Abstract
Evidence from neuroimaging studies suggests that the right hemisphere of the human brain might be more specialized for attention than the left hemisphere. However, differences between right and left hemisphere in the magnitude of hemodynamic activity (i.e., ‘functional asymmetry’) rarely have been explicitly examined in previous neuroimaging studies of attention. This study used a new voxel-based comparison method to examine hemispheric differences in the amplitude of the hemodynamic response in response to infrequent target, infrequent novel, and frequent standard stimuli during an event-related fMRI auditory oddball task in 100 healthy adult participants. Processing of low probability task-relevant target stimuli, or ‘oddballs’, and low probability task-irrelevant novel stimuli is believed to engage in orienting and attentional processes. It was hypothesized that greater right-hemisphere activation compared to left would be observed to infrequent target and novel stimuli. Consistent with predictions, greater right hemisphere than left frontal, temporal, and parietal lobe activity was observed for target detection and novelty processing. Moreover, asymmetry effects did not differ with respect to age or gender of the participants. The results (1) support the proposal that the right hemisphere is differentially engaged in processing salient stimuli and (2) demonstrate the successful use of a new voxel-based laterality analysis technique for fMRI data.
Keywords: Oddball, MRI, Brain, Asymmetry, Cognition
Attention is believed to involve a distinctive neural network that interacts with other brain systems to facilitate various cognitive processes. Although there are many different operational definitions of attention, it is generally agreed that attention functions to orient to sensory events, to detect specific signals for subsequent processing, and to maintain vigilance over time (Posner and Petersen, 1990). Previous studies have examined attention by measuring brain activity to target stimuli in the context of ‘oddball’ tasks. Oddball tasks require detection of infrequent target stimuli within the context of frequently presented standard stimuli (Polich and Kok, 1995; Sutton et al., 1965)—for example, the detection of an occasional high-pitched tone in a sequence of low-pitched tones. The process of detecting salient target stimuli is believed to require orienting and allocation of attention, while vigilance is required to maintain task performance. Brain activity during oddball tasks is frequently measured by averaging task-related electroencephalogram recordings to produce event-related potentials (ERPs). Detection of low probability target stimuli produces a characteristic ERP waveform that includes several meaningful components, including the mismatch negativity (MMN; Naatanen et al., 1992) related to sensory trace memory, the N2 related to matching stimuli to an internally generated contextual template (Gehring et al., 1992), the P3b, related to contextual updating of working memory processes (Donchin and Coles, 1988). The P3b is elicited by target stimuli and has a centro-parietal scalp topography, whereas infrequent task-irrelevant novel stimuli (e.g., non-repeating random or environmental noises) produce a P3 with a frontocentral distribution (i.e., P3a; Courchesne et al., 1975) that peaks earlier than the P3b. The different scalp distributions and peak latencies of the P3a and P3b suggest that each component reflects different cognitive processes. Specifically, novelty stimuli are thought to better represent an orienting response (Friedman et al., 1997), whereas target stimuli require additional cognitive processes required for conscious awareness of target identification.
Functional asymmetries can be defined as neural activity that is stronger in one cerebral hemisphere compared to the other. Such hemispheric asymmetries have been reported for several ERP components elicited by target and novel stimuli in oddball tasks. For example, Alexander et al. (1996) found that the P3 amplitude elicited by target and standard stimuli was relatively greater over the right hemisphere frontal and parietal sites than over the left. Alexander and colleagues also found that the N2 amplitude generally was larger over the right than left hemisphere lateral sites for target stimuli. These results are consistent with other suidies that examine oddball ERP topography (Cohen et al., 2002; Holinger et al., 1992; Karniski and Blair, 1989; Naumann et al., 1992; Oades et al., 1995). In addition, several ERP and magnetoencephalography studies suggest that MMN is greater in the right hemisphere, both at temporal and prefrontal foci (Alain et al., 1998; Deouell et al., 1998; Jemel et al., 2002; Levanen et al., 1996; Otten et al., 2000; Paavilainen et al., 1991; Rinne et al., 2000). However, it is difficult to identify the exact neural generators of scalp-recorded ERPs due to the limitations of ERP source localization techniques (Baillet and Garnero, 1997; Pascual-Marqui et al., 1994; Scherg and Von Cramon, 1986; Scherg et al., 1989) (i.e., the lack of a unique mathematical solution to the location, strength, or orientation of a putative neural source). This prevents the use of ERP data to determine exactly which brain regions may subserve a particular cognitive process.
Event-related functional magnetic resonance imaging (fMRl), on the other hand, can provide precise information about the spatial characteristics of neural activity in each hemisphere. To date, over a dozen fMRl experiments have employed oddball paradigms to examine attention-related neural activity (Bledowski et al., 2004a,b; Casey et al., 2001; Clark et al., 2000, 2001; Horovitz et al., 2002; Kiehl and Liddle, 2003; Kiehl et al., 2001a,b, in press; Kirino et al., 2000; Linden et al., 1999; McCarthy et al., 1997; Menon et al., 1997; Mulert et al., 2004; Opitz et al., 1999; Strange and Dolan, 2001). None of these studies explicitly examined whether functional asymmetry exists in measures of hemodynamic activity to target or novel stimuli, despite some evidence that suggests hemispheric differences. For example, Kiehl and colleagues report greater z and t scores representing activation magnitude to rare events in right superior, middle, and inferior frontal gyri, middle and inferior temporal gyri, and right cerebellum (Kiehl et al., 2001a,b) compared to left hemisphere areas. There also are reports of larger activation t scores to targets in right compared to left inferior parietal lobule/supramarginal gyms (Kiehl et al., in press; Menon et al., 1997). The results of other fMRl studies of attention also support the proposal that there may be a right-hemisphere functional asymmetry for attention. Activity in right-hemisphere brain structures is found in attention tasks involving voluntary control (Corbetta and Shulman, 2002) or shifting of attentional focus (Vandenberghe et al., 2001; Yantis et al., 2002), attention to objects (O'Craven et al., 1999) versus spatial location (Corbetta et al., 1993, 1995, 2000), or the modulation of sensory cortex activity with increased attention (Jancke et al., 1999; Pinsk et al., 2004). Although these tasks measure cognitive processes that are somewhat different from those conceptualized in oddball tasks, they provide additional evidence that right hemisphere brain areas are important to attention.
In summary, both ERP and fMRl studies of oddball tasks and other paradigms measuring attention processes support right hemisphere dominance for attentional processes. In particular, the right dorsolateral and ventrolateral prefrontal cortex, superior, middle and inferior temporal gyri, and inferior parietal lobule might play special roles in attention-related neural activity. The current experiment used a new technique that was developed to facilitate the analysis of fMRl data for functional asymmetries. This study compared the amplitude of the hemodynamic response between the two cerebral hemispheres to determine which brain structures show lateralized differences in hemodynamic activity-associated with processing salient target and novel stimuli. Participants included 100 healthy adults performing a three-stimulus auditory oddball task. Discriminating target and novel from standard stimuli should require greater right hemisphere activity, consistent with the view that processing of target and novel stimuli engages right hemisphere attentional resources. Therefore, it was hypothesized that there would be right-lateralized hemodynamic activity for both target and novel stimuli in lateral prefrontal cortex and temporal–parietal cortex (i.e., superior/middle temporal gyrus, inferior parietal lobule). It also was predicted that target detection, which requires a manual response with the right index linger, would be associated with stronger ipsilateral (i.e., right) cerebellar activity and contralateral (i.e., left) precentral and postcentral gyrus activity. This prediction is consistent with the known neuroanatomy of the motor system in right-handed individuals. We also examined the asymmetry indices for gender and age effects. However, no specific predictions were made for these latter analyses.
Methods
Participants
One-hundred healthy right-handed volunteers (53 men and 47 women, mean age 29.2 years (range: 18–62; SD 10.03) participated in the study (described in detail by Kiehl et al., in press). Participants were drawn from two sites: The University of British Columbia (UBC), Vancouver, BC and The Olin Neuropsychiatry Research Center at The Institute of Living/Hartford Hospital (IOL), Hartford, CT.1 All participants were free from serious medical problems, Axis I or Axis II psychopathology in the last 6 months, and visual or auditory sensory deficits. Participants provided written informed consent in protocols approved by each site's governing Institutional Review Board.
Procedure
The experimental task was a three-stimulus auditory oddball task previously used in both ERP and fMRl studies (Kiehl et al., 2001 a,b, in press). Participants were instructed to respond as quickly and as accurately as possible with their right index finger every time a target tone occurred and not to respond to the standard tones or the novel stimuli. The standard stimulus was a 1000 Hz tone (probability, P = 0.80), the target stimulus was a 1500 Hz tone (P= 0.10), and the novel stimuli (P = 0.10) were non-repeating random digital noises (e.g., tone sweeps, whistles). Target and novel stimuli were always preceded by at least 3 standard stimuli (range 3–5). Stimuli were presented for 200 ms with a 2000 ms stimulus onset asynchrony (SOA). The intervals between stimuli of interest (i.e., target and novel stimuli) were allocated in a pseudorandom manner in the range 6–10 s so as to ensure that these stimuli had equal probability of occurring at 0, 1, and 2 s after the beginning of a 3 s image acquisition period. As a result, the hemodynamic response to each type of stimulus of interest was sampled uniformly at 1 s intervals. Prior to beginning the task, each participant performed a practice block of 10 trials to ensure understanding of the instructions. A commercially available MRI compatible fiber-optic response device (Lightwave Medical, Vancouver, BC) was used to acquire behavioral responses. All participants reported that they could hear the stimuli and discriminate them from the background scanner noise.
Imaging parameters
For both sites, imaging was implemented on a standard clinical GE 1.5 T system fitted with a Horizon Echo-speed upgrade. The participants' heads were firmly secured using a custom head holder. Conventional spin-echo Tl-weighted sagittal localizers were acquired for use in prescribing the functional image volumes. Functional image volumes were collected with a gradient-echo sequence (TR/TE 3000/40 ms, flip angle 90°, FOV 24 × 24 cm, 64 × 64 matrix, 62.5 kHz bandwidth, 3.75 × 3.75 mm in plane resolution, 5 mm slice thickness, 29 slices) effectively covering the entire brain (145 mm). The two stimulus runs consisted of 167 time points, prefaced by a 12 s rest period that was collected to allow for Tl effects to stabilize. These initial four images were not included in any subsequent analyses.
Image processing
Functional images were reconstructed offline and reoriented to the anterior commissure posterior commissure (AC/PC) plane. Functional images from each run were corrected for motion artifacts using an algorithm unbiased by local signal changes (INRIAlign; Freire and Mangin, 2001; Freire et al., 2002). Translation and rotation corrections for each participant typically did not exceed half a voxel or 2.0°, respectively (visual inspection of the statistical parametric maps for the three participants whose movement exceeded half a voxel did not show any obvious artifact; these participants were retained in the current analysis.) Following realignment, a mean functional image was computed for each EP1 time series. The mean EP1 image was matched to the standard EPI template provided by Statistical Parametric Mapping 2 (SPM2), a software package used to examine fMRI time series data. Spatial transformation was calculated using a tailored algorithm with both linear and nonlinear components (Friston et al., 1995). The parameters from this spatial transformation were applied to the corresponding functional images to align them to standardized MNI space. The normalized data were smoothed (12 mm full-width half-maximum). A fifth-order infinite impulse response Butterworth low-pass filter with a cutoff of 0.16 Hz was applied to remove any high frequency noise associated with alterations in the applied radio frequency field.
Event-related responses to the target and novel stimuli were modeled using two gamma functions to represent (1) hemodynamic peak latency of 6 s and temporal variations around this peak, and (2) the small ‘overshoot’ of the hemodynamic response on recovery. The modeled composite hemodynamic response for each run was derived by extracting stimulus onset timings for only those events that each participant responded to correctly (e.g., targets with correct button-presses within 1500 ms post-stimulus, or correctly ignored novel stimuli within this 0–1500 ms window). Thus, every participant had an fMRI time series model specific to his or her behavioral response patterns. A high pass filter (cutoff period 128 s) was incorporated into the model to remove noise associated with low frequency confounds (e.g., respiratory artifact; scanner drift). Contrasts were specified that evaluated the effects of (1) standard stimuli relative to the implicit baseline, (2) target stimuli relative to the standard stimulus baseline, and (3) novel stimuli relative to the standard stimulus baseline. In order to reduce the impact of spatially varying hemodynamic delays as well as delays due to slice timing, the true amplitude of the hemodynamic response (a function of both the non-derivative and derivative terms) was calculated (Calhoun et al., 2004b). An image containing these amplitudes was then entered into the second level analyses (i.e., random effects analyses) for each comparison of interest. Importantly, no within session scaling (proportional scaling) was employed to avoid the well-known artifacts that it can produce (Aguirre et al., 1998; Desjardins et al., 2001).
Asymmetry index calculation
The first step in the asymmetry index calculation was to create a hemispherically symmetric template for spatial normalization. There are well-described differences in several brain structures between the left and the right hemispheres (Foundas et al., 1999; Giedd et al., 1999; Hellige et al., 1998; Penhune et al., 1996) making it necessary to take this anatomical variance into consideration when directly comparing functional or anatomical data between hemispheres. As described in previous reports (Liegeois et al., 2002; Rowan et al., 2004), an average approximation of brain morphology for both hemispheres can be constructed using two images derived from the MNI EPI template provided in SPM2. The symmetric template was constructed as the average of two images. The first image contained the left hemisphere of the standard MNI EPI template in both the left and in the right hemispheres (that is, the left hemisphere was “flipped” along the y axis). The second image was the right hemisphere of the standard EPI template, with its mirror in the left hemisphere space.
The second step was to determine for each individual participant the parameters required to spatially normalize images to the symmetric EPI template. These parameters were calculated from the mean of the original spatially normalized EPI time series and then were applied to images representing the amplitude of hemodynamic response for target, novel, and standard stimuli (a.k.a., beta and/or contrast images in SPM; but also see Calhoun et al., 2004a). This method compromises the exact mapping of MNI coordinates in SPM2. However, it provides a less anatomically biased way to compare activation between similar brain structures from each cerebral hemisphere.
The third step in the procedure was to create images representing the difference in the amplitude of the hemodynamic response between the two hemispheres. Amplitudes of the hemodynamic response for each voxel in the right hemisphere were subtracted from the corresponding voxel in the left hemisphere (L–R). An analogous subtraction (R–L) was computed for right hemisphere voxels. The resulting difference image contained positive voxel values in both hemispheres indicating where that hemisphere had greater activity than the other. However, it is important to note that the sign of each voxel in this hemispheric asymmetry image does not provide any information as to whether the difference results from positive or negative signal change (see Postprocessing masking, below). The images for all participants for each condition of interest were entered into a series of second-level (group) random effects SPM analyses.
Statistical analysis of hemispheric laterality images
One-sample t tests were employed to test study hypotheses regarding hemispheric differences in the amplitude of hemodynamic response for (1) standard stimuli versus the implicit baseline. (2) targets versus the standard baseline, and (3) novels versus the standard baseline. Supplementary analyses also were performed to examine the effects of gender and age on hemispheric functional asymmetry for each condition of interest. The effect of gender on hemispheric asymmetry was evaluated using two-sample t tests separately for standard, target, and novel stimuli hemispheric laterality images. The effects of age were examined by computing the correlation between age and the hemispheric asymmetry images. For all reported statistical test results, significant peak voxel coordinates, slope, and t score statistics are reported for local maxima. The slope is reported in order to provide information about the magnitude and direction of the effect. Unless otherwise noted, all reported effects and illustrations depict significant differences at a voxelwise threshold of P < 0.01 FWE, corrected for searching the whole brain.
Postprocessing masking
For any given asymmetry effect, differences between the two hemispheres could be the result of three separate conditions: (1) positive signal change (i.e., ‘activation’) in one hemisphere could be greater than activation in the other; (2) positive signal change in one hemisphere could be greater than negative signal change (i.e., ‘deactivation’) in the other; and (3) negative signal change in one hemisphere could be greater than negative signal change in the other. Because difference maps were created using simple subtraction, these lateralized fMRI amplitude maps are blind to these three possible patterns. To address this ambiguity, a set of masks was created to differentiate patterns of signal change associated with each of the three possible patterns. Three mean images were calculated from symmetrically normalized hemodynamic response amplitude images for the participant sample (one mean image each for standards, targets versus standards, and novels versus standards). The mean images were used to classify each possible pattern (e.g., right hemisphere positive signal change versus left hemisphere positive signal change). These masks were then applied to the results of the asymmetry statistical tests.
An additional complication is that, for any given condition of interest, significant group differences between hemispheres could be detected in voxels where a significant main effect does not exist in either hemisphere. The absence of a significant main effect in both hemispheres calls into question the meaning of finding a significant hemispheric difference in hemodynamic activity for that region. Two approaches were used to identify such regions. First, three separate one-sample t tesls examined images for each participant that represented the amplitude of the hemodynamic response to standard, target, or novel stimuli which had been spatially normalized to the symmetric EPI template. Data describing these main effects are reported for those regions where lateralized differences are found (see Tables 1 and 2, below) (for complete analyses of all main effects using the canonical MNI template, see Kiehl et al., in press). Second, another set of masks were created to assist visualization of which regions had a significant main effect in both hemispheres. For each participant, the values of images representing the amplitude of hemodynamic response to each condition of interest (i.e., standards, targets, and novels) were written out to two new images. One image had left-hemisphere activation mirrored on the right side. The second image had right-hemisphere activation mirrored on the left side. One-sample t tesls identified voxels that were significantly active in these images for the sample of 100 participants. For any condition, a voxel whose F statistic met the threshold P < .01 (FWE corrected for searching the whole brain) for either left-hemisphere or right-hemisphere activation was included into a mask. These masks then were applied to data from the functional asymmetry analyses in order to visualize which areas of significant hemodynamic asymmetry between the hemispheres occurred in regions with main effects to stimulus type (see Figs. 1 and 2, below).
Table 1.
Summary of the areas showing significantly lateralized activation in brain regions where the left hemisphere had greater amplitude of hemodynamic response than the right hemisphere in healthy adult participants (n = 100) for each condition of interest (standard stimuli versus implicit baseline, target versus standard stimuli, and novel versus standard stimuli)
| Left > right hemisphere |
Left hemisphere main effecta |
Right hemisphere main effecta |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Peak voxel |
Slope | t99 | Slope | t99 | Slope | t99 | ||
| x | y | z | |||||||
| Standard stimuli | |||||||||
| Inferior parietal lobule superior temporal gyrus | −48 | −40 | 16 | 0.53 | 8.51 | 0.39 | 4.93b | −0.14 | 1.86b |
| Inferior temporal gyrus | −48 | −16 | −16 | 0.52 | 6.69 | 0.25 | 2.73b | −0.27 | 3.09b |
| Targets vs. standard baseline | |||||||||
| Transverse temporal gyrus | −56 | −24 | 12 | 0.87 | 9.45 | 2.41 | 23.65 | 1.53 | 16.24 |
| Postcentral gyms | −40 | −24 | 56 | 1.39 | 13.79 | 1.90 | 18.75 | 0.52 | 5.41 |
| Superior parietal lobule | −24 | −48 | 60 | 0.43 | 7.24 | 1.21 | 13.80 | 0.78 | 10.67 |
| Left cerebellum | −24 | −72 | −36 | 0.45 | 9.22 | 1.55 | 16.68 | 1.10 | 13.26 |
| Novels vs. standard baseline | |||||||||
| Left cerebellum | −24 | −68 | −32 | 0.35 | 6.93 | 1.03 | 12.67 | 0.68 | 9.35 |
| −20 | −80 | −44 | 0.29 | 6.08 | 0.94 | 13.10 | 0.65 | 9.77 | |
Also shown are data for the main effect of the condition of interest in the same voxel coordinate and in the opposite hemisphere. β coefficients (slope) and t scores for each effect are shown. All t scores are at least FWE P < 0.01, corrected for searching the whole brain unless otherwise indicated.
Because these data were normalized to a symmetric template, the peak voxel slope and t score data may not exactly correspond to data at the same voxel coordinate presented in Kiehl et al. (in press), which were not normalized to a symmetric EPI template.
FEW p > 0.05, or non-significant, corrected for searching the whole brain.
Table 2.
Summary of the areas showing significantly lateralized activation in brain regions where the right hemisphere had greater amplitude of hemodynamic response than the left hemisphere in healthy adult participants (n = 100) for each condition of interest (standard stimuli versus implicit baseline, target versus standard stimuli, and novel versus standard stimuli)
| Right > left hemisphere |
Left hemisphere main effecta |
Right hemisphere main effecta |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Peak voxel |
Slope | t99 | Slope | t99 | Slope | t99 | ||
| x | y | z | |||||||
| Targets vs. standard baseline | |||||||||
| Superior frontal gyrus | 12 | 48 | 44 | 0.61 | 8.90 | 0.65 | 6.43 | 0.04 | 0.43b |
| 20 | 52 | 28 | 0.44 | 7.94 | 0.95 | 12.97 | 0.51 | 7.59 | |
| Medial frontal gyrus | 12 | 64 | 8 | 0.41 | 6.62 | 0.57 | 6.61 | 0.16 | 1.94b |
| Inferior frontal gyrus | 44 | 44 | 0 | 0.79 | 10.12 | 1.35 | 14.16 | 0.55 | 5.81 |
| 56 | 20 | 8 | 0.78 | 9.69 | 1.22 | 11.86 | 0.43 | 5.36c | |
| 48 | 28 | 8 | 0.53 | 7.66 | 0.93 | 11.50 | 0.40 | 5.84 | |
| Inferior parietal lobule | 52 | −56 | 44 | 0.50 | 6.19 | 1.04 | 11.68 | 0.54 | 6.86 |
| Precuneus | 40 | −72 | 40 | 0.61 | 7.40 | 0.51 | 5.72 | −0.10 | 0.98b |
| Middle temporal gyrus | 56 | −32 | −12 | 0.63 | 8.65 | 1.23 | 13.90 | 0.60 | 8.35 |
| Inferior temporal gyrus | 56 | −20 | −20 | 0.64 | 8.46 | 1.23 | 12.54 | 0.58 | 7.07 |
| Right cerebellum | 16 | −52 | −24 | 0.79 | 12.58 | 1.89 | 22.65 | 1.10 | 12.11 |
| 12 | −72 | −48 | 0.54 | 7.67 | 1.51 | 17.82 | 0.98 | 10.59 | |
| 20 | −60 | −52 | 0.38 | 6.11 | 1.05 | 12.09 | 0.67 | 7.89 | |
| Novels vs. standard baseline | |||||||||
| Superior frontal gyms | 20 | 24 | 52 | 0.31 | 6.68 | 0.36 | 6.69 | 0.05 | 0.99b |
| 16 | 52 | 36 | 0.45 | 7.47 | 0.69 | 8.46 | 0.24 | 3.44b | |
| Middle frontal gyrus | 48 | 20 | 28 | 0.59 | 7.45 | 1.23 | 14.59 | 0.64 | 7.77 |
| Inferior frontal gyms | 52 | 20 | 4 | 0.53 | 7.53 | 1.05 | 12.85 | 0.52 | 7.42 |
| Medial frontal gyrus | 8 | 64 | 12 | 0.42 | 6.85 | 0.25 | 2.99 | −0.17 | 2.03b |
| Middle temporal gyrus | 52 | −32 | −8 | 0.57 | 8.45 | 1.17 | 15.70 | 0.60 | 8.52 |
| Inferior temporal gyrus | 52 | −20 | −20 | 0.57 | 8.57 | 0.99 | 12.27 | 0.42 | 6.26 |
| Superior parietal lobule | 36 | −60 | 48 | 0.45 | 7.33 | 1.08 | 13.80 | 0.63 | 9.12 |
| Inferior parietal lobule | 40 | −72 | 44 | 0.52 | 6.31 | 0.82 | 10.93 | 0.30 | 3.34 |
Also shown are data for the main effect of the condition of interest in the same voxel coordinate and in the opposite hemisphere. β coefficients (slope) and t scores for each effect are shown. All t scores are at least FWE P < 0.01, corrected for searching the whole brain unless otherwise indicated.
Because these data were normalized to a symmetric template, the peak voxel slope and t-score data may not exactly correspond to data at the same voxel coordinate presented in (in press), which were not normalized to a symmetric EPI template.
FWE P > 0.05, or non-significant, corrected for searching the whole brain.
FWE P < 0.05, corrected for searching the whole brain.
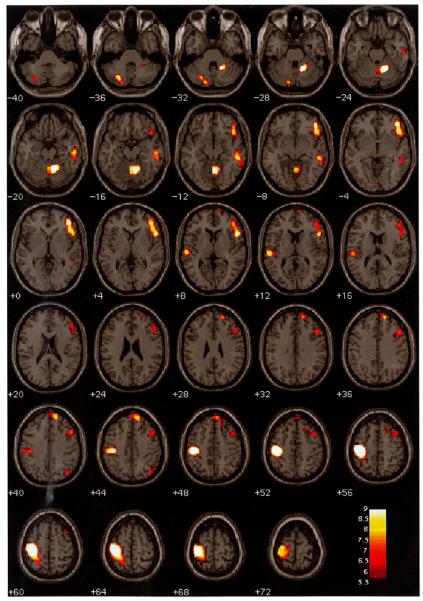
Fig. 1.
Illustration of the areas where there were significant lateralized differences in the amplitude of hemodynamic response to target stimuli versus the standard baseline (P < 0.01 FWE, corrected for searching the whole brain). Active voxels in the left hemisphere show L > R; active voxels in the right hemisphere show R > L. The figure is in neurological convention (that is, the left hemisphere is on the left).
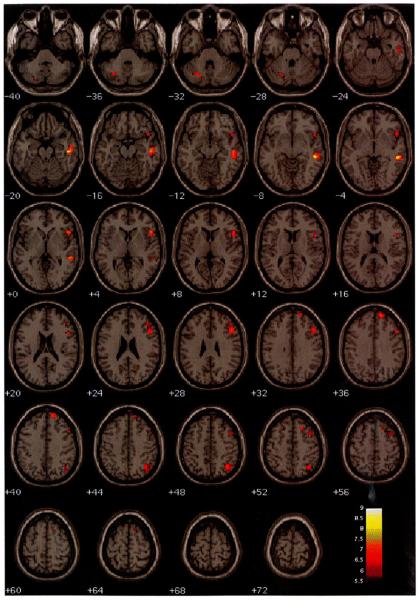
Fig. 2.
Illustration of the areas where there were significant lateralized differences in the amplitude of hemodynamic response to novel stimuli versus the standard baseline (P < 0.01 FWE, corrected for searching the whole brain). Active voxels in the left hemisphere show L > R; active voxels in the right hemisphere show R > L. The figure is in neurological convention (i.e., the left hemisphere is on the left).
Results
Sample characteristics, behavioral performance, and activation differences
As reported in Kiehl et al. (in press), there were no significant differences observed between sites for any demographic or behavioral measures. All subjects performed the task accurately and there were no significant differences in reaction time or accuracy across site, gender, or by age. Likewise, there were no noteworthy differences between genders or sites in hemodynamic activity to target, novel, or standard stimuli.
Hemispheric comparison of the standard stimuli relative to implicit baseline
Standard stimuli showed greater amplitude of the hemodynamic response in left hemisphere relative to right in inferior parietal lobule/superior temporal gyrus and inferior temporal gyrus (Tables 1 and 2). The functional asymmetry for inferior parietal lobe/superior temporal gyrus was the result of greater positive signal change (i.e., ‘activation’) in the right relative to left hemisphere. The asymmetry observed in inferior temporal gyrus activity was the result of positive signal change in the left hemisphere compared to negative signal change in the right. However, neither of these regions were identified in the main effect analysis as showing statistically significant signal change in either hemisphere (P > .05 FWE). There were no areas where right hemisphere hemodynamic activity was greater than left for processing standard stimuli.
Hemispheric comparison of target stimuli relative to the standard stimuli baseline
There was greater hemodynamic activity to target stimuli in right hemisphere frontal, temporal, and parietal lobe areas, including superior, inferior, and medial frontal gyri, middle and inferior temporal gyri, precuneus, and inferior parietal lobule (Tables 1 and 2; Fig. 1). There also was greater activity in the anterior lobe of the right cerebellum compared to left. Target stimuli elicited relatively greater hemodynamic activity in left hemisphere relative to right in postcentral gyrus, transverse temporal gyrus, superior parietal lobule, and the posterior lobe of the left cerebellum. Almost all significant differences between hemispheres were the result of ‘activation’ (i.e., positive signal change) differences. One region (right precuneus) showed negative signal change, but the statistic was non-significant (t = −0.98, ns). The right inferior frontal gyrus (Table 2) showed a main effect to targets at P < 0.05 FWE (peak voxel t score = 5.38 for this region, whereas P < .01 FWE threshold was t = 5.48).
Hemispheric comparison of novel stimuli relative to the standard stimuli baseline
Novel stimuli elicited relatively greater hemodynamic activity in the right hemisphere relative to the left in middle and inferior temporal gyri, and superior, middle, inferior, and medial frontal gyri (Tables 1 and 2; Fig. 2). In the parietal lobe, right hemisphere activation was greater than left in the superior parietal lobule and inferior parietal lobule. There was only one area in the left hemisphere where the amplitude of the hemodynamic response was greater than in the right hemisphere. This was observed in the posterior lobe of the left cerebellum. Except for one region in right medial frontal gyrus, all significant differences between hemispheres were the result of positive signal change ‘activation’ differences. The right medial frontal gyrus response to novel stimuli showed non-significant negative signal change (t = −2.03. ns).
Gender and age analyses
The results of two-sample t tests revealed no gender differences in the hemispheric laterality of hemodynamic response for standards, targets, or novel stimuli. The results of the correlational analysis likewise revealed no significant effect of age on standard, target, or novel hemodynamic asymmetry.
Discussion
The primary objective of the present study was to test the hypothesis that processing of target and novel stimuli would elicit greater hemodynamic activity in right hemisphere lateral prefrontal and temporal–parietal junction cortices than in corresponding sites in the left hemisphere. This was tested using a hemodynamic laterality index that was created to represent the difference between left- and right-sided amplitude of the hemodynamic response. In support of our experimental hypotheses, both target and novel stimuli elicited greater activity in several right hemisphere prefrontal cortex structures, including superior, middle, inferior, and medial frontal gyri. Greater right-hemisphere hemodynamic activity suggests that right hemisphere frontal structures may be relatively more important than left hemisphere structures in mediating cognitive processes elicited by oddball task performance. Because both target and novel stimuli engage orienting and attention processes, it is likely that these hemispheric asymmetries in prefrontal activation are related to attention.
These results are consistent with oddball ERP studies that reported greater right hemisphere than left N2 and P3 amplitude over anterior scalp regions (Alexander et al., 1996; Cohen et al., 2002; Holinger et al., 1992; Karniski and Blair, 1989; Naumann et al., 1992; Oades et al., 1995). During active attention tasks, the N2 to target stimuli is believed to reflect detection of some form of mismatch between an internally generated working memory template and stimulus characteristics (Gehring et al., 1992). The P3 is thought to reflect contextual updating of these working memory stimulus representations (Donchin and Coles, 1988). Working memory models are commonly subsumed in theoretical accounts of attention and attentional control. The present study found hemispheric differences in hemodynamic activity in brain areas often observed to be engaged in various attention and working memory processes. For example, ventrolateral prefrontal cortex is believed to be engaged during active comparison of target stimulus characteristics to a working memory template representation via interconnections with posterior temporal and parietal regions via strong bi-directional connections (Petrides, 1998). In the current study, both targets and novel stimuli showed relatively more right-sided activation in inferior frontal gyrus and inferior parietal lobule. However, the extent of cortex in the right ventrolateral prefrontal cortex compared to the left hemisphere appeared to be larger for targets compared to novel stimuli. Therefore, while both types of infrequent stimuli activate bilateral neural networks, cognitive processes related to salient target identification might depend more on right than left ventrolateral prefrontal cortex. Greater right hemisphere than left activation also was observed for both target and novel stimuli in right dorsolateral and polar prefrontal cortex. This suggests that the relatively greater right than left hemisphere activation in these areas is not dependent on either task salience or specific characteristics of deviant stimuli. Rather, these areas of greater right-sided activity might be primarily modulated by a generalized process of stimulus monitoring in working memory or stimulus categorization, respectively.
However, previous ERP and MEG studies raise the possibility that the greater right hemisphere activity observed in the current study may reflect the function of a mismatch detection neural network in inferior frontal cortex (Alain et al., 1998; Deouell et al., 1998; Di Salle et al., 2001; Giard et al., 1990; Muller et al., 2002; Naatanen et al., 1997; Paavilainen et al., 1991) and right temporal lobe (Alain et al., 1998; Deouell et al., 1998; Giard et al., 1990; Naatanen et al., 1997; Paavilainen et al., 1991). Alternatively, the lateralized activity may be related to cognitive phenomena reflected in other ERP components elicited by rare events during auditory oddball tasks. For example, Halgren and colleagues measured an N2a/P3a/SW waveform in widespread cortical areas elicited by both rare targets and distractor stimuli (Halgren et al., 1998). They attributed this triphasic waveform to a general attentional orienting. If BOLD measurements do accurately reflect the cognitive processes depicted by ERPs, the current results may indicate that several lateral cortical areas (i.e., right hemisphere inferior parietal and dorsolateral prefrontal) may make a larger contribution to attentional orienting compared to homologous left hemisphere structures. A similar argument implicates the right hemisphere intraparietal sulcus region for the P3b which is thought to be involved in attentional processing requiring cognitive control. It is noteworthy that spatiotemporal principal component analysis (PCA) of target stimuli extract components with a characteristic P3-like topography centered over right frontocentral (i.e., P3a) and right prefrontal (i.e., P3b) cortex (Spencer et al., 2001). This suggests that these PCA-derived waveforms may be most detectable in these right-sided cortical structures. However, this suggestion does not conclusively link the hemodynamic asymmetry reported in this experiment to P3 waveforms. In Spencer et al. (2001), the PCA also yielded a waveform characteristic of the slow wave ERP found in oddball tasks. Moreover, that particular analysis did not find a factor accounting for high variance in the data suggestive of the N2 ERP. Thus, further work is needed to link the hemodynamic phenomenon observed in the present data to detailed analyses of ERPs.
It is important to note that it is difficult to conclusively link blood oxygen level dependent (BOLD) measures of hemodynamic activity to specific ERPs measured by electrophysiological methods. Despite a convergence of results from many BOLD and ERP studies, it has not yet been irrefutably shown that cognitive phenomena observed in ERPs will be represented in BOLD signal measurements. Until such time that analyses which explicitly combine multimodal imaging data (e.g., Calhoun et al., 2005) clarify these issues, the precise functional contribution of right inferior frontal gyrus to specific target detection cognitive processes as described by ERPs will not be completely understood.
As expected, asymmetry analyses revealed a strong left hemisphere focus of hemodynamic activity in the postcentral gyrus (and ipsilateral cerebellum). This activity is likely due to the motor activity of the right index finger associated with the manual response to target stimuli. Also consistent with known anatomy of the descending motor neural pathway, these analyses found lateralized activity in the anterior lobe of the right cerebellum, which is ipsilateral to participants' response hand. Another finding was that an area of left posterior lobe cerebellum was relatively more active than the corresponding right hemisphere region for both targets and novels. The well-known involvement of the cerebellum in motor function makes it possible that that this area of relatively greater left cerebellum activity also relates to motor processes. However, this greater left than right cerebellum activity was found for both types of infrequent stimuli, but not for standards. An alternative interpretation is that the greater left cerebellum activity could be part of a corticocerebellar neural circuit involved in attention to rare events. In support of this idea, fMRI studies have found activity in the posterior cerebellum on attention tasks (Allen et al., 1997), and impairments in performance on attention-based cognitive tasks have been found in patients with cerebellar lesions (Gottwald et al., 2003). Courchesne and Allen (1997) have proposed a key role for the cerebellum in preparing and regulating other neural systems of the brain in the service of higher-order cognitive function such as attention.
The current results indicate that there are no differences in the lateralization of brain activity due to gender or age in the current sample, consistent with previous ERP studies that did not observe significant age-related changes in hemispheric laterality in healthy young to middle-aged adults (Anderer et al., 1996; Fabiani et al., 1998; Friedman et al., 1993; Goodin et al., 1978; Iragui et al., 1993; Pfefferbaum et al., 1984; Vesco et al., 1993). In general, age-related ERP changes appear to be most observable in samples of older adults and typically take the form of an ‘anteriorization’ of activation focus (Anderer et al., 1996; Sangal and Sangal, 1996). The absence of age-related differences in the present study may be due to the fact that the sample was comprised of predominantly young to late middle-aged adults (i.e., ages 18 to 62). Few studies have examined the effect of gender on target or novel stimuli ERP topography. Limited findings suggest there are few, if any, gender differences in healthy persons' oddball ERP topography (Sangal and Sangal, 1996). Insofar as neural activity measured by either EEG and fMRI is relatively stable across healthy persons (Alexander et al., 1996; Kiehl et al., in press), it comes as no great surprise that the lack of lateralized differences in such activity likewise are stable across basic demographic factors.
There are both similarities and differences between previously described methods for testing lateralization of function in fMRI data and the voxel-based approach described here. One approach used previously involves identifying region-by-condition interactions based on counting the number of activated voxels within a specific region of interest from each cerebral hemisphere. As discussed in previous voxel-based asymmetry studies (Liegeois et al., 2002; Rowan et al., 2004), the results of that approach strongly depend on the statistical threshold used to choose what represents an ‘activated’ voxel. Lower thresholds may inflate the count of activated voxels within a region-of-interest, whereas too stringent thresholds may mask small regions of true brain function. Furthermore, regions-of-interest are typically defined arbitrarily, which can pose problems when considering individual differences in cerebral morphology or activation foci. Voxel-based approaches provide a direct statistical test on data without the need to arbitrarily set regions or thresholds. Some elements of previously described voxel-based approaches (Liegeois et al., 2002; Rowan et al., 2004; Salmond et al., 2000) were used in this analysis (e.g., use of a symmetric template for spatial normalization, the “flipping” of images so that left and right hemispheres can be compared). However, the present method differs in a number of important ways. Previously reported methods applied the “flipping” and spatial normalization steps to the EPI time series data. In the present approach, the calculations to create images representing functional asymmetry either can be applied to the time series or can be applied to images representing the amplitude of the hemodynamic response for each participant. The latter option (illustrated by the current analysis) is computationally less intensive and can be readily applied to the results of existing statistical analyses without need for extensive reprocessing. In contrast to previous approaches, the current method does not rely on conjunction analysis techniques. Instead, this method uses random effects group level analysis models, which incorporate between-subject variation into their statistical inference. Because the individual subject asymmetry images are computed separately then entered into a second-level random-effects model, this approach provides flexibility for additional types of group level statistics.
Conclusion
In summary, the results provide evidence for greater right-hemisphere hemodynamic activity to rare target and novel stimuli. Our data also suggest that neither age nor gender alters hemispheric differences in the amplitude of the hemodynamic response to oddball stimuli for healthy young and middle-age adults. This experiment demonstrates that a voxel-based asymmetry index based on a simple left versus right difference is an effective tool. It appears to detect differences in neural activity occurring in homologous structures from both hemispheres as well as to effectively characterize strongly lateralized processes. Because this approach is general, it can easily be used to search for hemispheric differences in hemodynamic activity elicited by other types of paradigms and can be applied to various types of group-level analyses (i.e., one-sample t tests, two-sample t tests, correlational analyses, etc.).
Acknowledgments
We appreciate the assistance given by Jana Schaich-Borg in preparing the manuscript. An SPM2 toolbox that contains routines used in this study is available from the authors at http://www.nrc-iol.org/resources/onrcsoftware.htm. This work was supported by Hartford Hospital Open Grant Competition Award (KAK), a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (KAK) and NIMH grants 1 R01 MH0705539-01 (KAK) and 1 R01 MH072681-01 (KAK). MCS was supported by 1 K23 MH70036-01A1.
Footnotes
It should be noted that a previous report using these data explicitly test for differences between the two sites. No meaningful differences were found for any main effect of the auditory oddball lask (Kiehl et al., in press).
References
- Aguirre GK, Zarahn E, D'Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. NeuroImage. 1998;8:302–306. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Alain C, Cortese F, Picton TW. Event-related brain activity associated with auditory pattern processing. NeuroReport. 1998;9:3537–3541. doi: 10.1097/00001756-199810260-00037. [DOI] [PubMed] [Google Scholar]
- Alexander JE, Bauer LO, Kuperman S, Morzorati S, O'Connor SJ, Rohrbaugh J, Porjesz B, Begleiter H, Polich J. Hemispheric differences for P300 amplitude from an auditory oddball task. Int. J. Psychophysiol. 1996;21:189–196. doi: 10.1016/0167-8760(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Anderer P, Semlitsch HV, Saletu B. Multichannel auditory event-related brain potentials: effects of normal aging on the scalp distribution of N1, P2, N2 and P300 latencies and amplitudes. Electroencephalogr. Clin. Neurophysiol. 1996;99:458–472. doi: 10.1016/s0013-4694(96)96518-9. [DOI] [PubMed] [Google Scholar]
- Baillet S, Garnero L. A Bayesian approach to introducing anatomo-fiinctional priors in the EEG/MEG inverse problem. IEEE Trans. Med. Imag. 1997;44:374–385. doi: 10.1109/10.568913. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DE. Attentional systems in target and distractor processing: a combined ERP and fMRI study. NeuroImage. 2004a;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DE. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J. Neurosci. 2004b;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pekar JJ. A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magn. Reson. Imaging. 2004a;22:1181–1191. doi: 10.1016/j.mri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Stevens MC, Pearlson GD, Kiehl KA. fMRI analysis with the general linear model: removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. NeuroImage. 2004b;22:252–257. doi: 10.1016/j.neuroimage.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Giuliani N, Pekar JJ, Pearlson GD, Kiehl KA. A method for multimodal analysis of independent source differences in schizophrenia: combining gray matter structural and auditory oddball functional data. Hum. Brain Map. doi: 10.1002/hbm.20166. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, Noll DC. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum. Brain Mapp. 2001;13:26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J. Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R. Paradigm-dependent modulation of event-related fMRI activity evoked by the oddball task. Hum. Brain Mapp. 2001;14:116–127. doi: 10.1002/hbm.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HL, Ji J, Chorlian DB, Begleiter H, Porjesz B. Alcohol-related ERP changes recorded from different modalities: a topographic analysis. Alcohol. Clin. Exp. Res. 2002;26:303–317. [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev., Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J. Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learn. Mem. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr. Clin. Neurophysiol. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Deouell LY, Bentin S, Giard MH. Mismatch negativity in dichotic listening: evidence for interhemispheric differences and multiple generators. Psychophysiology. 1998;35:355–365. [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional magnetic resonance imaging analyses. NeuroImage. 2001;13:751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Di Salle F, Formisano E, Seifritz E, Linden DE, Scheffler K, Saulino C, Tedeschi G, Zanella FE, Pepino A, Goebel R, Marciano E. Functional fields in human auditory cortex revealed by time-resolved fMRI without interference of EPI noise. NeuroImage. 2001;13:328–338. doi: 10.1006/nimg.2000.0683. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988;11:357–374. [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Foundas AL, Faulhaber JR, Kulynych JJ, Browning CA, Weinberger DR. Hemispheric and sex-linked differences in Sylvian fissure morphology: a quantitative approach using volumetric magnetic resonance imaging. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999;12:1–10. [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. NeuroImage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans. Med. Imag. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30:383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An overview of age-related changes in the scalp distribution of P3b. Electroencephalogr. Clin. Neurophysiol. 1997:104. doi: 10.1016/s0168-5597(97)00036-1. Evoked. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum. Brain Mapp. 1995;2:165–189. [Google Scholar]
- Gehring WJ, Gratton G, Coles MGH, Donchin E. Probability effects on stimulus evaluation and response processes. J. Exp. Psychol. Hum. Percept. Perform. 1992;18:198–216. doi: 10.1037/0096-1523.18.1.198. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Pans T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Squires KC, Henderson BH, Starr A. Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalogr. Clin. Neurophysiol. 1978;44:447–458. doi: 10.1016/0013-4694(78)90029-9. [DOI] [PubMed] [Google Scholar]
- Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia. 2003;41:1452–1460. doi: 10.1016/s0028-3932(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr. Clin. Neurophysiol. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Hellige JB, Taylor KB, Lesmes L, Peterson S. Relationships between brain morphology and behavioral measures of hemispheric asymmetry and interhemispheric interaction. Brain Cogn. 1998;36:158–192. doi: 10.1006/brcg.1997.0951. [DOI] [PubMed] [Google Scholar]
- Holinger DP, Faux SF, Shenton ME, Sokol NS, Seidman LJ, Green AI, McCarley RW. Reversed temporal region asymmetries of P300 topography in left- and right-handed schizophrenic subjects. Electroencephalogr. Clin. Neurophysiol. 1992;84:532–537. doi: 10.1016/0168-5597(92)90042-a. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Skudlarski P, Gore JC. Correlations and dissociations between BOLD signal and P300 amplitude in an auditory oddball task: a parametric approach to combining fMRI and FRP. Magn. Reson. Imaging. 2002;20:319–325. doi: 10.1016/s0730-725x(02)00496-4. [DOI] [PubMed] [Google Scholar]
- Iragui VJ, Kutas M, Mitchiner MR, Hillyard SA. Effects of aging on event-related brain potentials and reaction times in an auditory oddball task. Psychophysiology. 1993;30:10–22. doi: 10.1111/j.1469-8986.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Jancke L, Mirzazade S, Shah NJ. Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci. Lett. 1999;266:125–128. doi: 10.1016/s0304-3940(99)00288-8. [DOI] [PubMed] [Google Scholar]
- Jemel B, Achenbach C, Mullen BW, Ropcke B, Oades RD. Mismatch negativity results from bilateral asymmetric dipole sources in the frontal and temporal lobes. Brain Topogr. 2002;15:13–27. doi: 10.1023/a:1019944805499. [DOI] [PubMed] [Google Scholar]
- Karniski W, Blair RC. Topographical and temporal stability of the P300. Electroencephalogr. Clin. Neurophysiol. 1989;72:373–383. doi: 10.1016/0013-4694(89)90043-6. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. Reproducibility of the hemodynamic response to auditory oddball stimuli: a six-week test–retest study. Hum. Brain Mapp. 2003;18:42–52. doi: 10.1002/hbm.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. An event-related fMRI study of visual and auditory oddball tasks. J. Psychophysiol. 2001a;21:221–240. [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001b;38:133–142. [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson GP, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. NeuroImage. doi: 10.1016/j.neuroimage.2004.12.035. in press. [DOI] [PubMed] [Google Scholar]
- Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. J. Neurosci. 2000;20:6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanen S, Ahonen A, Hari R, McEvoy L, Sams M. Deviant auditory stimuli activate human left and right auditory cortex differently. Cereb. Cortex. 1996;6:288–296. doi: 10.1093/cercor/6.2.288. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha-Khadem F, Baldeweg T. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. NeuroImage. 2002;17:1861–1867. doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Voellinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb. Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J. Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A. Combined event-related fMRI and EEG evidence for temporal–parietal cortex activation during target detection. NeuroReport. 1997;8:3029–3037. doi: 10.1097/00001756-199709290-00007. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, Juckel G, Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. NeuroImage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Muller BW, Juptner M, Jentzen W, Muller SP. Cortical activation to auditory mismatch elicited by frequency deviant and complex novel sounds: a PET study. NeuroImage. 2002;17:231–239. doi: 10.1006/nimg.2002.1176. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Teder W, Alho K, Lavikainen J. Auditory attention and selective input modulation: a topographical ERP study. NeuroReport. 1992;3:493–496. doi: 10.1097/00001756-199206000-00009. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, Iivonen A, Vainio M, Alku P, Ilmoniemi RJ, Luuk A, Allik J, Sinkkonen J, Alho K. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Naumann E, Huber C, Maier S, Plihal W, Wustmans A, Diedrich O, Bartussek D. The scalp topography of P300 in the visual and auditory modalities: a comparison of three normalization methods and the control of statistical type II error. Electroencephalogr. Clin. Neurophysiol. 1992;83:254–264. doi: 10.1016/0013-4694(92)90119-3. [DOI] [PubMed] [Google Scholar]
- Oades RD, Zerbin D, Dittmann-Balcar A. The topography of event-related potentials in passive and active conditions of a 3-tone auditory oddball test. Int. I. Neurosci. 1995;81:249–264. doi: 10.3109/00207459509004890. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Downing PE, Kanwisher N. fMRI evidence for objects as the units of attentional selection. Nature. 1999;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, Von Cramon DY, Kruggel F. Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology. 1999;36:142–147. doi: 10.1017/s0048577299980848. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Alain C, Picton TW. Effects of visual attentional load on auditory processing. NeuroReport. 2000;11:875–880. doi: 10.1097/00001756-200003200-00043. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Alho K, Reinikainen K, Sams M, Naatanen R. Right hemisphere dominance of different mismatch negativities. Electroencephalogr. Clin. Neurophysiol. 1991;78:466–479. doi: 10.1016/0013-4694(91)90064-b. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. Interhemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans. Cereb. Cortex. 1996;6:661–672. doi: 10.1093/cercor/6.5.661. [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. In: Roberts AC, et al., editors. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford Univ. Press; Oxford: 1998. pp. 103–116. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials: I. Normal aging. Electroencephalogr. Clin. Neurophysiol. 1984;59:85–103. doi: 10.1016/0168-5597(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, Kastner S. Push–pull mechanism of selective attention in human extrastriate cortex. J. Neurophysiol. 2004;92:622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300s: an integrative review. Biological. Psychiatry. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Naatanen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. NeuroImage. 2000;12:14–19. doi: 10.1006/nimg.2000.0591. [DOI] [PubMed] [Google Scholar]
- Rowan A, Liegeois F, Vargha-Khadem F, Gadian D, Connelly A, Baldeweg T. Cortical lateralization during verb generation: a combined ERP and fMRI study. NeuroImage. 2004;22:665–675. doi: 10.1016/j.neuroimage.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Gadian DG, Friston KJ. Detecting bilateral abnormalities with voxel-based morphometry. Hum. Brain Mapp. 2000;11:223–232. doi: 10.1002/1097-0193(200011)11:3<223::AID-HBM80>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangal B, Sangal JM. Topography of auditory and visual P300 in normal adults. Clin. Electroencephalogr. 1996;27:145–150. doi: 10.1177/155005949602700307. [DOI] [PubMed] [Google Scholar]
- Scherg M, Von Cramon D. Evoked dipole source potentials of the human auditory cortex. Electroencephalogr. Clin. Neurophysiol. 1986;65:344–360. doi: 10.1016/0168-5597(86)90014-6. [DOI] [PubMed] [Google Scholar]
- Scherg M, Vajsar J, Picton TW. A source analysis of the late human auditor, evoked potentials. J. Cogn. Neurosci. 1989;1:336–355. doi: 10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus. 2001;11:690–698. doi: 10.1002/hipo.1084. [DOI] [PubMed] [Google Scholar]
- Sutton S, Baren M, Zubin J, John ER. Evoked potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM. Functional specificity of superior parietal mediation of spatial shifting. NeuroImage. 2001;14:661–673. doi: 10.1006/nimg.2001.0860. [DOI] [PubMed] [Google Scholar]
- Vesco KK, Bone RC, Ryan JC, Polich J. P300 in young and elderly subjects: auditory frequency and intensity effects. Electroencephalogr. Clin. Neurophysiol. 1993;88:302–308. doi: 10.1016/0168-5597(93)90054-s. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat. Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]