Abstract
Calorie restriction (CR) enhances immune response and prolongs life span in animals. However, information on the applicability of these results to humans is limited. T-cell function declines with age. We examined effects of CR on T-cell function in humans. Forty-six overweight, nonobese participants aged 20–42 years were randomly assigned to 30% or 10% CR group for 6 months. Delayed-type hypersensitivity (DTH), T-cell proliferation (TP), and prostaglandin E2 (PGE2) productions were determined before and after CR. DTH and TP to T-cell mitogens were increased in both groups over baseline (p ≤ .019). However, number of positive responses to DTH antigens (p = .016) and TP to anti-CD3 reached statistical significance only after 30% CR (p = .001). Lipopolysaccharide-stimulated PGE2 was reduced in both groups but reached statistical significance after 30% CR (p ≤ .029). These results, for the first time, show that 6-month CR in humans improves T-cell function.
Keywords: Calorie restriction, T cell, Immune response, Aging, Obesity
LONG-TERM calorie restriction (CR) has been shown to prolong life in rodents and other shorter lived species (1). However, whether CR is effective in prolonging life in humans is not known. Because conducting studies to determine the effect of CR on life span in humans is not feasible, surrogate measures have to be used. One of the hallmarks of aging is the well-characterized impaired regulation of the immune response. This decline in immune function contributes to the increased incidence of infectious, inflammatory, and neoplastic diseases observed in elderly participants as well as their prolonged postillness recovery periods. Prospective studies indicate a higher incidence of morbidity and mortality in elderly participants with low delayed-type hypersensitivity (DTH), an in vivo measure of cell-mediated immune response (2–6).
Different cells of the immune system contribute to the impaired immunity of old age, but T cells are shown to be the major contributor (7–9). In vivo, T-cell–dependent functions, such as DTH (6,10), and response to T-cell–dependent vaccines (11,12) are depressed with age. In vitro, several measures of T-cell function have been shown to decline with age (13–20). One such measure that consistently has been shown to exhibit an age-associated decrease across all species is the proliferative response of T cells to T-cell mitogens, phytohemagglutinin A (PHA) and concanavalin A (Con A), and to anti–T-cell receptor antibody (anti-CD3) (8,13,21–23).
The alteration in T-cell–mediated function has been attributed to intrinsic changes in T cells themselves (13–20,24–32) as well as an increase in the production of prostaglandin E2 (PGE2) (33), a T-cell–suppressive factor. An age-associated increase in production of PGE2 and its contribution to reduced antibody production, DTH, and lymphocyte proliferation have been reported (10,33–35).
CR has been shown to significantly affect many age-sensitive immunologic responses (36–39) in animal models, but there is little information on the effect of CR on the immune response of humans. The splenocyte response to T-cell mitogens, antibody and interleukin-2 production, response to interleukin-2, mixed lymphocyte reaction, and T-cell cytotoxicity have all been shown to be enhanced by CR (36–39). It is also interesting to note that CR has been shown to reduce production of PGE2, a T-cell–suppressive factor, in both mice and rats (40,41). Thus, immune responses, particularly that of T-cell mediated and its regulators, lend themselves as appropriate biologic markers to validate the CR effects in humans. The aim of this study was to determine, for the first time, the effect of 6 months of CR on the T-cell–mediated response in humans using selected in vivo and in vitro measures of T-cell function, which have consistently exhibited age- and CR-induced changes in animal models.
METHODS
Study Participants
This study was performed as part of the Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy trial (Clinical trial No. NCT00099099) conducted at the Human Nutrition Research Center on Aging at Tufts University with approval from the Tufts-New England Medical Center Human Investigation Review Committee. The details of the study protocol, participant randomization, and treatment arms have been published elsewhere (42,43). Briefly, the study consisted of a 6-week baseline period during which weight maintenance energy requirements were established and baseline measures of all study outcomes were obtained. This period was followed by a 6-month phase during which time participants were randomly assigned to the 30% or 10% CR group and all food was provided to the participants based on their randomization. The 10% CR group rather than a control group was included to mimic the CR studies in rodents where ad-libitum feeding causes weight gain, so 10% CR was used to keep the weight constant in the control group. Forty-six healthy women and men aged 20–42 years with body mass index (BMI) in the range 25–29.9 kg/m2 were recruited to participate in this study. Individuals 20–42 years of age were chosen because although CR has been shown to be effective starting in early through midlife in animal species, it may be less effective if started late in life.
Eligibility was determined by normal health history questionnaires, physical and psychological examinations, and blood and urine tests. Exclusion criteria included high physical activity levels, smoking, alcoholism, weight fluctuations (>15 lb in the past year), inability to accurately complete a dietary record (>70% or >130% of estimated energy requirements), and any anticipated lifestyle changes (such as pregnancy, relocation). Volunteers who had known serious disorders that affect longevity, energy metabolism, body composition, and immune responsiveness, including diabetes, cancer, heart disease, cachexia, eating disorder, depression, alcoholism, inflammatory disorders, abnormal kidney, liver and thyroid function, and AIDS, were also excluded from the study. Prior to enrollment, all eligible volunteers were shown typical menus from the study to rule out food allergies and major food dislikes that could potentially affect compliance to the CR protocol. Informed consent was obtained from all participants prior to participation in the study, and the participants were provided with a stipend.
Measurement of DTH Response
DTH was determined using the Mantoux test, which utilized three skin test antigens including Tuberculin (Tubersol; Aventis Pasteur, Swiftwater, PA), Candida albicans (Candin; Allermed Laboratories, San Diego, CA), and Trichophyton species (Trichophyton mentagrophytes in conjunction with Trichophyton rubrum; Hollister-Stier Labs, Spokane, WA) as well as a negative control (0.9% normal saline; Bound Tree Medical, Dublin, OH). Antigens were employed in a standard volume of 0.1 mL except Tetanus toxoid (Aventis Pasteur), with a volume of 0.025 mL (0.2 limit of flocculation units per dose), and were injected intradermally at separate sites on the volar surface of the forearm in a clean area free of hair or acneiform rash. The same investigator evaluated the DTH response for all participants. We measured the vertical and horizontal diameters of induration after 24 and 48 hours and considered the reaction positive when the mean value was 5 mm or higher. Averages for each individual antigen were calculated, and a composite score, based on the results of all of the antigens in each participant, was determined.
Lymphocyte Proliferation
A 10-mL venous blood sample was drawn from each participant, after an overnight fast, into a heparinized tube. Lymphocyte proliferation was measured by [3H]-thymidine incorporation following stimulation with anti-CD3 (anti–T-cell receptor) and T-cell mitogens Con A and PHA using a modified whole-blood assay as previously described (41). Briefly, diluted whole blood (1:10 in RPMI 1640 medium) was incubated in 96-well round-bottom plates (Nunc, Roskilde, Denmark) in the presence or absence of Con A (Sigma, St Louis, MO) or PHA (Difco Laboratories, Detroit, MI), both at 25 μg/mL, or anti-CD3 (BD PharMingen, San Diego, CA), at 0.1 μg/mL for 72 hours. Cultures were pulsed with 0.5 μCi [3H]-thymidine (Perkin Elmer, Shelton, CT) during the final 4 hours of incubation. The cells were harvested onto glass fiber filter mats (Wallac, Gaithersburg, MD) by a Tomtec harvester (Wallac), and cell proliferation was quantified as the amount of [3H]-thymidine incorporation into DNA as determined by liquid scintillation counting in a 1205 Betaplate counter (Wallac). The counter had an efficiency of more than 50% for [3H]-thymidine. Results are expressed as counts per minute.
PGE2 Production
Diluted whole blood (1:8 in RPMI 1640 medium) was incubated in the presence of lipopolysaccharide (LPS) at 1 μg/mL for 48 hours. Cell-free supernatants were collected at the end of incubation, and PGE2 was measured using radioimmunoassay as described previously (44).
Statistical Analysis
Data are presented as means ± standard errors of the mean. Because the data were not normally distributed, statistically significant differences at p ≤ .05 were assessed by Wilcoxon signed rank tests for within-group differences and Mann–Whitney rank sum tests for between-group differences using Systat 10 statistical software (Systat, Evanston, IL). In addition, correlations between changes in different immune responses with those of BMI were analyzed using Spearman correlation tests.
RESULTS
Study Participants
Forty-six overweight (BMI = 27.9 ± 1.5 kg/m2) nonobese men and women aged 20–40 years (M ± SD = 35 ± 5 years) were randomly assigned to a 30% (N = 34, men:women = 8:26) or 10% (N = 12, men:women = 4:8) CR group. There were no statistically significant differences in any of the demographic variables such as weight or height at baseline between the participants randomized to the two levels of CR (p = .12–.92; data not shown). The mean percentage weight lost during CR was lower in the 10% CR group (M ± SD, −6.97 ± 6.40) compared with the 30% CR group (−10.20 ± 3.90), but the difference did not reach statistical significance (p = .08) (45). However, using doubly labeled water method, to assess the percentage of CR in participants, we observed that mean %CR was significantly different between the two CR groups during this time (19.9 ± 15.5 in the 10% CR group vs 30.7 ± 10.9 in the 30% CR group; p = .02) (45).
DTH Response
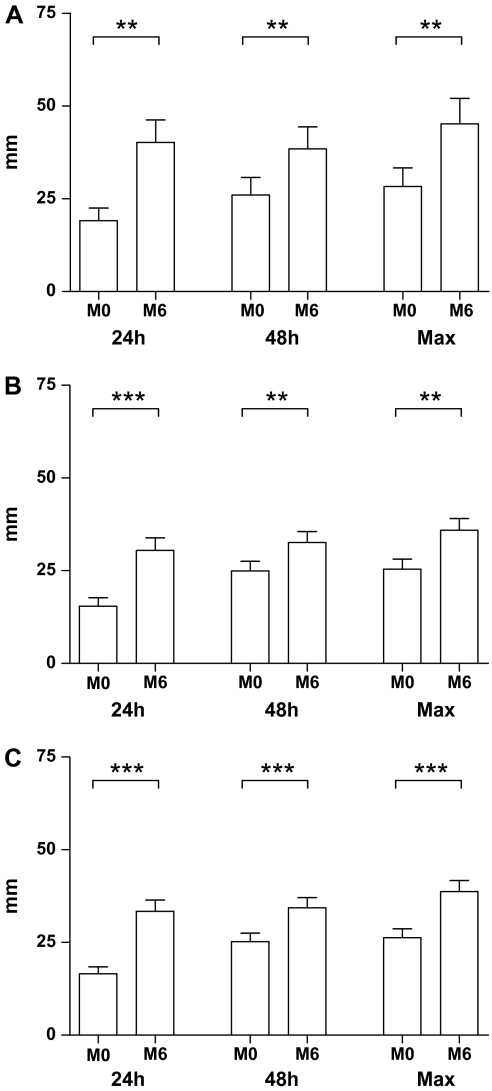
DTH response was increased in both the 10% CR group (Figure 1A; p = .008, .004, and .004, for 24 h, 48 h, and the maximum induration from the two time points, respectively) and the 30% CR group (p = .001, .010, and .002 for 24 h, 48 h, and the maximum induration from the two time points, respectively; Figure 1B) compared with their baselines. There was no statistically significant difference in the change in diameter of induration between the 10% and 30% CR groups. Thus, the results from the two groups were combined. As can be seen in Figure 1C, when the two groups are combined, there continues to be a significant increase in DTH (p ≤ .001) for 24 hours, 48 hours, and the maximum induration from the two time points. However, only the participants in the 30% CR group exhibited a significant 22% increase in the number of positive responses to antigens compared with their baseline levels (p = .016; Table 1). This significant change was also observed when the data from the two groups were combined (p = .008; Table 1).
Figure 1.
Effect of calorie restriction (CR) on delayed-type hypersensitivity (DTH) in humans. Participants were administered a Mantoux test with three recall antigens and a control at baseline (M0) and following 6 months (M6) of 10% (A) or 30% (B) CR as described in the Methods section. The response of the two CR groups combined is represented in (C). Data are mean ± standard error of the mean of the diameter of induration measured at 24 hours, 48 hours, or the maximum response of the two time points. N = 12 for the 10% and 34 for the 30% group and 46 for the combined group. **p = .01–.001 and ***p < .001 for differences of DTH responses between the baseline and after 6-month CR with Wilcoxon signed rank tests.
Table 1.
Effect of 10% and 30% CR on Response to DTH Antigens per Person Using the Mantoux Test
| 10% CR | 30% CR | All Participants | |
| M0* | 0.344 ± 0.05 | 0.328 ± 0.03 | 0.333 ± 0.02 |
| M6* | 0.400 ± 0.06 | 0.399 ± 0.03 | 0.399 ± 0.03 |
| Percent changes (%) | 16 | 22 | 20 |
| p Value† | 1.000 | .016 | .008 |
Notes: CR, calorie restriction; DTH, delayed-type hypersensitivity.
Mean ± standard deviation of the ratio of number of positive antigen responses to the total number of antigens administered.
Significant increase of responses to positive antigens per person between the baseline (M0) and after 6 months (M6) of CR analyzed by Wilcoxon signed rank test.
Lymphocyte Proliferation
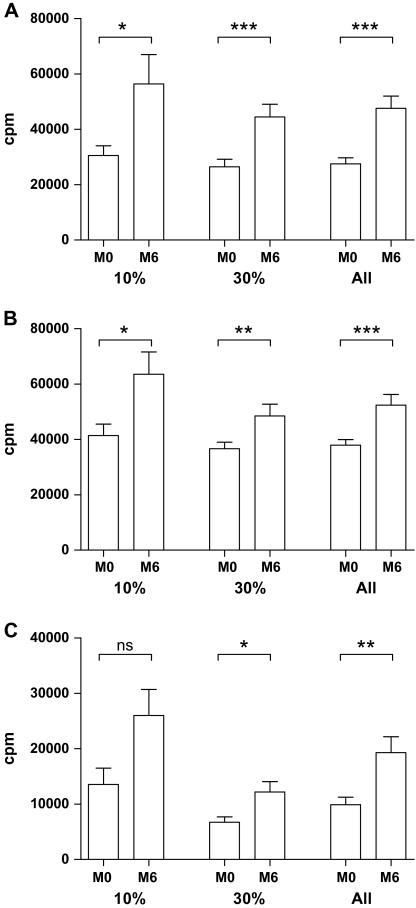
There was a significant increase in Con A–stimulated lymphocyte proliferation in both the 10% (p = .010) and 30% (p < .001) CR groups (Figure 2A). In addition, significant increases in proliferative responses were observed in response to another T-cell mitogen, PHA, in both the 10% (p = .019) and 30% (p = .009) CR groups (Figure 2B). Although both groups exhibited increase over their respective baselines in proliferative response to anti-CD3, the increase reached statistical significance in the 30% CR group only (p = .001; Figure 2C). When the two groups were combined, there continued to be a significant increase in responses to all three stimuli following CR (p < .05).
Figure 2.
Effect of 10% and 30% calorie restriction (CR) on lymphocyte proliferation. Fasting whole blood was stimulated by mitogens 25 μg/mL concanavalin A (Con A) (A), 25 μg/mL phytohemagglutinin A (B), or 0.1 μg/mL anti-CD3 (C) for 72 hours, and proliferation was measured by [3H]-thymidine incorporation as described in the Methods section. *p ≤ .05–.01, **p = .01–.001, and ***p < .001 for differences of lymphocyte proliferation between the baseline (M0) and after 6-month (M6) CR by Wilcoxon signed rank tests.
PGE2 Production
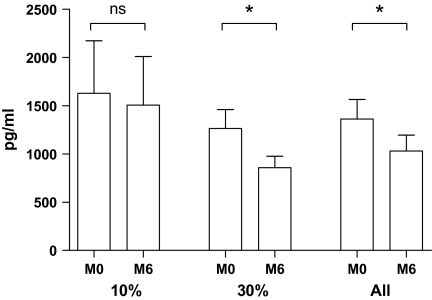
LPS-stimulated PGE2 production decreased significantly only in the 30% CR group (p = .020; Figure 3). Although PGE2 production was lower following 10% CR, the change did not reach statistical significance (Figure 3). However, when the two groups were combined, there continued to be a significant reduction due to CR (p = .029).
Figure 3.
Effect of 10% and 30% calorie restriction (CR) on LPS-stimulated prostaglandin E2 (PGE2) production. Whole blood was stimulated with 1 μg/mL LPS for 48 hours. PGE2 in supernatant was measured as described in the Methods section. *p = .05–.01 and ns = nonsignificant for differences of PGE2 production between the baseline (M0) and after 6-month (M6) CR with Wilcoxon signed rank tests.
Correlation Between Change in BMI and Different Immune Parameters
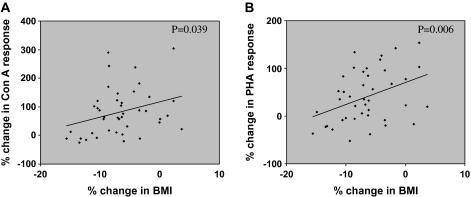
Changes in different immune parameters were correlated with BMI. We observed a significant correlation between changes in BMI and change in response to T-cell mitogens, Con A (p = .039) and PHA (p = .006; Figure 4). No significant correlation, however, was observed between changes in BMI and changes of PGE2 production, or proliferative responses to anti-CD3, or DTH (data not shown).
Figure 4.
Correlation between calorie restriction (CR)–induced percent changes in body mass index (BMI) in relation to percent changes of concanavalin A (Con A) response (A) and percent changes of phytohemagglutinin A (PHA) response (B). The p values were determined using Spearman correlation tests.
DISCUSSION
Studies suggest that CR is the only identified natural regimen to increase longevity in all experimental models tested, including yeast, helminthes, fruit flies, mice, rodents, and nonhuman primates (46–48), and has been suggested to potentially provide health benefits in humans (49). It is also well established that immune response and resistance to diseases decline with aging. Studies in several experimental animal models demonstrate that CR improves several aspects of the immune system, including the ability of T cells to respond to different stimuli, whereas reduces PGE2 production (39,50–53). CR has been shown to partially reverse these age-associated changes by mechanisms yet to be elucidated. There is currently no well-controlled human study that demonstrates the interaction between CR and the immune markers. In this article, for the first time, we report that 6 months of CR in overweight adult humans improves ex vivo and in vivo measures of T-cell–mediated function. The DTH response has been long utilized as an overall indicator of the robustness of cell-mediated immunity. In the elderly participants, altered T-cell–mediated immunity is reflected in their inability to mount a DTH response (4,6). In this study, we demonstrate that DTH response as well as the proliferative response of T cells to T-cell mitogens and T-cell receptor antibody was significantly increased in both the 30% and 10% CR groups compared with their baseline. However, a statistically significant increase in the proliferative response to anti-CD3 as well as a significant reduction in PGE2 was only observed in the 30% CR group. Although these latter observations suggest more effectiveness of 30% CR compared with 10% CR in modulating the immune response, it could also be due to the lower number of participants in the 10% CR group and larger variability observed in these measurements. Regardless, these results suggest that the CR-induced improvements in T-cell function reported in animals (50,54) are reproducible in humans with as low as 10% CR. In addition, similar to reports in rodents (41), we observed a significant reduction in PGE2 production in humans. The decline in PGE2 production following CR in humans might partly contribute to the CR-induced enhancement of T-cell function as PGE2 has been shown to be a negative regulator of T cells. The underlying mechanism of CR-induced reduction in PGE2 is not known at this time and might be either due to decreased availability of the precursor substrate (arachidonic acid) or a change in expression/activity of the enzymes involved in PGE2 production, that is, cyclooxygenases and PGE2 synthase. Other CR-induced effects such as changes in the ratio of naive to memory T cells or molecular changes in specific subpopulations of T cells could contribute to the enhancement of T-cell function observed in this study. These alternative mechanisms need to be explored in future investigations.
When the change in immune parameters was correlated with that of BMI, a significant correlation was observed for some, but not all, parameters, suggesting that a threshold might exist between weight reduction induced by CR and some of the immunologic measures or that some measures of the immune response might be more sensitive to the changes in BMI.
As indicated in the Methods section, we utilized a 10% CR group in place of a control group to mimic CR studies conducted in mice. Because we did not have a no-CR group, one could argue that the benefits of the 30% CR group might have been greater if compared with 0% CR. On the other hand, it could be argued that the results observed are due to seasonal variation, which might have occurred regardless of CR intervention. This is unlikely as participants were enrolled from October 2002 to December 2003 in a random fashion. Furthermore, we observed a correlation between changes in BMI and lymphocyte proliferation, further indicating that the observed changes are due to reduction in caloric intake. In addition, in several other studies in which we have had a placebo control group and using the same design and measures of immune response, we have not observed a significant change in the placebo group compared with their baseline (41,55,56).
Although the study was not designed to investigate age and sex differences in response to CR, we analyzed the data based on sex and age (≤30 and >30 years old). At baseline, female participants produced significantly more PGE2 than male participants in both the 10% and 30% CR groups (1650.1 ± 253.7 vs 575.4 ± 122.3, p = .003, respectively). CR decreased PGE2 production in both male and female participants, but this decrease reached statistical significance in female participants only (p = .030). The lack of a significant impact on PGE2 production in male participants might be due to a much lower number of male than female participants. PGE2 production tended to be higher in participants who were more than 30 years old compared with those who were 30 years old or younger (1454.4 ± 248.6 vs 1031.2 ± 245.8, p = .112, respectively). Higher PGE2 production by macrophage of old mice compared with young mice has been previously reported (34,57). CR reduced PGE2 production in both groups, but this reduction reached statistical significance in those older than 30 years only (p = .048). The lack of a significant effect in the younger age group could be due to the much lower number of participants in this age group.
At baseline, DTH was significantly lower in female participants compared with male participants (20.6 ± 2.1 vs 37.6 ± 3.8, p = .001, respectively). CR increased DTH in both male and female participants, but the increase reached statistical significance in female participants only (p = .06 and .001 in male participants and female participants, respectively). Lack of a significant effect of CR in male participants could be due to a much lower number of male than female participants. There was no significant difference in proliferative responses to T-cell mitogens or anti-CD3 between male and female participants at baseline. CR improved immune response in both male and female participants. The T-cell–proliferative responses and DTH tended to be lower in participants with more than 30 years of age; however, CR improved TP in both age groups.
Because of the small number of participants in some of the subanalyses, these results need to be interpreted with caution.
In conclusion, we show, for the first time, that CR significantly improves T-cell–mediated function in adult humans and this effect might be in part mediated through a reduction in PGE2 production. Further studies are needed to determine the effect of longer term CR on immune response of humans as well as a more comprehensive assessment of underlying mechanisms.
FUNDING
National Institute on Aging (NGA-3U01-AG20478); United States Department of Agriculture (contract 58-1950-9-001); International Nutrition Foundation–Ellison Medical Foundation Scholarship.
References
- 1.Weindruch R, Walford RL, Fligiel S, et al. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 2.Christou NV, Meakins JL, Gordon J, et al. The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg. 1995;222(4):534–546. doi: 10.1097/00000658-199522240-00011. discussion 546–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn JR, Hohl CA, Buckley CE., III The relationship between cutaneous cellular immune responsiveness and mortality in a nursing home population. J Am Geriatr Soc. 1983;31(12):808–809. doi: 10.1111/j.1532-5415.1983.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 4.Roberts-Thomson IC, Whittingham S, Youngchaiyud U, et al. Ageing, immune response, and mortality. Lancet. 1974;2(7877):368–370. doi: 10.1016/s0140-6736(74)91755-3. [DOI] [PubMed] [Google Scholar]
- 5.Rodysill KJ, Hansen L, O’Leary JJ. Cutaneous-delayed hypersensitivity in nursing home and geriatric clinic patients. Implications for the tuberculin test. J Am Geriatr Soc. 1989;37(5):435–443. doi: 10.1111/j.1532-5415.1989.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 6.Wayne SJ, Rhyne RL, Garry PJ, et al. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 1990;45(2):M45–M48. doi: 10.1093/geronj/45.2.m45. [DOI] [PubMed] [Google Scholar]
- 7.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273(5271):70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 8.Murasko DM, Nelson BJ, Matour D, et al. Heterogeneity of changes in lymphoproliferative ability with increasing age. Exp Gerontol. 1991;26(2–3):269–279. doi: 10.1016/0531-5565(91)90020-m. [DOI] [PubMed] [Google Scholar]
- 9.Makinodan T. Cellular basis of immunologic aging. In: Schimke RT, editor. Biological Mechanisms in Aging. Boston, MA: USDA; 1981. pp. 488–500. 1981. [Google Scholar]
- 10.Goodwin JS, Searles RP, Tung KS. Immunological responses of healthy elderly population. Clin Exp Immunol. 1982;48(2):403–410. [PMC free article] [PubMed] [Google Scholar]
- 11.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(suppl 1):S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 13.Larbi A, Dupuis G, Khalil A, et al. Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal. 2006;18(7):1017–1030. doi: 10.1016/j.cellsig.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Gillis S, Kozak R, Durante M, et al. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981;67(4):937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoman ML, Weigle WO. Cell-mediated immunity in aged mice: an underlying lesion in IL 2 synthesis. J Immunol. 1982;128(5):2358–2361. [PubMed] [Google Scholar]
- 16.Karanfilov CI, Liu B, Fox CC, et al. Age-related defects in Th1 and Th2 cytokine production by human T cells can be dissociated from altered frequencies of CD45RA+ and CD45RO+ T cell subsets. Mech Ageing Dev. 1999;109(2):97–112. doi: 10.1016/s0047-6374(99)00030-5. [DOI] [PubMed] [Google Scholar]
- 17.Haynes L, Eaton SM, Burns EM, et al. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100(25):15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swain S, Clise-Dwyer K, Haynes L. Homeostasis and the age-associated defect of CD4 T cells. Semin Immunol. 2005;17(5):370–377. doi: 10.1016/j.smim.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RA, Berger SB, Burke DT, et al. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunol Rev. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 20.Adolfsson O, Huber BT, Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J Immunol. 2001;167(7):3809–3817. doi: 10.4049/jimmunol.167.7.3809. [DOI] [PubMed] [Google Scholar]
- 21.Makinodan T, Kay MM. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- 22.Murasko DM, Nelson BJ, Silver R, et al. Immunologic response in an elderly population with a mean age of 85. Am J Med. 1986;81(4):612–618. doi: 10.1016/0002-9343(86)90546-2. [DOI] [PubMed] [Google Scholar]
- 23.Douziech N, Seres I, Larbi A, et al. Modulation of human lymphocyte proliferative response with aging. Exp Gerontol. 2002;37(2–3):369–387. doi: 10.1016/s0531-5565(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 24.Kubo M, Cinader B. Polymorphism of age-related changes in interleukin (IL) production: differential changes of T helper subpopulations, synthesizing IL 2, IL 3 and IL 4. Eur J Immunol. 1990;20(6):1289–1296. doi: 10.1002/eji.1830200614. [DOI] [PubMed] [Google Scholar]
- 25.Miller RA. Aging and immune function. Int Rev Cytol. 1991;124:187–215. doi: 10.1016/s0074-7696(08)61527-2. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Beckman I, Ahern M, et al. A comprehensive analysis of peripheral blood lymphocytes in healthy aged humans by flow cytometry. Immunol Cell Biol. 1993;71(pt 6):549–557. doi: 10.1038/icb.1993.61. [DOI] [PubMed] [Google Scholar]
- 27.Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996;169(2):185–195. doi: 10.1006/cimm.1996.0109. [DOI] [PubMed] [Google Scholar]
- 28.Tamir A, Eisenbraun MD, Garcia GG, et al. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165(3):1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 29.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166(5):3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 30.Nagel JE, Chopra RK, Chrest FJ, et al. Decreased proliferation, interleukin 2 synthesis, and interleukin 2 receptor expression are accompanied by decreased mRNA expression in phytohemagglutinin-stimulated cells from elderly donors. J Clin Invest. 1988;81(4):1096–1102. doi: 10.1172/JCI113422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller RA. Aging and immune function. In: Paul WE, editor. Fundamental Immunology. Philadelphia, PA: Lippincott-Raven; 1999. pp. 947–966. [Google Scholar]
- 32.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169(9):5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 33.Beharka AA, Wu D, Han SN, et al. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93(1–3):59–77. doi: 10.1016/s0047-6374(96)01819-2. [DOI] [PubMed] [Google Scholar]
- 34.Hayek MG, Mura C, Wu D, et al. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159(5):2445–2451. [PubMed] [Google Scholar]
- 35.Bartocci A, Maggi FM, Welker RD, et al. Age-related immunosuppression: putative role of prostaglandins. In: Powles TJ, Backman RS, Honn KV, Ramwell P, editors. Prostaglandins and Cancer. New York: Alan R. Liss; 1982. pp. 725–730. [Google Scholar]
- 36.Weindruch R. Immunogerontologic outcomes of dietary restriction started in adulthood. Nutr Rev. 1995;53(4 pt 2):S66–S70. doi: 10.1111/j.1753-4887.1995.tb01519.x. discussion S70–S61. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes G, Venkatraman JT, Turturro A, et al. Effect of food restriction on life span and immune functions in long-lived Fischer-344 × Brown Norway F1 rats. J Clin Immunol. 1997;17(1):85–95. doi: 10.1023/a:1027344730553. [DOI] [PubMed] [Google Scholar]
- 38.Esquifino AI, Cano P, Jimenez V, et al. Experimental allergic encephalomyelitis in male Lewis rats subjected to calorie restriction. J Physiol Biochem. 2004;60(4):245–252. doi: 10.1007/BF03167069. [DOI] [PubMed] [Google Scholar]
- 39.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93(1–3):87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 40.Venkatraman J, Fernandes G. Modulation of age-related alterations in membrane composition and receptor-associated immune functions by food restriction in Fischer 344 rats. Mech Ageing Dev. 1992;63(1):27–44. doi: 10.1016/0047-6374(92)90014-5. [DOI] [PubMed] [Google Scholar]
- 41.Meydani SN, Lipman R, Blumberg JB, et al. Dietary energy restriction decreases ex vivo spleen prostaglandin E2 synthesis in Emory mice. J Nutr. 1990;120(1):112–115. doi: 10.1093/jn/120.1.112. [DOI] [PubMed] [Google Scholar]
- 42.Pittas AG, Roberts SB, Das SK, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity (Silver Spring) 2006;14(12):2200–2209. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 43.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85(4):1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 44.Hayek MG, Meydani SN, Meydani M, et al. Age differences in eicosanoid production of mouse splenocytes: effects on mitogen-induced T-cell proliferation. J Gerontol. 1994;49(5):B197–B207. doi: 10.1093/geronj/49.5.b197. [DOI] [PubMed] [Google Scholar]
- 45.Das SK, Saltzman E, Gilhooly CH, et al. Low or moderate dietary energy restriction for long-term weight loss: what works best? Obesity. 2009 doi: 10.1038/oby.2009.120. doi:10.1038/oby.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78(3):361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 47.Lane MA, Tilmont EM, De Angelis H, et al. Short-term calorie restriction improves disease-related markers in older male rhesus monkeys (Macaca mulatta) Mech Ageing Dev. 2000;112(3):185–196. doi: 10.1016/s0047-6374(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 48.Roth GS, Ingram DK, Lane MA. Calorie restriction in primates: will it work and how will we know? J Am Geriatr Soc. 1999;47(7):896–903. doi: 10.1111/j.1532-5415.1999.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 49.Fontana L, Meyer TE, Klein S, et al. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101(17):6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jolly CA. Dietary restriction and immune function. J Nutr. 2004;134(8):1853–1856. doi: 10.1093/jn/134.8.1853. [DOI] [PubMed] [Google Scholar]
- 51.Messaoudi I, Warner J, Fischer M, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103(51):19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nikolich-Zugich J, Messaoudi I. Mice and flies and monkeys too: caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40(11):884–893. doi: 10.1016/j.exger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Spaulding CC, Walford RL, Effros RB. The accumulation of non-replicative, non-functional, senescent T cells with age is avoided in calorically restricted mice by an enhancement of T cell apoptosis. Mech Ageing Dev. 1997;93(1–3):25–33. doi: 10.1016/s0047-6374(96)01808-8. [DOI] [PubMed] [Google Scholar]
- 54.Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008;84(4):882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277(17):1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 56.Meydani SN, Ribaya-Mercado JD, Russell RM, et al. Vitamin B-6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am J Clin Nutr. 1991;53(5):1275–1280. doi: 10.1093/ajcn/53.5.1275. [DOI] [PubMed] [Google Scholar]
- 57.Claycombe KJ, Wu D, Nikolova-Karakashian M, et al. Ceramide mediates age-associated increase in macrophage cyclooxygenase-2 expression. J Biol Chem. 2002;277(34):30784–30791. doi: 10.1074/jbc.M204463200. [DOI] [PubMed] [Google Scholar]