Abstract
Purpose
To evaluate the effectiveness of the Convergence Insufficiency Treatment Trial (CITT) placebo therapy program in maintaining masking of patients randomized to the office-based treatment arms, determine whether demographic variables affect masking, and determine whether perception of assigned treatment group was associated with treatment outcome or adherence to treatment.
Methods
Patients (n = 221, ages, 9–17 years) were randomized to one of four treatment groups, two of which were office-based and masked to treatment (n = 114). The placebo therapy program was designed to appear to be real vergence/accommodative therapy, without stimulating vergence, accommodation, or fine saccades (beyond levels of daily visual activities). After treatment, patients in the office-based groups were asked whether they thought they had received real or placebo therapy and how confident they were in their answers.
Results
Ninety-three percent of patients assigned to real therapy and 85% assigned to placebo therapy thought they were in the real therapy group (P = 0.17). No significant differences were found between the two groups in adherence to the therapy (P ≥ 0.22 for all comparisons). The percentage of patients who thought they were assigned to real therapy did not differ by age, sex, race, or ethnicity (P > 0.30 for all comparisons). No association was found between patients' perception of group assignment and symptoms or signs at outcome (P ≥ 0.38 for all comparisons).
Conclusions
The CITT placebo therapy program was effective in maintaining patient masking in this study and therefore may have potential for use in future clinical trials using vergence/accommodative therapy. Masking was not affected by demographic variables. Perception of group assignment was not related to symptoms or signs at outcome (ClinicalTrials.gov number, NCT00338611).
Vergence/accommodative therapy is a form of active vision therapy/orthoptics often prescribed for the treatment of convergence insufficiency (CI). CI is a common binocular vision disorder that frequently causes symptoms such as double vision, sore eyes, blurred vision, and/or headaches with near work (e.g., reading).1–7 The effectiveness of vergence/accommodative therapy has been challenged due to lack of controlled studies,8,9 because only a few studies have incorporated a placebo therapy group. The studies that have included a placebo arm focused on specific aspects of therapy, had small sample sizes, and did not evaluate whether placebo therapy was effective as a control.10–14 Although controversy remains regarding the existence and/or impact of a placebo effect,15–24 it is generally accepted that patients may show clinical improvements due to the natural history of the disease, regression to the mean, and/or nonspecific treatment effects (e.g., patient–provider interaction and/or the patient's belief in the effectiveness of the treatment) in addition to true treatment effects.16–19,25 Therefore, including a placebo (or control) arm has become the standard in randomized treatment trials to control for bias as well as potential placebo effects.15,23 A placebo treatment is generally defined as a harmless treatment that simulates the real therapy under investigation.15,25,26
Office-based vergence/accommodative therapy for the treatment of symptomatic CI typically involves the controlled manipulation of accommodative demand, vergence demand, and/or target distance under the guidance of a therapist.27 It is possible that aspects related to administering therapy (such as the therapist–patient interaction and the patient's expectation that the treatment will be effective) may also affect treatment outcome. Recently, the Convergence Insufficiency Treatment Trial (CITT) Group designed a placebo therapy program to appear to be real vergence/accommodative therapy, without stimulating vergence, accommodation, or fine saccades (beyond levels of daily visual activities). This placebo program was used in a multicenter randomized controlled clinical trial pilot and was found to be effective in maintaining masking of 61 patients aged 9 to 30 years who were randomized to the office-based treatment arms.28–30
A modified version of this placebo therapy program was incorporated into the full-scale randomized CITT comparing the effectiveness of home-based pencil push-ups, home-based computer vergence/accommodative therapy and pencil pushups, office-based vergence/accommodative therapy with home reinforcement, and office-based placebo therapy as treatments for symptomatic CI.31 This study showed that 12 weeks of office-based vergence/accommodative therapy resulted in a significantly greater proportion of children being classified as having a successful or improved outcome in symptoms and clinical signs of convergence ability (near point of convergence and positive fusional vergence) when compared with home-based pencil push-ups, home-based computer vergence/accommodative therapy and pencil push-ups, and office-based placebo therapy (73% vs. 43%, 33%, and 35%, respectively).32 Although the placebo therapy arm was found to be effective in maintaining masking in the CITT pilot, its effectiveness in maintaining masking in the full-scale CITT has not been thoroughly investigated. Furthermore, the sample size of the pilot study was insufficient to examine the potential effect of (1) demographic variables on masking and (2) patient perception of group assignment on outcome or adherence. For example, the results of the pilot study showed that although most patients thought they had received real therapy, adults were less sure about their answer than children.30 In addition, ethnic and racial differences in response to placebo have been reported.33,34 It is not known whether older children were less sure than younger children or if sex, race, or ethnicity influenced masking. Previous research has suggested that the level of symptoms at outcome may influence subjects' perception of their treatment group (with improvement being associated with assignment to real therapy).35 Therefore, the objectives of this study were to (1) describe the modified CITT placebo program; (2) evaluate its effectiveness in maintaining patient masking in patients who were randomized to office-based therapy (real or placebo) in the full scale clinical trial; (3) determine whether age, sex, race, or ethnicity affected masking; and (4) determine whether patients' perception of their assigned treatment group was associated with their treatment outcome or adherence to treatment.
Materials and Methods
The study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, Department of Health and Human Services, and was conducted by the CITT Investigator Group at nine clinical sites (see the Appendix). The protocol- and Health Insurance Portability and Accountability Act (HIPAA)–compliant informed consent forms were approved by the institutional review boards at participating sites. Research adhered to the tenets of the Declaration of Helsinki. A parent or guardian (referred to subsequently as “parent”) of each study patient gave written informed consent and the children gave written assent. Study oversight was provided by an independent data and safety monitoring committee.
Subjects and Outcome Measures
The major eligibility criteria included children ages 9 to 17 years, exophoria at near greater than at distance (by ≥4Δ), a receded near-point of convergence break (≥6 cm), insufficient positive fusional convergence at near (i.e., failing Sheard's criterion36 or minimum positive fusional vergence of ≤15Δ base-out blur or break), and a symptomatic score (≥16) on the Convergence Insufficiency Symptom Survey (CISS). A complete listing of the eligibility and exclusion criteria have been reported previously.28,29,31 Eligibility/baseline testing included the CISS, cover testing at distance and near, near point of convergence, and positive fusional vergence at near and was administered by CITT-trained and -certified ophthalmologists or optometrists. The standardized protocols for these procedures have been described previously31 and are briefly described here. The CISS score (derived from a patient-reported symptom questionnaire) was the primary outcome measure in the CITT.1,2,28,29,31,37 The CISS queries the patient regarding approximately 15 symptoms that may be experienced when reading or doing other near work and quantifies the response to each item from 0 (never) to 4 (always). The range of possible scores is 0 to 60, with a higher score indicating a higher level of symptoms. The CISS was administered before any other testing and repeated after all testing was completed with the average of the two scores used for analysis. Secondary outcome measures were near point of convergence and positive fusional vergence at near. Near point of convergence was measured three times by bringing a target containing a single column of letters (20/30 equivalent at 40 cm) slowly toward the child until the child reported that the letters appeared to become two or the examiner noted an eye turn out. Positive fusional vergence was measured three times with a horizontal prism bar while the patient fixated a target of a single column of letters (20/30 equivalent at 40 cm).
Treatment Programs
Enrolled patients were randomly assigned with equal probability to one of four treatment groups: home-based pencil push-ups, home-based computer vergence/accommodative therapy and pencil pushups, office-based vergence/accommodative therapy with home reinforcement, and office-based placebo therapy. Randomization was achieved on the study's Web site by using randomly selected blocks of four or eight, with a separate sequence of computer-generated random numbers for each clinical site.
Each patient knew whether he or she had been assigned to an office-based or home-based therapy group. However, patients assigned to the two office-based treatment groups were not told whether they were assigned to real or placebo therapy. The two home-based treatment groups will not be discussed further, because the focus of this article is to evaluate masking in the two office-based groups.
The treatment programs were 12 weeks in duration with monthly masked examinations and a masked primary outcome examination conducted at the end of the 12-week therapy program. Both office-based groups received weekly (60-minute) in-office therapy visits administered by a trained therapist and were prescribed home therapy procedures to be performed 5 days per week to supplement the in-office therapy. Office visits were scheduled so as to prevent any interaction between patients. All patients completed home logs to show adherence to prescribed home therapy procedures. Therapists reviewed the logs and encouraged adherence. The therapists had to have undergone training in vergence/accommodative therapy and therefore could not be masked to the patient's treatment group assignment (real or placebo). However, the therapists were instructed to encourage and provide positive reinforcement to all patients in the same manner regardless of treatment assignment. Therapists were observed performing both real and placebo therapy during certification and a site visit to ensure that procedures were performed according to protocol and that encouragement/positive reinforcement was provided in a similar manner to both groups. Therapists also participated in monthly therapist conference calls.
The real office-based therapy program consisted of standard vergence/accommodative therapy techniques for the treatment of CI and has been described.28,29,31 The placebo therapy program included 16 in-office therapy procedures and four home reinforcement therapy procedures that were designed to look like real vergence/accommodative therapy procedures but did not stimulate vergence, accommodation, or fine saccadic eye movements beyond normal daily visual activities. Five procedures were performed during each office therapy visit and two procedures were assigned for home reinforcement therapy each week. Placebo procedures included traditional vergence/accommodative therapy procedures modified to be monocular rather than binocular (e.g., Brock string), binocular procedures modified so that there was no alteration of vergence demand (e.g., computer orthopter, stereoscope), procedures using lenses with no dioptric power (plano or yoked prism lenses), and computer visual perceptual therapy with filter glasses. Placebo therapy procedures also included testing procedures that did not require significant demand on the vergence, accommodative, or fine saccadic eye movement systems (e.g., ductions, Bailey-Lovie acuity testing, after image testing, Hess Lancaster screen testing, modified Thorington phoria testing, and double Maddox rod cyclophoria testing). To further simulate real therapy, we designed some procedures to have increasing levels of difficulty. As in real therapy, patients frequently wore filter glasses and were told that the glasses ensured that both eyes were being used together. In addition, goals (such as improving how the eyes work together as a team) were established for each placebo procedure, and the therapist told the patient the goal of each procedure before beginning the technique to motivate the patient and simulate real therapy.
Therapists estimated the adherence of each office-based patient to in-office therapy procedures and the home reinforcement therapy procedures using the scale 0%, 1% to 24%, 25% to 49%, 50% to 74%, 75% to 99%, or 100%. At the end of treatment, patients in both office-based therapy groups were asked: (1) “Which treatment (placebo or real) do you think you received?” and, (2) “How sure are you about your answer?” Patients responded to the latter question with “very sure,” “pretty sure, “somewhat sure,” “a little sure,” or “not at all sure.”
Statistical Analysis
All statistical analyses were performed with commercial software (SAS ver. 9.1; SAS Institute, Cary, NC). For dichotomous outcome measures, χ2 tests were used to test for associations. Associations with ordinal outcomes were performed with a Kruskal-Wallis test, which is the nonparametric equivalent to an analysis of variance (ANOVA). ANOVA methods were used to compare continuous outcomes between levels.
Results
There were no significant differences between groups in age, sex, race, or ethnicity.32 The mean age at enrollment was approximately 12 years in both the real therapy (mean, 12.0; SD 2.6) and the placebo therapy (mean, 11.8, SD 2.2) group (P = 0.60). Slightly more than three fourths (77.8%) of the subjects in the placebo therapy group were 9 to 13 years of age, compared with 71.2% in the real therapy group (P = 0.42). There was a slightly higher but nonsignificant percentage of females in the real therapy group (67.8% compared to 59.3%, P = 0.35). There was no difference in race between the two groups (P = 0.27). Sixty percent of subjects in the real therapy group were white and 24% were African American. In the placebo therapy group, 46% were white and 37% were African American. There was also no difference in the percentage of Hispanic or Latino in the two therapy groups (P = 0.24). The primary outcome examination was completed by 59 (98%) of 60 patients assigned to real therapy and all 54 (100%) patients assigned to placebo therapy (Fig. 1).
Figure 1.
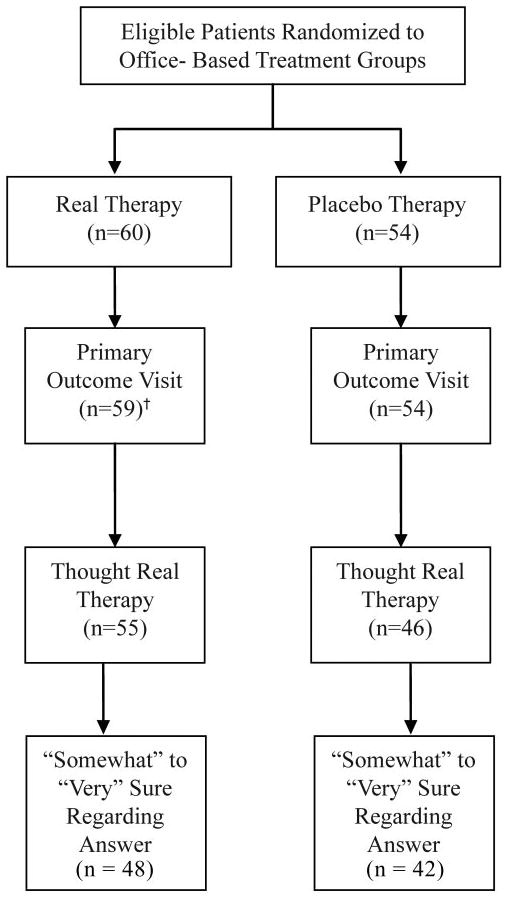
Flow of office-based patients in the CITT. Patients assigned to the two home-based treatments are not included (n = 107). †One missed visit.
Therapists' ratings of adherence to in-office therapy procedures and home reinforcement therapy procedures showed no significant differences between the two office-based groups (office: P ≥ 0.22 for all comparisons; home: P ≥ 0.45 for all comparisons; Table 1).
Table 1.
Percentage of Office-Based Therapy Patients Estimated by Their Therapists to Adhere to Their Prescribed Therapy Procedures at Least 75% of the Time
| Real Therapy Group | Placebo Therapy Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | P | |||||||
| Office therapy procedures | |||||||||||
| 4-Week visit estimate | 59 | 98.3 | 54 | 98.2 | 0.99 | ||||||
| 8-Week visit estimate | 60 | 100 | 54 | 96.3 | 0.22 | ||||||
| Home reinforcement therapy procedures | |||||||||||
| 4-Week visit estimate | 57 | 94.7 | 53 | 98.1 | 0.62 | ||||||
| 8-Week visit estimate | 60 | 91.7 | 52 | 96.2 | 0.45 | ||||||
| 12-Week visit estimate | 58 | 91.4 | 54 | 87.0 | 0.46 | ||||||
Patients' Perception of Therapy Group Assignment
All patients who completed the 12-week primary outcome visit responded to the two questions concerning the group to which they thought they had been assigned and their level of confidence in their answer. Ninety-three percent (55/59) of the patients assigned to real therapy and 85% (46/54) assigned to placebo therapy thought they had been assigned to real therapy (P = 0.17). The proportion of patients who perceived that they had been assigned to real therapy did not differ by age (9–13 years vs. 14–17 years), sex, race, or ethnicity (P > 0.30 for all comparisons). Patients' perception of whether they received real or placebo therapy was not related to improvements in symptoms (CISS score) and in clinical signs (near point of convergence and positive fusional vergence) at outcome (placebo therapy group: P ≥ 0.26 for all comparisons; real therapy group: P ≥ 0.41 for all comparisons; both groups: P ≥ 0.38 for all comparisons).
Patients' Level of Confidence in Their Perception of Therapy Type Received
Most patients in both groups were “somewhat sure,” “pretty sure,” or “very sure” that they had been assigned to real therapy (real: 87%; placebo: 91%; P = 0.19) (Fig. 2). Patients assigned to real therapy were significantly more confident of their answer than those assigned to the placebo group, irrespective of accuracy regarding perception of treatment group assignment (P = 0.047; Table 2). Patients' level of confidence was not significantly related to whether the patient was correct in his or her perception of group assignment (P = 0.16; Table 2).
Figure 2.
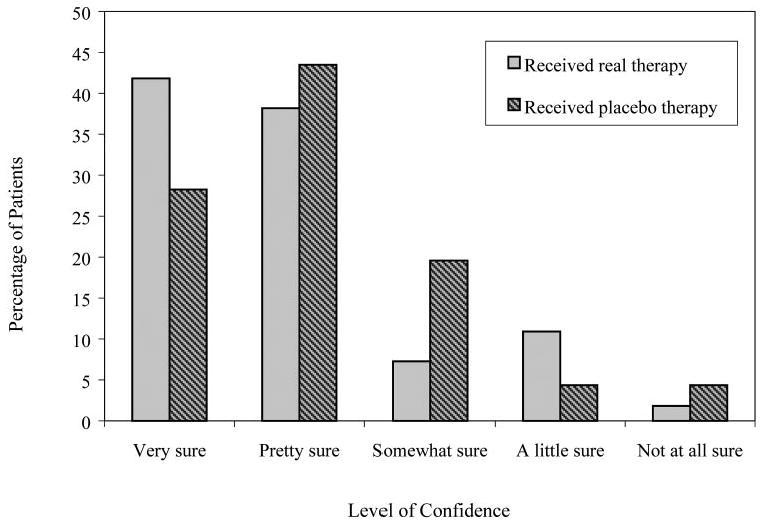
Level of confidence, by actual therapy, of patients who thought they had received real therapy.
Table 2.
Relation between Patients' Level of Confidence in Perceived Treatment and Accuracy of Perception
| Level of Confidence (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Very Sure | Pretty Sure | Somewhat Sure | A Little Sure | Not at All Sure |
| Actual group* | |||||
| Real | 39.0 | 37.3 | 10.2 | 11.9 | 1.7 |
| Placebo | 24.1 | 37.0 | 20.4 | 9.3 | 9.3 |
| Perception | |||||
| Incorrect | 26.0 | 42.0 | 22.0 | 6.0 | 4.0 |
| Correct | 36.5 | 33.3 | 9.5 | 14.3 | 6.4 |
Irrespective of accuracy regarding perception of treatment group.
Patients who thought they had received real therapy were significantly more sure of their answers than were those who thought they received placebo therapy (regardless of true group assignment; P < 0.0001). Of the patients who thought they had received real therapy, 89% were “somewhat sure,” “pretty sure,” or “very sure” of their answers (includes 87% [48/55]) of patients assigned to real therapy and 91% (42/46) of patients assigned to placebo therapy; Fig. 2). In contrast, only 42% of the patients who thought they had received placebo therapy were “somewhat sure,” “pretty sure,” or “very sure” that they had been assigned to placebo therapy (includes 75% [3/4]) of patients assigned to real therapy and 25% [2/8] of patients assigned to placebo therapy).
For patients who thought they had been assigned to real therapy (irrespective of assigned treatment group), we explored the relationship between their level of confidence (how sure the patient was that the therapy was real) and (1) adherence to home therapy and (2) changes in symptoms (CISS score) or clinical signs (near point of convergence and positive fusional vergence). There was no significant relationship between how sure a patient was that he or she had received real therapy and reported adherence to home therapy (P = 0.91; Table 3) or changes in symptoms or clinical signs (P ≥ 0.23 for all comparisons; Table 4).
Table 3.
Adherence to Home Reinforcement Therapy Procedures as Reported on the Home Log Forms for Office-Based Patients Who Thought They Were Receiving Real Therapy, by Level of Confidence
| Percentage Adherence | ||||
|---|---|---|---|---|
| Level of Confidence | Mean | SD | ≥ 75% | ≥ 90% |
| Not at all sure (n = 3) | 86.30 | 23.7 | 90.9 | 81.8 |
| A little sure (n = 8) | 96.75 | 7.6 | ||
| Somewhat sure (n = 13) | 88.85 | 19.9 | 76.9 | 76.9 |
| Pretty sure (n = 41) | 93.49 | 12.1 | 92.7 | 80.5 |
| Very sure (n = 36) | 92.11 | 15.1 | 88.9 | 77.8 |
Table 4.
Change from Baseline to Week 12 for Each Outcome by Level of Confidence
| Change in CISS* | Change in NPC (cm)† |
Change in PFV (Δ)‡ |
||||
|---|---|---|---|---|---|---|
| Level of Confidence | Mean | SD | Mean | SD | Mean | SD |
| Patients assigned to real therapy and who thought they were receiving real therapy | ||||||
| Not at all sure (n = 1) | −13.57 | 11.0 | −10.93 | 7.1 | 17.63 | 10.4 |
| A little sure (n = 6) | ||||||
| Somewhat sure (n = 4) | −5.88 | 10.3 | −10.13 | 13.3 | 21.08 | 20.1 |
| Pretty sure (n = 21) | −14.90 | 10.7 | −8.29 | 4.5 | 20.52 | 13.6 |
| Very sure (n = 23) | −17.13 | 14.1 | −10.3 | 6.7 | 18.39 | 12.3 |
| ANOVA results§ | F = 1.00, P = 0.38 | F = 0.68, P = 0.51 | F = 0.15, P = 0.86 | |||
| Patients assigned to placebo therapy but who thought they were receiving real therapy | ||||||
| Not at all sure (n = 2) | −7.88 | 8.5 | −1.00 | 6.7 | 7.98 | 3.0 |
| A little sure (n = 2) | ||||||
| Somewhat sure (n = 9) | −9.61 | 4.6 | −3.39 | 5.2 | 8.00 | 5.9 |
| Pretty sure (n = 20) | −8.43 | 9.6 | −6.53 | 7.7 | 7.54 | 9.7 |
| Very sure (n = 13) | −6.77 | 10.1 | −3.42 | 6.2 | 5.39 | 8.0 |
| ANOVA results§ | F = 0.24, P = 0.79 | F = 1.54, P = 0.23 | F = 0.39, P = 0.68 | |||
Negative change in CISS represents an improvement/decrease of symptoms.
Negative change in near point of convergence (NPC) represents an improvement/decrease in NPC.
Positive change in positive fusional vergence (PFV) represents an improvement/increase in PFV.
After combining the “not at all.” “a little,” and “somewhat” sure categories due to the small number of patients in these groups.
Discussion
We evaluated the effectiveness of the CITT placebo therapy program in the full-scale CITT in maintaining masking of patients randomized to the office-based treatment arms of real vergence/accommodative therapy and placebo therapy by asking patients at treatment completion whether they thought they had received real or placebo therapy. We found no difference between groups in how likely patients were to think they had been assigned to real therapy. Although patients assigned to real therapy were significantly more confident of their answers than those assigned to the placebo group (regardless of whether the patient was accurate in his or her perception), most of the patients in both groups were “somewhat sure,” “pretty sure,” or “very sure” that they had been assigned to real therapy (real: 87%; placebo: 91%). Therefore, the results of this investigation are in agreement with the results of the CITT pilot study in which 95% of patients assigned to real therapy and 83% assigned to placebo therapy thought that they were in the real therapy group and most patients who thought they had received real therapy, were “somewhat sure,” “pretty sure,” or “very sure” of their answer (90% assigned to real therapy and 89% assigned to placebo therapy).28–30 This suggests that the inability to mask the therapists did not hinder masking and that trained therapists are able to perform therapy with a similar level of encouragement and positive reinforcement for both placebo and real therapy. The fact that the patients' level of confidence was not significantly related to the accuracy of perception of treatment group assignment also indicates that the placebo therapy program was effective in masking the enrolled patients.
There was no difference between groups in completion rate of the primary outcome examination or in adherence to therapy protocols. The CITT pilot study showed that although most patients thought that they had received real therapy, the adults were less sure about their answers than were the children.28–30 These results showed no relation between a child's age (9–13 years vs. 14–17 years) and his or her perception of treatment assignment. In addition, ethnic and racial differences in response to placebo have been reported.33,34 In this study, these variables were not associated with the patients' perception of assigned treatment group. It has been reported that the severity of symptoms at an outcome visit may influence the perception of treatment group (with improvement being associated with assignment to real therapy).35 However, in this study improvement in symptoms and clinical signs did not appear to influence the CITT patients' treatment group perception.
Although one might expect adherence to be related to level of confidence that the assigned treatment was real, in this study the level of confidence was not related to adherence to therapy for the patients who thought they were receiving real therapy. The lack of any differences in adherence may be attributable to the fact that most patients were “somewhat” to “very” sure they were receiving real therapy and therefore there was good treatment compliance in both office-based groups.
One might hypothesize that if there were a placebo effect influencing outcomes, it would be strongest in patients who were most certain that they were receiving real therapy. Therefore, the lack of a significant association between level of confidence (how sure the patient was that the assigned treatment was real) and changes in symptoms and clinical signs at outcome would argue against the existence of a significant placebo effect. However, it is possible that no differences were seen, because patients were well masked and most were “somewhat” to “very” certain that they were receiving real therapy. Regardless, the placebo serves as a control against which active treatments can be evaluated.
Conclusion
These findings demonstrate the effectiveness of patient masking in the placebo treatment arm of the CITT and the feasibility of including a placebo therapy control group in future clinical trials of vergence/accommodative therapy in children. Masking was not affected by patient demographics. Perception of group assignment was not related to symptoms or signs at outcome.
Acknowledgments
The authors thank Karla Zadnik, PhD, and Israel A Goldberg, PhD, for advice and help in the development of the research design for this study.
Supported by Grants U10EY014713, U10EY014659, U10EY014716, U10EY014715, U10EY014709, U10EY014710, U10EY014676, U10EY014706, and U10EY014712 from the National Eye Institute. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
Appendix
Writing Committee
Marjean Kulp, G. Lynn Mitchell, Eric Borsting, Mitchell Scheiman, Susan Cotter, Michael Rouse, Susanna Tamkins, Brian G. Mohney, Andrew Toole, Kathleen Reuter.
The Convergence Insufficiency Treatment Trial Investigator Group
Clinical Sites
Sites are listed in order of the number of patients enrolled in the study, with the number of patients enrolled listed in parentheses preceded by the site name and location. Abbreviations designating the roles of personnel are PI, principal investigator; SC, coordinator; E, examiner; and VT, therapist.
Bascom Palmer Eye Institute, Miami, FL (35): Susanna Tamkins (PI), Hilda Capo (E), Mark Dunbar (E), Craig McKeown (Co-PI), Arlanna Moshfeghi (E), Kathryn Nelson (E), Vicky Fischer (VT), Adam Perlman (VT), Ronda Singh (VT), Eva Olivares (SC), Ana Rosa (SC), Nidia Rosado (SC), and Elias Silver-man (SC).
SUNY College of Optometry, New York, NY (28): Jeffrey Cooper (PI), Audra Steiner (E, Co-PI), Marta Brunelli (VT), Stacy Friedman (VT), Steven Ritter (E), Lily Zhu (E), Lyndon Wong (E), Ida Chung (E), Kaity Colon (SC), and Ashley Fazarry (SC).
UAB School of Optometry, Birmingham, AL (28): Kristine Hopkins (PI), Marcela Frazier (E), Janene Sims (E), Marsha Swanson (E), Katherine Weise (E), Adrienne Broadfoot (VT, SC), Michelle Anderson (VT), Catherine Baldwin (SC), and Leslie Simms (SC).
NOVA Southeastern University, Fort Lauderdale, FL (27): Rachel Coulter (PI), Deborah Amster (E), Gregory Fecho (E), Tanya Mahaphon (E), Jacqueline Rodena (E), Mary Bartuccio (VT), Yin Tea (VT), and Annette Bade (SC).
Pennsylvania College of Optometry, Philadelphia, PA (25): Michael Gallaway (PI), Brandy Scombordi (E), Mark Boas (VT), Tomohiko Yamada (VT), Ryan Langan (SC), Ruth Shoge (E), and Lily Zhu (E).
The Ohio State University College of Optometry, Columbus, OH (24): Marjean Kulp (PI), Michelle Buckland (E), Michael Earley (E), Gina Gabriel (E), Aaron Zimmerman (E), Kathleen Reuter (VT), Andrew Toole (VT), Molly Biddle (SC), and Nancy Stevens (SC)
Southern California College of Optometry, Fullerton, CA (23): Susan Cotter (PI), Eric Borsting (E), Michael Rouse (E), Carmen Barnhardt (VT), Raymond Chu (VT), Susan Parker (SC), Rebecca Bridgeford (SC), Jamie Morris (SC), Javier Villa-lobos (SC), and Jessica Chang (E).
Ratner Children's Eye Center, La Jolla, CA (17): David Granet (PI), Lara Hustana (E), Shira Robbins (E), Erica Castro (VT), and Cintia Gomi (SC).
Mayo Clinic, Rochester, MN (14): Brian G. Mohney (PI), Jonathan M Holmes (E), Melissa Rice (VT), Virginia Karlsson (VT), Becky Nielsen (SC), Jan Sease (SC), and Tracee Shevlin (SC).
CITT Study Chair
Mitchell Scheiman (Chair), Karen Pollack (Coordinator), Susan Cotter, (Vice Chair), Richard Hertle (Vice Chair), and Michael Rouse (Consultant).
CITT Data Coordinating Center
Gladys Lynn Mitchell (PI), Tracy Kitts (Project Coordinator), Melanie Bacher (Programer), Linda Barrett (Data Entry), Loraine Sinnott (Biostatistician), Kelly Watson (Student's worker), and Pam Wessel (Office Associate).
National Eye Institute
Maryann Redford, Paivi Miskala.
CITT Executive Committee
Mitchell Scheiman, G. Lynn Mitchell, Susan Cotter, Richard Hertle, Marjean Kulp, Maryann Redford, and Michael Rouse.
Data and Safety Monitoring Committee
Marie Diener-West (Chair), The Rev. Andrew Costello, William V. Good, Ron D. Hays, Argye Hillis (through March 2006), and Ruth Manny.
Footnotes
Disclosure: M. Kulp, None; G.L. Mitchell, None; E. Borsting, None; M. Scheiman, None; S. Cotter, None; M. Rouse, None; S. Tamkins, None; B.G. Mohney, None; A. Toole, None; K. Reuter, None
For reprints contact: Mitchell Scheiman, Pennsylvania College of Optometry, 1200 West Godfrey Avenue, Philadelphia, PA 19141; mscheiman@salus.edu.
References
- 1.Borsting EJ, Rouse MW, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003;80:832–838. doi: 10.1097/00006324-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Rouse MW, Borsting EJ, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalmic Physiol Opt. 2004;24:384–390. doi: 10.1111/j.1475-1313.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 3.Borsting E, Rouse MW, Deland PN, et al. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74:25–34. [PubMed] [Google Scholar]
- 4.Duke-Elder S, Wybar K. Ocular Motility and Strabismus. System of Ophthalmology. Vol. 6. St. Louis: Mosby; 1973. [Google Scholar]
- 5.Rouse MW, Borsting E, Hyman L, et al. Frequency of convergence insufficiency among fifth and sixth graders. The Convergence Insufficiency and Reading Study (CIRS) group. Optom Vis Sci. 1999;76:643–649. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Daum KM. Convergence insufficiency. Am J Optom Physiol Opt. 1984;61:16–22. doi: 10.1097/00006324-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Cooper J, Duckman R. Convergence insufficiency: incidence, diagnosis, and treatment. J Am Optom Assoc. 1978;49:673–680. [PubMed] [Google Scholar]
- 8.Keogh BK, Pelland M. Vision training revisited. J Learn Disabil. 1985;18:228–236. doi: 10.1177/002221948501800410. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp GR. Optometric vision training. Pediatrics. 1986;77:121–124. [PubMed] [Google Scholar]
- 10.Cooper J, Selenow A, Ciuffreda KJ, et al. Reduction of asthenopia in patients with convergence insufficiency after fusional vergence training. Am J Optom Physiol Opt. 1983;60:982–989. doi: 10.1097/00006324-198312000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Daum K. Double-blind placebo-controlled examination of timing effects in the training of positive vergences. Am J Optom Physiol Opt. 1986;63:807–812. doi: 10.1097/00006324-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Cooper J, Feldman J. Operant conditioning of fusional convergence ranges using random dot stereograms. Am J Optom Physiol Opt. 1980;57:205–213. doi: 10.1097/00006324-198004000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Cooper J, Feldman J, Selenow A, et al. Reduction of asthenopia following accommodative facility training. Am J Optom Physiol Opt. 1987;64:430–436. doi: 10.1097/00006324-198706000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sterner B, Abrahamsson M, Sjostrom A. The effects of accommodative facility training on a group of children with impaired relative accommodation–a comparison between dioptric treatment and sham treatment. Ophthalmic Physiol Opt. 2001;21:470–476. doi: 10.1046/j.1475-1313.2001.00615.x. [DOI] [PubMed] [Google Scholar]
- 15.Margo CE. The placebo effect. Surv Ophthalmol. 1999;44:31–44. doi: 10.1016/s0039-6257(99)00060-0. [DOI] [PubMed] [Google Scholar]
- 16.de la Fuente-Fernandez R, Schulzer M, Stoessl AJ. The placebo effect in neurological disorders. Lancet Neurol. 2002;1:85–91. doi: 10.1016/s1474-4422(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 17.Thomas K. General practice consultations: is there any point in being positive? BMJ. 1987;294:1200–1203. doi: 10.1136/bmj.294.6581.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas KB. The placebo in general practice. Lancet. 1994;344:1066–1067. doi: 10.1016/s0140-6736(94)91716-7. [DOI] [PubMed] [Google Scholar]
- 19.Gryll SL, Katahn M. Situational factors contributing to the placebos effect. Psychopharmacology (Berl) 1978;57:253–261. doi: 10.1007/BF00426747. [DOI] [PubMed] [Google Scholar]
- 20.Kienle GS, Kiene H. The powerful placebo effect: fact or fiction? J Clin Epidemiol. 1997;50:1311–1318. doi: 10.1016/s0895-4356(97)00203-5. [DOI] [PubMed] [Google Scholar]
- 21.Greene PJ, Wayne PM, Kerr CE, et al. The powerful placebo: doubting the doubters. Adv Mind Body Med. 2001;17:298–307. discussion 312–318. [PubMed] [Google Scholar]
- 22.Kirsch I, Scoboria A. Apples, oranges, and placebos: heterogeneity in a meta-analysis of placebo effects. Adv Mind Body Med. 2001;17:307–309. discussion 312–318. [PubMed] [Google Scholar]
- 23.Hrobjartsson A, Gotzsche PC. An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1595–1601. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 24.Hrobjartsson A. What are the main methodological problems in the estimation of the placebo effects? J Clin Epidemiol. 2002;55:430–435. doi: 10.1016/s0895-4356(01)00496-6. [DOI] [PubMed] [Google Scholar]
- 25.Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA. 1994;271:1609–1614. [PubMed] [Google Scholar]
- 26.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciuffreda K. The scientific basis for and efficacy of optometric vision therapy in nonstrabismic accommodative and binocular vision disorders. Optometry. 2002;73:735–762. [PubMed] [Google Scholar]
- 28.Scheiman M, Mitchell GL, Cotter S, et al. A randomized clinical trial of treatments for convergence insufficiency in children. Arch Ophthalmol. 2005;123:14–24. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 29.Scheiman M, Mitchell GL, Cotter S, et al. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optom Vis Sci. 2005;82:583–595. doi: 10.1097/01.opx.0000171331.36871.2f. [DOI] [PubMed] [Google Scholar]
- 30.Kulp M, Borsting E, Mitchell G, et al. Feasibility of using placebo vision therapy in a multicenter clinical trial. Optom Vis Sci. 2008;85:255–261. doi: 10.1097/OPX.0b013e318169288a. [DOI] [PubMed] [Google Scholar]
- 31.Convergence Insufficiency Treatment Trial Study Group. The Convergence Insufficiency Treatment Trial: design, methods, and baseline data. Ophthalmic Epidemiol. 2008;15:24–36. doi: 10.1080/09286580701772037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Convergence Insufficiency Treatment Trial Study Group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008;126:1336–1349. doi: 10.1001/archopht.126.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg SC, Schooler NR, Davidson EM, Kayce MM. Sex and race differences in response to drug treatment among schizophrenics. Pychopharmacologia. 1966;9:31–47. doi: 10.1007/BF00427702. [DOI] [PubMed] [Google Scholar]
- 34.Wagner GJ, Maguen S, Rabkin JG. Ethnic differences in response to fluoxetine in a controlled trial with depressed HIV-positive patients. Psychiatr Serv. 1998;49:239–240. doi: 10.1176/ps.49.2.239. [DOI] [PubMed] [Google Scholar]
- 35.Vitiello B, Davis M, Greenhill LL, Pine DS. Blindness of clinical evaluators, parents, and children in a placebo-controlled trial of fluvoxamine. J Child Adolesc Psychopharmacol. 2006;16:219–225. doi: 10.1089/cap.2006.16.219. [DOI] [PubMed] [Google Scholar]
- 36.Sheard C. Zones of ocular comfort. Am J Optom Physiol Opt. 1930;7:9–25. [Google Scholar]
- 37.Rouse M, Borsting E, Mitchell GL, et al. Validity of the Convergence Insufficiency Symptom Survey: a confirmatory study. Optom Vis Sci. 2009;86:357–363. doi: 10.1097/OPX.0b013e3181989252. [DOI] [PMC free article] [PubMed] [Google Scholar]


