Abstract
Caries Detector staining reveals 4 zones in dentin containing caries lesions, but characteristics of each zone are not well-defined. We therefore investigated the physical and microstructural properties of carious dentin in the 4 different zones to determine important differences revealed by Caries Detector staining. Six arrested dentin caries lesions and 2 normal controls were Caries-Detector-stained, each zone (pink, light pink, transparent, apparently normal) being analyzed by atomic force microscopy (AFM) imaging for microstructure, by AFM nano-indentation for mechanical properties, and by transverse digital microradiography (TMR) for mineral content. Microstructure changes, and nanomechanical properties and mineral content significantly decreased across zones. Hydrated elastic modulus and mineral content from normal dentin to pink Caries-Detector-stained dentin ranged from 19.5 [10.6-25.3] GPa to 1.6 [0.0-5.0] GPa and from 42.9 [39.8-44.6] vol% to 12.4 [9.1-14.2] vol%, respectively. Even the most demineralized pink zone contained considerable residual mineral.
Keywords: dentin, caries, mineral, mechanical properties, atomic force microscopy
Introduction
Current management of caries involves non-invasive techniques and maximum conservation of tooth structure. Differentiation between heavily infected outer carious dentin and demineralized, affected inner dentin reduces the risk of pulp exposure, maximizing reparative potential (McComb, 2000). Different layers of dentin caries lesions have been classified by clinical and laboratory techniques (Fusayama and Terachima, 1972; Anderson et al., 1985), but recommendations may conflict or overlap. Therefore, it is essential that the nature of and variations in such lesions be understood.
Caries-staining products have been developed (Fusayama and Terachima, 1972) to assist clinicians during caries removal. Although the biochemical principle of staining carious dentin has been reported (Ohgushi and Fusayama, 1975; Kuboki et al., 1977; Kuboki et al., 1983), it remains unclear what characteristics of the lesion are stained, or how staining is related to microstructural features of various caries lesion zones. Not all stainable dentin is infected (Kidd et al., 1993), but the absence of stain does not ensure bacterial elimination (Anderson et al., 1985).
Caries staining characteristics have been described as pink, light pink, transparent, and apparently normal dentin, and nano-indentation properties of moderately active and arrested caries lesions have been compared (Zheng et al., 2003). Nanohardness values for intertubular dentin increased from the pink zone to the apparently normal dentin layer (outer to inner). In another study, mechanical properties across dentin caries lesions decreased as the lesion surface was approached (Angker et al., 2004b).
Hydration and dehydration affect dentin mechanical properties, especially demineralized dentin (Kinney et al., 1993, 1996; Marshall, 1993). Therefore, properties measured under hydrated conditions provide more realistic estimates of those found in vivo (Marshall et al., 2001).
The hypothesis for this study was that Caries Detector staining allows for the discrimination of carious zones with distinct microstructural characteristics, mechanical properties, and mineral contents. We investigated the zones revealed by Caries Detector staining in arrested caries lesions. We related these zones to their microstructural features as determined by atomic force microscopy (AFM), nanomechanical properties as determined by AFM nano-indentation, and mineral concentration.
Materials & Methods
Tooth Selection and Sample Preparation
Teeth were collected following protocols approved by the UCSF Committee on Human Research, and informed consent was obtained for the use of human tissues. Eight freshly extracted, unrestored third molars (6 carious, 2 non-carious controls), obtained from research participants requiring extractions for dental treatment, were sterilized by gamma radiation (White et al., 1994), then stored in Hanks’ balanced salt solution at 4ºC (Habelitz et al., 2002). After the tooth was longitudinally sectioned through the lesion center by means of a water-cooled saw (Isomet low-speed saw; Buehler, Lake Bluff, IL, USA), a tooth with an obvious lesion appearing to extend 50-75% through the dentin thickness was eligible for the lesion group (Zheng et al., 2003). Longitudinal sections (2 mm thick) through the center of the lesion were further sectioned from occlusal surface to pulp chamber to produce 2 x 2 x ~8 mm sticks. Similar sticks were sectioned from normal dentin controls. The surface was polished with waterproof SiC papers, under running water, with grit sizes of 600 to 1200, and 1- and 0.25-µm diamond pastes. This prepared surface was stained by Caries Detector (Kuraray, Osaka, Japan). Six lesions exhibiting apparently arrested caries (discolored, firm consistency, lightly Caries-Detector-stained) were identified for the lesion group. Digital images of each prepared surface were recorded.
Classification of Dentin Caries Lesion Zones
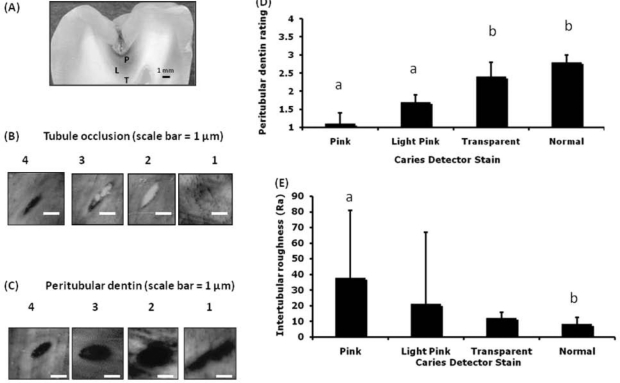
Four zones were identified in the lesions, based on their optical appearance and degree of staining: pink, light pink, transparent, and apparently normal. A representative section through an arrested dentin caries lesion can be seen (Fig. 1A), showing each Caries-Detector-stained zone, before being further sectioned into sticks.
Figure 1.
Carious morphological features. (a) Representative section through an arrested dentin caries lesion showing each Caries Detector zone, pink (P), light pink (L), transparent (T), and apparently normal (N). AFM height images (n = 61) showing examples of (b) tubule occlusion (TO) (scale bar = 1 µm), 4 = open, 3 = partial, 2 = closed, 1 = covered; (c) peritubular dentin (PT) (scale bar = 1 µm), 4 = complete, 3 = partial, 2 = none, 1 = distorted (images 30 x 30 µm); (d) PT rating means for each CD stain (n = 8) with 95% confidence boundaries indicated; (e) intertubular roughness (Ra) measurement means of CD stain zones (n = 8), with 95% confidence boundaries indicated. (Different letters a, b indicate non-overlapping confidence bounds corresponding to significant differences; error bars show 95% confidence bounds.)
Carious Dentin Microstructure Analysis by AFM
Using the Triboscope (Hysitron, Minneapolis, MN, USA) indenter tip of the AFM (Nanoscope III, Digital Instruments, Santa Barbara, CA, USA), we took a 30 µm by 30 µm AFM image of each area before and after nano-indentation, to examine the dentin microstructure in each area. Microstructural criteria included tubule occlusion, peritubular dentin, and intertubular roughness.
For each AFM image, the total number of tubules was determined. To assess tubule occlusion, we gave each tubule a rating between 4 (open) and 1 (completely closed) (Fig. 1B). For peritubular dentin assessment, the ratings were 4 (complete peritubular dentin surrounding the lumen), 3 (lumen partially surrounded), 2 (no peritubular dentin, but tubule shape was undistorted), and 1 (if no peritubular dentin and the tubule had a distorted shape) (Fig. 1C). This peritubular dentin rating system accounted for the difference between a loss of peritubular dentin in the initial stages of demineralization and the tubule distortion characteristic of more advanced demineralization caused by dentin caries. Average ratings for tubule occlusion and peritubular dentin were determined for each AFM image. These ratings were then related to additional physical and mechanical properties and Caries Detector stain.
Intertubular surface roughness (root mean square roughness, Ra) of the peaks and valleys of the analyzed area was measured with the roughness analysis option from AFM software (Nanoscope III, version 5.12r3, Digital Instruments, Santa Barbara, CA, USA) (Oliveira et al., 2003). Three 5 µm by 5 µm images of intertubular dentin were chosen from the same images as used for tubule occlusion and peritubular dentin analyses.
Measurement of Reduced Elastic Modulus and Hardness
Reduced elastic modulus and hardness along the stick used for the microstructural evaluation were determined by an AFM modified with a Triboscope nano-indenter (Hysitron, Minneapolis, MN, USA), as previously described (Balooch et al., 1998; Marshall et al., 2001). Nano-indentations were performed with a Berkovich diamond tip, both dry and in a liquid cell filled with de-ionized water, with a trapezoidal force profile with peak loads between 50 µN and 500 µN, and three-second indentation times. Each indentation yielded a load-deformation curve, from which the reduced elastic modulus, E, and hardness, H, were determined according to the following equations (Doerner and Nix, 1986):
where S represents the slope of the unloading curve based on the method of Oliver and Pharr (1992), a is the indentation contact area, and Fmax is the maximum force. Calibration used a silica standard as previously described (Marshall et al., 2004). Five indentations were made in intertubular dentin at ~ 350-µm intervals along a line from near the pulp chamber to the carious occlusal surface. We collected AFM images before and after indentations to ensure that they were uniform, well-defined, and within intertubular dentin.
Measurement of Mineral Concentration
We used a custom-built digital transverse microradiography (TMR) system to measure mineral concentration in the dentin caries lesions (sections approx. 200 mm thick), and the equipment and technique have been described previously (Darling et al., 2006). The vol% mineral of each section was determined with a calibration curve of x-ray intensity vs. sample thickness created with sound enamel sections of 86.3 ± 1.9% vol% mineral, varying from 50 to 300 mm in thickness. The calibration curve was validated via comparison with cross-sectional microhardness measurements. The vol% mineral determined by microradiography for section thicknesses ranging from 50 to 300 mm was highly correlated with the vol% mineral determined by microhardness, r2 = 0.99.
Statistical Analysis
We calculated means and 95% confidence intervals of each property (Ra, modulus, hardness, and vol% mineral) for each zone with a bootstrap sampling approach, stratifying on sample (selecting one measurement per zone per sample with replacement, calculating the mean, repeating 20,000 times, and determining the 5% and 95% bounds). We used Pearson’s correlation coefficient (r) to assess the relationship of the properties (elastic modulus and hardness) with vol% mineral, with Spearman’s rank (rs) for ordinal data to assess the relationship of the properties (Ra, elastic modulus, hardness, and vol% mineral) with stain (zone). We assessed the relationship of peritubular dentin and tubule occlusion with Caries Detector stain with rs, using a bootstrap procedure to select one tubule per stick and calculating rs; this was repeated 20,000 times, and the distribution of rs was determined with the mean and 5% and 95% bounds reported.
Results
As expected, with increasing levels of demineralization, peritubular dentin rating, mechanical properties, and mineral content decreased (Table). Peritubular dentin rating varied significantly according to Caries Detector stain (Table, Fig. 1D). The correlation coefficient between peritubular dentin and Caries Detector stain was rs = 0.58 (bootstrap 95% CI, 0.20, 0.82), indicating a significant association, and that between tubule occlusion and Caries Detector stain was 0.36 (bootstrap 95% CI, -0.09, 0.72), which was not significant. Mean intertubular Ra of the pink zone was significantly greater than that in the normal zone, as indicated by the non-overlapping confidence bounds (Table, Fig. 1E).
Table.
Means According to Caries Detector Stain*
| Caries Detector Stain |
||||
|---|---|---|---|---|
| Feature | Normal | Transparent | Light Pink | Pink |
| Peritubular dentin rating | 2.8 [2.7-3.0]a | 2.4 [2.1-2.8]a | 1.7 [1.6-1.9]b | 1.1 [1.0-1.3]b |
| Tubule occlusion rating | 3.3 [3.1-3.4]a | 2.9 [2.6-3.2] | 2.6 [2.4-2.8]b | 2.1 [1.6-2.7]b |
| Intertubular Ra (nm) | 8.3 [4.6-12.7]a | 12.3 [8.8-16.2] | 21.1 [6.7-67.0] | 37.8 [15.5-80.9]b |
| Elastic modulus dry (GPa) | 23.8 [21.8-26.4] | 18.1 [11.3-25.3] | 17.1 [12.0-22.8] | 11.4 [0.1-23.2] |
| Elastic modulus wet (GPa) | 19.5 [10.6-25.3]a | 14.4 [1.2-26.7] | 9.8 [2.3-17.8] | 1.6 [0.0-5.0]b |
| Hardness dry (GPa) | 1.63 [0.95-2.49]a | 0.65 [0.24-1.25] | 0.52 [0.30-0.88]b | 0.31 [0.01-0.50]b |
| Hardness wet (GPa) | 0.87 [0.10-1.20]a | 0.58 [0.00-1.00] | 0.63 [0.05-1.10] | 0.05 [0.00-0.10]b |
| Mineral content (vol%) | 42.9 [39.8-44.6]a | 37.0 [36.9-37.1]a | 26.2 [19.8-32.3]c | 12.4 [9.1-14.2]d |
Characterization criteria means (n = 8). Bootstrap mean and 95% confidence boundaries are shown. Different letters a, b, c, d indicate non-overlapping confidence bounds corresponding to significant differences between stains.
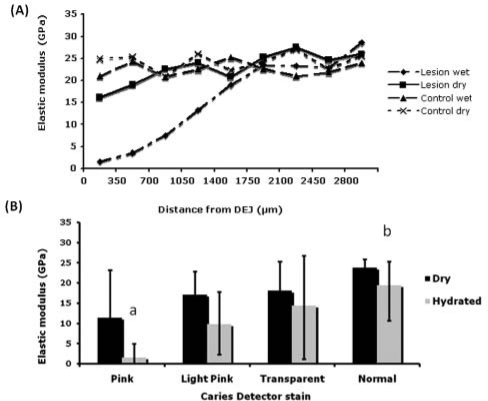
A typical elastic modulus profile of normal control dentin and a dentin caries lesion, along lines starting 3 mm below the DEJ, shows the decreased values in the demineralized zones (Fig. 2A). Dry modulus values were significantly correlated with the ordinal Caries Detector stain (normal, transparent, and light pink) (rs = 0.59; bootstrap 95% CI, 0.12, 0.91) (Fig. 2B). Hydrated elastic modulus values were significantly less in pink than in normal, as indicated by non-overlapping 95% CIs (Fig. 2B). Hardness values are shown in the Table.
Figure 2.
Nanomechanical properties of dentin caries lesions—AFM nano-indentation in hydrated and dry conditions. (a) Elastic modulus (E) in GPa of 1 lesion, under hydrated and dry conditions, and control (normal dentin). (b) E for each CD stain (n = 8) (Different letters a, b indicate non-overlapping confidence bounds corresponding to significant differences; error bars show 95% confidence bounds.)
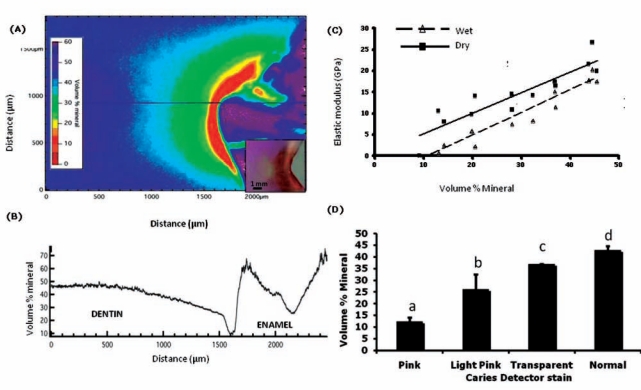
A microradiograph (Fig. 3A) from a representative dentin caries lesion with a vol% mineral line profile (Fig. 3B) through the lesion center shows mineral variations across the lesion. Vol% mineral differed significantly between Caries Detector stain zones, as indicated by non-overlapping bootstrap 95% CIs (Fig. 3C). Mineral content was significantly correlated with dry elastic modulus (r = 0.71; bootstrap 95% CI, 0.20, 1.00).
Figure 3.
Digital transverse microradiography (TMR). (a) Vol% mineral color gradient TMR image showing a representative dentin caries lesion; inset shows corresponding CD-stained lesion. (b) Line profile showing vol% mineral along the line shown in (a), vs. distance in µm along lesion from above pulp to just above DEJ; (c) mineral content vs. E hydrated and dry (n = 8) [correlation (r) significant for E dry, p < 0.05]; and (d) vol% mineral averages by CD stain (n = 8). (Different letters a, b, c, d indicate non-overlapping confidence bounds corresponding to significant differences; error bars show 95% confidence bounds.)
Discussion
Previous studies have suggested that mechanical properties of a calcified tissue are associated with mineral content (Featherstone et al., 1983; Arends and ten Bosch, 1992; Kodaka et al., 1992; Kinney et al., 1996; Angker et al., 2004b). It has been reported that mechanical properties are significantly affected by wet or dry testing conditions (Angker et al., 2004a). In our study, elastic modulus of hydrated dentin could better distinguish between normal and pink stains than could dry nano-indentation, perhaps because the dentin was hydrated when it was Caries-Detector-stained. Hardness of both wet and dry dentin showed significant differences between normal and pink stain. Interestingly, dry nano-indentation measurements correlated better with Caries Detector stain, perhaps because of less variability.
A biochemical mechanism has been suggested for the stainability of carious dentin based on the remaining collagen that has been demineralized by cariogenic acids. The inner layer of carious dentin that stains most dramatically (pink zone) has decreased cross-links (Kuboki et al., 1977), allowing the stain to penetrate more efficiently. Furthermore, in one study, collagen was stainable only when demineralized by a cariogenic acid, which may indicate bacterial involvement in Caries Detector stainability (Kuboki et al., 1983), although there is disagreement about the correlation between stain and level of bacterial infection (Kidd et al., 1993; Zacharia and Munshi, 1995). Our data are consistent with the idea that altered collagen cross-linking may contribute to increased stainability of the pink zone, and decreased mechanical properties. However, the exposure to cariogenic acids of the pink zone may simply remove more mineral, causing increased porosity, and hence increased stainability.
In this study, the Caries Detector stain sometimes gave false-positives in normal dentin near the pulp chamber, as well as false-negatives in slightly demineralized dentin that had not become porous enough to pick up the stain. These discrepancies agree with a previous report (McComb, 2000). The inconsistency of carious dentin staining could result from increased or decreased porosity, unrelated to mechanical properties, collagen structure, or microstructure, but instead caused by variations in tubule density.
Intertubular dentin modulus is a major determinant of overall elastic properties of dentin, and there is minimal contribution from the peritubular dentin properties (Kinney et al., 1996). Nano-indentations were made in intertubular dentin for accurate assessment of the mechanical properties of bulk dentin. However, the presence or absence of peritubular dentin is also important, since its dissolution is an early microstructural indicator of demineralization (Marshall et al., 1998). Likewise, tubule occlusion by mineral deposits due to demineralization/remineralization cycles during the caries process (ten Cate et al., 1988; Featherstone et al., 1990; Featherstone, 2004) is an indicator of transparent dentin formation (Fusayama, 1991). Therefore, we analyzed AFM images from the nano-indented areas and rated each tubule for peritubular dentin and tubule occlusion. While peritubular ratings were significantly related to Caries Detector stain, tubule occlusion was not. Tubule occlusion was most apparent in transparent dentin, leading to its transparent optical appearance. While tubule coverage and distortion were observed in Caries-Detector-stained dentin, these tubules were not filled, but had loosely attached mineral or organic material.
Our study showed that when the dentin was stained by the Caries Detector, peritubular dentin disappeared, perhaps because the lack of peritubular dentin widened the tubules and intertubular dentin had been partially demineralized and was more porous. While peritubular dentin ratings were a good indicator of demineralization, they could not distinguish between partially demineralized (light pink) dentin and the more demineralized pink zone. Intertubular roughness (Ra) measurements with AFM showed that Ra could also distinguish between normal and pink zones.
Caries Detector stain may not differentiate remineralizable demineralized dentin from that which should be removed. Thus, a greater understanding of the Caries Detector staining process is needed to improve its clinical utility. This study emphasized the importance of measuring dentin properties in hydrated conditions. Dry measurements of modulus or hardness may result in misleadingly high values in the more demineralized zones, but may produce less variable measures.
A significant finding of this study is that the most demineralized (pink) zone of these dentin caries lesions contained about 25% of the mineral of normal dentin. Previous studies of bone have shown that the mineral phase is partitioned between intrafibrillar mineral and extrafibrillar mineral within the spaces separating the fibrils (Landis et al., 1996). Extrafibrillar mineral makes up as much as 75% of the total mineral (Bonar et al., 1985), leaving 25% intrafibrillar mineral. Therefore, the pink zone may contain remaining intrafibrillar mineral, which may have important consequences for remineralization and the restoration of mechanical properties (Kinney et al., 2003).
In conclusion, the characterization of Caries-Detector-stained zones from arrested dentin caries lesions described here indicates different mechanical properties and microstructure between and among zones, which is valuable for caries diagnosis. However, analysis of our data suggests that the pink zone, which is commonly removed by the clinician, could contain residual mineral, and thus may be worth retaining.
Footnotes
The authors acknowledge G. Nonomura for tooth collection and support from NIH/NIDCR grants T32 DE 00736, P01 DE09859, and R01 DE16849.
References
- Anderson MH, Loesche WJ, Charbeneau GT. (1985). Bacteriologic study of a basic fuchsin caries-disclosing dye. J Prosthet Dent 54: 51-55 [DOI] [PubMed] [Google Scholar]
- Angker L, Nijhof N, Swain MV, Kilpatrick NM. (2004a). Influence of hydration and mechanical characterization of carious primary dentine using an ultra-micro indentation system (UMIS). Eur J Oral Sci 112: 231-236 [DOI] [PubMed] [Google Scholar]
- Angker L, Nockolds C, Swain MV, Kilpatrick N. (2004b). Correlating the mechanical properties to the mineral content of carious dentine—a comparative study using an ultra-micro indentation system (UMIS) and SEM-BSE signals. Arch Oral Biol 49: 369-378 [DOI] [PubMed] [Google Scholar]
- Arends J, ten Bosch JJ. (1992). Demineralization and remineralization evaluation techniques. J Dent Res 71 (Spec Iss):924-928 [DOI] [PubMed] [Google Scholar]
- Balooch M, Wu-Magidi IC, Lundkvist AS, Balazs A, Marshall SJ, Marshall GW, et al. (1998). Viscoelastic properties of demineralized human dentin in water with AFM-based indentation. J Biomed Mater Res 40:539-544 [DOI] [PubMed] [Google Scholar]
- Bonar LC, Lees S, Mook HA. (1985). Neutron diffraction studies of collagen in fully mineralized bone. J Mol Biol 181: 265-270 [DOI] [PubMed] [Google Scholar]
- Darling CL, Huynh GD, Fried D. (2006). Light scattering properties of natural and artificially demineralized dental enamel at 1310 nm. J Biomed Opt 11: 34023, 1-11 [DOI] [PubMed] [Google Scholar]
- Doerner MF, Nix WD. (1986). A method for interpreting the data from depth-sensing indentation instruments. J Mater Res 1:601-609 [Google Scholar]
- Featherstone JD. (2004). The caries balance: the basis for caries management by risk assessment. Oral Health Prev Dent 2 (Suppl 1):259-264 [PubMed] [Google Scholar]
- Featherstone JD, ten Cate JM, Shariati M, Arends J. (1983). Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res 17: 385-391 [DOI] [PubMed] [Google Scholar]
- Featherstone JD, Glena R, Shariati M, Shields CP. (1990). Dependence of in vitro demineralization of apatite and remineralization of dental enamel on fluoride concentration. J Dent Res 69 (Spec Iss):620-625 [DOI] [PubMed] [Google Scholar]
- Fusayama T. (1991). Intratubular crystal deposition and remineralization of carious dentin. J Biol Buccale 19:255-262 [PubMed] [Google Scholar]
- Fusayama T, Terachima S. (1972). Differentiation of two layers of carious dentin by staining. J Dent Res 51: 866. [DOI] [PubMed] [Google Scholar]
- Habelitz S, Marshall GW, Jr, Balooch M, Marshall SJ. (2002). Nanoindentation and storage of teeth. J Biomech 35: 995-998 [DOI] [PubMed] [Google Scholar]
- Kidd EA, Joyston-Bechal S, Beighton D. (1993). Microbiological validation of assessments of caries activity during cavity preparation. Caries Res 27: 402-408 [DOI] [PubMed] [Google Scholar]
- Kinney JH, Balooch M, Marshall GW, Marshall SJ. (1993). Atomic-force microscopic study of dimensional changes in human dentine during drying. Arch Oral Biol 38: 1003-1007 [DOI] [PubMed] [Google Scholar]
- Kinney JH, Balooch M, Marshall SJ, Marshall GW, Jr, Weihs TP. (1996). Hardness and Young’s modulus of human peritubular and intertubular dentine. Arch Oral Biol 41: 9-13 [DOI] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. (2003). The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res 82:957-961 [DOI] [PubMed] [Google Scholar]
- Kodaka T, Debari K, Yamada M, Kuroiwa M. (1992). Correlation between microhardness and mineral content in sound human enamel (short communication). Caries Res 26: 139-141 [DOI] [PubMed] [Google Scholar]
- Kuboki Y, Ohgushi K, Fusayama T. (1977). Collagen biochemistry of the two layers of carious dentin. J Dent Res 56:1233-1237 [DOI] [PubMed] [Google Scholar]
- Kuboki Y, Liu CF, Fusayama T. (1983). Mechanism of differential staining in carious dentin. J Dent Res 62:713-714 [DOI] [PubMed] [Google Scholar]
- Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. (1996). Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc Res Tech 33: 192-202 [DOI] [PubMed] [Google Scholar]
- Marshall GW., Jr (1993). Dentin: microstructure and characterization. Quintessence Int 24: 606-617 [PubMed] [Google Scholar]
- Marshall GW, Jr, Wu-Magidi IC, Watanabe LG, Inai N, Balooch M, Kinney JH, et al. (1998). Effect of citric acid concentration on dentin demineralization, dehydration, and rehydration: atomic force microscopy study. J Biomed Mater Res 42: 500-507 [DOI] [PubMed] [Google Scholar]
- Marshall GW, Habelitz S, Gallagher R, Balooch M, Balooch G, Marshall SJ. (2001). Nanomechanical properties of hydrated carious human dentin. J Dent Res 80: 1768-1771 [DOI] [PubMed] [Google Scholar]
- Marshall GW, Marshall SJ, Balooch M, Kinney JH. (2004). Evaluating demineralization and mechanical properties of human dentin with AFM. Methods Molec Biol 242:141-159 [DOI] [PubMed] [Google Scholar]
- McComb D. (2000). Caries-detector dyes—how accurate and useful are they? J Can Dent Assoc 66: 195-198 [PubMed] [Google Scholar]
- Ohgushi K, Fusayama T. (1975). Electron microscopic structure of the two layers of carious dentin. J Dent Res 54:1019-1026 [DOI] [PubMed] [Google Scholar]
- Oliveira SS, Pugach MK, Hilton JF, Watanabe LG, Marshall SJ, Marshall GW., Jr (2003). The influence of the dentin smear layer on adhesion: a self-etching primer vs. a total-etch system. Dent Mater 19: 758-767 [DOI] [PubMed] [Google Scholar]
- Oliver WC, Pharr GM. (1992). An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7:1564-1583 [Google Scholar]
- ten Cate JM, Timmer K, Shariati M, Featherstone JD. (1988). Effect of timing of fluoride treatment on enamel de- and remineralization in vitro: a pH-cycling study. Caries Res 22: 20-26 [DOI] [PubMed] [Google Scholar]
- White JM, Goodis HE, Marshall SJ, Marshall GW. (1994). Sterilization of teeth by gamma radiation. J Dent Res 73: 1560-1567 [DOI] [PubMed] [Google Scholar]
- Zacharia MA, Munshi AK. (1995). Microbiological assessment of dentin stained with a caries detector dye. J Clin Pediatr Dent 19:111-115 [PubMed] [Google Scholar]
- Zheng L, Hilton JF, Habelitz S, Marshall SJ, Marshall GW. (2003). Dentin caries activity status related to hardness and elasticity. Eur J Oral Sci 111: 243-252 [DOI] [PubMed] [Google Scholar]