Abstract
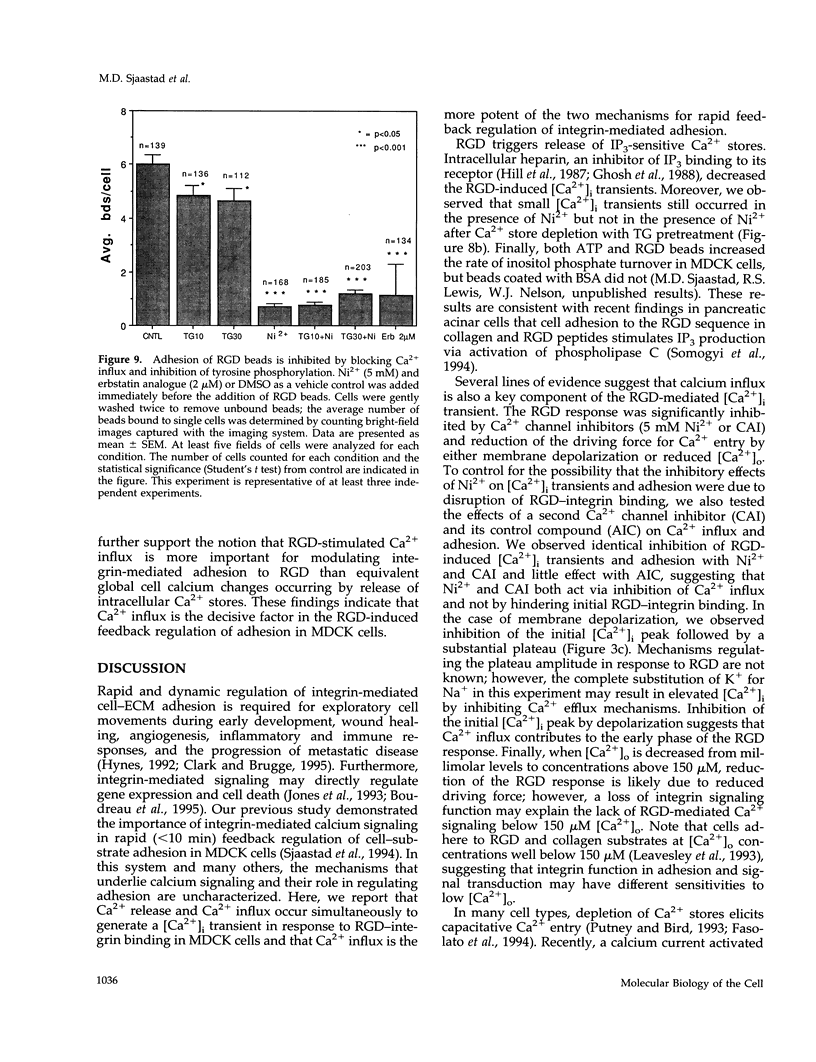
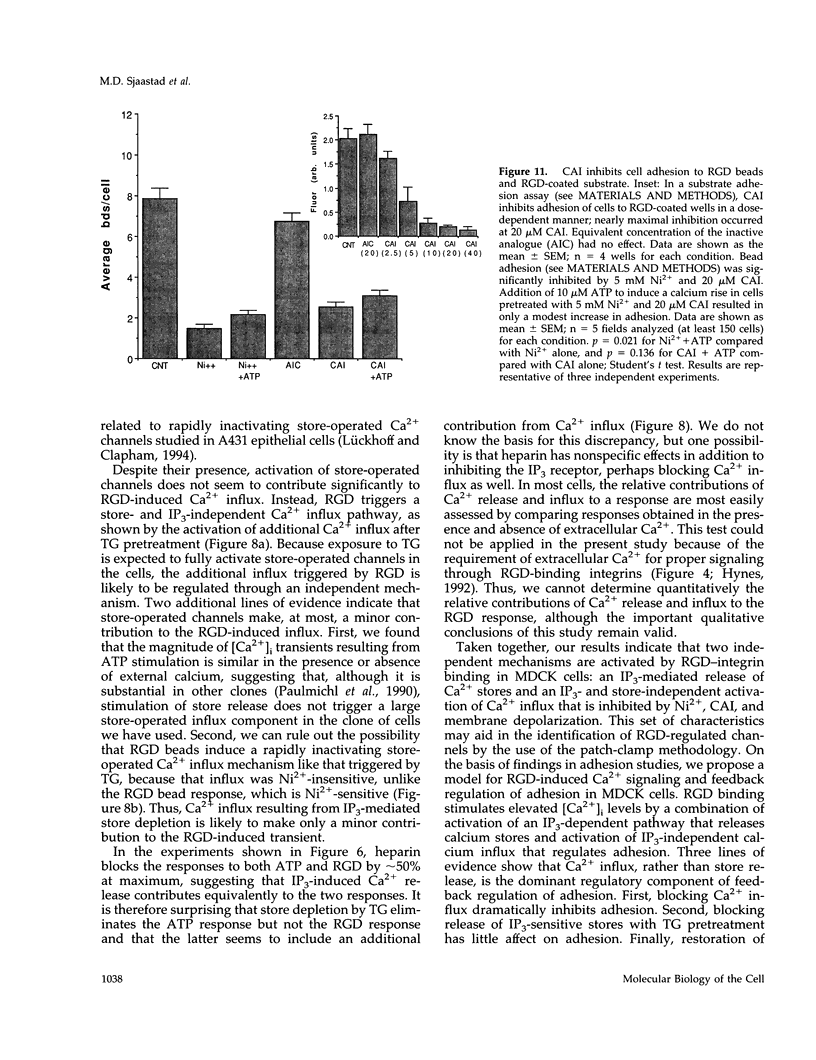
Peptides containing Arg-Gly-Asp (RGD) immobilized on beads bind to integrins and trigger biphasic, transient increases in intracellular free Ca2+ ([Ca2+]i) in Madin-Darby canine kidney epithelial cells. The [Ca2+]i increase participates in feedback regulation of integrin-mediated adhesion in these cells. We examined influx pathways and inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ store release as possible sources of the [Ca2+]i rise. The RGD-induced [Ca2+]i response requires external Ca2+ (threshold approximately 150 microM), and its magnitude is proportional to extracellular calcium. RGD-induced transients were attenuated by Ca2+ channel inhibitors (Ni2+ and carboxy-amidotriazole) or by plasma membrane depolarization, indicating that Ca2+ influx contributes to the response. Loading cells with heparin reduced the size of RGD-induced [Ca2+]i transients, indicating that IP3-mediated release of Ca2+ from stores may also contribute to the RGD response. Depletion of Ca2+ stores with thapsigargin activated Ni(2+)-sensitive Ca2+ influx that might also be expected to occur after IP3-mediated depletion of stored Ca2-. However, RGD elicited a Ni(2+)-sensitive Ca2+ influx even after pretreatment with thapsigargin, indicating that Ca2+ influx is controlled by a mechanism independent of IP3-mediated store depletion. We conclude that RGD-induced [Ca2+]i transients in Madin-Darby canine kidney cells result primarily from the combination of two distinct mechanisms: 1) IP3-mediated release of intracellular stores, and 2) activation of a Ca2+ influx pathway regulated independently of IP3 and Ca2+ store release. Because Ni2+ and carboxy-amidotriazole inhibited adhesion, whereas store depletion with thapsigargin had little effect, we suggest that the Ca2+ influx mechanism is most important for feedback regulation of integrin-mediated adhesion by increased [Ca2+]i.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo A. G., Campanero M. R., Sánchez-Mateos P., Zapata J. M., Ursa M. A., del Pozo M. A., Sánchez-Madrid F. Induction of tyrosine phosphorylation during ICAM-3 and LFA-1-mediated intercellular adhesion, and its regulation by the CD45 tyrosine phosphatase. J Cell Biol. 1994 Sep;126(5):1277–1286. doi: 10.1083/jcb.126.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R. A., Schieven G. L., Watson S. P. Collagen stimulates tyrosine phosphorylation of phospholipase C-gamma 2 but not phospholipase C-gamma 1 in human platelets. FEBS Lett. 1994 Oct 17;353(2):212–216. doi: 10.1016/0014-5793(94)01037-4. [DOI] [PubMed] [Google Scholar]
- Boitano S., Dirksen E. R., Sanderson M. J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992 Oct 9;258(5080):292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Sympson C. J., Werb Z., Bissell M. J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995 Feb 10;267(5199):891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Montgomery A. M., Rosenfeld M., Reisfeld R. A., Hu T., Klier G., Cheresh D. A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994 Dec 30;79(7):1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chenu C., Colucci S., Grano M., Zigrino P., Barattolo R., Zambonin G., Baldini N., Vergnaud P., Delmas P. D., Zallone A. Z. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol. 1994 Nov;127(4):1149–1158. doi: 10.1083/jcb.127.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Integrins and signal transduction pathways: the road taken. Science. 1995 Apr 14;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol. 1992 Oct;4(5):772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Delles C., Haller T., Dietl P. A highly calcium-selective cation current activated by intracellular calcium release in MDCK cells. J Physiol. 1995 Aug 1;486(Pt 3):557–569. doi: 10.1113/jphysiol.1995.sp020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Silverstein S. C. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990 Feb-Mar;11(2-3):57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- Dietl P., Völkl H. Maitotoxin activates a nonselective cation channel and stimulates Ca2+ entry in MDCK renal epithelial cells. Mol Pharmacol. 1994 Feb;45(2):300–305. [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Ma A. L., Liotta L. A., Kohn E. C. The antiproliferative and antimetastatic compound L651582 inhibits muscarinic acetylcholine receptor-stimulated calcium influx and arachidonic acid release. J Pharmacol Exp Ther. 1991 Jun;257(3):967–971. [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Ginsberg M. H., Du X., Plow E. F. Inside-out integrin signalling. Curr Opin Cell Biol. 1992 Oct;4(5):766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Hendey B., Maxfield F. R. Regulation of neutrophil motility and adhesion by intracellular calcium transients. Blood Cells. 1993;19(1):143–164. [PubMed] [Google Scholar]
- Hill T. D., Berggren P. O., Boynton A. L. Heparin inhibits inositol trisphosphate-induced calcium release from permeabilized rat liver cells. Biochem Biophys Res Commun. 1987 Dec 31;149(3):897–901. doi: 10.1016/0006-291x(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Prusty D., Frangioni J. V., Cragoe E. J., Jr, Lechene C., Schwartz M. A. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol. 1990 May;110(5):1803–1811. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. L., Schmidhauser C., Bissell M. J. Regulation of gene expression and cell function by extracellular matrix. Crit Rev Eukaryot Gene Expr. 1993;3(2):137–154. [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Grosmaire L. S., Ledbetter J. A., Damle N. K. Beta 2-integrin LFA-1 signaling through phospholipase C-gamma 1 activation. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke R. L., Yebra M., Bayna E. M., Cheresh D. A. Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. J Cell Biol. 1994 Nov;127(3):859–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn E. C., Alessandro R., Spoonster J., Wersto R. P., Liotta L. A. Angiogenesis: role of calcium-mediated signal transduction. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1307–1311. doi: 10.1073/pnas.92.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn E. C., Felder C. C., Jacobs W., Holmes K. A., Day A., Freer R., Liotta L. A. Structure-function analysis of signal and growth inhibition by carboxyamido-triazole, CAI. Cancer Res. 1994 Feb 15;54(4):935–942. [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Kornberg L., Juliano R. L. Signal transduction from the extracellular matrix: the integrin-tyrosine kinase connection. Trends Pharmacol Sci. 1992 Mar;13(3):93–95. doi: 10.1016/0165-6147(92)90034-4. [DOI] [PubMed] [Google Scholar]
- Leavesley D. I., Schwartz M. A., Rosenfeld M., Cheresh D. A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993 Apr;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989 Nov;1(1):99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Calcium channels activated by depletion of internal calcium stores in A431 cells. Biophys J. 1994 Jul;67(1):177–182. doi: 10.1016/S0006-3495(94)80467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. V., Premack B. A., Gardner P. Flash photolysis of caged inositol 1,4,5-trisphosphate activates plasma membrane calcium current in human T cells. J Biol Chem. 1993 Feb 25;268(6):3889–3896. [PubMed] [Google Scholar]
- McNamee H. P., Ingber D. E., Schwartz M. A. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993 May;121(3):673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Akiyama S. K., Yamada K. M. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995 Feb 10;267(5199):883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Reenstra W. W., Machen T. E. Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am J Physiol. 1989 Feb;256(2 Pt 1):C241–C251. doi: 10.1152/ajpcell.1989.256.2.C241. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1986 Nov;103(5):1751–1765. doi: 10.1083/jcb.103.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Modulation of fodrin (membrane skeleton) stability by cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1987 Jun;104(6):1527–1537. doi: 10.1083/jcb.104.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Katagiri Y., Faull R. J., Peter K., Tamura R., Quaranta V., Loftus J. C., Shattil S. J., Ginsberg M. H. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994 Mar;124(6):1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian G. K., Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994 Mar;107(Pt 3):561–576. [PubMed] [Google Scholar]
- Ozaki Y., Satoh K., Yatomi Y., Miura S., Fujimura Y., Kume S. Protein tyrosine phosphorylation in human platelets induced by interaction between glycoprotein Ib and von Willebrand factor. Biochim Biophys Acta. 1995 Apr 13;1243(3):482–488. doi: 10.1016/0304-4165(94)00178-z. [DOI] [PubMed] [Google Scholar]
- Pavalko F. M., Otey C. A. Role of adhesion molecule cytoplasmic domains in mediating interactions with the cytoskeleton. Proc Soc Exp Biol Med. 1994 Apr;205(4):282–293. doi: 10.3181/00379727-205-43709. [DOI] [PubMed] [Google Scholar]
- Pelletier A. J., Bodary S. C., Levinson A. D. Signal transduction by the platelet integrin alpha IIb beta 3: induction of calcium oscillations required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transfected cells. Mol Biol Cell. 1992 Sep;3(9):989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper G., Ingber D. E. Rapid induction and isolation of focal adhesion complexes. Biochem Biophys Res Commun. 1993 Jun 15;193(2):571–578. doi: 10.1006/bbrc.1993.1662. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993 Oct;14(5):610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Noble N. A., Kagami S., Border W. A. Integrins. Kidney Int Suppl. 1994 Jan;44:S17–S22. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sastry S. K., Horwitz A. F. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993 Oct;5(5):819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Parsons J. T. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994 Oct;6(5):705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Schoenenberger C. A., Zuk A., Zinkl G. M., Kendall D., Matlin K. S. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J Cell Sci. 1994 Feb;107(Pt 2):527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Denninghoff K. Alpha v integrins mediate the rise in intracellular calcium in endothelial cells on fibronectin even though they play a minor role in adhesion. J Biol Chem. 1994 Apr 15;269(15):11133–11137. [PubMed] [Google Scholar]
- Schwartz M. A., Ingber D. E. Integrating with integrins. Mol Biol Cell. 1994 Apr;5(4):389–393. doi: 10.1091/mbc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993 Feb;120(4):1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar G., Davison I., Helfrich M. H., Mason W. T., Horton M. A. Integrin receptor-mediated mobilisation of intranuclear calcium in rat osteoclasts. J Cell Sci. 1993 May;105(Pt 1):61–68. doi: 10.1242/jcs.105.1.61. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Haimovich B., Cunningham M., Lipfert L., Parsons J. T., Ginsberg M. H., Brugge J. S. Tyrosine phosphorylation of pp125FAK in platelets requires coordinated signaling through integrin and agonist receptors. J Biol Chem. 1994 May 20;269(20):14738–14745. [PubMed] [Google Scholar]
- Sjaastad M. D., Angres B., Lewis R. S., Nelson W. J. Feedback regulation of cell-substratum adhesion by integrin-mediated intracellular Ca2+ signaling. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8214–8218. doi: 10.1073/pnas.91.17.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi L., Lasić Z., Vukicević S., Banfić H. Collagen type IV stimulates an increase in intracellular Ca2+ in pancreatic acinar cells via activation of phospholipase C. Biochem J. 1994 May 1;299(Pt 3):603–611. doi: 10.1042/bj2990603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C. H., Schmidhauser C., Kobrin M., Bissell M. J., Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol. 1993 Jan;120(1):253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan C., Plantavid M., Bachelot C., Grondin P., Breton M., Mauco G., Lévy-Toledano S., Caen J. P., Chap H. Involvement of platelet glycoprotein IIb-IIIa (alpha IIb-beta 3 integrin) in thrombin-induced synthesis of phosphatidylinositol 3',4'-bisphosphate. J Biol Chem. 1991 Dec 15;266(35):23554–23557. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993 Oct 15;268(29):21459–21462. [PubMed] [Google Scholar]
- Wu Y. Y., Lin M. C. Induction of differentiation in v-Ha-ras-transformed MDCK cells by prostaglandin E2 and 8-bromo-cyclic AMP is associated with a decrease in steady-state level of inositol 1,4,5-trisphosphate. Mol Cell Biol. 1990 Jan;10(1):57–67. doi: 10.1128/mcb.10.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Dualistic nature of adhesive protein function: fibronectin and its biologically active peptide fragments can autoinhibit fibronectin function. J Cell Biol. 1984 Jul;99(1 Pt 1):29–36. doi: 10.1083/jcb.99.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko A. D., Liu D. Y., Eierman D., Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimolo Z., Wesolowski G., Tanaka H., Hyman J. L., Hoyer J. R., Rodan G. A. Soluble alpha v beta 3-integrin ligands raise [Ca2+]i in rat osteoclasts and mouse-derived osteoclast-like cells. Am J Physiol. 1994 Feb;266(2 Pt 1):C376–C381. doi: 10.1152/ajpcell.1994.266.2.C376. [DOI] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]


