Abstract
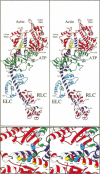
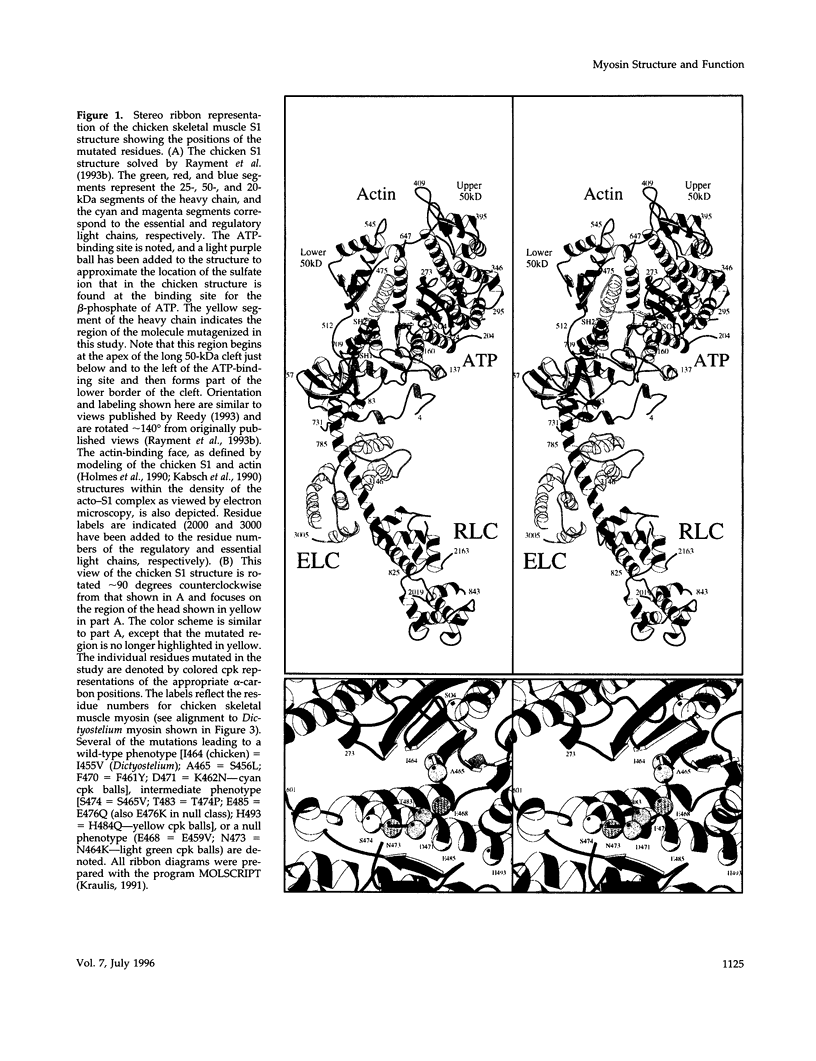
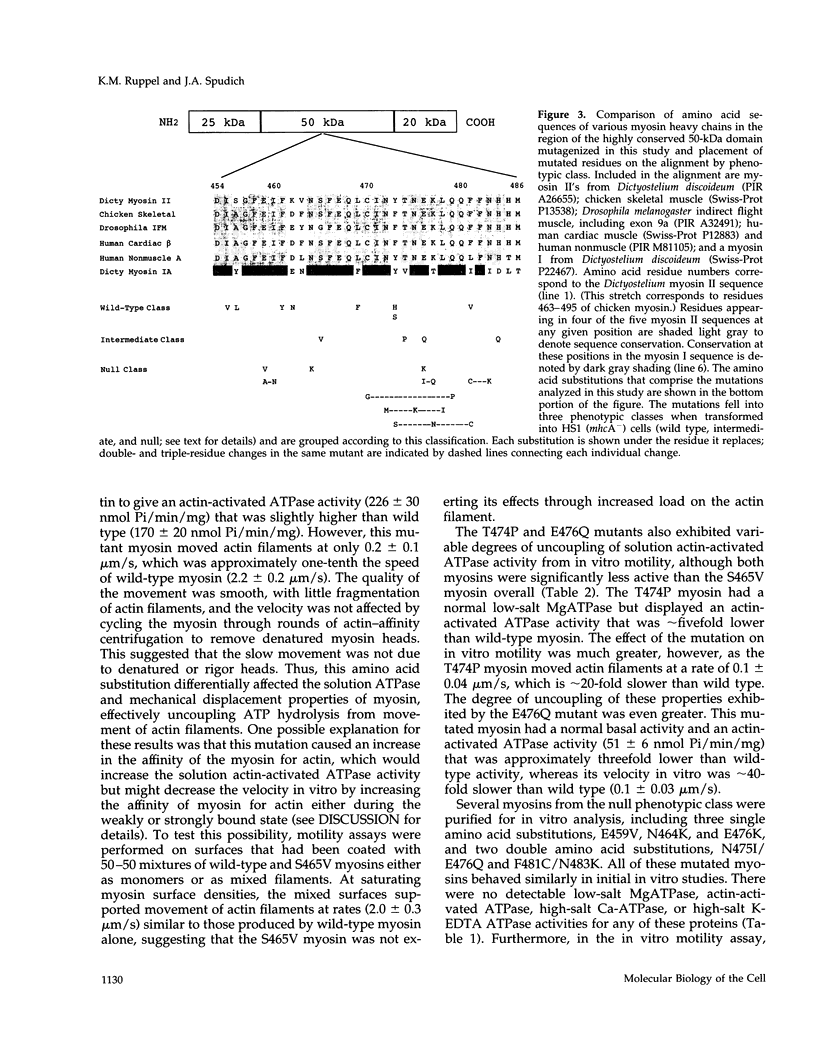
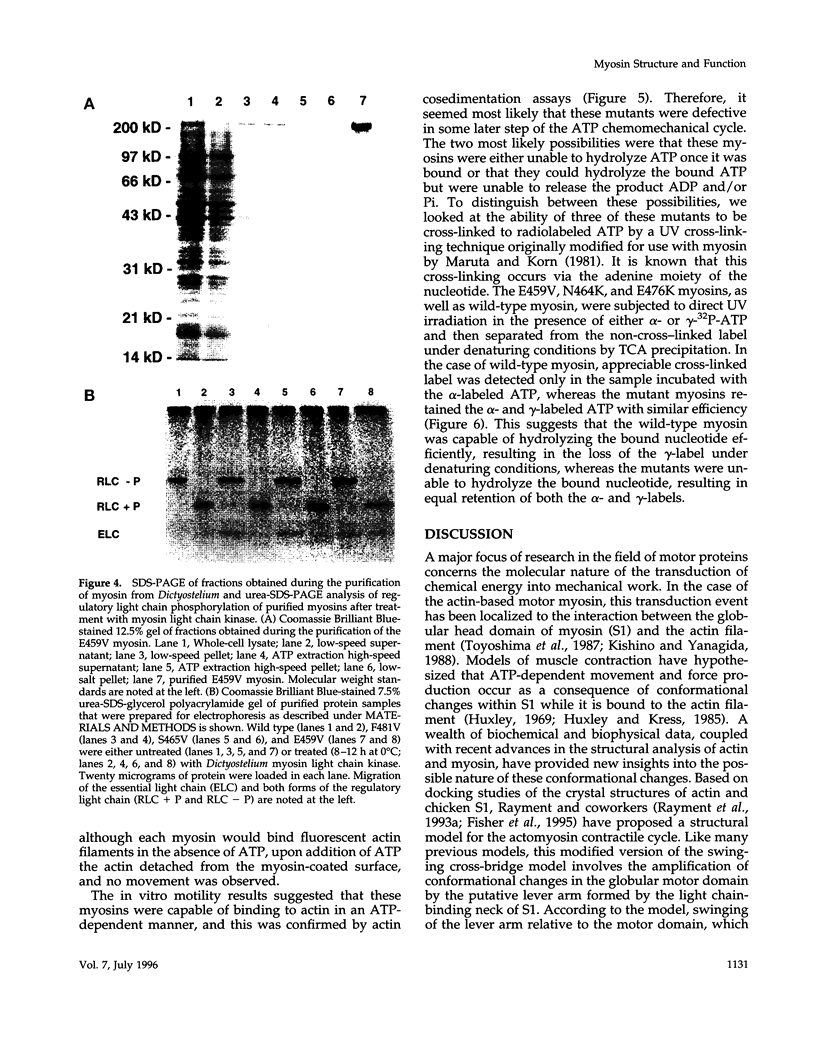
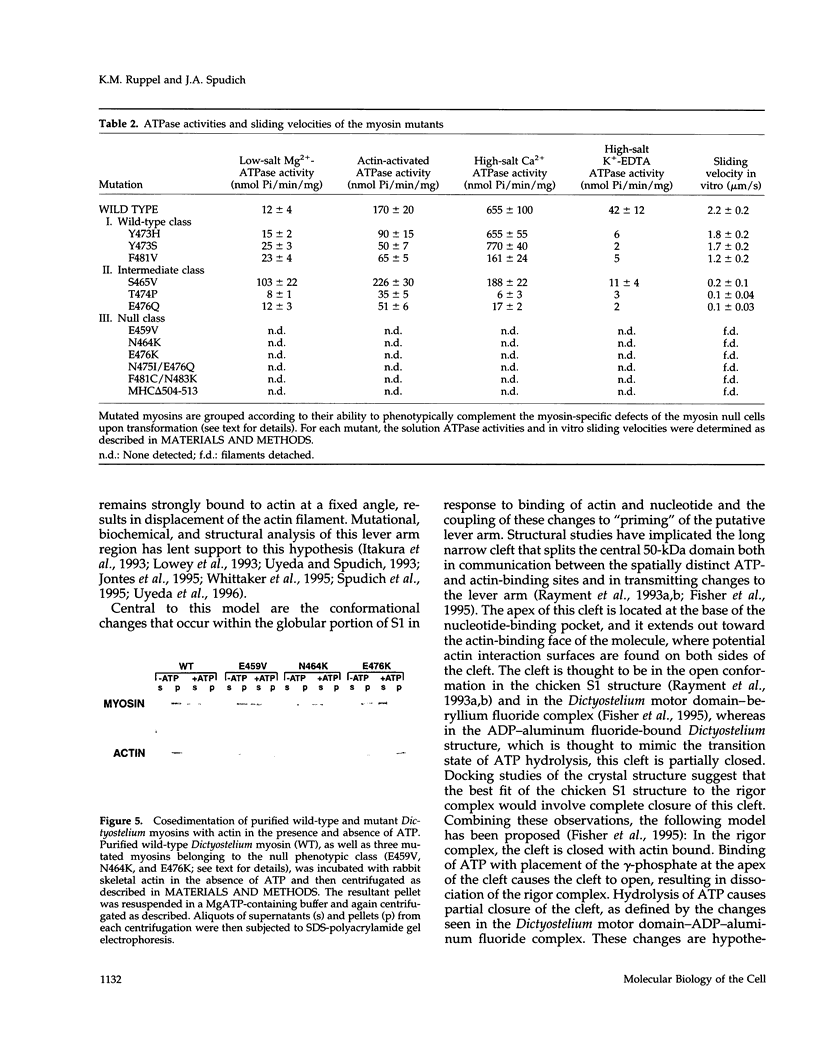
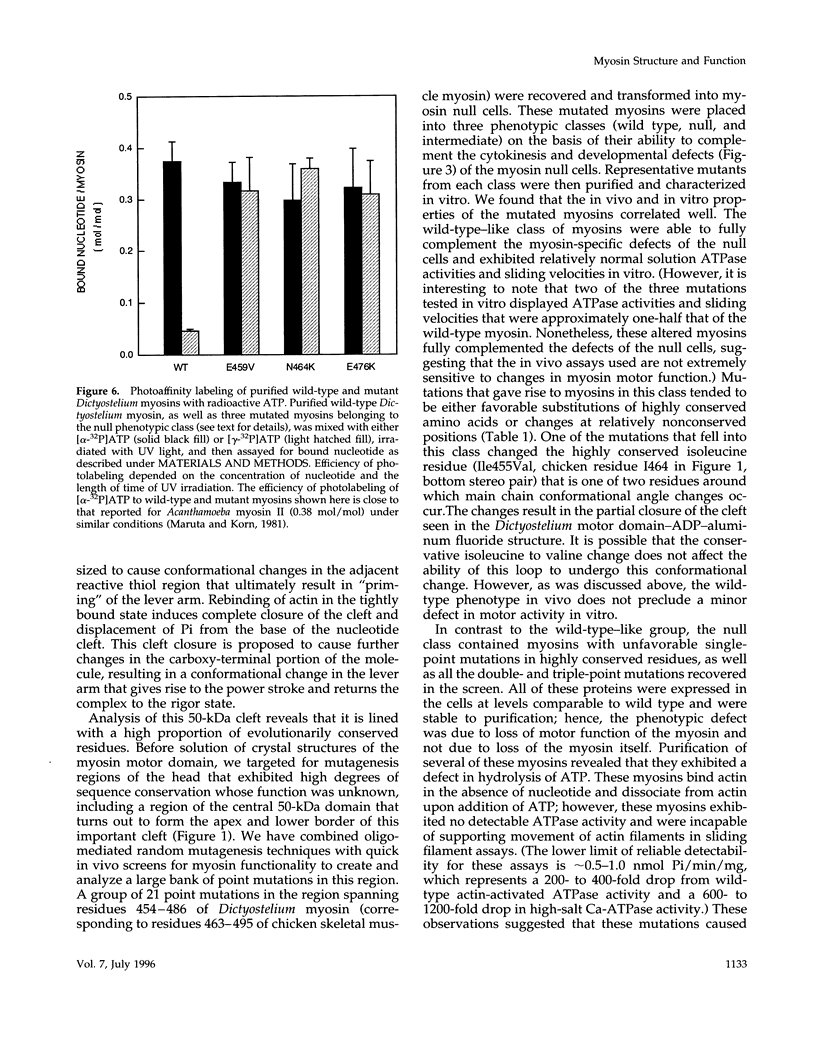
We used random mutagenesis to create 21 point mutations in a highly conserved region of the motor domain of Dictyostelium myosin and classified them into three distinct groups based on the ability to complement myosin null cell phenotypes: wild type, intermediate, and null. Biochemical analysis of the mutated myosins also revealed three classes of mutants that correlated well with the phenotypic classification. The mutated myosins that were not fully functional showed defects ranging from ATP nonhydrolyzers to myosins whose enzymatic and mechanical properties are uncoupled. Placement of the mutations onto the three-dimensional structure of myosin showed that the mutated region lay along the cleft that separates the active site from the actin-binding domain and that has been shown to move in response to changes at the active site. These results demonstrate that this region of myosin plays a key role in transduction of chemical energy to mechanical displacement.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botts J., Thomason J. F., Morales M. F. On the origin and transmission of force in actomyosin subfragment 1. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2204–2208. doi: 10.1073/pnas.86.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. Isolation and characterization of myosin from amoebae of Dictyostelium discoideum. J Mol Biol. 1974 Jun 25;86(2):209–222. doi: 10.1016/0022-2836(74)90013-8. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Lee R. J., Spudich J. A. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993 Oct 22;75(2):363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Manstein D. J., Spudich J. A. Complementation of myosin null mutants in Dictyostelium discoideum by direct functional selection. Dev Biol. 1990 Feb;137(2):359–367. doi: 10.1016/0012-1606(90)90260-p. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Titus M. A., Manstein D. J., Ruppel K. M., Spudich J. A. Molecular genetic tools for study of the cytoskeleton in Dictyostelium. Methods Enzymol. 1991;196:319–334. doi: 10.1016/0076-6879(91)96029-q. [DOI] [PubMed] [Google Scholar]
- Griffith L. M., Downs S. M., Spudich J. A. Myosin light chain kinase and myosin light chain phosphatase from Dictyostelium: effects of reversible phosphorylation on myosin structure and function. J Cell Biol. 1987 May;104(5):1309–1323. doi: 10.1083/jcb.104.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Itakura S., Yamakawa H., Toyoshima Y. Y., Ishijima A., Kojima T., Harada Y., Yanagida T., Wakabayashi T., Sutoh K. Force-generating domain of myosin motor. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1504–1510. doi: 10.1006/bbrc.1993.2422. [DOI] [PubMed] [Google Scholar]
- Jontes J. D., Wilson-Kubalek E. M., Milligan R. A. A 32 degree tail swing in brush border myosin I on ADP release. Nature. 1995 Dec 14;378(6558):751–753. doi: 10.1038/378751a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kronert W. A., O'Donnell P. T., Bernstein S. I. A charge change in an evolutionarily-conserved region of the myosin globular head prevents myosin and thick filament accumulation in Drosophila. J Mol Biol. 1994 Feb 25;236(3):697–702. doi: 10.1006/jmbi.1994.1182. [DOI] [PubMed] [Google Scholar]
- Kubalek E. W., Uyeda T. Q., Spudich J. A. A Dictyostelium myosin II lacking a proximal 58-kDa portion of the tail is functional in vitro and in vivo. Mol Biol Cell. 1992 Dec;3(12):1455–1462. doi: 10.1091/mbc.3.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature. 1993 Sep 30;365(6445):454–456. doi: 10.1038/365454a0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Titus M. A., De Lozanne A., Spudich J. A. Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 1989 Mar;8(3):923–932. doi: 10.1002/j.1460-2075.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Maruta H., Korn E. D. Direct photoaffinity labeling by nucleotides of the apparent catalytic site on the heavy chains of smooth muscle and Acanthamoeba myosins. J Biol Chem. 1981 Jan 10;256(1):499–502. [PubMed] [Google Scholar]
- Patterson B., Spudich J. A. Cold-sensitive mutations of Dictyostelium myosin heavy chain highlight functional domains of the myosin motor. Genetics. 1996 Jun;143(2):801–810. doi: 10.1093/genetics/143.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz G., Spudich J. A., Parham P. Monoclonal antibodies against seven sites on the head and tail of Dictyostelium myosin. J Cell Biol. 1985 Apr;100(4):1016–1023. doi: 10.1083/jcb.100.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reedy M. K. Myosin-actin motors: the partnership goes atomic. Structure. 1993 Sep 15;1(1):1–5. doi: 10.1016/0969-2126(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Ruppel K. M., Uyeda T. Q., Spudich J. A. Role of highly conserved lysine 130 of myosin motor domain. In vivo and in vitro characterization of site specifically mutated myosin. J Biol Chem. 1994 Jul 22;269(29):18773–18780. [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Goodson H. V. Motor proteins 2: myosin. Protein Profile. 1995;2(12):1323–1423. [PubMed] [Google Scholar]
- Spudich J. A., Finer J., Simmons B., Ruppel K., Patterson B., Uyeda T. Myosin structure and function. Cold Spring Harb Symp Quant Biol. 1995;60:783–791. doi: 10.1101/sqb.1995.060.01.084. [DOI] [PubMed] [Google Scholar]
- Spudich J. A. In pursuit of myosin function. Cell Regul. 1989 Nov;1(1):1–11. doi: 10.1091/mbc.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Sutoh K., Tokunaga M., Wakabayashi T. Electron microscopic mappings of myosin head with site-directed antibodies. J Mol Biol. 1989 Mar 20;206(2):357–363. doi: 10.1016/0022-2836(89)90485-3. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Abramson P. D., Spudich J. A. The neck region of the myosin motor domain acts as a lever arm to generate movement. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4459–4464. doi: 10.1073/pnas.93.9.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda T. Q., Kron S. J., Spudich J. A. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J Mol Biol. 1990 Aug 5;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Ruppel K. M., Spudich J. A. Enzymatic activities correlate with chimaeric substitutions at the actin-binding face of myosin. Nature. 1994 Apr 7;368(6471):567–569. doi: 10.1038/368567a0. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Spudich J. A. A functional recombinant myosin II lacking a regulatory light chain-binding site. Science. 1993 Dec 17;262(5141):1867–1870. doi: 10.1126/science.8266074. [DOI] [PubMed] [Google Scholar]
- Warshaw D. M., Desrosiers J. M., Work S. S., Trybus K. M. Smooth muscle myosin cross-bridge interactions modulate actin filament sliding velocity in vitro. J Cell Biol. 1990 Aug;111(2):453–463. doi: 10.1083/jcb.111.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker M., Wilson-Kubalek E. M., Smith J. E., Faust L., Milligan R. A., Sweeney H. L. A 35-A movement of smooth muscle myosin on ADP release. Nature. 1995 Dec 14;378(6558):748–751. doi: 10.1038/378748a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]