Abstract
Genetic manipulations of Mn superoxide dismutase (MnSOD), SOD2 expression have demonstrated that altering the level of MnSOD activity is critical for cellular function and life span in invertebrates. In mammals, Sod2 homozygous knockout mice die shortly after birth, and alterations of MnSOD levels are correlated with changes in oxidative damage and in the generation of mitochondrial reactive oxygen species. In this study, we directly tested the effects of overexpressing MnSOD in young (4–6 months) and old (26–28 months) mice on mitochondrial function, levels of oxidative damage or stress, life span, and end-of-life pathology. Our data show that an approximately twofold overexpression of MnSOD throughout life in mice resulted in decreased lipid peroxidation, increased resistance against paraquat-induced oxidative stress, and decreased age-related decline in mitochondrial ATP production. However, this change in MnSOD expression did not alter either life span or age-related pathology.
Keywords: Oxidative damage, Mn superoxide dismutase, Pathology, Aging
THE oxidative stress theory of aging proposes that the life span of an organism is determined by the age-related loss of physiological function caused by the progressive accumulation of oxidative damage. In 1972, Harman (1) modified his original theory to incorporate the contribution of mitochondria to oxidative stress and proposed the mitochondrial theory of aging. The core principle of the theory is that the generation of reactive oxygen species (ROS) and the subsequent oxidative damage to key mitochondrial components, such as protein, lipids, and DNA, lead to an overall decline in cellular function and ultimately determines the life span of an organism (1). Since the original proposal by Harman, significant amounts of correlative data have been accumulated in support of the oxidative stress theory of aging.
Mn superoxide dismutase (MnSOD) is an essential mitochondrial antioxidant enzyme that scavenges superoxide anions (O2·−) generated in the respiratory chain as a by-product of mitochondrial respiration (2,3). MnSOD is located in the mitochondrial matrix and, because of its rapid reaction with O2·−, is thought to be a first-line defense against mitochondrial oxidative damage. The potential importance of MnSOD in aging has been highlighted by studies showing that mutations in the insulin or insulin-like growth factor-1 (IGF-1) signaling pathway lead to increased life span in invertebrates (4,5). Early studies showed that the daf-2 mutants in Caenorhabditis elegans upregulate the expression of the sod-3 gene, that is, the gene for MnSOD, through an insulin-like signaling pathway (6). Using DNA microarray analysis, Kenyon’s laboratory (7) subsequently found that the expression of the sod-3 and the ctl-1 and ctl-2 genes, which code for catalase in C. elegans, was upregulated in these long-lived mutants and met their criteria for playing a role in extended life span. There is also evidence that some mouse models with mutations in the insulin or IGF-1 system show alterations in the expression of MnSOD and resistance to oxidative stress. For example, Yamamoto and colleagues (8) reported that Klotho transgenic mice, which have increased life span, showed increased expression of MnSOD that was correlated with increased resistance to oxidative stress. Taguchi and colleagues (9) reported that mice in which the gene for insulin receptor substrate 2 (Irs2) was conditionally knocked out and lived longer and had higher levels of MnSOD in their brain. Ames dwarf mice, which have low circulating levels of IGF-1, also show increased superoxide dismutase (SOD) activity in several tissues (10), and Baba and colleagues (11) showed that mice deficient in the insulin receptor have increased activity of MnSOD that correlates with their increased resistance to oxidative stress. Therefore, these observations have been often used to argue that the upregulation of MnSOD by reduced insulin or IGF-1 signaling may play an important role in the mechanism(s) responsible for the increased resistance to oxidative stress and increased life span observed in animal models with mutations in the insulin or IGF-1 signaling pathway.
Studies using genetic manipulations to alter the level of MnSOD activity have demonstrated that regulating O2·− levels is critical for maintenance of cellular function and limits the life span of an organism. For example, in Saccharomyces cerevisiae, deletion of the gene MnSOD dramatically accelerates chronological aging and overexpression of MnSOD increases chronological life span (12,13). Tower’s group reported a similar extension of life span by overexpressing MnSOD in Drosophila melanogaster (14). In mice, two independent laboratories have shown that the deletion of MnSOD is lethal (15,16); mice exhibit oxidative damage and die shortly after birth (15,16). Conversely, overexpressing MnSOD has been reported to protect against mitochondrial apoptosis and oxidative damage by generating less mitochondrial ROS (17,18).
The purpose of this study was to test the effects of overexpressing MnSOD on mitochondrial function, levels of oxidative stress or damage, and life span in mice. We show that an approximately twofold increase in MnSOD expression throughout life resulted in a slight decrease in oxidative damage and enhanced resistance against oxidative stress. However, overexpressing MnSOD did not alter either life span or age-related pathology in these mice.
MATERIALS AND METHODS
Animals
The Sod2 transgenic (Tg) mice used in this study were obtained from Dr. Epstein’s laboratory as described by Raineri and colleagues (19). A 13-kb genomic Sod2 clone isolated from C57BL/6J mice, which encompassed 2 kb of the native Sod2 promoter, was used to generate Sod2 Tg mice. Subsequent generations of Sod2 Tg mice were bred by mating male Sod2 Tg mice to female wild-type (WT) C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, ME). The mice were genotyped at 4–5 weeks of age by polymerase chain reaction analysis of DNA obtained from tail clips as previously described (16). The mice were maintained under pathogen-free barrier conditions in a temperature-controlled environment and fed a commercial mouse chow (Teklad Diet LM485) ad libitum. For tissue collection, mice were euthanized by CO2 inhalation followed by cervical dislocation. Hind limb skeletal muscle was used for mitochondrial isolation and enzymatic assays.
For the life span and all other analyses, male WT and Sod2 Tg mice were housed four per cage following weaning and fed commercial mouse chow (Teklad Diet LM485) ad libitum. WT and Tg mice were assigned to survival groups at 2 months of age and allowed to live out their entire life span. There was no censoring of the WT or the Sod2 Tg mice when measuring survival. The life span for individual Sod2 Tg mice and WT mice was determined by recording the age of spontaneous death; and the median, mean, 10th percentile, and maximum survivals were calculated for each group. All procedures followed the guidelines approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System, Audie L. Murphy Division.
Isolation of the Skeletal Muscle Mitochondria
Mitochondria were purified from whole-hind limb skeletal muscle according to Chappell and Perry (20) and Ernster and Nordenbrand (21), as described previously (22). Hind limb skeletal muscles, gastrocnemius, tibialis anterior, and vastus lateralis were excised, weighed, bathed in 150 mM KCl, and placed in Chappell–Perry buffer with the protease nagarse. The minced skeletal muscle was homogenized, the homogenate was centrifuged for 10 minutes at 600g, and the supernatant passed through cheesecloth and centrifuged at 14,000g for 10 minutes. The resulting pellet was washed once in modified Chappell–Perry buffer with 0.5% bovine serum albumin (BSA) and once in modified Chappell–Perry buffer without BSA. Mitochondria were used immediately. Protein concentration was measured by the Bradford method (Bio-Rad, Richmond, CA).
MnSOD Activity Assay
MnSOD was measured in tissue extracts from brain, kidney, liver, and heart of male WT and Sod2 Tg mice using native gels as previously described by Van Remmen and colleagues (23). Extracts containing 40–80 μg protein were separated on a 10% polyacrylamide gel, and the gel was soaked in a solution containing nitroblue tetrazolium, riboflavin, and N,N,N,N, tetramethyl ethylene diamine (TEMED). In the presence of SOD, the riboflavin is activated to oxidize an electron donor (TEMED). The gel image was captured with a digital-camera imager system (ImageMaster VDS; Amersham Pharmacia Biotech, Piscataway, NJ) and analyzed using ImageQuant Software (Sunnyvale, CA) to quantify the intensity of the regions representing the MnSOD activity.
Mitochondrial H2O2 Release
Mitochondrial ROS production was measured with the Amplex Red-horseradish peroxidase method (Molecular Probes, Eugene, OR) (24). Horseradish peroxidase (HRP, 2 U/mL) catalyses the H2O2-dependent oxidation of nonfluorescent Amplex Red (80 μM) to fluorescent resorufin red (24). Thirty-seven units per milliliter CuZnSOD was added to convert all O2·− into H2O2, a necessity because O2·− reacts very rapidly with HRP and HRP-Compound I, resulting in an underestimation of the actual rate of H2O2 production (25,26). Therefore, our results reflect the sum of both superoxide and H2O2 production and are referred to as ROS rather than H2O2 production (27). Fluorescence was followed at an excitation wavelength of 545 nm and an emission wavelength of 590 nm using a Fluoroskan Ascent type 374 multiwell plate reader (Labsystems, Helsinki, Finland). The slope of the increase in fluorescence is converted to the rate of H2O2 production with a standard curve. All assays were performed at 37°C in black 96-well plates with 5 mM succinate plus rotenone and 5 mM glutamate plus malate as complex I- and complex II-linked substrates, respectively. For each assay, one reaction well contained buffer only and another contained buffer with mitochondria to estimate the mitochondria without substrate (state 1) (28). The reaction buffer consisted of 125 mM KCl, 10 mM 2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), 5 mM MgCl2, and 2 mM K2HPO4, pH 7.4. Data are expressed in pmole H2O2/min/mg mitochondrial protein.
Superoxide Production
Superoxide production was measured indirectly by inhibition of aconitase activity, as described by Gardner (29,30) and Muller and colleagues (22). Briefly, aconitase activity was quantified by measuring the reduction of NADP+. Mitochondria (∼0.4 mg of protein/mL) were aliquoted in 96-well plates in 100 μL at pH 7.44 in 125 mM KCl, 10 mM HEPES, 5 mM MgCl2, and 2 mM K2HPO4 and incubated at 30°C up to 40 minutes. Substrate (5 mM glutamate or malate) was then added as indicated. For each experiment, 200 U/mL SOD (from bovine erythrocytes; Sigma, St. Louis, MO) and 900 U/mL catalase were also added to eliminate extramitochondrial H2O2 and superoxide. Incubation was stopped, and aconitase activity measurements were begun by the addition of 1 volume (100 μL) of 50 mM Tris, 0.6 mM MnCl2, 60 mM citrate, 0.2% Triton X-100, 100 μM NADP+, and 1 U of isocitrate dehydrogenase. Fluorometric measurements (excitation at 355 nm and emission at 460 nm) were then started immediately using a microplate reader. The “control or blank” to measure aconitase-independent NADP+ reduction consisted of the same buffer with isocitrate dehydrogenase omitted. The slope of the increase in NADPH fluorescence was taken as the amount of aconitase activity.
ATP Production
ATP production by isolated mitochondria was measured using a luminometric assay (ATP Bioluminescence Assay CLS II; Roche, Indianapolis, IN) that follows the change in luminescence at 560 nm. The assay utilizes the dependence of ATP in the light-emitting luciferase-catalyzed oxidation of luciferin. Mitochondrial proteins, in the presence of complex II-linked substrates (succinate with rotenone to inhibit reverse electron transfer through complex I), were mixed in the assay buffer (125 mM KCl, 10 mM HEPES, 5 mM MgCl2, and 2 mM K2HPO4, pH 7.44) and added to a 96-well plate. Luciferase (Roche) and 0.3 mM ADP were then added to start the reaction, and the rates of ATP production were determined at 560 nm using a Fluoroskan Ascent multiwell plate reader (Labsystems). The slope of the increase in luminescence is converted to the rate of ATP production with a standard curve.
Mitochondrial Respiration
Mitochondrial oxygen consumption was measured using a Clark electrode as originally described by Estabrook (31). The respiratory buffer consisted of 125 mM KCl, 10 mM HEPES, 5 mM MgCl2, and 2 mM K2HPO4, pH 7.44, with 0.3% BSA. State 3 respiration was induced with the addition of 0.3 mM ADP. The respiratory control ratio (RCR) was ∼10 with glutamate or malate as substrate, indicating intactness of the inner mitochondrial membrane.
Measurements of Oxidative Damage
Lipid peroxidation.—
F2-isoprostanes were measured in plasma and tissues using the gas chromatography–mass spectrometry method of Roberts and Morrow (32) as previously described (33). The levels of F2-isoprostanes are expressed as nanograms F2-isoprostanes per milliliter of plasma or per gram tissue.
Protein oxidation.—
Protein oxidation in tissues was determined by the level of protein carbonyls using an advanced variant (34) of the original method described by Ahn and colleagues (35). In brief, fresh tissue was homogenized in degassed 20 mM sodium phosphate buffer pH 6.0 containing 0.5 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA), and protease cocktail inhibitors (500 μM 4-(2-Aminoethyl)benzenesulfonyl fluoride-hydrochloride, HCl, 150 nM aprotinin, 0.5 mM EDTA, disodium salt, and 1 μM leupeptin hemisulfate). For the cytoplasmic fraction, the homogenate was centrifuged at 100,000g at 4°C for 1 hour and the supernatant was saved for further processing. One percent streptomycin sulfate was added to remove nucleic acids. The protein samples were then bubbled with nitrogen for 15 seconds at 25 kPa, followed by treatment with 0.3 M guanidine HCl for partial unfolding of the proteins in the sample. The hydrazine reagent, fluorescein-5-thiosemicarbazide (FTC; Molecular Probes; 1 mM), was added and incubated for 2 hours at 37°C in the dark. The excess unreacted FTC was removed by precipitation with equal volume of 20% trichloroacetic acid followed by washing four times with ethanol or ethyl acetate (1:1; v/v). Equal amounts of protein were loaded on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to resolve the FTC-labeled proteins. A fluorescence scan of the gel was then taken, which measures the amount of bound FTC, that is, the amount of protein carbonyls. The gel was then stained with Coomassie blue, and the protein concentration was determined. The carbonyl content of the protein samples was expressed as the ratio of FTC fluorescence (carbonyls) to Coomassie blue absorption (protein concentration).
Western Blot Analysis
CuZnSOD, glutathione peroxidase 1 (GPX1), and catalase levels were determined by Western blot analysis. Protein (40–80 μg) samples were separated on a 4%–20% SDS-PAGE gel and transferred onto a nitrocellulose membrane. Equal loading was confirmed visually with Ponceau S staining. Membranes were blocked in 5% nonfat milk in Tris-buffered saline (TBS) with 0.1% Tris-buffered saline Tween-20 (TBST) and incubated at 4°C in TBST overnight with the appropriate primary antibodies: CuZnSOD (1 μg/mL; Stressgen, Canada), GPX1 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), and catalase (1:5,000; Research Diagnostics, Minneapolis, MN). Following three washings using TBST, membranes were incubated with HRP-conjugated secondary antibodies (1:2,000; Santa Cruz Biotechnology). The protein band was visualized using the ECL Plus Kit (Amersham Pharmacia Biotech), and the intensities of the bands were quantified using ImageQuant v5.0 (Molecular Dynamics, Amersham, UK). Western blot data were analyzed by normalizing protein bands to levels of skeletal muscle actin (Sigma) as loading control.
Sensitivity of Murine Embryonic Fibroblasts and Mice to Paraquat
Timed mating of male Sod2 Tg mice and female WT mice was carried out to generate Sod2 Tg and WT embryos. Murine embryonic fibroblasts (MEFs) were derived from 13- to 14-day mouse embryos, and the sensitivity of MEFs to paraquat was determined as previously described (36). MEFs were seeded (2 × 104 cells/well) in 48-well plates in Dulbecco's modified eagle medium/F12 supplemented with 10% fetal bovine serum and incubated at 37°C with 5% CO2. After 24 hours, the cells were treated with various concentrations of paraquat (50–400 μM) and cell viability was assessed by the neutral red assay (37). All treatments were performed in triplicate, and each experiment was repeated with cells isolated from three animals.
The sensitivity of whole animals to oxidative stress was determined using paraquat. Paraquat was dissolved in 0.9% saline (25 mg/mL) and injected intraperitoneally at the dosage of 50 mg/kg body weight. The mice were then followed periodically over 7 days and deaths recorded. At the end of 7 days, all the remaining mice were terminated.
Pathological Assessment of Mice
Mice found dead in the survival study were removed immediately from the cage to minimize autolysis, and necropsy was performed. Organs and tissues were excised and preserved in 10% buffered formalin: brain, pituitary gland, heart, lung, trachea, thymus, aorta, esophagus, stomach, small intestine, colon, liver, pancreas, spleen, kidneys, urinary bladder, reproductive system (prostate, testes, epididymis, and seminal vesicles), thyroid gland, adrenal glands, parathyroid glands, psoas muscle, knee joint, sternum, and vertebrae. Any other tissue with gross lesions was also excised. The fixed tissues were processed conventionally, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin–eosin. Although autolysis of varying severity occurred, it did not prevent the histopathological evaluation of lesions. Diagnosis of each histopathological change was made with histological classifications in aging mice described by Bronson and Lipman (38). A list of pathological lesions was constructed for each mouse that included both neoplastic and nonneoplastic diseases.
The probable cause of death for each mouse was determined by the severity of diseases found by necropsy as assessed independently by two pathologists. For neoplastic diseases, cases that had grades 3 and 4 lesions were categorized as “death by neoplastic lesions. “For nonneoplastic diseases, cases that had a severe lesion, for example, grade 4, associated with other histopathological changes (pleural effusion, ascites, congestion, and edema in lung) were categorized as death by the nonneoplastic lesion. In more than 90% of the cases, there was agreement by the two pathologists. In cases in which the pathologists disagreed or in which no disease was considered severe enough, the cause of death was categorized as unknown.
RESULTS
Characterization of Sod2 Tg Mice
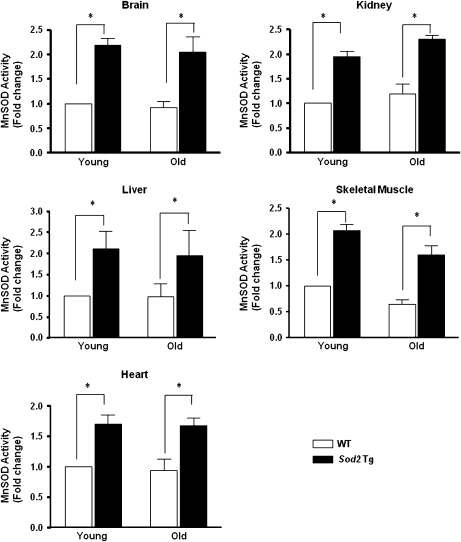
To show that MnSOD is overexpressed throughout the life span of the transgenic mice, we measured the enzymatic activity of MnSOD in tissues from both young (4–6 months) and old (26–28 months) WT and Sod2 Tg mice. As shown in Figure 1, the activity of MnSOD is increased approximately twofold in the brain, kidney, liver, heart, and skeletal muscle of Sod2 Tg mice. We measured MnSOD activity and protein content in mitochondria isolated from a few tissues in WT and Sod2 Tg mice and found a similar level of overexpression in the Sod2 Tg mice. Furthermore, we found no evidence that mitochondrial content was altered in the Sod2 Tg mice as measured by marker proteins (i.e., cytochrome c, COX IV, citrate synthase) that correlate with mitochondrial content (data not shown). To determine whether overexpression of MnSOD had an effect on other components of the antioxidant defense system, we measured the levels of CuZnSOD, GPX1, and catalase in skeletal muscle from young and old Sod2 Tg and WT mice. As shown in Table 1, the levels of all three proteins were similar regardless of age or genotype. Therefore, overexpression of MnSOD did not downregulate the expression of other antioxidant enzymes.
Figure 1.
MnSOD activity in various tissues of wild-type (WT) and Sod2 Tg mice. The activity of MnSOD was measured in tissue homogenates isolated from brain, kidney, liver, skeletal muscle, and heart of young and old WT (open bar) and Sod2 Tg (solid bar) mice, determined using native gels described in the Materials and Methods section. The data were obtained from four to six mice per group and expressed as mean ± SEM. The MnSOD activity for young WT mice was normalized to 1. Data were statistically analyzed using two-way analysis of variance with the Bonferroni test mice; an asterisk denotes those values that are significantly different from young WT mice at the p < .05 level.
Table 1.
Antioxidant Enzyme Levels in Young and Old WT and Sod2 Tg Mice
| CuZnSOD | GPX1 | Catalase | |
| Young WT | 556 ± 29 | 0.127 ± 0.008 | 1.15 ± 0.10 |
| Young Sod2 Tg | 523 ± 25 | 0.121 ± 0.010 | 1.26 ± 0.13 |
| Old WT | 557 ± 22 | 0.116 ± 0.007 | 1.25 ± 0.08 |
| Old Sod2 Tg | 538 ± 28 | 0.121 ± 0.003 | 1.67 ± 0.17 |
Note: CuZnSOD, GPX1, and catalase levels were determined in skeletal muscle of young (4–5 months) and old (26–28 months) WT and Sod2 Tg mice by Western blot analysis as described in the Materials and Methods section (Unit: OD/mg protein). Data shown are the mean ± SEM for four animals per group and were analyzed using analysis of variance with Bonferroni’s post hoc test.
We compared body weight and food consumption of Sod2 Tg and age-matched WT mice and found no changes in either body weight (Table 2) or food consumption (data not shown). Additionally, no differences in the tissue weights were observed with age or genotype in liver, spleen, kidney, and brain between the two groups. However, age-associated changes were seen in heart and skeletal muscle (Table 2). The decrease in skeletal muscle mass with age is a common biomarker of aging that is believed to occur because of increased oxidative damage or stress that the data to be presented subsequently demonstrate (39). As can be seen in Table 2, a significant decline in muscle mass of gastrocnemius or plantaris and tibialis anterior was observed with age. These data are consistent with other reports showing that gastrocnemius and tibialis anterior, which contain a high content of fast twitch fibers, are more susceptible to age-related muscle loss (27). In contrast, we found that the soleus, which is predominately slow fiber, showed no age-related decline in muscle mass, which is consistent with previous reports (40,41). However, despite evidence for an increase in ROS, overexpressing MnSOD had no effect on the age-related decline in mass in the gastrocnemius or plantaris and tibialis anterior muscles (Table 2).
Table 2.
Total BW and Tissue Weights in Young and Old WT and Sod2 Tg Mice
| Skeletal Muscle |
|||||||||
| BW | Liver | Spleen | Kidney | Heart | Brain | Soleus | Gast/pln | TA/EDL | |
| Young WT | 26.2 ± 0.4 | 1.24 ± 0.03 | 0.09 ± 0.01 | 0.32 ± 0.01 | 0.11 ± 0.001 | 0.45 ± 0.00 | 0.013 ± 0.002 | 0.291 ± 0.005 | 0.172 ± 0.003 |
| Young Sod2 Tg | 25.9 ± 0.5 | 1.32 ± 0.06 | 0.07 ± 0.00 | 0.32 ± 0.01 | 0.12 ± 0.001 | 0.43 ± 0.00 | 0.017 ± 0.000 | 0.282 ± 0.012 | 0.172 ± 0.005 |
| Old WT | 31.1 ± 0.9* | 1.98 ± 0.30 | 0.10 ± 0.01 | 0.44 ± 0.03 | 0.14 ± 0.001* | 0.45 ± 0.01 | 0.013 ± 0.001 | 0.191 ± 0.010* | 0.134 ± 0.009* |
| Old Sod2 Tg | 29.1 ± 1.0* | 1.41 ± 0.15 | 0.11 ± 0.02 | 0.42 ± 0.02 | 0.15 ± 0.01* | 0.46 ± 0.01 | 0.014 ± 0.002 | 0.203 ± 0.017* | 0.142 ± 0.009* |
Note: The BW and the weight of various tissues from young (4–5 months) and old (26–28 months) WT and Sod2 Tg mice were collected from five to six mice per group and were analyzed using analysis of variance with Bonferroni’s post hoc test. Asterisks denote a statistically significant difference between young and old mice at the p < .05 level. BW = body weight; Gast/pln = gastrocnemius/plantaris, TA/EDL = tibialis anterior/extensor digitorum longus; WT = wild type.
ROS Production in Skeletal Muscle Mitochondria
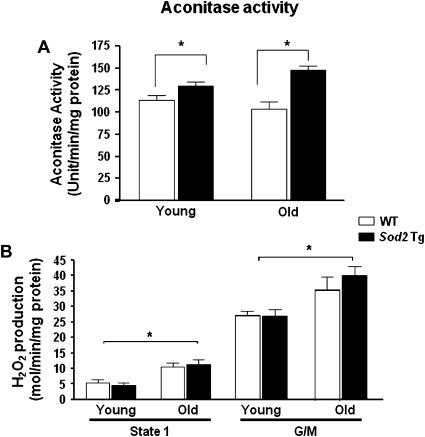
To test whether an increased level of MnSOD decreases mitochondrial ROS generation, we measured the O2·− levels and H2O2 release in skeletal muscle mitochondria from young and old mice (Figure 2). Aconitase activity in the mitochondria was used as an indirect measure of O2·− levels because the Fe-S clusters in aconitase are highly sensitive to reaction of O2·− (29,30). Compared with WT mice, mitochondrial aconitase activity in Sod2 Tg mice (Figure 2A) was significantly higher in both young and old animals, and this increase was greater in the old (∼40%) than in young (∼13%) mice.
Figure 2.
Aconitase activity and H2O2 generation in skeletal muscle mitochondria from wild-type (WT) and Sod2 Tg mice. Aconitase activity (A) and H2O2 production (B) were determined in mitochondria isolated from skeletal muscle of young and old WT (open bars) and Sod2 Tg mice (solid bars). The data are the mean of four to six animals ± SEM and were analyzed by the nonparametric test of analysis of variance (with Bonferroni’s post hoc). The asterisk denotes a statistically significant difference between WT and Sod2 Tg mice (aconitase activity) and young and old mice (H2O2 production) at the p < .05 level.
The rate of H2O2 release from mitochondria was measured in the absence of added respiratory substrates (state 1) and in the presence of 5 mM glutamate plus malate. In state 1 (Figure 2B), H2O2 production from skeletal muscle mitochondria was increased ∼90% with age, consistent with what we have previously observed (27). MnSOD overexpression did not alter the rate of H2O2 release in either age group. Similarly, using complex I-linked substrate glutamate and malate (Figure 2C), H2O2 levels were significantly increased (∼50%) with age, but no difference in H2O2 was observed between Sod2 Tg and age-matched WT mice.
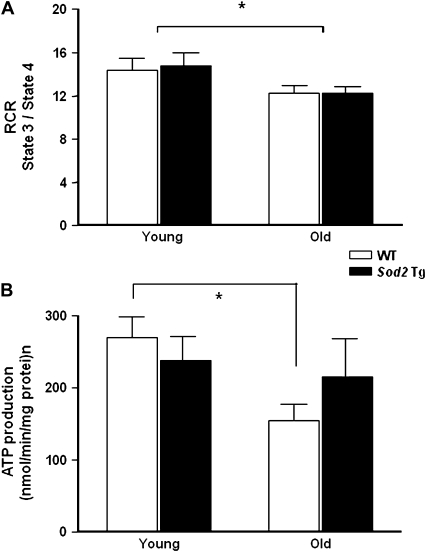
Mitochondrial Function
We next assessed mitochondrial function by measuring the RCR and ATP production from mitochondria isolated from skeletal muscle. The RCR decreased significantly with age (10%) but was unaffected by MnSOD overexpression (Figure 3A). ATP production by isolated mitochondria was measured in the presence of mitochondrial complex II-linked substrates, succinate or rotenone (Figure 3B). In agreement with our previous report (27), ATP production from the skeletal muscle mitochondria showed a significant decline with age using complex II-linked substrate. Similar results were observed using glutamate or malate (data not shown). In contrast, mitochondria from Sod2 Tg mice did not show a significant decrease with age in ATP production, and there was no significant difference in ATP production by mitochondria from Sod2 Tg and WT at the two ages studied.
Figure 3.
Mitochondrial respiration and ATP production in skeletal muscle mitochondria from wild-type (WT) and Sod2 Tg mice. The mitochondrial respiration control ratio (A) and ATP production (B) were determined in mitochondria isolated from skeletal muscle of young and old WT (open bars) and Sod2 Tg (solid bars) mice using complex II-linked substrate. The data are the mean of six animals ± SEM and were analyzed by the nonparametric test of analysis of variance (with Bonferroni’s post hoc). The asterisk denotes a statistically significant difference between young and old mice at the p < .05 level.
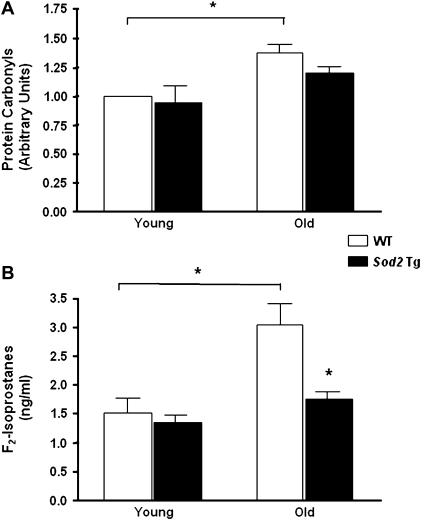
Oxidative Damage in Skeletal Muscle of Young and Old WT and Sod2 Tg Mice
Several groups, including ours, have shown that oxidative damage to protein, lipid, and DNA in skeletal muscle increases with age (27). To test whether the Sod2 Tg mice are protected against protein oxidation with age, we measured the level of protein carbonyls from the cytosolic fraction of skeletal muscle. Protein carbonyls were significantly elevated with age in WT mice (Figure 4A). In contrast, we did not observe a statistically significant increase in carbonyls with age in the Sod2 Tg mice. Although there was a trend toward a decrease in protein carbonyl levels in the old Sod2 Tg compared with old WT mice (p = .08), the protein carbonyl levels were not statistically different between the WT and the Sod2 Tg mice in either age group (Figure 4A). Next, we assessed oxidative damage to lipids by measuring F2-isoprostanes in the skeletal muscle (Figure 4B). Unlike other lipid peroxidation derivatives, F2-isoprostanes are stable and provide excellent biomarkers of lipid peroxidation. We found that with age, skeletal muscle from WT mice exhibited a twofold increase in F2-isoprostane levels. However, the F2-isoprostane levels did not increase significantly with age in the Sod2 Tg mice, and in old mice, the levels of F2-isoprostanes were reduced significantly (43%) in the Sod2 Tg mice compared with WT mice.
Figure 4.
Oxidative damage in skeletal muscle from young and old wild-type (WT) and Sod2 Tg mice. Protein carbonyl (A) and F2-isoprostane (B) levels were measured in skeletal muscle from young and old mice of Sod2 Tg (solid bars) and WT (open bars) mice. Protein carbonyl levels were determined as described by (42) and F2-isoprostanes determined as described by (43). Data shown are the mean ± SEM for six animals per group and were analyzed using analysis of variance with Bonferroni’s post hoc test. The asterisks denote a statistically significant difference at the p < .05 level.
Sensitivity of WT and Sod2 Tg Mice to Oxidative Stress
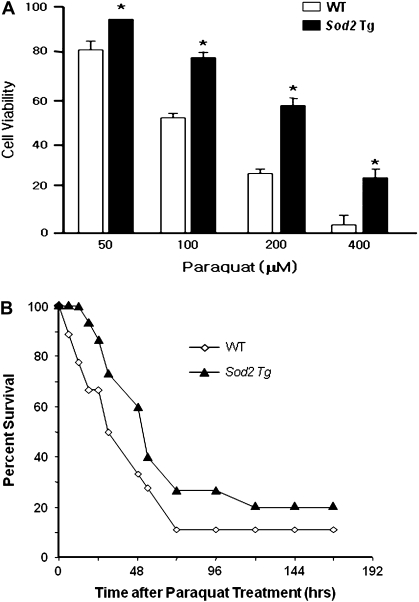
To determine whether the overexpression of MnSOD affected the sensitivity of the mice to oxidative stress, we tested the sensitivity of MEFs and whole animals to paraquat. Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) is a bipyridyl compound that is widely used as a redox cycler to stimulate superoxide production in organisms, cells, and mitochondria. A recent study demonstrated that complex I toward the mitochondrial matrix is the major site of paraquat-induced superoxide generation (44,45). Figure 5A shows the sensitivity of MEFs from WT and Sod2 Tg mice to paraquat at concentrations of 100–400 μM. The cell viability of MEFs from the Sod2 Tg mice was significantly higher than MEFs isolated from WT mice at all doses studied, and at the highest paraquat concentration tested, MEFs from Sod2 Tg mice were fourfold more resistant to paraquat toxicity than MEFs from WT mice. Figure 5B shows the sensitivity of WT and Sod2 Tg mice to a lethal dose of paraquat. Over the 7-day period following the administration of paraquat, the deaths in the Sod2 Tg mice were 10%–30% less than in the WT mice, and the log-rank test showed that the survival curves for the WT and Sod2 Tg mice were significantly different. Thus, both at the level of the cell and the whole organism, we found that overexpressing MnSOD approximately twofold had a significant effect on resistance to oxidative stress.
Figure 5.
Effect of overexpressing MnSOD on sensitivity to oxidative stress. (A) Primary cultures of murine embryonic fibroblasts (MEFs) isolated from Sod2 Tg and wild-type (WT) mice were treated with various doses of paraquat for 48 hours. Cell viability was measured by the neutral red assay as described in the Materials and Methods section. All values represent the mean ± SEM from three different animals. The data were analyzed by a two-way analysis of variance with a follow-up Tukey’s multiple range test. The asterisk denotes those values that are significantly different at p < .05 between MEFs isolated from Sod2 Tg compared with WT mice. (B) Paraquat (50 mg/kg) was administered to 12 WT (open diamonds) and 13 Sod2 Tg (solid triangles) mice, and the survival of the mice was followed over 7 days. The survival curves were statistically analyzed using the log-rank test and shown to be significant at the p < .05 level.
Life Span and the End-of-Life Pathology of Sod2 Tg Mice
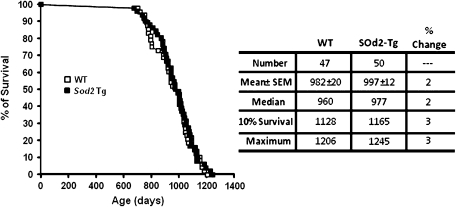
To determine whether overexpressing MnSOD throughout life span has any effect on aging, we first measured the survival of the WT and Sod2 Tg mice housed under barrier conditions. Figure 6 shows the survival data and curves for the Sod2 Tg and WT mice. The mean survival of the male WT mice in our study was well over 32 months (982 days). This is extremely long lived for C57BL/6 mice but is not unexpected because we measure life span under optimal conditions that minimize potential complications from a poor environment (47). We observed no significant differences between the survival curves for the Sod2 Tg and WT mice or between the mean, median, and 10% (when 90% of the mice died) survivals of these mice. Thus, overexpression of MnSOD had no significant effect on the life span of the mice.
Figure 6.
Life span of Sod2 Tg and WT mice. The survival curves of wild-type (WT; open symbols) and Sod2 Tg (solid symbols) mice were obtained from 50 male Sod2 Tg and 47 male WT mice. The survival data in the table are expressed in days. Mean survival (±SEM) for each group was compared with the WT group by performing a Student’s t test upon log-transformed survival times from the respective groups. The mean, median, 10%, and maximum survivals for each group were compared with the WT group using a score test adapted from Wang and colleagues (67).
To determine whether overexpressing MnSOD has an impact on the pathologic lesions leading to death, we conducted a comprehensive end-of-life pathological analysis of the WT and Sod2 Tg mice in the survival groups. As shown in Table 3, the probable causes of death for the WT and Sod2 Tg mice were similar. As expected for mice in the C57BL/6 background (48,49), the majority of fatal tumors in both WT and Sod2 Tg mice was lymphoma. The proportions of mice that died from neoplastic diseases were ∼62% for WT and 50% for the Sod2 Tg mice; however, this difference was not statistically significant. There were no changes in the average of age of tumor development between genotypes, 933 and 922 days for WT and Sod2 Tg mice, respectively; and the percentage of tumor bearing mice was 62% for WT and 65% for Sod2 Tg mice. We also studied the effect of overexpressing MnSOD on the incidence of multiple tumors in the mice. The WT mice showed an average of 0.73 tumors per mouse compared with 0.71 for the Sod2 Tg mice. The major nonneoplastic pathology observed in the WT and Sod2 Tg mice was glomerulonephritis, which was similar in frequency in WT and Sod2 Tg mice. Although the Sod2 Tg mice showed a higher incidence of nonneoplastic pathology, 50% versus 38%, this difference was not statistically significant.
Table 3.
The Probable Causes of Death for WT and Sod2 Tg Mice
| WT (%) | Sod2 Tg (%) | |
| Neoplasm | 29 (62) | 26 (50) |
| Lymphoma | 22 (47) | 22 (42) |
| Hemangioma | 3 (6) | 2 (4) |
| Others | 4 (8) | 2 (4) |
| Nonneoplasm | 18 (38) | 26 (50) |
| Glomerulonephritis | 10 (21) | 11 (21) |
| Acidophilic macrophage pneumonia | 3 (6) | 2 (4) |
| Others | 5 (11) | 13 (25) |
Note: Pathological analyses were determined in mice that died in the life span study as described in the Materials and Methods section. The data were obtained from 47 WT and 52 Sod2 Tg mice. WT = wild type.
DISCUSSION
Our rationale for studying the effect of overexpressing MnSOD on life span of mice originated from three areas. First, Tower’s laboratory showed that overexpressing MnSOD up to 75% in Drosophila increased life span by 15% (14). Second, Rabinovitch’s group showed that overexpressing catalase in mitochondria increased the life span of mice by 21% (50), suggesting that an enhanced mitochondrial antioxidant defense system is important in increasing longevity. Third, studies with C. elegans mutants in insulin or IGF-1 signaling suggest that the induction of MnSOD, one of the target genes that are upregulated through a reduction in insulin or IGF-1 signaling, is important in the increased resistance to stress and increased life span (8,9).
In the current study, we used Sod2 Tg mice generated by Epstein’s laboratory that overexpress MnSOD (19). We show here that the Sod2 Tg mice expressed MnSOD approximately twofold more than the WT mice in all tissues examined over the life span of the transgenic mice. Because there was no significant decrease in any of the other major antioxidant enzymes (GPX1, catalase, and CuZnSOD), the Sod2 Tg mice would be expected to have an enhanced antioxidant defense system with an increased ability to detoxify ROS, especially (O2·−). The Sod2 Tg mice showed no overt phenotype, and their body and tissue weights and food consumption were similar to those of their WT littermates. Because MnSOD is a key component of the mitochondrial antioxidant defense system, we also were interested in how overexpressing MnSOD affects various functions of mitochondria isolated from the skeletal muscle of young and old mice. As expected, we observed a reduction in superoxide anion levels (as measured indirectly by reduction in aconitase activity) in the mitochondria isolated from Sod2 Tg mice compared with WT mice. However, no changes were seen in mitochondrial H2O2 generation. In an agreement with these observations, in our previous report showed that, Sod2 heterozygous knockout mice (Sod2+/−) which have an ∼50% reduction in MnSOD activity, mitochondrial H2O2 production was not significantly different in both young (3–5 months) and old (26–29 months) skeletal muscle. Although it is logical to think that the majority of mitochondrial H2O2 production is directly proportional to levels of MnSOD activity, several possible reasons may explain why we did not observe any differences in H2O2 production between WT and Sod2 Tg mice. Because the probe we used to detect H2O2 (Amplex Red) is membrane impermeable and because H2O2 can freely diffuse in and out of membranes, we may be detecting only a small portion of H2O2 released from the mitochondria. For example, if there were an approximately twofold increase in H2O2 at the matrix side of mitochondria due to MnSOD overexpression, other mitochondrial H2O2 detoxifying enzymes such as peroxiredoxin 3 (PRX3), glutathione peroxidase 1 (GPX1), and GPX4 may act prior to the release of H2O2 from the mitochondria. Additionally, we assessed the level of superoxide in vivo by reduction in aconitase activity, whereas the rate of H2O2 production was measured solely in vitro in isolated mitochondria. Furthermore, it is also feasible to think that the mitochondrial substrates we add to enhance electron transfer may not be ideally mimicking the in vivo mitochondrial environment.
Previous studies with long-lived invertebrates (42,51), dwarf mice (43), and caloric-restricted rodents (46) have shown a strong correlation between resistance to oxidative stress and increased longevity. We found that MEFs from Sod2 Tg mice were more resistant to paraquat treatment than cells from WT mice. In support of this observation, when cadmium-induced toxicity was measured in similar fashion, Sod2 Tg MEFs showed approximately twofold higher resistance compared with WT MEFs (data not shown). Moreover, when a lethal dose of paraquat was injected intraperitoneally to assess toxicity at an organism level, Sod2 Tg mice exhibited a higher survival rate compared with age-matched WT littermates. It is noteworthy that most of the differences in survival occur within 24 hours after injection, and survival rates were similar in both groups after 24 hours. In our previous study, we did not observe acute early deaths in same strain of WT mice (C57BL/6J) (52). This discrepancy could be due to the gender difference in two studies as Holzenberger and colleagues (53) reported that survival in paraquat-induced toxicity is significantly different in male and female mice.
Hu and colleagues (54) also reported that overexpressing MnSOD in another transgenic mouse reduced the age-related increase in ROS levels in brain using dihydroethidium. We also measured the effect of overexpressing MnSOD on the age-related increase in oxidative damage in skeletal muscle, using protein carbonyls as a marker for protein oxidation and F2-isoprostanes as an indicator of lipid peroxidation. In agreement with the previous findings (55), we found that although protein carbonyls were significantly elevated with age in the skeletal muscle of WT mice, they were not in Sod2 Tg mice. Moreover, MnSOD overexpression had a dramatic effect on lipid peroxidation. In WT mice, we observed an approximately twofold increase in F2-isoprostanes with age in skeletal muscle that was completely attenuated in the Sod2 Tg mice.
Because Sod2 Tg mice are more resistant to oxidative stress and show reduced oxidative damage, one would predict that transgenic mice overexpressing MnSOD would show an increase in life span. Recently, Hu and colleagues (54) reported that overexpression of MnSOD increased the life span of transgenic mice. In this study, the complementary DNA to Sod2 was expressed using a β-actin promoter (twofold to fourfold increase, except liver) in B6C3 mice. Using 30 WT and 24 transgenic mice, Hu and colleagues (54) reported that the transgenic mice overexpressing MnSOD showed a 18% increase in maximum life span (1,095 vs 1,290 days). However, there was no statistical analysis of the survival curves or survival data, and the mean survival for the transgenic mice overexpressing MnSOD was only 4% longer than the WT mice (828 vs 864 days). In our present study, we showed no statistical difference in the survival between WT and Sod2 Tg mice; for example, neither the mean, median, nor 10% survival were significantly different for WT and Sod2 Tg mice. Although the maximum survival of the Sod2 Tg mice (1,245 days) in our study was similar to the maximum life span of the transgenic mice (1,290 days) in the study by Hu and colleagues (54), the mean survival of WT and Sod2 Tg mice in our study (982 and 997 days, respectively) was ∼15% longer than the mean survival of the mice in the study by Hu and colleagues (54). In our study, we used more than 45 mice per group to measure life span, which gives us sufficient power to detect a 10% difference in mean life span (47). As can be seen from our survival data, the mice in our study were long lived, for example, a mean life span of 982 days for the male WT mice, which is longer than historically reported for C57BL/6 mice (56,57) and longer than reported for the animal colonies maintained by National Institute on Aging (58) or the C57BL/6 mice maintained by Jackson Laboratories (59). By maximizing the life span of the mice, potential genotype and environmental interactions are minimized, and the results should reflect a more accurate measurement of the effect of the manipulation, in this case the overexpression of MnSOD, on aging. Our survival data show that overexpressing MnSOD had no effect on life span, one important measure of aging. Our pathological data on the WT and Sod2 Tg mice also show that overexpressing MnSOD had no effect on aging.
These data, when combined with an earlier study in which we showed that the life span of Sod2+/− mice was not altered (60), show that varying MnSOD levels fourfold (from 50% in the Sod2+/− mice to 200% in the Sod2 Tg mice) has no detectable effect on the life span of mice maintained under barrier conditions. Nevertheless, the level of MnSOD expression is correlated with how well the mouse can handle acute oxidative stress such as is caused by paraquat: Sod2+/− mice are more sensitive (60) and Sod2 Tg mice are more resistant (61). Our survival data in mice differed from those from Drosophila that showed that overexpressing MnSOD increased life span (14). It also should be noted that observations of overexpressing CuZnSOD increased the life span of Drosophila (14,62) were not replicated in mice (63) (data from our laboratory submitted for publication). Therefore, it is likely that the effect of overexpressing the SODs is species dependent, with overexpression of either MnSOD or CuZnSOD increasing the life span of Drosophila but not of mice. These data point to the importance of genetic manipulations that extend life span in invertebrates may not be applicable in a mammalian system.
It is interesting to note that Schriner and colleagues (50) reported that transgenic mice overexpressing catalase in mitochondria showed an increase in life span in contrast to our study showing that overexpressing endogenous mitochondrial MnSOD had no affect on life span. In addition, a recent report by Treuting and colleagues (64) reported that the mitochondrial-targeted catalase transgenic mice showed reduced malignant nonhematopoetic tumor burden and reduced cardiac lesions at end-of-life pathology. In contrast, we did not observe a significant difference in tumor burden in the Sod2 Tg mice compared with the WT. These data suggest that mitochondrial H2O2 levels are more important than mitochondrial O2·− levels in aging. For example, the reduced levels of mitochondrial H2O2 may play an important role in aging as a signaling molecule (65). Although a small portion of mitochondrial O2·− can leak out of the mitochondria (66), the majority of O2·− anions that are generated by the mitochondrial electron transport chain are rapidly dismutated by SOD to H2O2. In contrast, H2O2 has relatively long half-life and can easily diffuse across the mitochondrial membrane making it a potentially important molecule for cell signaling (65).
FUNDING
National Institutes of Health grants (P01AG19316, P01AG020591, R37AG026557 to A.R.), the San Antonio Nathan Shock Aging Center (1P30-AG13319), and VA Merit grants (A.R. and H.V.R.) and the Reserve Educational Assistance Program from the Department of Veteran Affairs.
Acknowledgments
The authors would like to acknowledge the assistance of Marian Sabia, Jay Cox, Vivian Diaz, and Amanda Jernigan for excellent mouse husbandry. We would also like to express our thanks to Alex Bokov for assistance in analyzing the survival data.
References
- 1.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 2.Fridovich I. The trail to superoxide dismutase. Protein Sci. 1998;7:2688–2690. doi: 10.1002/pro.5560071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 4.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 5.Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–175. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- 6.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 7.Murphy CT, McCarroll SA, Bargmann CI, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 10.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- 11.Baba T, Shimizu T, Suzuki Y, et al. Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J Biol Chem. 2005;280:16417–16426. doi: 10.1074/jbc.M500924200. [DOI] [PubMed] [Google Scholar]
- 12.Longo VD, Gralla EB, Valentine JS. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J Biol Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 13.Longo VD, Liou LL, Valentine JS, Gralla EB. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch Biochem Biophys. 1999;365:131–142. doi: 10.1006/abbi.1999.1158. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebovitz RM, Zhang H, Vogel H, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 17.Silva JP, Shabalina IG, Dufour E, et al. SOD2 overexpression: enhanced mitochondrial tolerance but absence of effect on UCP activity. EMBO J. 2005;24:4061–4070. doi: 10.1038/sj.emboj.7600866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motoori S, Majima HJ, Ebara M, et al. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res. 2001;61:5382–5388. [PubMed] [Google Scholar]
- 19.Raineri I, Carlson EJ, Gacayan R, et al. Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility. Free Radic Biol Med. 2001;31:1018–1030. doi: 10.1016/s0891-5849(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 20.Chappell JB, Perry SV. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature. 1954;173:1094–1095. doi: 10.1038/1731094a0. [DOI] [PubMed] [Google Scholar]
- 21.Ernster L, Nordenbrand K. Skeletal muscle mitochondria. Meth Enzymol. 1967;10:86–94. [Google Scholar]
- 22.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 23.Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363:91–97. doi: 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 25.Kettle AJ, Carr AC, Winterbourn CC. Assays using horseradish peroxidase and phenolic substrates require superoxide dismutase for accurate determination of hydrogen peroxide production by neutrophils. Free Radic Biol Med. 1994;17:161–164. doi: 10.1016/0891-5849(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 26.Bielski BHJ. Reactivity of HO2·/O2·- radicals in aqueous solution. J Phys Chem Ref Data. 1985;14:1041–1091. [Google Scholar]
- 27.Mansouri A, Muller FL, Liu Y, et al. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127:298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 29.Gardner PR. Aconitase: sensitive target and measure of superoxide. Meth Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 30.Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 31.Estabrook RW. Mitochondrial respiratory control and the polarographic measurement of ADP: O ratios. Meth Enzymol. 1974;10:41–47. [Google Scholar]
- 32.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- 33.Ward WF, Qi W, Van Remmen H, Zackert WE, Roberts JL, Richardson A. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. J. Gerontol. 2005;60(7):847–851. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri AR, de Waal EM, Pierce A, Van Remmen H, Ward WF, Richardson A. Detection of protein carbonyls in aging liver tissue: a fluorescence-based proteomic approach. Mech Ageing Dev. 2006;127:849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Ahn B, Rhee SG, Stadtman ER. Use of fluorescein hydrazide and fluorescein thiosemicarbazide reagents for the fluorometric determination of protein carbonyl groups and for the detection of oxidized protein on polyacrylamide gels. Anal Biochem. 1987;161:245–257. doi: 10.1016/0003-2697(87)90448-9. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Siu B, Ho YS, et al. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 37.Repetto G, Sanz P, Repetto M. Comparative in vitro effects of sodium arsenite and sodium arsenate on neuroblastoma cells. Toxicology. 1994;92:143–153. doi: 10.1016/0300-483x(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 38.Bronson RT, Lipman RD. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- 39.Muller FL, Song W, Liu Y, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 40.Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- 41.McKiernan SH, Bua E, McGorray J, Aiken J. Early-onset calorie restriction conserves fiber number in aging rat skeletal muscle. FASEB J. 2004;18:580–581. doi: 10.1096/fj.03-0667fje. [DOI] [PubMed] [Google Scholar]
- 42.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 43.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 44.Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 45.Farrington JA, Ebert M, Land EJ, Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973;314:372–381. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- 46.Weraarchakul N, Strong R, Wood WG, Richardson A. The effect of aging and dietary restriction on DNA repair. Exp Cell Res. 1989;181:197–204. doi: 10.1016/0014-4827(89)90193-6. [DOI] [PubMed] [Google Scholar]
- 47.Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 49.Frith CH, Wiley LD. Morphologic classification and correlation of incidence of hyperplastic and neoplastic hematopoietic lesions in mice with age. J Gerontol. 1981;36:534–545. doi: 10.1093/geronj/36.5.534. [DOI] [PubMed] [Google Scholar]
- 50.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine lifespan by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 51.Johnson TE, Henderson S, Murakami S, et al. Longevity genes in the nematode Caenorhabditis elegans also mediate increased resistance to stress and prevent disease. J Inherit Metab Dis. 2002;25:197–206. doi: 10.1023/a:1015677828407. [DOI] [PubMed] [Google Scholar]
- 52.Van Remmen H, Qi W, Sabia M, et al. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med. 2004;36:1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 54.Hu D, Cao P, Thiels E, et al. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng J, Navratil M, Thompson LV, Arriaga EA. Estimating relative carbonyl levels in muscle microstructures by fluorescence imaging. Anal Bioanal Chem. 2008;391:2591–2598. doi: 10.1007/s00216-008-2187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith GS, Walford RL, Mickey MR. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F 1 hybrids. J Natl Cancer Inst. 1973;50:1195–1213. doi: 10.1093/jnci/50.5.1195. [DOI] [PubMed] [Google Scholar]
- 57.Storer JB. Longevity and gross pathology at death in 22 inbred mouse strains. J Gerontol. 1966;21:404–409. doi: 10.1093/geronj/21.3.404. [DOI] [PubMed] [Google Scholar]
- 58.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 59.Kunstyr I, Leuenberger HG. Gerontological data of C57BL/6J mice. I. Sex differences in survival curves. J Gerontol. 1975;30:157–162. doi: 10.1093/geronj/30.2.157. [DOI] [PubMed] [Google Scholar]
- 60.Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 61.Klivenyi P, St Clair D, Wermer M, et al. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol Dis. 1998;5:253–258. doi: 10.1006/nbdi.1998.0191. [DOI] [PubMed] [Google Scholar]
- 62.Phillips JP, Parkes TL, Hilliker AJ. Targeted neuronal gene expression and longevity in Drosophila. Exp Gerontol. 2000;35:1157–1164. doi: 10.1016/s0531-5565(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 63.Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of Cu, Zn superoxide dismutase does not extend life span in mice. J Gerontol A Biol Sci Med Sci. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- 64.Treuting PM, Linford NJ, Knoblaugh SE, et al. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813–822. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 65.Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52:3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- 66.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 67.Wang C, Li Q, Redden DT, Weindruch R, Allision DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]