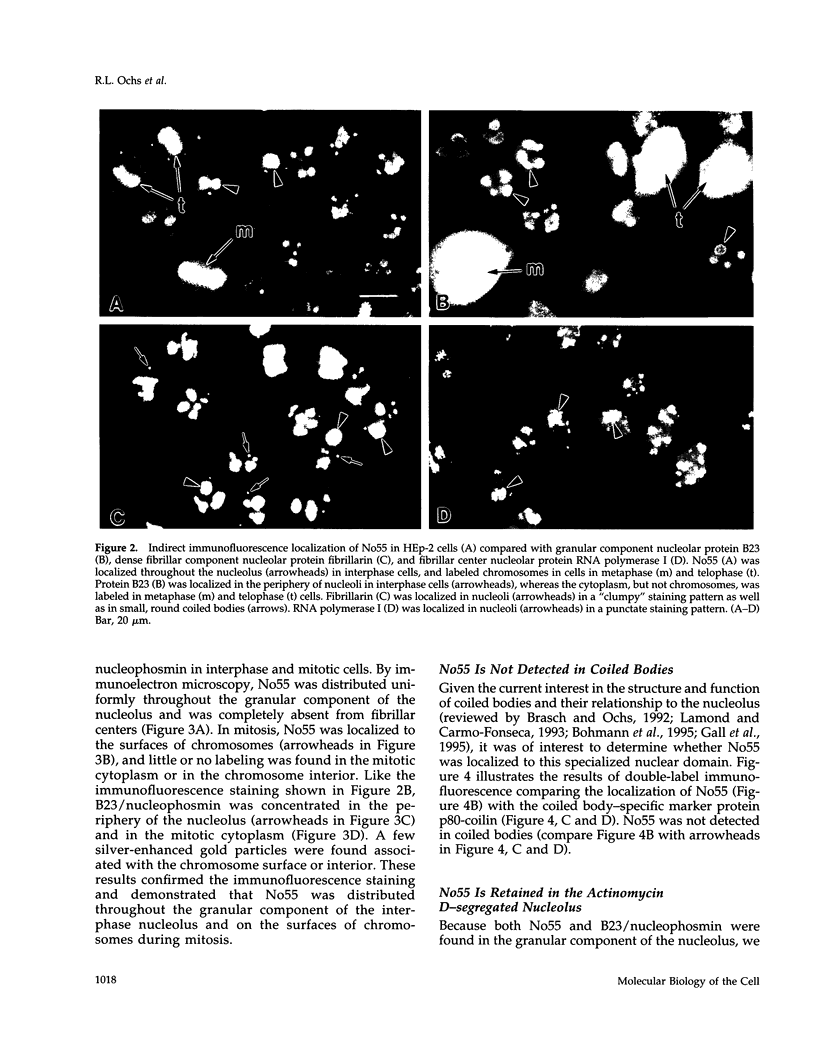
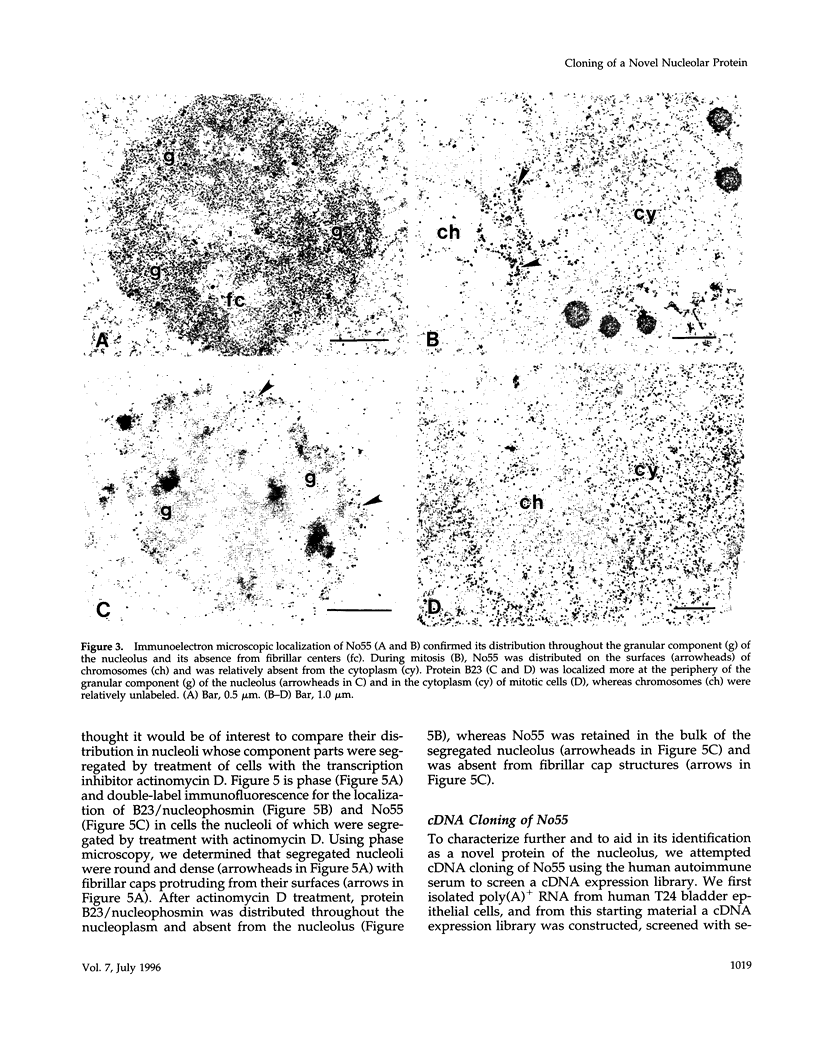
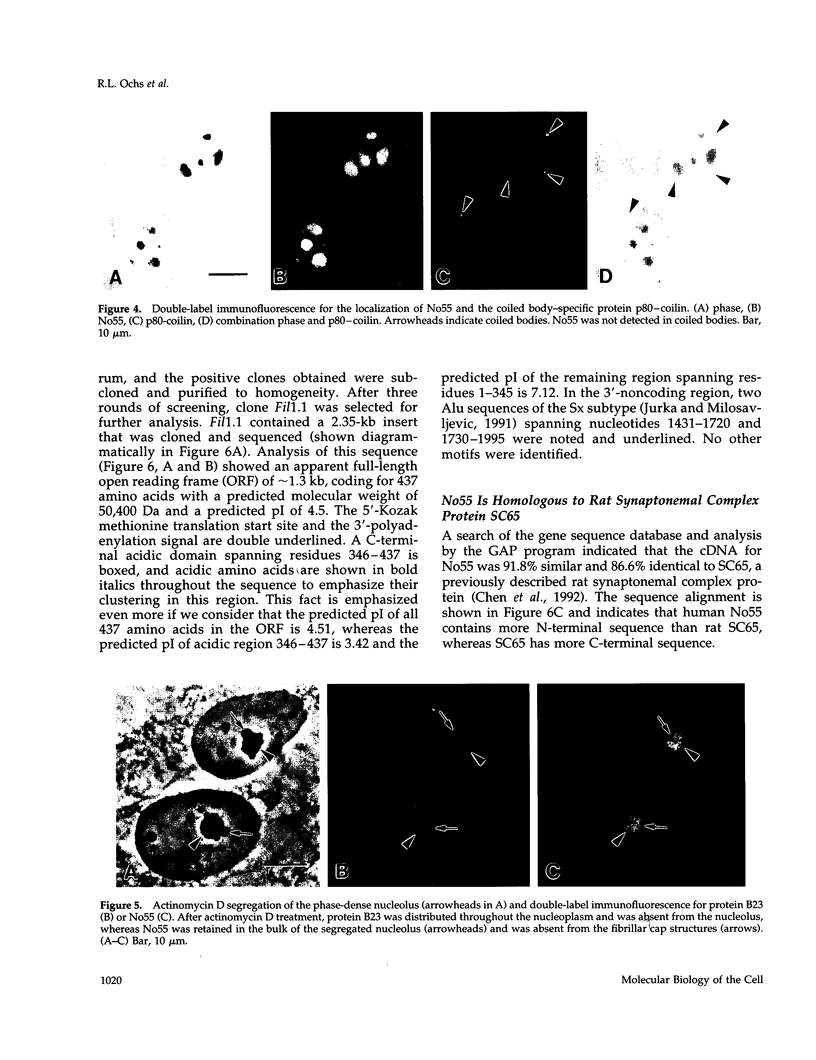
In an initial study of anti-nuclear antibodies in the chronic inflammatory bladder disease interstitial cystitis, we reported that 7% of interstitial cystitis patients studied had autoantibodies to the nucleolus. We now report that, using an autoimmune serum from a patient with interstitial cystitis, we have identified and partially characterized a novel protein with an M(r) of approximately 55 kDa (hereafter referred to as No55) localized to the granular component of the nucleolus. No55 was initially characterized by diffuse nucleolar immunofluorescence staining in interphase cells and by Western blotting as a 55-kDa doublet on whole-cell extracts. During mitosis, No55 was associated with chromosomes and appeared in prenucleolar bodies during telophase, but it did not colocalize with p80-coilin in coiled bodies. Immunoelectron microscopy revealed that No55 was localized uniformly throughout the granular component of the nucleolus compared with a more peripheral localization of nucleolar granular component protein B23. On segregation of the nucleolus with actinomycin D, No55 remained with the granular component of the segregated nucleolus, whereas protein B23 was found predominantly in the nucleoplasm. Finally, a cDNA expression library was screened with the human autoantibody against No55, and a 2.4-kb insert was isolated, subcloned to homogeneity, and then sequenced. Analysis of this sequence showed an open reading frame of approximately 1.3 kb coding for 437 amino acids with a predicted molecular weight of 50 kDa. A search of the gene sequence database indicated homology with SC65, a rat synaptonemal complex protein. Therefore, on the basis of molecular weight, nucleolar sublocalization, response to actinomycin D, and cDNA sequence determination, No55 is a novel protein of the interphase nucleolus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade L. E., Tan E. M., Chan E. K. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1947–1951. doi: 10.1073/pnas.90.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann K., Ferreira J., Santama N., Weis K., Lamond A. I. Molecular analysis of the coiled body. J Cell Sci Suppl. 1995;19:107–113. doi: 10.1242/jcs.1995.supplement_19.16. [DOI] [PubMed] [Google Scholar]
- Brasch K., Ochs R. L. Nuclear bodies (NBs): a newly "rediscovered" organelle. Exp Cell Res. 1992 Oct;202(2):211–223. doi: 10.1016/0014-4827(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Pearlman R. E., Moens P. B. Isolation and characterization of a cDNA encoding a synaptonemal complex protein. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1030–1038. doi: 10.1139/o92-147. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser M. E., Moses M. J. Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griseus). IV. Light and electron microscopy of synapsis and nucleolar development by silver staining. Chromosoma. 1980;76(1):1–22. doi: 10.1007/BF00292222. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Bernat R. L. Chromosomal passengers: toward an integrated view of mitosis. Chromosoma. 1991 Mar;100(3):139–146. doi: 10.1007/BF00337241. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Tsvetkov A., Wu Z., Murphy C. Is the sphere organelle/coiled body a universal nuclear component? Dev Genet. 1995;16(1):25–35. doi: 10.1002/dvg.1020160107. [DOI] [PubMed] [Google Scholar]
- Gautier T., Dauphin-Villemant C., André C., Masson C., Arnoult J., Hernandez-Verdun D. Identification and characterization of a new set of nucleolar ribonucleoproteins which line the chromosomes during mitosis. Exp Cell Res. 1992 May;200(1):5–15. doi: 10.1016/s0014-4827(05)80065-5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D., Gautier T. The chromosome periphery during mitosis. Bioessays. 1994 Mar;16(3):179–185. doi: 10.1002/bies.950160308. [DOI] [PubMed] [Google Scholar]
- Heyting C., Dietrich A. J., Moens P. B., Dettmers R. J., Offenberg H. H., Redeker E. J., Vink A. C. Synaptonemal complex proteins. Genome. 1989;31(1):81–87. doi: 10.1139/g89-016. [DOI] [PubMed] [Google Scholar]
- Ierardi L. A., Moss S. B., Bellvé A. R. Synaptonemal complexes are integral components of the isolated mouse spermatocyte nuclear matrix. J Cell Biol. 1983 Jun;96(6):1717–1726. doi: 10.1083/jcb.96.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H., Ochs R. L., Kiyosawa K., Furuta S., Nakamura R. M., Tan E. M. Nucleolar antigens and autoantibodies in hepatocellular carcinoma and other malignancies. Am J Pathol. 1992 Apr;140(4):859–870. [PMC free article] [PubMed] [Google Scholar]
- Jiménez-García L. F., Segura-Valdez M. L., Ochs R. L., Rothblum L. I., Hannan R., Spector D. L. Nucleologenesis: U3 snRNA-containing prenucleolar bodies move to sites of active pre-rRNA transcription after mitosis. Mol Biol Cell. 1994 Sep;5(9):955–966. doi: 10.1091/mbc.5.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J., Milosavljevic A. Reconstruction and analysis of human Alu genes. J Mol Evol. 1991 Feb;32(2):105–121. doi: 10.1007/BF02515383. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Carmo-Fonseca M. The coiled body. Trends Cell Biol. 1993 Jun;3(6):198–204. doi: 10.1016/0962-8924(93)90214-l. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Meier U. T., Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994 Dec;127(6 Pt 1):1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Heyting C., Dietrich A. J., van Raamsdonk W., Chen Q. Synaptonemal complex antigen location and conservation. J Cell Biol. 1987 Jul;105(1):93–103. doi: 10.1083/jcb.105.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Pearlman R. E. Satellite DNA I in chromatin loops of rat pachytene chromosomes and in spermatids. Chromosoma. 1989 Oct;98(4):287–294. doi: 10.1007/BF00327315. [DOI] [PubMed] [Google Scholar]
- Neal D. E., Jr, Dilworth J. P., Kaack M. B. Tamm-Horsfall autoantibodies in interstitial cystitis. J Urol. 1991 Jan;145(1):37–39. doi: 10.1016/s0022-5347(17)38241-1. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Smetana K. Detection of fibrillarin in nucleolar remnants and the nucleolar matrix. Exp Cell Res. 1991 Dec;197(2):183–190. doi: 10.1016/0014-4827(91)90421-p. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Stein T. W., Jr, Peebles C. L., Gittes R. F., Tan E. M. Autoantibodies in interstitial cystitis. J Urol. 1994 Mar;151(3):587–592. doi: 10.1016/s0022-5347(17)35023-1. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Stein T. W., Jr, Tan E. M. Coiled bodies in the nucleolus of breast cancer cells. J Cell Sci. 1994 Feb;107(Pt 2):385–399. doi: 10.1242/jcs.107.2.385. [DOI] [PubMed] [Google Scholar]
- Ochs R., Lischwe M., O'Leary P., Busch H. Localization of nucleolar phosphoproteins B23 and C23 during mitosis. Exp Cell Res. 1983 Jun;146(1):139–149. doi: 10.1016/0014-4827(83)90332-4. [DOI] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Reimer G., Rose K. M., Scheer U., Tan E. M. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987 Jan;79(1):65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi Y., Powers J., Bolla R. I., Van Nguyen T., Schlessinger D. Proteins and RNA in mouse L cell core nucleoli and nucleolar matrix. Biochemistry. 1986 Sep 23;25(19):5745–5751. doi: 10.1021/bi00367a059. [DOI] [PubMed] [Google Scholar]
- Smith A., Benavente R. Identification of a structural protein component of rat synaptonemal complexes. Exp Cell Res. 1992 Feb;198(2):291–297. doi: 10.1016/0014-4827(92)90382-i. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mühlen C. A., Tan E. M. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum. 1995 Apr;24(5):323–358. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]
- von Wettstein D., Rasmussen S. W., Holm P. B. The synaptonemal complex in genetic segregation. Annu Rev Genet. 1984;18:331–413. doi: 10.1146/annurev.ge.18.120184.001555. [DOI] [PubMed] [Google Scholar]