Abstract
The high mortality rate due to ovarian cancer is attributed to the lack of an effective early detection method. Due to the non-specificity of symptoms at early stage, most of the ovarian cancer cases are detected at late stages. This makes the access to women with early stage disease problematic and presents a barrier to development and validation of tests for detection of early stage of ovarian cancer in humans. Animal models are used to elucidate disease etiologies and pathogenesis that are difficult to study in humans. Laying hen is the only available animal that develops ovarian cancer spontaneously; however, detail information on ovarian tumor histology is not available. The goal of this study was to determine the histological features of malignant ovarian tumors in laying hens. A total of 155 young and old (1-5 years of age) laying hens (Gallus domesticus) were selected randomly and evaluated gross and microscopically for the presence of ovarian tumors. Histological classification of tumors with their stages and grades were performed with reference to those for humans. Similar to humans, all four types including serous, endometrioid, mucinous and clear cell or mixed carcinomas were observed in hen ovarian tumors. Some early neoplastic as well as putative ovarian lesions were also observed. Similarities in histology, metastasis and stages of hen ovarian cancer to those of humans demonstrate the feasibility of the hen model for additional delineation of the mechanism underlying ovarian carcinogenesis, preclinical testing of new agents for the prevention and therapy of this disease.
Keywords: ovarian cancer, preclinical model, laying hen, tumor histology
Ovarian cancer (OVCA) is a fatal disease of women with the highest mortality rate of all gynecological malignancies. Approximately 70% of women with OVCA die of this disease (1, 2). Survival is high in women who present with early stage disease(3, 4). The lack of specific symptoms, the relative inaccessibility of the ovaries deep in the pelvis, and the absence of specific marker(s) represent barriers for early detection (5, 6). In most cases, OVCA is diagnosed at a late stage(3). Furthermore, our understanding of the early pathogenesis of OVCA has been hindered by the lack of sufficient number of patients with early stage disease(3, 4, 7). Animal models are used to elucidate disease etiologies and pathogenesis that are difficult to study in humans. Although large domestic mammals including bovine have similar reproductive traits and develop OVCA spontaneously similar to humans, the low incidence rate, multiple pregnancies, longer gestation and lactation period make them an inappropriate model for human OVCA. On the other hand, a number of rodent models, induced or genetically manipulated, have been developed and used successfully to elucidate some aspects of OVCA. However, the non-spontaneous nature of many of these models of OVCA limits their clinical relevance (8, 9). Chickens (Gallus domesticus) are the most widely available avian species and develop spontaneous OVCA with a high incidence rate(10, 11). Therefore, the laying hen is an appropriate animal model for the study of human OVCA.
Commercial egg laying hens (strains of Single Comb White Leghorn) attain sexual maturity (start laying eggs) at 20-22 weeks of age. They reach peak egg production at 30-32 weeks of age (12). Hens maintain a high laying rate (>90%) during the first year of lay (an average hen lays >280 eggs) and then egg production declines slowly indicating a decrease in ovarian function (12). In the chicken, only the left ovary and oviduct become functional. A fully functional left ovary in young healthy laying hens of commercial strains contains 5 or 6 large preovulatory follicles arranged in a hierarchy based on their size (termed hierarchical follicles). The ovulatory cycle in hens ranges from 24-26 hours depending on the age of the hens (e.g., shorter in young laying hens and longer in older hens) (12). Following ovulation of the largest follicle (F1), the second largest follicle becomes the largest, the third one becomes the fourth and so on and a small developing follicle is recruited from the pool into the hierarchy (Fig. 1A). Similar to humans, both the follicular development and ovulatory cycles are under the control of pituitary gonadotrophins and ovarian steroids (12, 13). Following ovulation, the egg passes through the oviduct and the remaining tissue of the ovulated follicle, now called postovulatory follicle, functions as an endocrine organ. Because the laying hen is an oviparous animal, the postovulatory follicle degrades within 3-4 days following ovulation. Therefore, similarities in some features of the reproductive physiology between humans and hens, wide availability and easy accessibility make the hen an extraordinary animal to be explored as a model of human OVCA.
FIGURE 1.
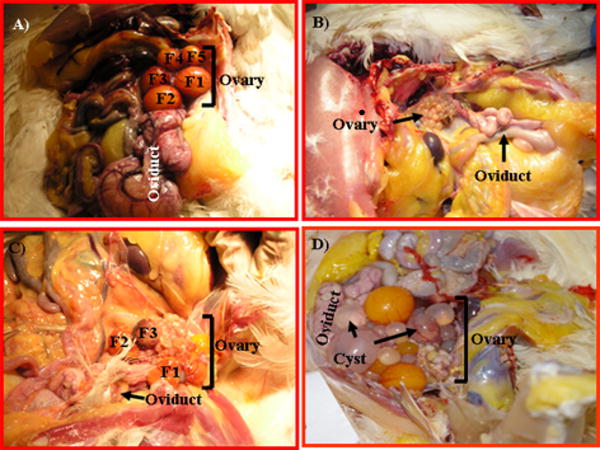
Ovarian morphology in laying hens without obvious tumors. A, Ovary of a normally laying hen. The ovary contains a set of 5 large preovulatory hierarchical (see the text for terminology) follicles (F1-F5) and small developing follicles. B, Ovary of a hen that ceased laying. As compared with the normal ovary, no large preovulatory hierarchical follicles are seen, although a few small follicles are present. The oviduct also regressed in size significantly. The regressed ovary and oviduct indicate that ovarian function is reduced, and this hen is out of egg laying. C, Ovarian failure in a laying hen. Although the ovary contains large preovulatory hierarchical follicles, all of them are either hemorrhagic or atretic. The oviduct appears normal. D, Polycystic ovary syndrome in a laying hen. Although some preovulatory follicles are seen, multiple cysts of varying size with or without hemorrhagic spots are present.
The incidence of spontaneous ovarian carcinoma and its epidemiology in laying hens were first described in two reports 20 years ago; however, those studies were performed from an agricultural interest rather than a biomedical perspective (10, 11). Therefore, both reports lacked detailed information on tumor types and their stages, and as such are irrelevant to clinical applications. As egg production decreases and OVCA incidence increases with aging of hens, poultry farmers seldom maintain hens older than 2 years. Because older hens are not profitable, those reports were largely ignored by the poultry industry. Hence, avian researchers did not pursue studies on OVCA in hens further. However, interest in laying hen OVCA is increasing as a few recent reports have shown it to be similar to human OVCA. Ovarian tumors in hens express several molecular markers including cytokeratin, Epidermal Growth Factor receptor (EGFR), Tag 72, Proliferating Cell Nuclear Antigen (PCNA), TGF-a and CA125 similar to humans(14, 15). Moreover, treatment of hens with progesterone reduced the incidence of OVCA by 40% and progesterone has been suggested as a preventive agent for OVCA in hens (16). This is similar to the epidemiological association of high progesterone states such as pregnancy and birth control pill use with reduced risk of OVCA in humans (17-19). Also, DNA damage to ovarian surface epithelial cells as a result of frequent rupture due to ovulation in hens corresponds to the number of lifetime ovulations in humans (20). Furthermore, similar to humans, hens of different genetic background (strains) have different rates of OVCA incidence (21). However, this information will be of limited value in clinical settings as none of the reports described these OVCA-associated features in hens relative to tumor types and their stages. No detailed report on the tumor stages and histopathological features including tumor types in hens with OVCA and their similarities to human OVCA is available.
Because laying hens are being considered as a feasible preclinical model for testing emerging chemotherapeutic agents, the precise identification of tumor types and their stages will be important for determining drug efficacy. In addition, most of the studies on hen OVCA were performed without distinguishing ovary from oviduct as the primary site. This further corroborates the urgency of a detailed study distinguishing the origin of hen OVCA, tumor types and their stages. All this information will be of enormous help to understand the etiology, pathophysiology, drug testing, and design treatment regimen of OVCA and will form the basis for clinical studies.
Laying hens may also offer an invaluable opportunity to explore the putative precursor lesions related to OVCA. The study of the precursor(s) of ovarian carcinoma in humans is complicated because the ovaries are not readily accessible for screening. Ovarian carcinomas are often large and present in advanced stage, obliterating or rendering unrecognizable any precursor that may have been present. Therefore, the goals of this exploratory study were to classify the histological types of malignant ovarian tumors and their stages in spontaneous OVCA in laying hens. Additionally, putative precursor lesions of OVCA in hens were also examined.
Materials and Methods
Animals
A total of 155 young (n=14, 1 to 1.5 years old with more than 5 eggs in a sequence) and old (n=141, 2 to 5 years old with 3-5 eggs in sequence) Single Comb White Leghorn laying hens (W/96 strain) were reared at the University of Illinois at Urbana-Champaign (UIUC) Poultry Research Farm. Hens were provided with commercial layer ration and water ad libitum and kept under 14h: 10h light and dark regimen. Egg production and mortality rates were recorded on a daily basis. All animal handling and husbandry practices were performed according to the Institutional Animal Care and Use Committee approved protocol.
Gross evaluation
Hens were examined for abnormal ovarian morphology upon euthanasia and following features were noted:
Primary ovarian carcinomas: To distinguish carcinomas of ovarian origin from that of oviductal origin, oviducts of hens were excised and the mucosal layer (inner layer) was examined for the presence of solid tissue masses or tumors. Large tumors and small nodules of solid masses of various sizes were seen in the oviducal mucosal layer in some hens (Fig. 3b and c) and these hens were excluded from the study. Metastatic carcinomas to the ovary were identified by the presence of intact large preovulatory hierarchical follicles. In hens with cancers metastasized to ovary, large preovulatory follicles were mostly unaffected indicating that the entire ovary was not involved by tumor (metastatic) and egg production in these hens was normal. These hens were also excluded from the study.
Tumor staging: Staging of the tumors was performed with reference to the International Federation of Gynecology and Obstetrics (FIGO, Rio de Janeiro 1988) (22) staging Systems for Ovarian Cancer in Humans (Table 1).
Non-tumor ovarian pathology: Non-tumor ovarian abnormalities were characterized by cysts, atretic large preovulatory follicles and regressed ovaries.
FIGURE 3.
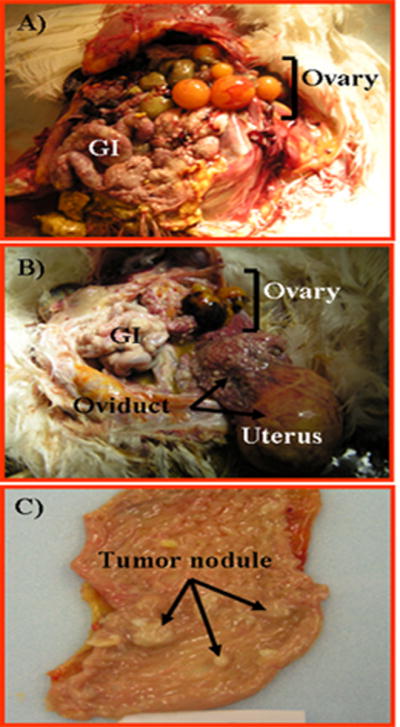
Tumor metastasized to the ovary in hens. A, Tumor of GI tract metastasized to the ovary. Although tumor growth and seeding are seen in the intestine, all the large preovulatory follicles remain uninvolved, and no ascites is seen. B, A case of oviductal tumor metastasized to the ovary. A solid tumor mass is seen in the uterus with the malignant seeding on the upper part of the tract, intestine, and the ovary with the large preovulatory follicle atretic with hemorrhagic spots. C, Early tubal tumor in the reproductive tract. Tumor nodules of various sizes are seen in the magnum (egg albumen–secreting part) of the oviduct.
Table 1.
Staging of ovarian cancer in laying hens
| Stages | Characteristic features |
|---|---|
| Stage I | Growth limited to the ovary; very little or no ascites |
| Stage II | Tumor extended or metastasized to the oviduct, moderate ascites |
| Stage III | Tumor seeding metastasized to the pelvic organs; peritoneal and abdominal implants; gastrointestinal tract and superficial liver metastasis with profuse ascites. |
| Stage IV | Tumor metastasized to distant organs including liver parenchyma and lung; multiple solid tumors in mother organs with profuse ascites. |
Microscopic Pathology
Tumor, non-tumor abnormal and normal ovaries of hens as well as ovarian tissues of women were collected and immediately fixed in 10% buffered formalin. Tissues were processed for routine histology. Sections of 5 μm thickness were cut and stained with hematoxylin and eosin and examined under light microscopy. Tumor types were classified according to the WHO criteria used for human OVCA.
Results
Ovaries of young and some of the old laying hens were more functional as determined by their egg laying rates. Some old hens had reduced ovarian function and laid fewer eggs irregularly whereas some other old hens ceased laying eggs. Physical examination before euthanasia revealed that most of these hens had a distended abdomen which suggested the presence of ascites.
Gross evaluation
Non-tumor ovarian abnormalities
The ovaries of all young (n= 14) and old hens with a higher egg laying rate (n=89, with normal ovaries) had a set of 5 or more large preovulatory hierarchical follicles without gross abnormality of any organ including the ovary and oviduct (Fig. 1A). Occasionally, ovulated ova were present in the oviducts. These hens showed no abnormality in the mucosal layers of the oviducts and therefore were considered healthy and normal. Non-tumor ovarian abnormalities in hens (n= 14) including regressed reproductive tract, atresia of large preovulatory hierarchical follicles and polycystic ovarian abnormalities were determined based on their gross appearance and not on histology. In hens with regressed reproductive tracts, both the ovary and oviduct were fully regressed though the ovaries in some hens contained a few small follicles (Fig. 1B). While regression and rejuvenation of the reproductive tract are common physiological phenomena in hens, regression may also be an earlier stage of OVCA initiation including intraepithelial neoplasia. In some hens, all of the large preovulatory follicles were hemorrhagic and atretic (Fig. 1C). Although atresia of stromal follicles is a natural event both in aves and mammals, atresia of large preovulatory hierarchical follicles is an abnormal condition in laying hens. Multiple cysts of various sizes were seen in the ovary of some laying hens (Fig.1D). Although the presence of one or two cysts in the normal ovaries are not rare but the presence of multiple cysts indicates ovarian abnormality.
Primary ovarian carcinomas and their staging in hens
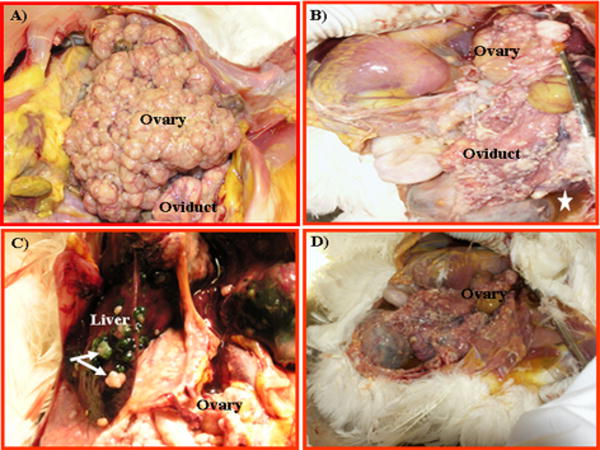
Primary ovarian carcinomas were distinguished from that of secondary carcinoma to the ovary using the criteria described in the Methods section. Staging of ovarian carcinomas in hens was performed with reference to the FIGO system for human OVCA with emphasis on: location of tumors, presence or absence of metastasis and peritoneal ascites. Similar to humans, all four stages (stage I to stage IV) of OVCA were seen in hens (n=30). In hens with Stage I (n=5) OVCA, tumors were confined to the ovary, appeared firm and resembled cauliflower-like nodules with no, or minimal ascites (Fig. 2A). In hens with Stage II (n=5) OVCA, tumors were metastasized to the oviduct with occasional seeding of the pelvic sidewall with moderate ascites (Fig. 2B). In hens with Stage III (n=13) OVCA, tumors were metastasized to both abdominal and peritoneal organs including small and large intestine, mesentery, undersurface of the diaphragm and surface of the liver with moderate to profuse ascites (Fig. 2C). At the time of necropsy, multiple hens had evidence of carcinomatosis and massive ascites consistent with Stage IV (n=7). Tumors at this stage were metastasized to most of the pelvic, abdominal and thoracic organs including liver, spleen and lung (Fig. 2D).
FIGURE 2.
Primary malignant ovarian tumors in hens. A, Stage I ovarian cancer. The tumor is limited to the ovary only with no large preovulatory hierarchical follicles. The solid tissue mass resembles a cauliflower without any noticeable ascites. B, Stage II primary ovarian cancer. The tumor is metastasized to the oviduct with a little ascites, but other organs appear uninvolved (asterisk). C, Stage III primary OVCA. The tumor is metastasized to abdominal organs including the GI tract accompanied by profuse ascites (not seen in the figure). White and greenish tumor seeding is seen on the superficial layer of liver (arrows). D, Stage IV OVCA. The tumor is metastasized to distant organs with profuse ascites. Multiple solid tumor masses are seen.
Metastatic tumor to the ovary (secondary ovarian cancer)
In most of the cases, primary ovarian cancers in hens were associated with atresia of large preovulatory hierarchical follicles. In the present study 8 hens had secondary ovarian carcinoma. In hens with primary gastrointestinal (GI, 3 hens) cancer, only a portion of the ovary appeared solid while the large preovulatory follicles remained uninvolved and the hens were laying regularly (Fig. 3A). The GI tracts in these hens were hardened, coiled, and tumor seeding and masses were found both outside and inside the wall of the tract. In 3 hens, oviducal tumors had metastasized to the ovary and large preovulatory follicles had become atretic appearing as hemorrhagic spots. These tumors had solid masses both on the exterior wall (serous like) and in the mucosal layers of uterus and some seeding on the upper part of the reproductive tract (Fig. 3B). In addition, early oviductal tumors were identified in the excision of oviductal mucosa and they (in 2 hens) were incidental findings (Figure 3C).
Histology
Primary ovarian epithelial carcinomas
Epithelial ovarian carcinomas were classified based on the cellular subtypes and patterns of cellular differentiation with reference to OVCA tumor types in humans. Four histological types of ovarian malignant tumors (n=26, including 18 well differentiated and 8 poorly differentiated tumors) resembling those of human OVCA showing low (G1) or high (G2-3) morphology were observed.
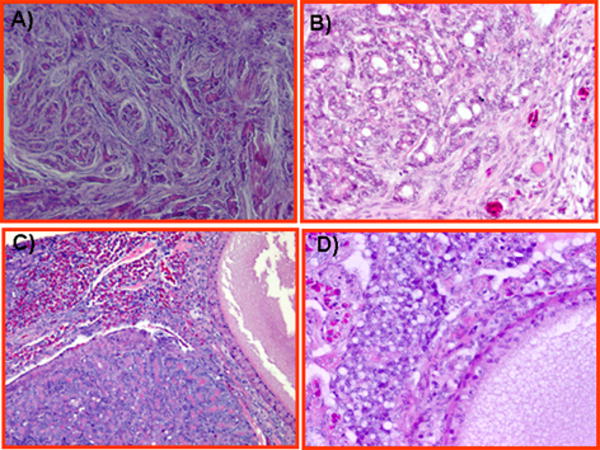
Well differentiated ovarian epithelial tumors (6 serous, 6 endometrioid; 5 mucinous) were found in hens with OVCA. Tumors with features similar to serous ovarian carcinomas in human had marked nuclear atypia and papillary structures. In most cases the architecture was characterized by labyrinth of slit like glands or lacelike papillary folding with large pleomorphic nuclei containing mitotic figures (Fig. 4A). Some of these tumors displayed papillary-like features with fibrovascular cores lined by atypical epithelial cells. Tumors resembling human endometrioid carcinomas were generally characterized by a complex glandular architecture, cribriform foci and nuclear atypia with a brisk mitotic rate. The glands contained a single layer of epithelial cells with mitosis and sharp luminal margins (Fig. 4B). A few cases showed glands lined by columnar epithelium with apparent cytoplasmic mucin compatible with mucinous differentiation. Features reminiscent of human ovarian mucinous carcinomas were observed in hens as crowded glands that merged together without intervening stroma forming clusters surrounded by a fibromascular layer. The tumor displayed columnar epithelium with intercalated ciliated goblet cells. The nuclei were separated from the basement membrane and had moved towards the apical surface with occasional stratification, mitotic figures and luminal secretion (Fig. 4C).
FIGURE 4.
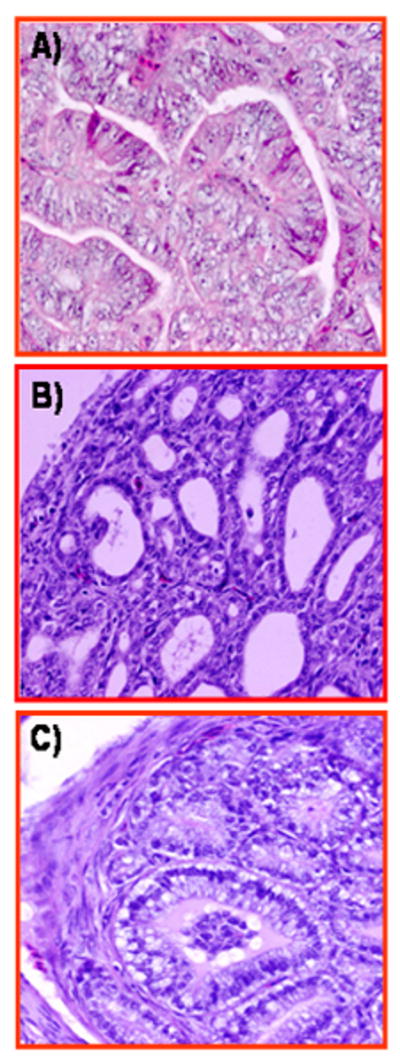
Histological types of well-differentiated malignant ovarian tumors in hens. A, Ovarian serous carcinoma showing sheets of lacelike papillary folding and cells with large pleomorphic nuclei with mitotic bodies. B, Ovarian endometrioid carcinoma in hens with confluent back-to-back glands. Glands contain a single layer of epithelial cells with sharp luminal margin. C, Ovarian mucinous carcinoma with crowded glands in clusters without intervening stroma surrounded by a fibromuscular layer. The epithelium contains columnar and intercalated ciliated goblet cells. The nuclei are separated from the basement membrane and have moved toward the apical surface with occasional stratification and luminal secretion. Hematoxylin and eosin, original magnification ×40.
In addition to low grade (well differentiated) carcinomas, high grade (moderate to poorly differentiated) ovarian tumors were also seen in hen ovaries albeit with low frequency (n=8, 1 serous, 2 endometrioid, 4 mucinous, 2 clear cell). Poorly differentiated carcinoma with serous-like feature displayed extensive solid areas composed of sheets of malignant cells and occasional slit like spaces containing cells with marked nuclear pleomorphism (Fig. 5A). A few tiny glands were present without any papillae. Poorly differentiated endometrioid like carcinomas were characterized by a solid growth pattern with complex glands and microglandular foci (Fig. 5B). Nuclear polymorphism, mitotic activity and necrosis were marked. Poorly differentiated “mucinous like” carcinomas were characterized by confluent microglandular architecture of cribriform foci displaying mucinous cells with marked nuclear atypia and no intervening stroma (Fig. 5C). Several marked eosinophilic foci were also characteristic features of these tumors. In poorly differentiated cancer with “clear cell like” feature, vacuolated cells with abundant clear cytoplasm and pleomorphic nuclei, and a brisk mitotic rate invaded the stroma and theca layer of stromal follicles (Fig. 5D). Deposition of eosinophilic hyalinized matrix in the stroma was also present.
FIGURE 5.
Histological types of poorly differentiated ovarian epithelial carcinoma in hens. A, Poorly differentiated ovarian serous carcinoma showing solid areas composed of slitlike sheets containing cells with high-grade nuclear atypia. A few tiny glands are also seen without any papillae. B, Poorly differentiated ovarian endometrioid carcinomas showing complex glandular and microglandular patterns. Nuclear polymorphism, mitotic activities, and necrosis are marked. C, Poorly differentiated ovarian mucinous carcinomas showing confluent microglandular architecture in a cribriform pattern with a high grade of nuclear atypia and no intervening stroma. Moderate to strong eosinophilic reactions are also seen in the stroma. D, Poorly differentiated ovarian clear cell carcinoma showing vacuolated cells containing high-grade nuclear atypia that invade the stroma and theca layer of stromal follicles. Deposition of eosinophilic hyalinized matrix in the stroma and necrotic bodies are also seen. Hematoxylin and eosin, original magnification ×40.
Malignant mixed tumors (n=4) of two epithelial cell types were also identified. Although mixed “serous” and endometrioid mixed ovarian carcinomas were not seen in this study, “mucinous” and endometrioid or “mucinous” and clear cell mixed carcinomas were found with equal frequency (2 hens with each type) (micrographs are not shown).
Early neoplastic progression
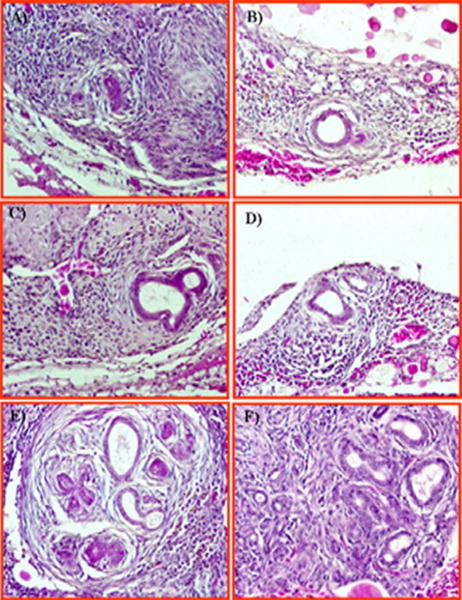
In some hens (n=9) with regressed ovaries, microscopic examination showed microscopic changes consistent with nascent neoplasia and malignant progression leading to tumor development (Fig. 6A-B). These microscopic carcinomas were unanticipated because there were no gross abnormalities. Focal lesions were formed in the stroma below the ovarian surface and appeared as a solid sheet of condensed granules with eosinophilic staining (Fig. 6B). Small cysts with or without outpouches and developing glandular structures with a single layer of epithelial cells with pleomorphic nuclei similar to endometrioid tumors are seen inside the focal lesions in the ovarian stroma of some hens (Fig. 6C-F).
FIGURE 6.
Early neoplastic progression leading to ovarian carcinoma in hen. A, Focal lesions are seen in the stroma below the ovarian surface and appear as a solid sheet of condensed granules with eosinophilic stain. B-D, Cysts developed from focal lesions are seen as dividing cells and out-pouches. E, Multiple cystic division and formation of additional condensed eosinophilic glands. F, Cysts lined with a single layer of epithelial cells arising from focal lesions appear to be an early stage of ovarian endometrioid neoplasm with pleomorphic nuclei neoplasm. Hematoxylin and eosin, original magnification ×40.
Putative precursor lesions
Microscopic evaluations of the ovaries of some hens (n=5) with non-tumor ovarian abnormalities (no grossly visible tumor) revealed a spectrum of histological abnormalities which are similar to those observed in the vicinity of m alignant ovaries. These microscopic abnormalities were similar to those described as tumor associated putative precursor lesions in humans. The normal ovarian epithelial layer in hens consists of a single layer of columnar epithelial cells. However, these columnar epithelial cells in several hens with non-tumor ovarian abnormalities had a rounded phenotype with mitotic figures were present suggesting a pre-malignancy (Figure 7A). Marked epithelial dysplasia with stromal invaginations was seen in ovaries of a few of these hens with non-tumor ovarian abnormalities (Fig. 7B). Simple glands lined by a single layer of rounded epithelial cells in the cortex beneath the ovarian surface were commonly seen in hens with non-tumor ovarian abnormalities (Fig.7C).
FIGURE 7.
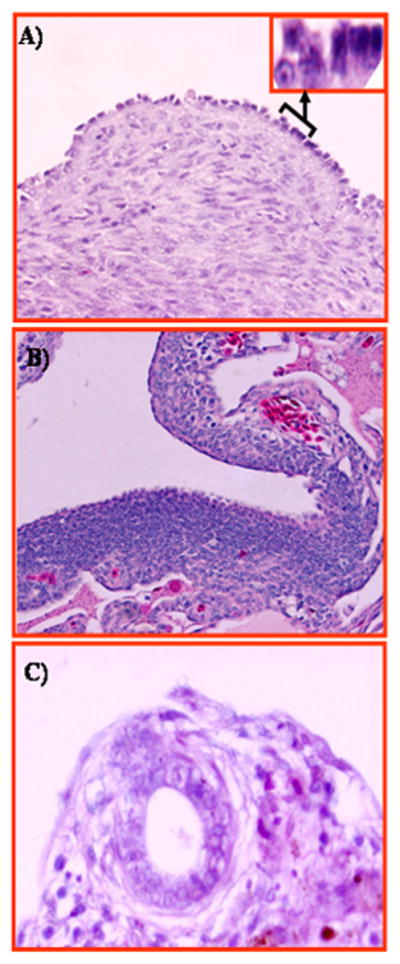
Figure 7. Putative precursor lesions of ovarian carcinoma in hens. A, Section of hen ovary with non-tumor abnormalities showing transformation of the ovarian surface epithelial layer from single columnar epithelial cells to a rounded phenotype with mitotic nuclei consistent with a malignant potential. Inset showing magnified (120×) view of transformed and normal surface epithelial cells. B, Section of hen ovary with non-tumor abnormalities. Marked epithelial dysplasia is seen in the surface layer with stromal invaginations. C, Section of hen ovary with non-tumor abnormalities showing inclusion cysts in the cortex beneath the ovarian surface epithelium containing epithelial cells of rounded phenotype. H &E, 40×.
Discussion
This report is the first detailed and comprehensive review of the histological types and characteristic features of OVCA staging in laying hens, a spontaneous model for human OVCA. Some of the putative preneoplastic ovarian lesions in laying hens were also demonstrated in this study. The findings of the present study show remarkable similarities in the histological types and stages of epithelial tumors of the ovary as well as their putative precursor lesions of OVCA in hens to that in humans.
Dissimilarities in the histopathology of OVCA between rodents and humans limit the use of rodents as an animal model of human OVCA which is also the reason for exploring new animal models in which OVCA has a similar histopathology to those seen in humans. Similarities in the association of OVCA with circulating anti-tumor antibodies (23) and the similar expression of some OVCA markers between hens and humans (14, 15) has led us to study whether histological types of hen ovarian tumors resemble those of humans. Four histological types of hen OVCA including: serous, endometrioid, mucinous, clear cell and their differentiation (Grades 1, 2, 3) are somewhat similar to their human counterparts. Ovarian tumors of mixed histopathology (two histotypes in the same specimen) were also observed in some hens. Similarities in tumor histology will facilitate the use of laying hens to improve our understanding of tumor biology in humans, explore new drugs to develop treatment modalities or to improve existing ones. Moreover, the ovary is a complex organ and its tumor types are varied. Because ovarian tumors are relatively uncommon and include several types, they are difficult to diagnose without proper experience. Thus the histologic diagnosis may therefore be compromised. Therefore, hen ovarian tumors, in addition to preclinical drug testing may also contribute to our comprehension of OVCA histopathology and diagnosis of OVCA as they are similar to humans.
Similar to histological types, tumor staging plays a key role in devising the treatment path and much is unknown about the specifics of effective drug therapy in relation to OVCA stages. In the current study, hen ovarian tumors were staged according to the FIGO classification for humans. Similar to humans, all four stages (stage I to stage IV) of tumor progression ranging from confinement in the ovary to distant metastases were observed in hens with OVCA. One of the most intriguing similarities between hen and humans is the association of advanced stage OVCA with profuse ascites. Because the laying hen has only one functional ovary, the staging criteria relative to the contra-lateral ovary in humans is not applicable in hens. Nonetheless, the laying hen can be utilized to determine the stage related efficacy and specificity of drugs with their prognostic value and can constitute the basis of clinical studies.
The discovery of microscopic malignant tumors in regressed ovaries (which functionally resemble the postmenopausal ovary in women) was of interest. These tumors were not anticipated and not uncovered until extensive microscopic examination of sections made from all areas of the ovary. This observation suggests that a perfunctory analysis of prophylactically removed ovaries could, in some cases, fail to detect small malignant tumors. If such were the case and these tumors had acquired early metastasizing potential, metastatic cells that metastasized from these tumors could explain the discovery of peritoneal carcinomatosis subsequent to prophylactic oophorectomy (24-26). These abnormalities can be identified and treated in the early stages of carcinogenesis, in order to prevent the development of invasive cancer.
One of the challenges related to the early detection and prevention of ovarian cancer has been the uncertainty as to whether a premalignant or precursor lesion in the pathway to the development of clinical OVCA exists. In other organ systems, such lesions or well-defined series of morphologic changes are recognized to occur that are critical to the success of early detection programs (e.g., cervical intraepithelial neoplasia for carcinoma of uterine cervix, ductal carcinoma in situ for breast carcinoma and advanced adenomatous polyps in colorectal cancer) (27-29). The candidates for the precursors of ovarian cancer include epithelial dysplasia of the surface epithelium or germinal inclusion cysts. Alternatively, carcinomas could also arise directly from the surface epithelium without an intermediate precursor lesion (25). It is conceivable that all these mechanisms account for ovarian carcinomas. As reported in humans, a series of putative precursor lesions like surface epithelial transformation, inclusion cysts and epithelial dysplasia were seen in hens in the present study. The identification of a premalignant lesion may improve the effectiveness of early detection screening. Therefore, the laying hen may also provide a better understanding of the putative precursor lesions leading to OVCA.
The controversy regarding the existence of morphologic precursors may in a large part be due to the fact that ovarian cancer is most frequently diagnosed at a late stage. Hence, the opportunity to examine a large number of early stage ovarian cancers in which it might be possible to see these changes repeatedly and document a consistent pattern of transition between benign and malignant ovarian surface epithelium is rare. This lack of information in turn has obviously constrained our understanding as to the most frequent sequence of morphologic changes that occur as clinical ovarian cancers develop. Moreover, the morphological precursors of clinical ovarian cancer in humans are not well established. Previous investigations with OVCA patients aimed at defining the types of lesions that lead to ovarian cancer have involved a variety of approaches, including the examination of the contralateral ovaries in patients with unilateral ovarian cancer or ovaries that contain stage I tumors (30-32). We believe this study complements previous investigations that some precursor lesions precede OVCA and cancers of other organs in humans as well as in hens. Through access to hens, their ovaries can be examined in vivo where there is a very high probability that malignant transformation will occur sometime during the animal's lifetime. For the first time, we developed in vivo imaging of hen ovaries by transvaginal grey scale and Doppler ultrasound which allows us to detect very early lesions based on their Doppler blood flow velocity indices (33). Therefore, the laying hen also offers a unique opportunity for preclinical development of an effective early detection of OVCA by evaluating changes in the tissue morphology in association with changes in ovarian vascularity.
One of the few limitations of this study is that hens with a low egg laying rate were selected and thus this study did not represent a totally blinded study. From our previous experience, we know that hens with reduced (or ceased) egg production are more prone to develop primary OVCA than those of high laying rate. This study was not intended to report the incidence rate of spontaneous OVCA in hens but our goal was to define the histological types of ovarian tumors and their stages. Therefore, we decided to obtain as many hens with potential OVCA as possible. In addition, we could not clearly determine all the sub-stages within a stage in hens as can be done in humans. One of the reasons for this is that laying hens do not possess a right ovary and hence staging (sub-stages of Stage I and II) in relation to the status of the contra-lateral ovary is not possible. Moreover, the lymph nodes in chicken are not as well organized as in humans and hence the nodal involvement in hen OVCA metastasis was not confirmed. Nevertheless these limitations do not reduce the feasibility of this model because sub-stages within a stage do not generally constitute significant differences either in diagnosis or drug efficacy.
In conclusion, this study confirmed that ovarian cancer in hens occurs spontaneously and demonstrated that their histological types as well as stages are similar to humans. This study additionally showed that similar to humans, several precursor lesions also exist in hens. The similarity in histology, metastasis and stages of hen OVCA to those of humans demonstrates the feasibility of the hen model for additional delineation of the mechanism underlying ovarian carcinogenesis. The laying hen model could be used for preclinical testing of new agents for the prevention and therapy of this disease. Thus this study will contribute to the establishment of laying hen as the preclinical model of human ovarian cancer.
Acknowledgments
This study was supported by NIH R01AI055060 (JL), the Daniel F. and Ada L. Rice Foundation (JL), the Ovarian Cancer Survivor Network (JL), POCRC, SPORE # P50 CA83636, Joy Piccolo O'Connell/Gravers Award, Segal Family Foundation, and DOD OC073325 (JL) and OC050091 (DBH).
References
- 1.Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Goodman MT, Howe HL. Descriptive epidemiology of ovarian cancer in the United States, 1992-1997. Cancer. 2003;97(10 Suppl):2615–30. doi: 10.1002/cncr.11339. [DOI] [PubMed] [Google Scholar]
- 3.Goodman MT, et al. Stage at diagnosis of ovarian cancer in the United States, 1992-1997. Cancer. 2003;97(10 Suppl):2648–59. doi: 10.1002/cncr.11347. [DOI] [PubMed] [Google Scholar]
- 4.Ries LA. Ovarian cancer. Survival and treatment differences by age. Cancer. 1993;71(2 Suppl):524–9. doi: 10.1002/cncr.2820710206. [DOI] [PubMed] [Google Scholar]
- 5.Bast RC, Jr, et al. Early detection of ovarian cancer: promise and reality. Cancer Treat Res. 2002;107:61–97. doi: 10.1007/978-1-4757-3587-1_3. [DOI] [PubMed] [Google Scholar]
- 6.Pepe MS, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93(14):1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 7.Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19(1):3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Stakleff KD, Von Gruenigen VE. Rodent models for ovarian cancer research. Int J Gynecol Cancer. 2003;13(4):405–12. doi: 10.1046/j.1525-1438.2003.13317.x. [DOI] [PubMed] [Google Scholar]
- 9.Vanderhyden BC, Shaw TJ, Ethier JF. Animal models of ovarian cancer. Reprod Biol Endocrinol. 2003;1:67. doi: 10.1186/1477-7827-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damjanov I. Ovarian tumours in laboratory and domestic animals. Curr Top Pathol. 1989;78:1–10. doi: 10.1007/978-3-642-74011-4_1. [DOI] [PubMed] [Google Scholar]
- 11.Fredrickson TN. Ovarian tumors of the hen. Environ Health Perspect. 1987;73:35–51. doi: 10.1289/ehp.877335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahr JM, Palmar SS. The influence of aging on ovarian function. CRC Critical Reviews in Poultry Biology. 1989;2(2):103–10. [Google Scholar]
- 13.Robinson FE, Etches RJ. Ovarian steroidogenesis during follicular maturation in the domestic fowl (Gallus domesticus) Biol Reprod. 1986;35(5):1096–105. doi: 10.1095/biolreprod35.5.1096. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Burford C, et al. Immunohistochemical expression of molecular markers in an avian model: a potential model for preclinical evaluation of agents for ovarian cancer chemoprevention. Gynecol Oncol. 2001;81(3):373–9. doi: 10.1006/gyno.2001.6191. [DOI] [PubMed] [Google Scholar]
- 15.Jackson E, et al. CA125 expression in spontaneous ovarian adenocarcinomas from laying hens. Gynecol Oncol. 2007;104(1):192–8. doi: 10.1016/j.ygyno.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Barnes MN, et al. A pilot study of ovarian cancer chemoprevention using medroxyprogesterone acetate in an avian model of spontaneous ovarian carcinogenesis. Gynecol Oncol. 2002;87(1):57–63. doi: 10.1006/gyno.2002.6806. [DOI] [PubMed] [Google Scholar]
- 17.Ness RB, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155(3):217–24. doi: 10.1093/aje/155.3.217. [DOI] [PubMed] [Google Scholar]
- 18.Runnebaum IB, Stickeler E. Epidemiological and molecular aspects of ovarian cancer risk. J Cancer Res Clin Oncol. 2001;127(2):73–9. doi: 10.1007/s004320000153. [DOI] [PubMed] [Google Scholar]
- 19.Terry KL, et al. Genetic variation in the progesterone receptor gene and ovarian cancer risk. Am J Epidemiol. 2005;161(5):442–51. doi: 10.1093/aje/kwi064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch WJ, Van Kirk EA, Alexander BM. DNA damages in ovarian surface epithelial cells of ovulatory hens. Exp Biol Med (Maywood) 2005;230(6):429–33. doi: 10.1177/15353702-0323006-11. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PA, Giles JR. Use of genetic strains of chickens in studies of ovarian cancer. Poult Sci. 2006;85(2):246–50. doi: 10.1093/ps/85.2.246. [DOI] [PubMed] [Google Scholar]
- 22.P S. FIGO Annual report on the results of treatment in gynecological cancer. J Epidemiol Biostat. 1998;3:1–168. [Google Scholar]
- 23.Barua A, et al. Anti-tumor antibodies and ovarian cancer in women and hens. American Association of Cancer Research: Molecular Diagnostics in Cancer Therapeutic Development, Maximizing Oppotunities for Individualized Treatment; Sept. 12-15, 2006; Chicago, IL. 2006. p. 40. Scholar-in-Training Award Winner, ( http://www.aacrmeetingabstracts.org/cgi/content/abstract/2006/2/A40) [Google Scholar]
- 24.Tobacman JK, et al. Intra-abdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2(8302):795–7. doi: 10.1016/s0140-6736(82)92681-2. [DOI] [PubMed] [Google Scholar]
- 25.Salazar H, et al. Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst. 1996;88(24):1810–20. doi: 10.1093/jnci/88.24.1810. [DOI] [PubMed] [Google Scholar]
- 26.Chen KT, Schooley JL, Flam MS. Peritoneal carcinomatosis after prophylactic oophorectomy in familial ovarian cancer syndrome. Obstet Gynecol. 1985;66(3 Suppl):93S–94S. [PubMed] [Google Scholar]
- 27.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 28.Castilla LH, et al. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994;8(4):387–91. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- 29.Wright TC, Kurman RJ, F A. Precancerous lesion of the cervix. In: K RJ, editor. Blaustein's Pathology of the female genetal tract. 5th. New York: Springer; 2001. pp. 253–324. [Google Scholar]
- 30.Mittal KR, et al. Contralateral ovary in unilateral ovarian carcinoma: a search for preneoplastic lesions. Int J Gynecol Pathol. 1993;12(1):59–63. doi: 10.1097/00004347-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Resta L, et al. Morphologic precursors of ovarian epithelial tumors. Obstet Gynecol. 1993;82(2):181–6. [PubMed] [Google Scholar]
- 32.Deligdisch L, et al. Ovarian dysplasia in epithelial inclusion cysts. A morphometric approach using neural networks. Cancer. 1995;76(6):1027–34. doi: 10.1002/1097-0142(19950915)76:6<1027::aid-cncr2820760617>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Barua A, et al. Detection of ovarian tumors in chicken by sonography: a step toward early diagnosis in humans. J Ultrasound Med. 2007;26(7):909–19. doi: 10.7863/jum.2007.26.7.909. [DOI] [PubMed] [Google Scholar]