Abstract
Tight junctions (TJs) play crucial roles in tissue homeostasis and inflammation through their roles in the control of paracellular transport and barrier function. There is evidence that these functions are compromised in older organisms, but the exact mechanisms leading to TJ deterioration are not well understood. Claudin proteins are a family of membrane proteins that constitute the structural barrier elements of TJs and therefore play a major role in their formation and function. Using immunohistochemistry and immunoblotting, we have studied the expression of six different claudin proteins (claudin-1, -2, -3, -4, -5, and -7) in three tissues (liver, kidney, and pancreas) of aging male and female mice. In general, we find an age-dependent decrease in the expression of several claudin proteins in all three tissues observed, although the exact changes are tissue specific. Our findings provide a possible basis for the decrease in tissue barrier function in older organisms.
Keywords: Claudin, Aging, Tight junction, Immunohistochemistry
THE tight junctions (TJs), present on epithelial and endothelial cells, are the apical-most structures of these cells and have crucial roles in providing a regulated barrier to paracellular transport between different fluid compartments (1,2). In addition, TJs are important in the maintenance of cell polarity through their ability to provide a fence function, restricting lateral diffusion in the plasma membrane (3,4). A number of proteins have been reported to be structural components or associated with TJs (1,2). In particular, occludin and claudin proteins are transmembrane proteins that are believed to have crucial roles in TJ formation and function. Although TJs can form in the absence of occludin, claudins are thought to be essential components of the TJ, forming the backbone of the structures (1,2). The claudin family of proteins consists of at least 23 members, and several studies have attributed the presence of particular claudin isoforms as the underlying basis for tissue-specific differences in permeability (2,5–7).
Diverse expression patterns of claudins in normal liver, kidney, and pancreas have been reported (8–11). Within any given tissue (with heterogeneous epithelial populations), claudin expression can be highly heterogeneous as well. For example, in pancreas, claudin-2 is located in the TJ of the duct epithelia while being absent in acinar cells (8). This pattern was inverted for claudin-5, whereas claudin-3 and -4 were expressed in the junctions of both cell types. In the liver, TJs play a crucial role in maintaining polarity among hepatocytes, and a previous study has shown a gradient expression of claudin-2 from portal to central hepatocytes (8). In the kidney, at least 11 different claudins are known to be expressed in various segments of the nephron (11). This claudin expression pattern is believed to be responsible for the unique permeability properties of various nephron segments and is therefore crucial for normal kidney function.
In the past several years, a large number of studies have demonstrated important roles for TJs in a number of physiological processes such as proliferation, differentiation, and immune functions (1,12). The importance of these functions is emphasized by the knockout of several TJ proteins, including claudin. For example, mice lacking functional Cldn1 die within 1 day of birth due to dehydration of the skin (13). Interestingly, Cldn6 transgenic mice exhibit a similar phenotype (14). Cldn5-deficient mice also die within days following birth and exhibit loosening of the blood– brain barrier (15). In humans, a number of diseases have been shown to be due to mutations in claudin genes. In particular, CLDN1 mutations have been shown to be associated with neonatal sclerosing cholangitis, probably because of decreased TJ functionality in the bile duct (16). In addition to these clear genetic studies, a large number of studies have implicated the deregulation of claudin expression in a number of conditions, including cancer. For example, aberrant expression of CLDN3 and CLDN4 has been shown in ovarian, breast, prostate, and pancreatic cancers (17).
TJ barrier function has been shown to be compromised during aging. Indeed, age-related increased leakiness of TJs and/or alterations in their biochemical components have been reported in the blood–brain barrier (18–20), lung (21), epididymis (22,23), kidney (24,25), and perineurium (26). However, the exact mechanisms that lead to this decrease in barrier function and whether this decrease in function can account for some of the aging phenotype remain to be determined. In this report, we study the levels of several key claudins in three different tissues of aging mice. We find that claudin expression is generally reduced with aging in all three tissues studied, providing a possible mechanism for the decrease in barrier function observed in various tissues of older individuals.
MATERIALS AND METHODS
Antibodies
For the immunohistochemistry experiments, polyclonal rabbit anti-claudin-1, -2, -3, -5, and -7 antibodies were purchased from Zymed (South San Francisco, CA) and rabbit anti-claudin-4 antibody from Abcam (Cambridge, UK). For immunoblotting, the following antibodies were used: anti-claudin-1 from Invitrogen (Camarillo, CA), anti-claudin-4 and anti glyceraldehyde 3-phosphate dehydrogenease (GAPDH) from Abcam, and anti-E-cadherin from BD Transduction Laboratories (San Jose, CA).
Tissue preparation and tissue microarray construction
As part of the National Institute on Aging Agemap project (27), male and female C57BL/6 mice were obtained from the NIA colony at 1 month, 6 months, 16 months, and 24 months of age. The liver, kidney, and pancreas were harvested from the mice and the tissues were fixed in formalin and embedded in paraffin. For each paraffin block, three 0.9-mm punches were taken and arrayed. The array was then embedded in paraffin, and 5-μm sections of the tissue array were cut and mounted onto glass slides.
Immunohistochemistry
The tissue microarray (TMA) slides were deparaffinized in xylene for 10 minutes followed by rehydration in graded ethanol (100%, 95%, 85%, and 75%) for 5 minutes each and washed in water. Antigens were exposed to heat using Target Retrieval Solution (Dako, Carpinteria, CA). Antigen-bound primary antibody was detected using streptavidin– biotin immunoperoxidase complex (Ultravision detection system; Lab Vision, Fremont, CA) and visualized using diaminobenzidine as chromogen. Nuclei were counterstained with hematoxylin. The antibodies against claudin-1, -2, -3, -4, -5, and -7 were applied at concentration of 5 μg/mL. For negative controls, absence of primary antibodies processed in parallel showed no positive staining. Images were acquired by Axiovision 3.1 software on a Zeiss Axiovert S100 microscope under 10× objective lens (Carl Zeiss, Thornwood, NY).
Protein expression scoring and analyses
Acquired images from TMA slides, using Zeiss Axioplasm (10×) were scored for protein expression using MCID core 7.0 version software (Interfocus Imaging, Cambridge, UK) whereby the staining was quantified and assigned arbitrary units. As previously reported (28), this approach can provide highly reproducible and reliable immunohistochemistry (IHC) staining assessment. The tissues from young and old mice, an average of three females and three males were used for analyses and three punches were taken from each tissue. For statistics, we used Student’s t test, two-tailed distribution.
Tissue fractionation and immunoblotting
Kidney and liver tissues from young (2-month-old) and old (17-month-old) mice were removed and immediately snap frozen in liquid nitrogen. The frozen tissues were then pulverized in a dry ice-cooled ore crusher. A portion of the tissue was then homogenized in 1 mL of cold buffer A (20 mM Tris, 10 mM ethylene glycol tetraacetic acid, 2.0 mM sodium ethylenediaminetetraacetic acid, 250 mM sucrose with Protease Inhibitor Cocktail Set III [Calbiochem, San Diego, CA] at 1:100 dilution, and Phosphatase Inhibitor Cocktail I [Sigma-Aldrich, St Louis, MO] and Phosphatase Inhibitor Cocktail II [Sigma-Aldrich, St Louis, MO] each at 1:100 dilution). The samples were sonicated for 1 minute at a low setting on ice and spun at 120,000 g for 1 hour at 4°C. The supernatant was collected as the cytosolic fraction and frozen immediately. One milliliter of cold buffer A + 1.0% Triton-X with the above protease and phosphatase inhibitors was used to resuspend the pellet containing the membrane fraction. This suspension was placed in an end-over-end shaker at 4°C for 1 hour and spun at 120,000 g for 60 minutes at 4°C. The supernatant was collected as the membrane fraction. All protein fractions were quantitated using the Bio-Rad Bradford protein assay reagent (Bio-Rad, Hercules, CA). The protein fractions were run on a 14% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting performed as previously described (29).
RESULTS
Claudins in aging liver
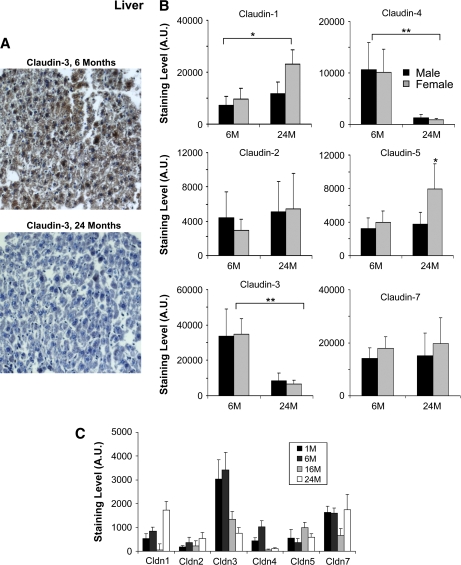
To investigate the changes in claudin expression that occur in the aging liver, liver tissues of young adult mice (6 months old) as well as older animals (24 months old) were studied by IHC for the presence of six different claudin proteins (claudin-1, -2, -3, -4, -5, and -7)(Figure 1). Figure 1A shows an example of claudin-3 staining in mouse liver at 6 and 24 months and demonstrates a significant decrease in older animals. Interestingly, much of the staining was detected in the cytoplasm. Quantitation of the IHC staining for all the claudins studied revealed the most significant changes in claudin expression to be decreases in claudin-3 and -4 in the liver of older mice (Figure 1B). Indeed, the levels of these claudins were reduced by more than fivefold in the hepatocytes of both male and female mice. Claudin-1 was found to be significantly upregulated in both older males and females, although the effect was more pronounced in females. Claudin-5 was elevated in the liver of older female mice. Claudin-2 and -7 did not appear significantly affected by aging in either sexes between 6 and 24 months.
Figure 1.
Immunohistochemistry analysis of selected claudins in aging mouse liver. (A) Representative staining for claudin-3 from liver sections of 6- and 24-month-old male mice. (B) Effects of aging on the expression of the indicated claudins. Results shown were quantitated using the MCID image analysis software and represent the average of nine sections (three liver punches from three mice) (*p < .05, **p < .001; brackets show the significance between age groups (both male and female) and the absence of a bracket indicates significance for one gender within the age group). The staining data are shown as arbitrary units (A.U.). Results are shown for 6- and 24-month-old mice. Black bars represent males and gray bars females. Claudin-3 and claudin-4 were significantly decreased in both male and female mice. Claudin-1 was increased in both males and females. (C) The effect of age and gender on claudin expression in mouse liver. The expression of the indicated claudins was examined in the liver of 1-month-old (1M), 6-month-old (6M), 16-month-old (16M), and 24-month-old (24M) mice. Again, claudin-3 and claudin-4 showed a significant decrease in older mice.
When looking at claudin expression at intermediate time points and as a composite of male and female mice, we found similar trends (Figure 1C). Both claudin-3 and -4 were decreased in older mice (16 and 24 months old) compared with younger mice (1 and 6 months old). Claudin-1 was strongly elevated at 24 months although the 16-month time point did not exhibit this increase. Claudin-7 appeared mostly unaffected by aging except for a noted decrease at 16 months. The other claudin staining did not exhibit significant changes. When males and females were taken as a group, the change in claudin-5 was not found to be significant, probably because of the fact that the change was only observed in females (Figure 1B).
Claudins in aging kidney
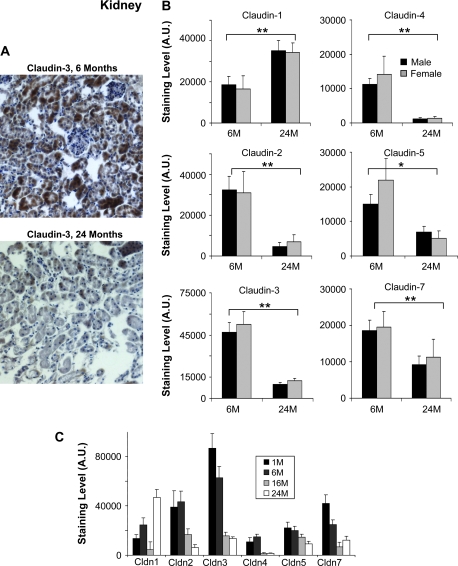
Kidney tissues (from the medulla) were then studied for changes in claudin expression in aging (Figure 2). Similar to our previous observations in liver, we found claudin-1 elevated and claudin-3 and -4 significantly decreased in the kidney of older mice (Figure 2B). However, in contrast to what we observed in liver, kidney claudins-2, -5, and -7 were also decreased significantly in older mice. Among the claudins studied, claudin-5 was the only claudin expressed in the glomeruli. Interestingly, we observed that claudin-5 staining was intense in the glomeruli of younger mice, but absent in older mice. As was observed in the liver, generally, the claudin staining appeared most intense in the cytoplasm of hepatocytes.
Figure 2.
Immunohistochemistry analysis of selected claudins in aging mouse kidney. (A) Representative staining for claudin-3 from kidney sections of 6- and 24-month-old male mice. Claudin-3 shows a significant decrease in older mice. (B) Effects of aging on the expression of the indicated claudins. Results shown were quantitated using the MCID image analysis software and represent the average of nine sections (three kidney punches from three mice) (*p < .05, **p < .001; brackets show the significance between age groups (both male and female) and the absence of a bracket indicates significance for one gender within the age group). The staining data are shown as arbitrary units (A.U.). Results are shown for 6- and 24-month-old mice. Black bars represent males and gray bars females. Claudin-2, -3, -4, -5, and -7 were significantly decreased in both male and female mice. Claudin-1 was increased in both males and females. (C) The effect of age and gender on claudin expression in mouse liver. The expression of the indicated claudins was examined in the liver of 1-month-old (1M), 6-month-old (6M), 16-month-old (16M), and 24-month-old (24M) mice. The same trends were observed when these additional time points were studied.
The same trends were observed when male and female mice were evaluated as a group (Figure 2C). Except for claudin-1, which exhibited an increase at 24 months, all the claudins were generally decreased in older mice (16 and 24 months old) compared with the younger mice (1 and 6 months old).
Claudins in aging pancreas
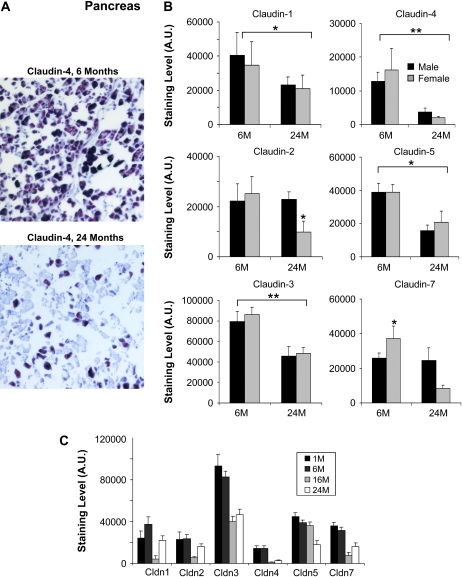
The impact of aging on pancreatic claudin expression was then evaluated (Figure 3). Claudin-4 was found expressed at relatively high levels in acinar cells of young mice and was significantly decreased in 24-month-old animals (Figure 3A). The pancreatic tissue punches observed did not have representative areas of the islet of Langerhans for all ages and hence were not included in the analysis. In addition to claudin-4, the levels of most claudin decreased significantly with age, except for claudin-2 and -7 where the decrease was observed only in females (Figure 3B). Again, when the male and female mice were examined as group at multiple points, most claudins exhibited a trend toward being expressed at lower levels in aged animals (Figure 3C). Overall, much of the claudin staining was cytoplasmic.
Figure 3.
Immunohistochemistry analysis of selected claudins in aging mouse pancreas. (A) Representative staining for claudin-4 from pancreas sections of 6- and 24-month-old male mice. Claudin-4 is clearly reduced in older mice. (B) Effects of aging on the expression of the indicated claudins. Results shown were quantitated using the MCID image analysis software and represent the average of nine sections (three pancreas punches from three mice) (*p < .05, **p < .001; brackets show the significance between age groups (both male and female) and the absence of a bracket indicates significance for one gender within the age group). The staining data are shown as arbitrary units (A.U.). Results are shown for 6- and 24-month-old mice. Black bars represent males and gray bars females. Claudin-1, -3, -4, and -5, and claudin-4 were significantly decreased in both male and female mice. Claudin-2 and -7 were significantly decreased in females. (C) The effect of age and gender on claudin expression in mouse liver. The expression of the indicated claudins was examined in the liver of 1-month-old (1M), 6-month-old (6M), 16-month-old (16M), and 24-month-old (24M) mice. All the claudin examined tended to be decreased in the pancreas of older mice.
Validation of staining patterns by immunoblotting
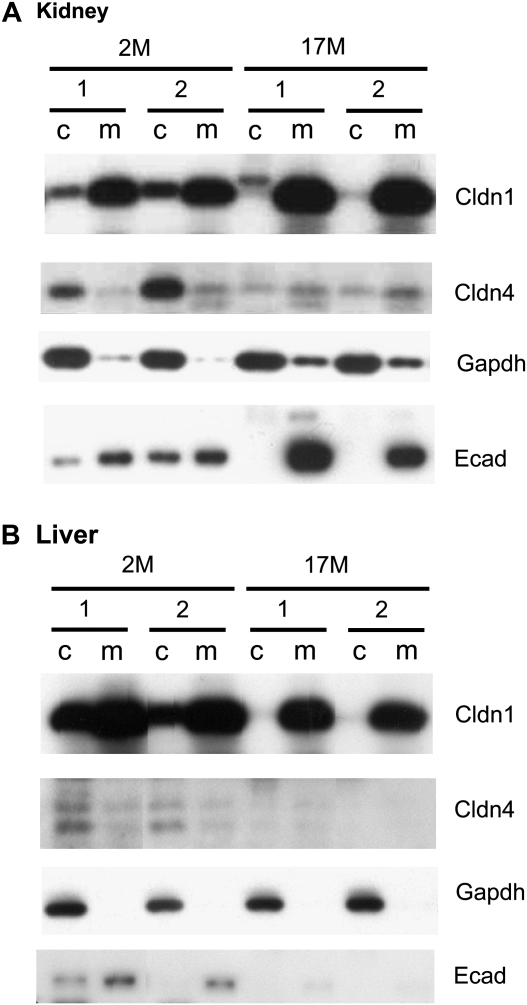
To validate the staining patterns observed by immunohistochemistry, we next performed immunoblotting for two claudins (claudin-1 and claudin-4) on kidney and liver tissues from young (2 months old) and old (17 months old) mice (Figure 4). Furthermore, to clarify the subcellular ocalization of these claudins, cellular fractionation was performed on the lysates and E-cadherin as well as GAPDH were included for membrane and cytoplasmic staining controls, respectively. In kidney, the claudin-1 levels were found elevated in older mice compared with the 2-month-old animals (Figure 4A). This result was consistent with our IHC findings (Figure 2B). The claudin-1 staining was mostly found at the membrane, although some signal was detected in the cytoplasmic fraction in the kidney of younger mice. However, this positive signal may have been due to some contamination, as suggested by the E-cadherin control included. In contrast, claudin-4 was markedly decreased in the kidney of older animals, which was consistent with our IHC results (Figure 2B). Interestingly, the claudin-4 protein in the kidney of young mice appears to be almost entirely cytoplasmic.
Figure 4.
Cellular fractionation and immunoblotting of aging mouse tissues. Kidney (A) and Liver (B) tissues were fractionated into cytoplasmic (c) and membrane (m) components before being run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Claudin-1 (Cldn1) and claudin-4 (Cldn4) expression patterns were observed. The lysates were also probed for glyceraldehyde 3-phosphate dehydrogenease (a cytoplasmic control) and E-cadherin (a membrane control). Claudin-1 is located predominantly in the membrane fraction and is increased in the kidney of older animals. Claudin-4, on the other hand, in mostly cytoplasmic and is decreased in both the kidney and liver of older mice.
By immunoblotting, claudin-1 did not appear to be significantly altered in the aging liver (Figure 4B). In contrast, claudin-4 was decreased to a point of being nondetectable in the liver of old animals. Similar to what we have observed in the kidney, claudin-1 appeared to be located mostly at the membrane, although claudin-4 was predominantly cytoplasmic.
DISCUSSION
Cellular senescence is a growth arrest program that limits the lifespan of mammalian cells and prevents unlimited cell proliferation. Although this process may be crucial in protecting organisms from cancer, it likely plays a significant role in aging (30). Aging in multicellular organisms is characterized by a progressive decline in multiple tissues and organs, ultimately leading to the death of the organism. The process of aging is now regarded as an extremely complex multifactorial phenomenon, and a full explanation of aging will likely involve multiple mechanisms operating at multiple levels of organization. At the cellular levels, the senescence theory, the oxidation theory, the “wear and tear” theory, and the apoptosis theory have all been proposed to explain tissue degeneration (31). These theories are not mutually exclusive and the process of aging is likely caused by multiple mechanisms. It has previously been suggested that one possible mechanism for tissue degeneration in aging is the decline in TJ integrity (32,33). In addition, it has been hypothesized that the increased systemic inflammation observed in aging may be due to a functional decline in TJ (34). The reduction in TJ function may be due to multiple factors, including a reduction or inappropriate expression of TJ components. Here, we show that several claudin proteins we studied were decreased during aging in three different tissues in mice. In particular, claudin-3 and -4 were decreased in all three tissues examined, whereas claudin-2, -5, and -7 were decreased in two of these tissues. Claudin-1 appeared to be an exception and was actually elevated in liver and kidney, and decreased in the pancreas. Overall, the picture that emerges is that claudin expression is significantly decreased in several aging tissues, suggesting a mechanism for a decrease in TJ function in aging.
Interestingly, much of the claudin IHC staining observed in the tissues included in this study is cytoplasmic (Figures 1A, 2A, and 3A). Cellular fractionation and immunoblotting confirmed this finding for claudin-4 in the kidney and liver of young mice (Figure 4). Although crucial in the formation and function of TJs, it has previously been shown that, under certain circumstances, claudin proteins can localize to the cytoplasm (35–37). The cytoplasmic localization may be important for the modulation of claudin function and has been shown to be regulated, at least in part, through phosphorylation (29,35,38,39). A cytoplasmic pool of claudin proteins may allow for rapid assembly of TJ structures in response to specific stimuli. A decrease in cytoplasmic claudins, as observed here in aging mice, may have a detrimental effect on the ability of the tissues to respond to changes in their environment and to adjust their barrier and reabsorptive properties. In any case, little is known about the exact roles of cytoplasmic claudin, although the current study reaffirms the presence of these proteins in the cytoplasm of various normal tissues.
Although there is a possibility that all TJ proteins are decreased nonspecifically during aging, the fact that certain claudins are elevated or unchanged in certain tissues argues against this possibility. It is well accepted that a change in claudin composition can greatly affect TJ functionality. It will therefore be important to ascertain these issues experimentally by manipulating claudin expression in the relevant tissues. For example, it would be extremely interesting to assess the effects of claudin-3 and claudin-4 reduction in normal liver, pancreas, and kidney using tissue-specific mouse transgenic knockouts. It may also be interesting to investigate claudin expression in calorie-restricted mice or midi-mice.
The complex claudin distribution observed in various tissues has been interpreted as playing a major functional role (8–11). Consequently, changes in the amounts and/or ratios of these claudins would significantly affect the barrier properties and functionality of these tissues. Consistent with this hypothesis, it was recently shown that an increase in claudin-2 in hepatocytes was associated with an enhanced barrier function that depended on molecular size (40). In addition, several mouse knockout models have demonstrated the importance of claudins in normal tissue homeostasis (13,15,41). Further experiments will be necessary to clearly establish the effects of the changes we have observed. We hypothesize that the alterations we report here account for some of the changes observed in TJ properties during aging. Although limited to three tissues (kidney, liver, and pancreas), this survey provides trends that we believe are likely to be preserved in several other tissues. It will be interesting to examine additional organs and tissues in mice as well as in humans for similar changes in claudin expression during aging.
In summary, we show for the first time that aging in mice is accompanied by a decrease in several claudins in multiple tissues. We hypothesize that some of the age-related changes in TJ function may be due to alteration in claudin expression in aging. A better understanding of the molecular changes that occur during aging may provide new opportunities to slow the aging process, or treat age-related diseases such as hypertension, atherosclerosis, kidney failure, and cancer.
FUNDING
This research was supported entirely by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Acknowledgments
We thank members of our laboratory for useful comments on the manuscript.
References
- 1.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 2.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 3.van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5:1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacallao R, Garfinkel A, Monke S, Zampighi G, Mandel LJ. ATP depletion: a novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J Cell Sci. 1994;107(pt 12):3301–3313. doi: 10.1242/jcs.107.12.3301. [DOI] [PubMed] [Google Scholar]
- 5.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 6.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 7.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 9.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkovetz DF. Tight junction claudins and the kidney in sickness and in health. Biochim Biophys Acta. 2009;1788:858–863. doi: 10.1016/j.bbamem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 13.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turksen K, Troy TC. Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development. 2002;129:1775–1784. doi: 10.1242/dev.129.7.1775. [DOI] [PubMed] [Google Scholar]
- 15.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadj-Rabia S, Baala L, Vabres P, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 18.Mooradian AD, Haas MJ, Chehade JM. Age-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) Mech Ageing Dev. 2003;124:143–146. doi: 10.1016/s0047-6374(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 19.Campbell SJ, Carare-Nnadi RO, Losey PH, Anthony DC. Loss of the atypical inflammatory response in juvenile and aged rats. Neuropathol Appl Neurobiol. 2007;33:108–120. doi: 10.1111/j.1365-2990.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 20.Virgintino D, Errede M, Robertson D, et al. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol. 2004;122:51–59. doi: 10.1007/s00418-004-0665-1. [DOI] [PubMed] [Google Scholar]
- 21.Tankersley CG, Shank JA, Flanders SE, et al. Changes in lung permeability and lung mechanics accompany homeostatic instability in senescent mice. J Appl Physiol. 2003;95:1681–1687. doi: 10.1152/japplphysiol.00190.2003. [DOI] [PubMed] [Google Scholar]
- 22.Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology. 2001;142:854–863. doi: 10.1210/endo.142.2.7975. [DOI] [PubMed] [Google Scholar]
- 23.Levy S, Robaire B. Segment-specific changes with age in the expression of junctional proteins and the permeability of the blood-epididymis barrier in rats. Biol Reprod. 1999;60:1392–1401. doi: 10.1095/biolreprod60.6.1392. [DOI] [PubMed] [Google Scholar]
- 24.Haddad M, Lin F, Dwarakanath V, Cordes K, Baum M. Developmental changes in proximal tubule tight junction proteins. Pediatr Res. 2005;57:453–457. doi: 10.1203/01.PDR.0000151354.07752.9B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes JL, Lamas M, Martin D, del Carmen Namorado M, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L. The renal segmental distribution of claudins changes with development. Kidney Int. 2002;62:476–487. doi: 10.1046/j.1523-1755.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- 26.Pummi KP, Heape AM, Grenman RA, Peltonen JT, Peltonen SA. Tight junction proteins ZO-1, occludin, and claudins in developing and adult human perineurium. J Histochem Cytochem. 2004;52:1037–1046. doi: 10.1369/jhc.3A6217.2004. [DOI] [PubMed] [Google Scholar]
- 27.Zahn JM, Poosala S, Owen AB, et al. AGEMAP: a gene expression database for aging in mice. PLoS Genet. 2007;3:e201. doi: 10.1371/journal.pgen.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Melo MR, Jr, Araujo Filho JL, Patu VJ, Machado MC, Mello LA, Carvalho LB., Jr Langerhans cells in cutaneous tumours: immunohistochemistry study using a computer image analysis system. J Mol Histol. 2006;37:321–325. doi: 10.1007/s10735-006-9056-3. [DOI] [PubMed] [Google Scholar]
- 29.D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–26240. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 30.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 31.Weinert BT, Timiras PS. Invited review: theories of aging. J Appl Physiol. 2003;95:1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- 32.Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging. 1981;2:283–291. doi: 10.1016/0197-4580(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 33.Mullin JM, Agostino N, Rendon-Huerta E, Thornton JJ. Keynote review: epithelial and endothelial barriers in human disease. Drug Discov Today. 2005;10:395–408. doi: 10.1016/S1359-6446(05)03379-9. [DOI] [PubMed] [Google Scholar]
- 34.Skrovanek S, Valenzano MC, Mullin JM. Restriction of sulfur-containing amino acids alters claudin composition and improves tight junction barrier function. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1046–R1055. doi: 10.1152/ajpregu.00072.2007. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:849–862. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hough CD, Sherman-Baust CA, Pizer ES, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–6287. [PubMed] [Google Scholar]
- 37.Lechpammer M, Resnick MB, Sabo E, et al. The diagnostic and prognostic utility of claudin expression in renal cell neoplasms. Mod Pathol. 2008;21:1320–1329. doi: 10.1038/modpathol.2008.116. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of claudin-4 and mediates paracellular permeability. J Biol Chem. 2005;280:42375–42382. doi: 10.1074/jbc.M503786200. [DOI] [PubMed] [Google Scholar]
- 39.D’Souza T, Indig FE, Morin PJ. Phosphorylation of claudin-4 by PKCepsilon regulates tight junction barrier function in ovarian cancer cells. Exp Cell Res. 2007;313:3364–3375. doi: 10.1016/j.yexcr.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imamura M, Kojima T, Lan M, et al. Oncostatin M induces upregulation of claudin-2 in rodent hepatocytes coinciding with changes in morphology and function of tight junctions. Exp Cell Res. 2007;313:1951–1962. doi: 10.1016/j.yexcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Gow A, Southwood CM, Li JS, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]