Abstract
Diseases of aging produce many alterations in the retina, but changes in growth factor signaling in normal aging are less characterized. This study investigated modifications in insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) signaling in the retina of Brown Norway × Fischer 344 F1 hybrid rats at 8, 22, and 32 months. Immunoblotting for proteins involved in IGF-1R signal transduction and electroretinograms were done to evaluate changes with aging. Aging produced a significant decrease in b-wave and oscillatory potential amplitudes in the retina. Aging produced increased phosphorylation of IGF-1R. Despite the increase in IGF-1R activity, insulin receptor substrate-1 (IRS-1) phosphorylation was significantly decreased with increasing age. Akt activity was significantly decreased at 22 and 32 months of age, resulting in increased cleaved caspase 3 levels. The results suggest that regulation of IRS-1 phosphorylation may modulate apoptotic rates in the aging retina, potentially preventing activation of vascular endothelial cell growth factor.
Keywords: IGF-1 receptor, Retina, Electroretinogram
WITH an ever-increasing number of baby boomers living longer, enhancing our understanding of the normal aging process in each organ becomes more important. Although it is clear that the rates of cancer and heart disease are increased with age, less attention has been focused on the changes in the visual system with age. The need to wear bifocals for reading is the most common age-associated change in the visual system due to alterations in the lens. Normal aging of the retina is associated with the loss of rod photoreceptors with age (1,2). In addition, hyperglycemia, which is more common due to advancing age, can induce formation of advanced glycation end products, potentially damaging the retina. We have previously reported that normal aging of the rat retina is associated with substantial changes to sympathetic neurotransmission, including a reduction in dopamine-β-hydroxylase and a concurrent increase in β1-adrenergic receptor protein (3). Previous work has also demonstrated that vascular endothelial cell growth factor (VEGF) protein is significantly increased in the retina of 32-month-old Brown Norway × Fischer 344 (BN × F344) F1 hybrid rats compared with their 8-month-old littermates (4). Because VEGF is associated with a number of age-related diseases due to its role in vascular growth, it has been the focus of development of a number of pharmaceutical agents.
Although VEGF is a key target for vascular changes, the regulation of VEGF activation also may offer insight into new targets for drug discovery. One key regulator of VEGF is insulin-like growth factor-1 (IGF-1). Work on IGF-1 receptor (IGF-1R) has previously been focused on its role in longevity as mutations in IGF-1R or its downstream signaling allow for increased life span (5,6). One mechanism for the increased life span of the IGF-1R mutants may be that these animals are resistant to a number of age-associated diseases involving cellular growth, such as cancer (7,8). These findings suggest that alterations in IGF-1R activity may produce changes in cellular function, linked to age-related diseases. The role of IGF-1R signaling in age-related disease is likely related to its ability to activate both the mitogen-activated protein kinase pathway and the phosphatidylinositol-3-kinase (PI3K) cascade (9,10). Some have shown that activation of IGF-1R can protect oligodendrocytes from apoptosis through activation of the PI3K pathway (11). Little has been done to investigate the balance of IGF-1R signaling in the retina with normal aging. Work has been done in the retina for IGF-1R signaling as a modulator of neovascularization in retinopathy of prematurity (12), diabetic retinopathy (13,14), and also in development (15), but to our knowledge, limited work has been done on the regulation of IGF-1R signaling in the retina with normal aging in vivo.
The goal of the present work was to investigate phosphorylation and activation of IGF-1R and its downstream intermediates in a rat model of normal aging. Investigations were done on retinal lysates from 8-, 22-, and 32-month-old Brown Norway × Fischer 344 F1 hybrid rats.
MATERIALS AND METHODS
Animals
Male BN × F344 F1 hybrid rats aged 8 months (n = 8, ∼20 years of age in human years), 22 months (n = 8, ∼50–60 years of age in human years), and 32 months (n = 8, ∼80–90 years of age in human years) purchased from the National Institute of Aging through Harlan were used to determine changes in IGF-1R signaling in the retina with age. This rat strain was used because they show less age-related pathologies and biological variability (16). Rats were anesthetized using a ketamine and xylazine mixture and the eyes were removed. The cornea was cut and the lens and vitreous removed. The retina was separated from the choroid and sclera and immediately placed into cold lysis buffer containing protease and phosphatase inhibitors. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center.
Functional Assessment
Electroretinogram (ERG) analyses were done to evaluate functional changes in the vision with age. For the ERG analyses, rats were dark adapted overnight. The following morning, the rats were anesthetized using an intraperitoneal injection of a ketamine and xylazine cocktail. The pupil of each eye was fully dilated using 1% tropicamide solution (Alcon, Ft. Worth, TX). To protect the eye and assist in maintaining a good electrical connection, a drop of methylcellulose solution was added to each eye (Celluvisc; Allergan, Irvine, CA). Body temperature was maintained at 37°C with a water-based heating pad. The ERG responses are recorded from both eyes simultaneously using platinum wire corneal electrodes, a forehead reference electrode, and ground electrode in the tail. The ERG stimuli were delivered via the Diagnosys LLC system. All animals tested recovered from anesthesia after the ERG recording sessions.
ERG responses are recorded in response to brief (4 milliseconds) white light emitting diode and then from the Xenon arc lamp delivered at 2.1-second intervals for dim stimuli and at 35-second frames for brighter stimuli. The range of stimulus intensities extended from −4.0 to 3.0 log (cd·s/m2) for analysis of the b-wave amplitudes. ERG waveforms are recorded with a bandwidth of 0.3–500 Hz and sampled at 2 kHz by a digital acquisition system (Diagnosys, Lowell, MA). Data were analyzed using the Espion System, which is provided by Diagnosys. Plots of intensity–response functions for the b-waves are fit to a hyperbolic (Naka–Rushton) function of the form
where R is the response amplitude at flash intensity I, Rmax is the asymptotic amplitude of the b-wave (in microvolts), K is the intensity at which b-wave amplitude is half of its asymptotic value (in candelas per square meter per second), and n determines the slope of the function at I = K.
For assessment of the oscillatory potentials (OPs), stimuli were administered at 3 log (cd·s/m2). Data analysis for the OPs was analyzed using the Fast Fourier Transform Algorithm for a time-based signal, which is a program within MatLab software (The MathWorks, Natick, MA), with a digital band-pass filter set for 60–300 Hz, and the peak wavelets of the four wavelets were measured from trough to peak (17,18).
Protein Measurements
Western blot analyses for IGF-1R and its downstream signaling intermediaries were done on retinal lysates as previously published (19). Primary antibodies used were phosphorylated IGF-1R (Tyr 1135/1136, 1:500; Cellular Signaling, Danvers, MA), total IGF-1R (1:500; Cellular Signaling), total Akt (1:500; Cellular Signaling), phosphorylated Akt (Ser 473, 1:500; Cellular Signaling), and cleaved caspase 3 (Asp 175; Cellular Signaling). For analyses of the data, mean densitometry values were obtained using the Kodak 5.0 software. The ratio of phosphorylated protein was compared to levels of total protein.
Immunoprecipitation for Phosphotyrosine
Once protein was collected into lysis buffer and assayed for protein content, 500 μg of total protein lysate was combined with 40 μL (1:250 dilution) of insulin receptor substrate (IRS)-1 or IRS-2 antibodies. After washing, protein A/G beads (Santa Cruz, Santa Cruz, CA) were added, and the immune complexes were allowed to bind at 4°C overnight. The following day, unbound proteins were removed by washing the beads, leaving the purified antibody–antigen complexes bound. Twenty microliters of sample buffer was added to the bead sample and boiled at 100°C for 7 minutes, spun briefly, and loaded onto 4%–12% tris–glycine gels for Western blotting. Blots were probed with 4G10 phosphotyrosine antibody (1:250; Millipore, Billerica, MA) to detect phosphorylated tyrosine residues within IRS-1 or IRS-2 protein complexes.
Enzyme-Linked Immunosorbent Assay Analyses
Enzyme-linked immunosorbent assay (ELISA) analyses were done for total IGF-1 levels (Immunodiagnostic Systems, Fountain Hills, AZ) and cleaved caspase 3 (PathScan Cleaved Caspase 3 ELISA; Cell Signaling) according to the manufacturer’s instructions, except that equal protein was loaded so that analyses could be done on optical density measurements.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Analysis
Retinal sections (5 μm) from 8-, 22-, and 32-month F344 × BN F1 hybrid rats were labeled using the DeadEnd Fluorometric terminal deoxynucleotidyl transferase dUTP nick end labeling analysis (TUNEL) System (Promega, Madison, WI). Sections were analyzed for fluorescein isothiocyanate (TdT) and tetramethyl rhodamine iso-thiocyanate (propidium iodide) and visualized using Nikon Eclipse 80i fluorescence microscope with NIS elements software. Sections were visualized at ×200 to give a global view of the retina.
Statistics Applied
For the analyses of phosphorylated and total protein levels, the mean densitometry values were obtained using Kodak 4.0 software. The ratio of phosphorylation to total protein was calculated and entered into Prism 4.0 software (GraphPad, San Diego, CA). A Mann–Whitney test was done to compare each age group against the mean from the 8-month group. For the ELISA analyses, the optical density numbers were entered into Prism and analyzed using a Mann–Whitney test. p Value of <.05 was taken to be significant.
RESULTS
Aging Causes a Significant Loss of B-Wave and OP Amplitude in the ERG
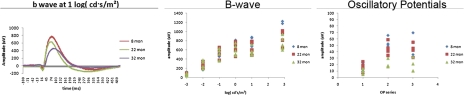
ERG analyses on 8-, 22-, and 32-month-old rats demonstrated that normal aging is associated with a significant loss of both B-wave and OP amplitudes (Figure 1). Many others have reported that the retina has decreased thickness with increasing age, which could reduce the amplitude of the B-wave and OPs (20,21).
Figure 1.
The left image is a representative waveform for each age group. The middle panel is the mean amplitudes for each age group for the B-wave. The right panel is the mean amplitude for the oscillatory potentials at the light intensities investigated. All animals were dark adapted overnight before electroretinogram analyses were completed. Light intensity is presented on the x-axis as log (cd·s/m2). N = 5 animals at each age.
IGF-1R Phosphorylation Is Significantly Increased With Age
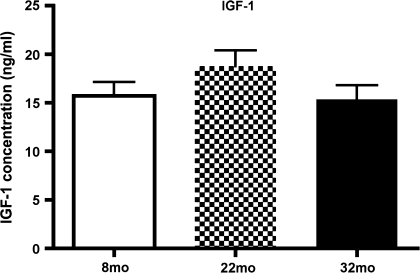
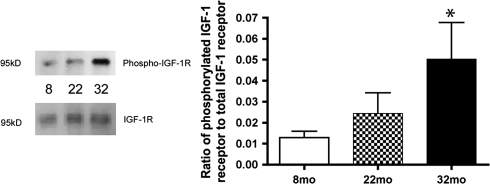
A common occurrence in aging is an alteration in growth factor levels and signaling. Because we have previously demonstrated that VEGF is increased in aging (4), we wanted to determine whether this is due to changes in IGF-1R signaling. Total levels of IGF-1 in the aging retina are not altered (Figure 2). Although it has been reported that serum levels of IGF-1 can change within specific time of the day, our data suggest that retinal levels of free IGF-1 are much more stable. The ratio of phosphorylated IGF-1R to total IGF-1R is significantly increased in 32-month-old rats compared with 8-month-old rats (Figure 3, p < .05 vs 8 months). Thus, in spite of limited changes in the level of available IGF-1 in the retina, receptor activity is increased.
Figure 2.
Enzyme-linked immunosorbent assay results for total insulin-like growth factor-1 (IGF-1) levels in retinal lysates. Aging does not significantly change total levels of IGF-1 in the retina. N = 5 animals.
Figure 3.
Bar graph of the ratio of phosphorylated insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) to total IGF-1R in retinal lysates from 8-, 22-, and 32-month-old F344 × BN F1 hybrid rats determined from Western blotting for both phosphorylated and total protein. Blot images are on the left, whereas means are combined into the bar graph. Aging results in a significant increase in IGF-1R phosphorylation. *p < .05 vs 8 months, N = 4 at each age.
Aging Results in a Significant Decrease in Tyrosine Phosphorylation of IRS-1 With No Change in IRS-2
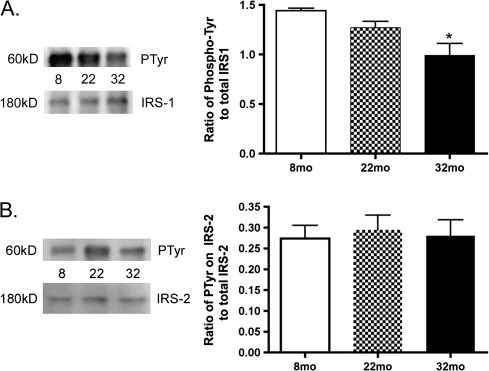
Because IGF-1R phosphorylation often leads to tyrosine phosphorylation of members of the IRS family, we next investigated IRS-1 and IRS-2 activity. Although total protein levels of IRS-1 were not altered with age, the phosphorylation of tyrosine residues is significantly reduced with increasing age (Figure 4A, p < .05 vs 8 months). This suggests that the increase in IGF-1R phosphorylation is not being transduced to activation of IRS-1 activity. In contrast to the age-related decrease in IRS-1 activity, no significant changes were observed in tyrosine phosphorylation of IRS-2 in the aging retina (Figure 4B).
Figure 4.
(A) The ratio of phosphorylated tyrosine on insulin receptor substrate-1 (IRS-1) to total IRS-1 levels in the retinal lysates from 8-, 22-, and 32-month-old F344 × BN F1 hybrid rats determined by immunoprecipitation experiments. Tyrosine phosphorylation is significantly decreased in 32-month-old retina. *p < .05 vs 8 months. (B) The ratio of phosphorylated tyrosine residues on IRS-2 to total IRS-2 levels in the retinal lysates determined by immunoprecipitation experiments. No changes were observed for IRS-2 tyrosine phosphorylation. N = 5 for each age for both proteins.
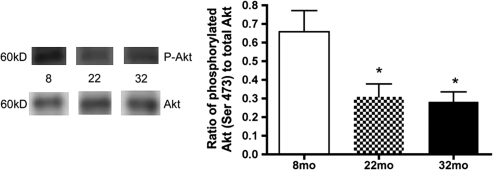
Serine Phosphorylation of Akt Is Significantly Reduced in Lysates From Aging Retina
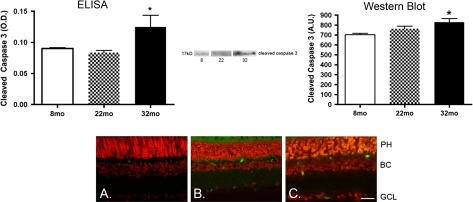
Likely due to the decrease in IRS-1 activity, phosphorylation of Akt is significantly decreased in retinal lysates from 22- and 32-month-old rat samples compared with younger animals (Figure 5, p < .05 vs 8 months). Because Akt is a key antiapoptotic factor, levels of cleaved caspase 3 in the aging samples are significantly increased in the aging retina (Figure 6, p < .05 vs 8 months). TUNEL labeling demonstrated that the apoptosis occurs primarily in the outer plexiform layer, with limited amounts in the ganglion cell layer. These results suggest that although IGF-1R phosphorylation is increased with age, this does not protect the aging retina from apoptosis. The increase in apoptosis noted by the increase in the cleavage of caspase 3 may explain the reduced amplitudes of the B-wave and OP in the ERG results.
Figure 5.
Serine 473 phosphorylation on Akt is significantly reduced in retinal lysates from 22- and 32-month-old rats compared with 8-month-old rats. Both phosphorylated protein and total protein levels were determined by Western blotting. *p < .05 vs 8 months, N = 5 at each age.
Figure 6.
On the top left are enzyme-linked immunosorbent assay (ELISA) analyses for cleaved caspase 3 in retinal lysates from 8-, 22-, and 32-month-old F344 × BN F1 hybrid rats. Aging is associated with an increase in the cleavage of caspase 3 in the retina. This result was verified by Western blot analyses for the cleaved caspase 3 band (top right). Terminal deoxynucleotidyl transferase dUTP nick end labeling Analysis (TUNEL) labeling is shown in the bottom panel for young (A), middle aged (B), and old (C) rats. Scale bar is 5 μm for the TUNEL image. *p < .05 vs 8 months. N = 6 for each age.
DISCUSSION
Increased vascular growth in the retina is associated with a number of diseases, including “wet” macular degeneration, diabetic retinopathy, and retinopathy of prematurity (12). This vascular growth often involves activation of VEGF, such that antagonism of VEGF activity in the retina is a new therapeutic strategy. Unfortunately, VEGF also has neuroprotective actions (22,23), which makes complete blockade produce potentially undesired side effects. Therefore, a more effective solution may be to determine a factor that can regulate VEGF actions and block its activation. One such factor would be IGF-1. Work on IGF-1R has previously been focused on its role in longevity as mutations in IGF-1R or its downstream signaling allow for increased life span (5,6), likely through its ability to regulate apoptosis. Although much is known on IGF-1 in the retina (24,25), much less is known on its signaling in the normal aging process. Because IGF-1R and insulin are involved in the aging process and utilize many of the same signaling molecules (26), we did investigate insulin receptor activity. We did not find significant changes in insulin receptor phosphorylation (data not shown). However, we did find that normal aging of the rat retina results in an increase in tyrosine phosphorylation of IGF-1R, in spite of no changes in total IGF-1 levels in the retina.
Although IGF-1R activity was increased in aging, tyrosine phosphorylation of IRS-1 was significantly reduced. This was an unexpected finding because increased IGF-1R normally would increase phosphorylation of IRS-1 proteins, leading to decreased apoptosis. Because we found that levels of cleaved caspase 3 were elevated, this would suggest that apoptosis is increased in the aging retina. This result agrees with our TUNEL and ERG findings, showing fluorescence in the outer plexiform layer and a decline in the amplitudes of the B-wave and OPs with age, respectively. Furthermore, we observed that Akt was reduced with increasing age in the retina. Therefore, in spite of the increased phosphorylation of IGF-1R, the other components of IGF-1R signal transduction cascade are significantly decreased in age.
One possible explanation for an increase in IGF-1R phosphorylation, but a decrease in IRS-1 tyrosine phosphorylation, could be increased inflammatory markers in aging. We have previously shown that aging of the retina is associated with an increase in prostaglandin E2 (PGE2) protein (27). This is not unexpected because PGE2 and other cytokines are increased with advancing age due to increased levels of reactive oxygen species (28). In the eye, the age-related macular degeneration study is designed to optimize the use of antioxidants to prevent or treat macular degeneration (29). Although it has not been demonstrated for PGE2, other cytokines, such as tumor necrosis factor-alpha (TNFα), are involved in the regulation of insulin/IGF-1 signaling through modulation of IRS-1 activity. In other cell types, activation of TNFα leads to serine phosphorylation of IRS-1 and IRS-2, thus promoting decreased insulin signaling (30–34). Because IGF-1R and insulin involve in the activation of IRS-1 leading to increased phosphorylation of PI3K and Akt, it is possible that the increase in PGE2 due to aging acts similar to TNFα to decrease tyrosine phosphorylation of IRS-1 in the retina. Although this is only one possibility, it certainly deserves further study, as it can link the increased inflammatory markers noted in the aging retina with altered growth factor signaling. Nonetheless, the decrease in IRS-1 activation in the aging retina may be a positive response of normal aging to prevent neovascularization. In this way, the ability to regulate IGF-1R signal transduction through IRS-1 may provide a novel site for therapeutic intervention to prevent increased VEGF levels and actions.
Inflammatory cytokines are not the only molecules that can regulate IRS-1 activation. Because IRS-1 activation lies downstream from both insulin receptor and IGF-1R phosphorylation, enzymes or chemicals that inhibit activation of the insulin/IGF-1R actions will block IRS-1 tyrosine phosphorylation and activities, such as phosphatases (35). Others have also reported that IRS proteins may undergo proteosome-mediated degradation rather than acute inhibition through serine phosphorylation (36). Although all these possibilities exist, the regulation of IRS-1 phosphorylation remains a key question for age-related studies of the retina.
In conclusion, this study demonstrates that normal aging of the retina produces increased IGF-1R phosphorylation. However, the increased IGF-1R activity is not antiapoptotic as activities of IRS-1 and Akt are reduced. The decrease in Akt phosphorylation likely leads to increased cleavage of caspase 3. The increase in cleaved caspase 3 may explain the age-related declines in B-wave and OPs that are observed in the rat retina. Further studies on the regulation of IRS-1 receptor phosphorylation in aging are warranted. These results suggest that the IGF-1R signaling through IRS-1 may be the critical deviation in the normal aging process, which could promote neovascular retinal disease in the aging retina if left uncontrolled to activate VEGF.
FUNDING
National Institutes of Health, National Institute of Aging (R01027827-03 to J.J.S.); Research to Prevent Blindness (William and Mary Greve Special Scholars Award to J.J.S.); NEI 1F31 EY19240 (R.J.W.); Research to Prevent Blindness (nonrestricted grant to Dr Barrett Haik, Chair), NEI Vision Core Grant: PHS 3P30 EY013080 (Dianna Johnson, PI).
References
- 1.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33(1):1–17. [PubMed] [Google Scholar]
- 2.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34(12):3278–3296. [PubMed] [Google Scholar]
- 3.Smith CP, Sharma S, Steinle JJ. Age-related changes in sympathetic neurotransmission in rat retina and choroid. Exp Eye Res. 2007;84(1):75–81. doi: 10.1016/j.exer.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Smith CP, Steinle JJ. Changes in growth factor expression in normal aging of the rat retina. Exp Eye Res. 2007;85(6):817–824. doi: 10.1016/j.exer.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holzenberger M. The gh/igf-i axis and longevity. Eur J Endocrinol. 2004;151(suppl 1):S23–S27. doi: 10.1530/eje.0.151s023. [DOI] [PubMed] [Google Scholar]
- 6.Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7(3):285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey MM, Ingram RL, Cashion AB, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (dmba)-induced mammary carcinogenesis. Endocrinology. 2002;143(10):4139–4142. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- 8.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120(4):449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Allen TR, Krueger KD, Hunter WJ, 3rd, Agrawal DK. Evidence that insulin-like growth factor-1 requires protein kinase c-epsilon, pi3-kinase and mitogen-activated protein kinase pathways to protect human vascular smooth muscle cells from apoptosis. Immunol Cell Biol. 2005;83(6):651–667. doi: 10.1111/j.1440-1711.2005.01387.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor JC, McCusker RH, Strle K, et al. Regulation of igf-i function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol. 2008;252((1–2)):191–210. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang Y, Zheng B, Fan LW, Rhodes PG, Cai Z. Igf-1 protects oligodendrocyte progenitors against tnfalpha-induced damage by activation of pi3k/akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55(11):1099–1107. doi: 10.1002/glia.20530. [DOI] [PubMed] [Google Scholar]
- 12.Smith LE. Igf-1 and retinopathy of prematurity in the preterm infant. Biol Neonate. 2005;88(3):237–244. doi: 10.1159/000087587. [DOI] [PubMed] [Google Scholar]
- 13.Seigel GM, Lupien SB, Campbell LM, Ishii DN. Systemic igf-i treatment inhibits cell death in diabetic rat retina. J Diabetes Complications. 2006;20(3):196–204. doi: 10.1016/j.jdiacomp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2(1):71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 15.Koriyama Y, Homma K, Sugitani K, et al. Upregulation of igf-i in the goldfish retinal ganglion cells during the early stage of optic nerve regeneration. Neurochem Int. 2007;50(5):749–756. doi: 10.1016/j.neuint.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Phelan JP, Austad SN. Selecting animal models of human aging: inbred strains often exhibit less biological uniformity than f1 hybrids. J Gerontol. 1994;49(1):B1–B11. doi: 10.1093/geronj/49.1.b1. [DOI] [PubMed] [Google Scholar]
- 17.Forte JD, Bui BV, Vingrys AJ. Wavelet analysis reveals dynamics of rat oscillatory potentials. J Neurosci Methods. 2007;169:191–200. doi: 10.1016/j.jneumeth.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Weymouth AE, Vingrys AJ. Rodent electroretinography: methods for extraction and interpretation of rod and cone responses. Prog Retin Eye Res. 2008;27(1):1–44. doi: 10.1016/j.preteyeres.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Steinle JJ. Sympathetic neurotransmission modulates expression of inflammatory markers in the rat retina. Exp Eye Res. 2007;84(1):118–125. doi: 10.1016/j.exer.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Hogg RE, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Retin Eye Res. 2006;25(3):249–276. doi: 10.1016/j.preteyeres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 21.DiLoreto D, Jr., Ison JR, Bowen GP, Cox C, del Cerro M. A functional analysis of the age-related degeneration in the fischer 344 rat. Curr Eye Res. 1995;14(4):303–310. doi: 10.3109/02713689509033530. [DOI] [PubMed] [Google Scholar]
- 22.Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-a is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous vegf is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo T, Vicent D, Suzuma K, et al. Knockout of insulin and igf-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest. 2003;111(12):1835–1842. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBosch BJ, Baur E, Deo BK, Hiraoka M, Kumagai AK. Effects of insulin-like growth factor-1 on retinal endothelial cell glucose transport and proliferation. J Neurochem. 2001;77(4):1157–1167. doi: 10.1046/j.1471-4159.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 26.Anisimov VN. Insulin/igf-1 signaling pathway driving aging and cancer as a target for pharmacological intervention. Exp Gerontol. 2003;38(10):1041–1049. doi: 10.1016/s0531-5565(03)00169-4. [DOI] [PubMed] [Google Scholar]
- 27.Steinle JJS, Sheena S, Smith CP, McFadyen-Ketchum LS. Normal aging involves modulation of specific inflammatory markers in the rat retina and choroid. J Gerontol A Biol Sci Med Sci. 2009;64:325–331. doi: 10.1093/gerona/gln052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barja G. Aging in vertebrates, and the effect of caloric restriction: a mitochondrial free radical production-DNA damage mechanism? Biol Rev Camb Philos Soc. 2004;79(2):235–251. doi: 10.1017/s1464793103006213. [DOI] [PubMed] [Google Scholar]
- 29.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 30.Paz K, Hemi R, LeRoith D, et al. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of irs-1 and irs-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem. 1997;272(47):29911–29918. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- 31.Hemi R, Paz K, Wertheim N, et al. Transactivation of erbb2 and erbb3 by tumor necrosis factor-alpha and anisomycin leads to impaired insulin signaling through serine/threonine phosphorylation of irs proteins. J Biol Chem. 2002;277(11):8961–8969. doi: 10.1074/jbc.M109391200. [DOI] [PubMed] [Google Scholar]
- 32.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3t3-l1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and glut4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272(2):971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil GS. The role of tnfalpha and tnf receptors in obesity and insulin resistance. J Intern Med. 1999;245(6):621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 34.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-jun nh(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of ser(307) J Biol Chem. 2000;275(12):9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Rodriguez A, Clampit JE, Escribano O, et al. Developmental switch from prolonged insulin action to increased insulin sensitivity in protein tyrosine phosphatase 1b-deficient hepatocytes. Endocrinology. 2007;148(2):594–608. doi: 10.1210/en.2006-0644. [DOI] [PubMed] [Google Scholar]
- 36.White MF. Irs proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283(3):E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]