Abstract
Objective
To review the maturational events that occur during prenatal and postnatal brain development and to present neuroimaging findings from studies of healthy individuals that identify the trajectories of normal brain development.
Method
Histological and postmortem findings of early brain development are presented, followed by a discussion of anatomical, diffusion tensor, proton spectroscopy, and functional imaging findings from studies of healthy individuals, with special emphasis on longitudinal data.
Results
Early brain development occurs through a sequence of major events, beginning with the formation of the neural tube and ending with myelination. Brain development at a macroscopic level typically proceeds first in sensorimotor areas, spreading subsequently and progressively into dorsal and parietal, superior temporal, and dorsolateral prefrontal cortices throughout later childhood and adolescence. These patterns of anatomical development parallel increasing activity in frontal cortices that subserves the development of higher-order cognitive functions during late childhood and adolescence. Disturbances in these developmental patterns seem to be involved centrally in the pathogenesis of various childhood psychiatric disorders including childhood-onset schizophrenia, attention-deficit/hyperactivity disorder, developmental dyslexia, Tourette’s syndrome, and bipolar disorder.
Conclusions
Advances in imaging techniques have enhanced our understanding of normal developmental trajectories in the brain, which may improve insight into the abnormal patterns of development in various childhood psychiatric disorders.
Keywords: normal brain development, neuroimaging, functional neuroimaging, cognitive development
Major advances in neuroimaging methods during the past 2 decades now permit detailed study of the maturation of the human brain. We aim to provide a review of the evidence for changes in brain structure and function that occur with advancing age in healthy individuals. The delineation of these normal maturational trajectories provides an invaluable and necessary template from which to identify deviant patterns of brain development in children who have neuropsychiatric disorders. We review the data for maturation of brain structure from fetal life through senescence. We then summarize findings from functional imaging studies that have assessed age-related changes in activity in neural systems that subserve higher-order cognitive functions throughout childhood and adulthood, particularly the development of the capacities for language development and for cognitive and emotional control.
IMAGING THE MAJOR EVENTS OF EARLY BRAIN DEVELOPMENT
Much of what we know about fetal and early postnatal brain development has been extrapolated either from histological studies in rodents or from sparse postmortem and imaging data in human and nonhuman primates. Practical and ethical concerns, as well as methodological limitations, have constrained studies of early development in both humans and primates.1,2 Because imaging data in the developing fetus, infant, and young child are so sparse, we review briefly what is known about the cellular bases of brain development during these ages because it helps to inform interpretation of what has been reported in imaging studies of early brain development.
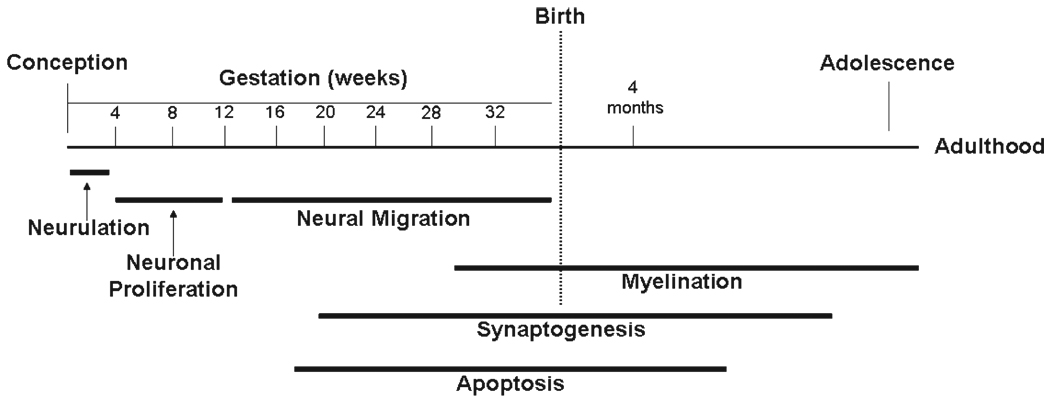
The central nervous system begins to develop in the human fetus 2 to 3 weeks after conception through the folding and fusion of ectoderm to create the neural tube.3 Around the fifth week of gestation, the neural tube closes and ectodermal tissues begin to differentiate into precursors of various brain structures according to their longitudinal and circumferential locations.2 The subsequent major maturational events of early brain development include the birth and death of neurons, neuronal migration, the elaboration and pruning of axons and dendritic arbors, the formation and elimination of synaptic contacts between neurons, metabolic and molecular interactions of neurons with glia, and myelination of axons. These are dynamic processes that have their own unique maturational time tables within and across brain regions (Fig. 1).
Fig. 1.
Major events during brain development. Brain development proceeds in a sequence that begins with neurulation, followed by neuronal proliferation, neural migration, and apoptosis. The sequence ends with synaptogenesis and myelination, which continue into adulthood. Reprinted with permission from the American Journal of Psychiatry (Copyright 1999), American Psychiatric Association.4
By the eighth week of gestation, stem cells in the ventricular (also called proliferative) zone, located adjacent to the precursor of the lateral ventricles, differentiate into the site for the division and origin of cortical and subcortical neurons.5–7 Between weeks 12 and 20 of gestation, neurons migrate from the ventricular zone and a newly formed adjacent region called the subventricular zone along a scaffolding of glial cells toward their destinations in the cortex (Fig. 2).8,9 Preliminary application of magnetic resonance imaging (MRI) to the study of human fetuses has shown visible evidence for the migration of neurons during the first half of gestation.10,11
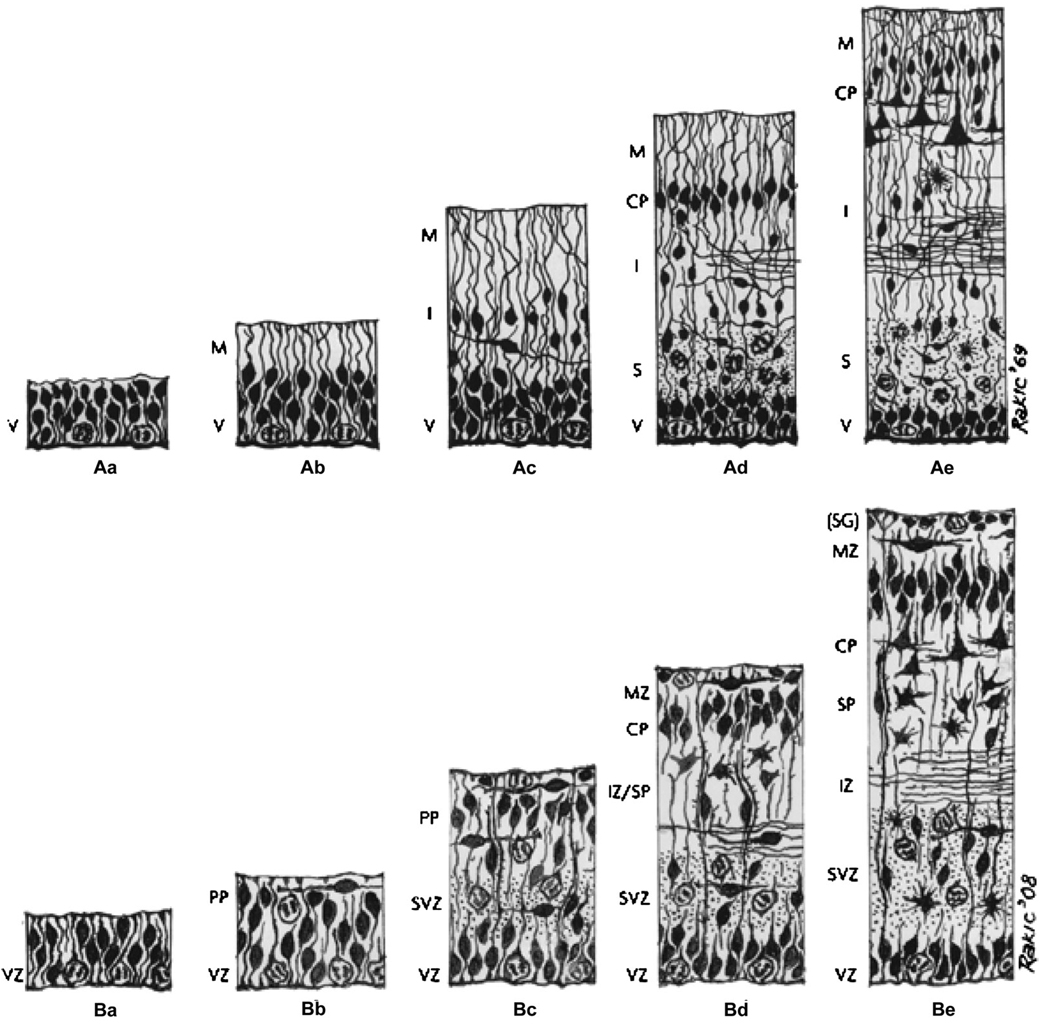
Fig. 2.
The Boulder Committee’s original 1970 model of human neocortical development and a 2008 revision. A, The Boulder Committee’s original summary diagram of neocortical development. B, A revised version published by Bystron et al in 2008. The figure depicts the sequence of developmental events at (a) embryonic day (E) 30, (b) E31–32, (c) E45, (d) E55. V/VZ = ventricular zone; M/MZ = marginal zone; I/IZ = intermediate zone; CP = cortical plate; S = subplate; PP = preplate; SVZ = subventricular zone; SG = subpial granular layer (part of the MZ). Reprinted with permission from Nature Publishing Group Macmillan Publishers Ltd.8
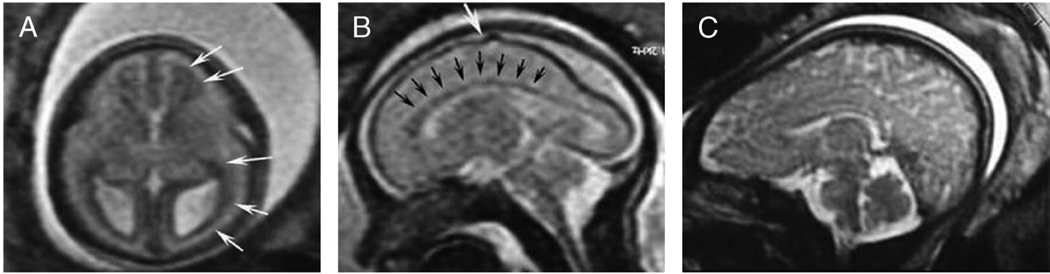
Between 8 and 16 weeks’ gestational age (GA), migrating neurons from the proliferative zones transiently connect to the subplate, a “waiting station” located directly underneath (i.e., on the ventricular side) of the cortical plate, the region that will become the cerebral cortex. Neurons in the subplate receive connections from afferent neurons originating in the thalamus, basal forebrain, and brainstem, temporary connections that are generated ahead of the correct cortical targets for these neural projections.2,12–16 At 17 weeks’ GA, this layer appears on human fetal MRI as a transient layer visible beneath the cortex (Fig. 3A).10 By 20 weeks’ GA, axons within the subplate break their connections there and form new, more permanent synapses within the cortical plate, thereby triggering the death of subplate neurons. This refinement of cortical connections reaches its peak at 24 to 28 weeks’ GA16 and coincides with increased functional organization of cortical circuits, as quantified by electrophysiological measurements in human infants.17 Simultaneously, the subplate ceases to appear on fetal MRI scans.10 Neurons in the cortical plate organize into vertical layers that are distinguishable by microscopic examination of postmortem tissue but that, thus far, have not been visualized successfully by in vivo imaging techniques. Starting around 20 weeks’ GA in humans, sulci and gyri become visible on fetal MRI (Fig. 3B).10
Fig. 3.
Fetus development during gestation and at full-term. A, At 17 weeks of gestation, a wide T2-hypointense band along the ventricles corresponds to the germinal matrix (white arrows). The brain is agyric. B, At 23 weeks of gestation, the germinal matrix is thinned (black arrows), and the first indentation of the cerebral sulcus is visible (white arrow). C, At full term, maximal infolding of the brain surface occurs and myelination advances.
The number of neurons in the human brain peaks around the 28th week of gestation at levels 40% greater than in the adult. Dendritic growth and arborization begin to accelerate rapidly at this time, 1,18–26 which corresponds with the disappearance of the proliferative zone and cortical subplate on MRI and with an increase in cortical thickness visible on both postmortem analysis and MRI (Fig. 3).10 Studies of the timing of dendritic growth and synaptogenesis across different brain regions have yielded variable findings. Studies of postmortem human brains have suggested that development in the frontal cortex is later than in more posterior regions of the brain, such as the visual cortex.27,28 In contrast, quantitative analyses of synaptogenesis in the motor, somatosensory, visual, prefrontal, and limbic cortices of macaque monkeys and human fetuses have indicated that the course of development is similar across these regions, particularly during the time of increasing synaptogenesis.29–33 Arborization of dendrites and axons is not visible directly on MRI, but they are believed to account for a significant portion of the increase in thickness of the cortex with age. The rate of synaptogenesis also increases rapidly at this time, peaking after the 34th week of gestation in humans at a rate of 40,000 new synapses per second.2 Synaptogenesis continues in postnatal life, although as the increasing rate of synapse elimination begins to overtake the declining rate of synapse formation, the net number of synapses begins to decrease at puberty.1,18,24–26 Age- related changes in positron emission tomography (PET) measures of glucose metabolism seem to parallel the rate of synaptogenesis in humans34,35 and in nonhuman primates, 36 suggesting that PET studies may provide an indirect measure of this neuronal process.
Myelination is accompanied by the proliferation and differentiation of oligodendrocytes that are necessary for neuronal insulation and metabolism. Myelination of the optic radiations and occipital white matter begins 1 to 2 months before birth in humans and extends gradually to the frontal lobe by 9 months’ postnatal age.2,37 Myelination in the cortex proceeds in a posterior-to-anterior direction and seems to follow maturation of functional circuits, with sensory pathways myelinating first, followed by motor pathways, and finally by as-sociation areas.28
Apoptosis (programmed cell death) and synaptic elimination are distinct processes that play important roles in the development of the primate brain during the second half of gestation and through the first few years of life.2 The exact timing and dynamics of these processes in humans are unknown, and in fact, no longitudinal studies of synapse formation or regression in humans have been reported. Consequently, whether synaptogenesis and the elimination of cells and synapses counterbalance one another between 2 and 7 years of age during a so-called plateau phase,2 or whether the net number of synapses increases through these years and peaks between 4 and 6 years, is unclear.38 What does seem clear, however, is that the regression of synapses predominates thereafter, being particularly prominent throughout adolescence.2 Synaptic pruning is currently not visible directly with neuroimaging, although the prominent thinning of frontal and parietal cortices during adolescence is thought likely to be attributable to synaptic pruning.1,39 Pathological synaptic pruning may contribute to the genesis of at least some psychiatric disorders, although this hypothesis has not yet been confirmed empirically.2,33,40
Studies of early human and nonhuman brain development have been conducted in temporal cross sections and in small samples. The methods of study have included postmortem analyses of the brains of fetuses and infants1,18,27,41–44 and PET measures of regional metabolism measures of regional metabolism.34–36 Studies of nonhuman primates have also used3 H-thymidine autoradiography, systematic autopsy of primate brains with neuronal labeling at various stages of development, and occasional analyses of molecular mediators.33 Each of these methods has significant limitations. Postmortem studies, by their very nature, and PET studies, because of the practical and ethical difficulties of repeated scanning in children, have been static and cross-sectional, although their interpretations are extrapolated to describe an ongoing dynamic, longitudinal process. Although the safety of MRI offers the promise of longitudinal studies, the number of repeated measures in fetal and early postnatal life has been small to date. The tedious and costly nature of all of these techniques has yielded small sample sizes and, given the large between-subject variation in most brain measures, has likely compromised the stability and applicability of most of the findings thus far for understanding the patterns of early brain maturation within individuals.
ANATOMICAL MRI STUDIES IN CHILDREN AND ADOLESCENTS
The first anatomical MRI studies of healthy brain development used techniques to measure regional brain volumes that first divide the brain into anatomical regions having presumably differing functional characteristics (e.g., the frontal and parietal lobes, or the hippocampus and amygdala) and then correlated the volumes of those regions with age. Findings from these cross-sectional studies revealed that volumes of cortical and subcortical gray matter decreased from childhood to adulthood when the effects of increasing whole-brain volumes with age were controlled.45,46 Other findings, however, indicated that portions of the temporal lobe, including the amygdala and hippocampus, increase in size through adolescence.47 Nevertheless, findings of gray matter reductions, coupled with simultaneous increases in white matter volumes, suggest that continued myelination during childhood and adolescence48 may account for age-related increases in white matter. Increasing myelination could also contribute to the decreases in gray matter volumes observed with advancing age if the myelination encroached on tissue at the periphery of the brain that was previously unmyelinated, 39 thereby reducing the amount of tissue that appears gray as opposed to white on MRI. Alternatively and perhaps more plausibly, changes in gray matter volumes with age may be caused by extensive synaptic pruning, consistent with the decreases in synaptic density that are reported to occur between 2 and 16 years of age.27,44
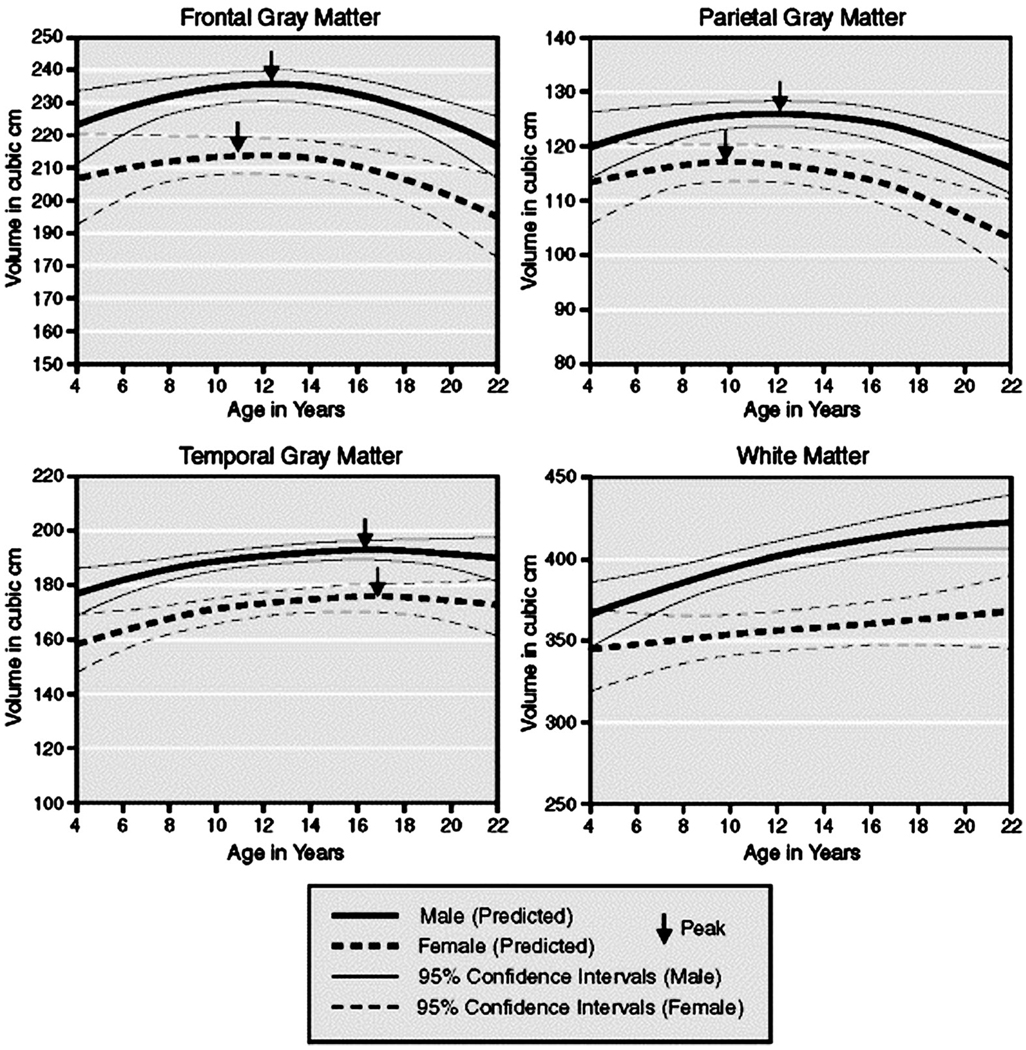
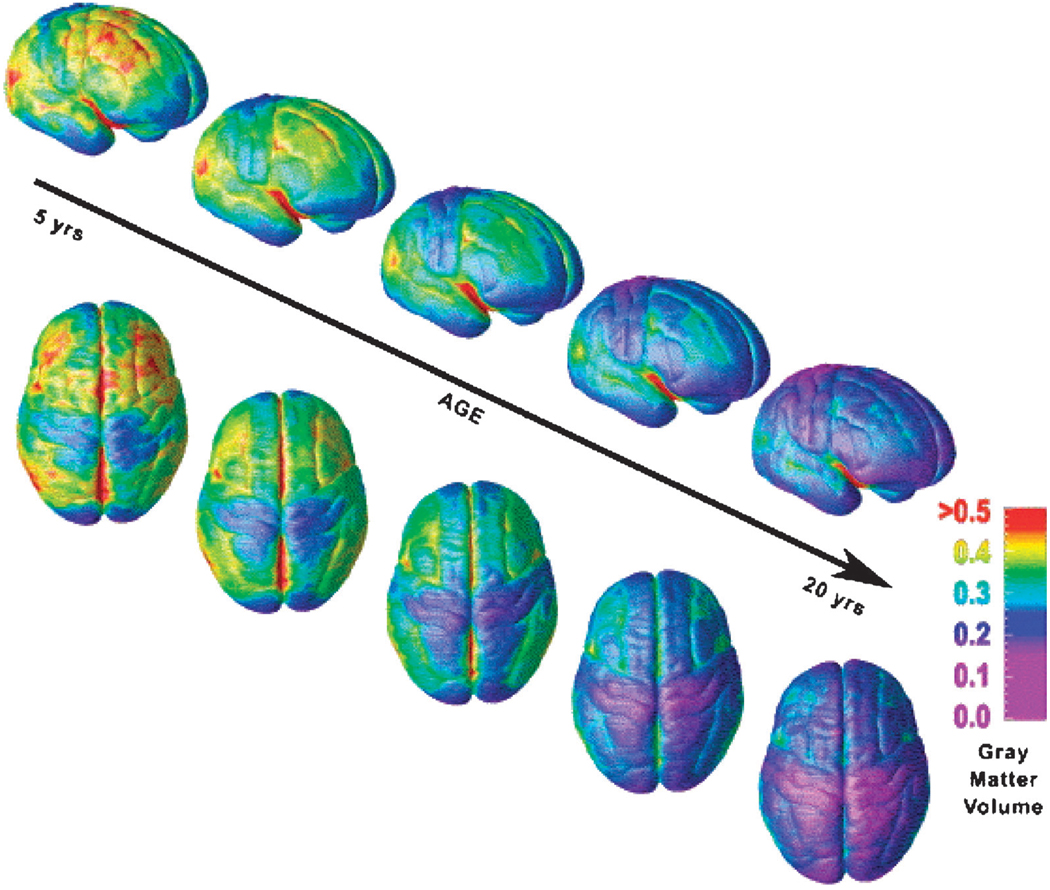
One of the first longitudinal studies of regional brain volumes in children was conducted by investigators in the Child Psychiatry Branch of the NIMH. Growth curves of volumes in various cerebral lobes were compiled for 145 children scanned at approximately 2-year intervals.49 These data included two scans each from 65 children, three scans each from 30 children, four scans from two children, and five scans from one child. Linear increases in white matter volume up to age 20 years were detected in all brain regions, whereas quadratic effects of age were detected for cortical gray matter volumes in frontal, parietal, and temporal lobes, indicating that gray matter volumes in those regions followed an “inverted U-shaped” developmental curve. Gray matter volumes increased before adolescence, and then they peaked at approximately 12 years of age in frontal and parietal cortices and at 16 years of age in the temporal lobe. Gray matter volumes decreased in all regions thereafter (Fig. 4). Although an oft-cited finding, the differences between these developmental peaks have not been assessed statistically and therefore require further study and confirmation.
Fig. 4.
Growth curves of gray and white matter volumes. Shown here is the predicted size with 95% confidence intervals for cortical gray matter in frontal, parietal, and temporal lobes. The arrows indicate the reported peak volumes in males and females from a study that included 243 scans from 89 males and 56 females, aged 4 to 22 years. Reprinted with permission from Nature Publishing Group, Macmillan Publishers Ltd.49
A more recent study from the same group of investigators used a finer-grained morphological analysis to quantify cortical development.50 Gray matter density was measured point by point on the surface of the brain in each lobe. A map was then constructed using 52 MRI scans from 13 children who were scanned at 2-year intervals during a 10-year period (Fig. 5). Changes in gray matter density, or the proportion of gray matter within a given volume of cortical tissue, were captured in a time-lapse movie for data acquired between 4 and 21 years of age (http://www.loni.ucla.edu/∼thompson/DEVEL/dynamic.html). These dynamic analyses showed that cortical thickness generally decreases with advancing age in “back-to-front” progression. Thinning of gray matter occurred first in sensorimotor areas, spreading subsequently and progressively into dorsal and parietal, superior temporal, and dorsolateral prefrontal cortices throughout later childhood and adolescence. Thus, cortical areas that subserve sensorimotor processes mature earliest, whereas those that mediate higher-order functions mature last. These findings are remarkably consistent with those reported in a cross-sectional study over a wider age range.51 The cortical thinning was interpreted as a likely consequence of the pruning of neural connections that has been documented in animal models of development.52 Unlike the prior study that defined coarse lobar divisions, however, this study used a finer-grained method of morphological analysis, although of a largely overlapping data set. It did not report age-related “peaks” in mean global volumes of gray matter but instead reported steady decreases in cortical density across adolescent development. The NIMH investigators who conducted these studies explained that, with more spatiotemporal detail, this newer method detected maturational trajectories that varied temporally across brain subregions and likely was responsible for the differing rates of change in these subregions from those reported in their prior volumetric study. When lobar volumes were measured, rates of growth and loss were likely averaged over large expanses of tissue, and this averaging may have distorted measures of overall rates of change within brain subregions. Nevertheless, subsequent studies by the same group of investigators applied complex growth models to the same overlapping data set and reported peaks in cortical thickness, with primary sensorimotor areas attaining their peak thickness before secondary areas, followed by higher-order association areas.53,54
Fig. 5.
Right lateral and dorsal views of the dynamic sequence of gray matter maturation over the cortical surface of the brain. This sequence was constructed from 52 MRI scans from 13 subjects who were scanned every 2 years during a 10-year period from ages 4 to 21 years. Red indicates more gray matter; blue, less gray matter. Gray matter wanes in a back-to-front wave as the brain matures and neural connections are pruned. Sensorimotor areas that subserve more basic functions mature earlier, whereas superior temporal and dorsolateral prefrontal areas that subserve higher-order functions mature later. MRI = magnetic resonance imaging. Reprinted with permission from the National Academy of Sciences.50
Other studies have used similar, spatially fine-grained methods of morphological analysis to measure changes in cortical thickness with development.39,55 One longitudinal study consisted of data from 45 children between the ages of 5 and 11 years who were scanned twice within a 2-year period. Changes in cortical thickness and overall brain size were assessed.39 Brain growth was most prominent in the prefrontal, temporal, and occipital regions bilaterally. Cortical thinning was observed in the right dorsolateral prefrontal and parieto-occipital regions, whereas cortical thickening was detected in classic language areas (Broca and Wernicke areas) of the frontal and temporal lobes. In a follow-up study of the same sample of children, gray matter thickening in the left inferior frontal gyrus was associated with improving phonological skills, but not with improving motor skills.56 These findings suggested that cortical thickening may represent the proliferation of synaptic connections that helps to form the neural networks that subserve the acquisition of skills required for the development of language in humans. Nevertheless, both cortical thinning and growth in regional brain volumes seemed to occur in the same regions (particularly in the right dorsolateral prefrontal and parieto-occipital cortices) and at the same time,39 suggesting myelination as one possible explanation for cortical thinning. In addition to synaptic pruning, which reduces the number of synapses in the brain, ongoing myelination of axons by oligodendrocytes would likely increase overall brain size.48 Increasing myelination would also contribute to regional cortical thinning if the axons within the cortical gray matter that were unmyelinated earlier in childhood become myelinated later with advancing age. Consistent with this interpretation of increasing myelination rather than synaptic pruning as the cause of regional thinning of the cortical mantle, thinning of the left dorsal frontal and parietal cortices correlated with improved performance on various cognitive tasks,39 suggesting that progressive myelination and its associated cortical thinning could be responsible for the improvements in cognitive performance that suggested improved neural processing within prefrontal and parietal cortices.
These finer-grained morphological techniques are now used frequently to study the differences in the trajectories of brain development of healthy children from those afflicted with neuropsychiatric disorders, such as childhood-onset schizophrenia (COS)57,58 or attention-deficit/hyperactivity disorder (ADHD).59,60 In a longitudinal study of 12 patients with COS and 12 healthy comparison subjects who were scanned at 2-year intervals during adolescence, accelerated gray matter loss was detected, progressing in a back-to-front direction, which was similar to the pattern observed during normal brain maturation.50 These findings suggested that gray matter loss in COS may reflect an exaggeration of the normal brain maturational process of synaptic pruning, perhaps supporting a hypothesis of “excessive pruning,”61 whereby a fault in synaptic elimination during adolescence is thought to produce schizophrenia, consistent with recent evidence that cortical thickness in COS predicts clinical outcome in adulthood.58 In contrast, findings from a recent longitudinal study of a large sample of children and adolescents with ADHD revealed a delayed, rather than a premature, pattern of cortical development relative to healthy controls that was related to clinical outcome.60 This maturational delay was most prominent in prefrontal regions that mediate the higher-order, cognitive functions that are known to be impaired in ADHD.62
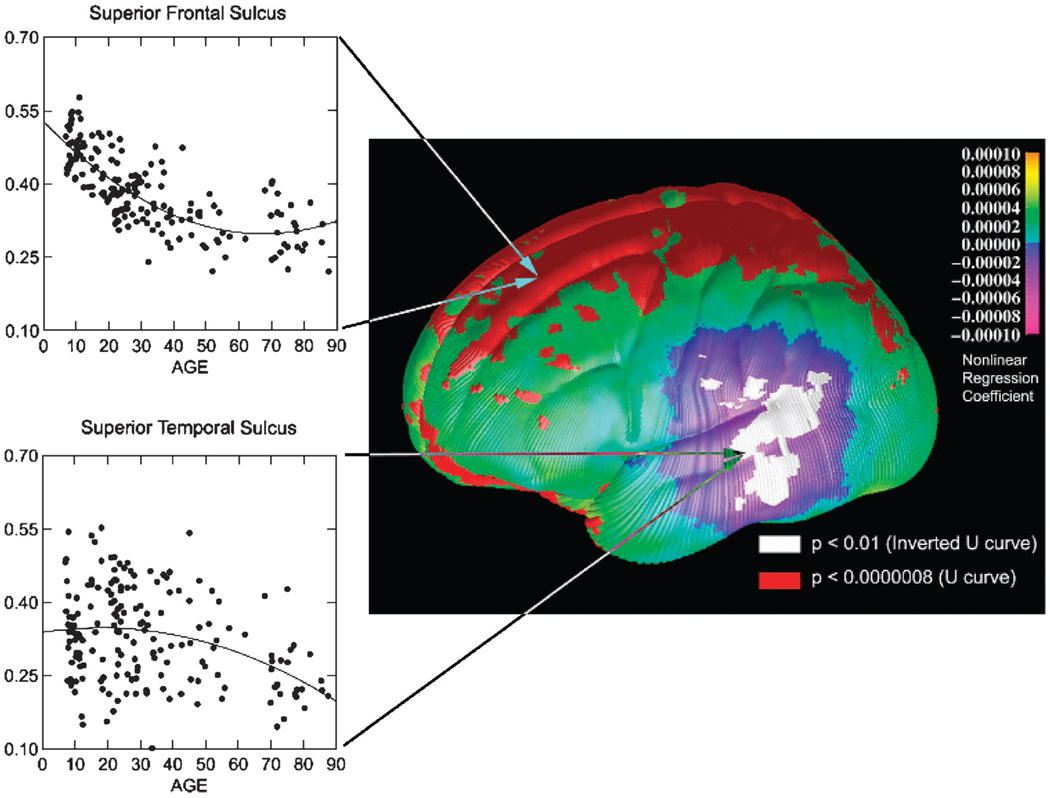
Understanding whether normative maturation of brain structure extends into later life requires studying individuals whose ages vary widely. Information gleaned from a study of brain maturation across the life span has important implications for theories of cognitive development and of cognitive decline with aging. Findings from a study of 176 healthy individuals ranging in age from 7 to 87 years revealed that the trajectories of maturational changes described above likely continue beyond adolescence and into young adulthood.51 A nonlinear decrease in cortical thickness with age was detected over the dorsal frontal and parietal cortices, with the most rapid thinning continuing until age 45 years, after which the cortical thickness remained generally constant (Fig. 6). Because the total volume of white matter continued to increase until the fifth decade, this decrease in gray matter density could have been caused by the progressive myelination of the underlying cortices, rather than by synaptic pruning. Alternatively, this decrease may have been caused by an age-related decrease in neuropil (the unmeylinated dendrites, axonal arbors, and synapses within the gray matter), consistent with findings of a rapid decrease in the total number of synapses with increasing age in nonhuman primates.63 In addition, cortical thinning that began at approximately 45 years and that progressed quickly was detected in the posterior temporal cortices of the left hemisphere51 (Fig. 6), consistent with evidence that some language functions, such as language production and word retrieval, decline during normal aging.64
Fig. 6.
Age effects on gray matter density on the lateral brain surface between childhood and old age. Shades of green/yellow represent positive partial regression coefficients for the quadratic term (U-shaped curves with respect to age), and shades of blue/purple represent negative coefficients (inverted U-shaped curves). Regions in red correspond to regression coefficients that showed significant positive nonlinear age effects, and regions in white showed significant negative nonlinear age effects. Scatterplots of age effects with the best-fitting quadratic regression line are shown for sample surface points in the superior frontal sulcus (top) and the superior temporal sulcus (bottom) representative of the positive (U-shaped) and negative (inverted U-shaped) nonlinear age effects. Gray matter thinning over dorsal frontal and parietal cortices occurs rapidly during adolescence until age 45 years, whereas progressive thinning in posterior temporal cortices begins around age 45 years. Reprinted with permission from Nature Publishing Group, Macmillan Publishers Ltd.51
The extended age range used in this cross-sectional study allowed for longitudinal inferences regarding the development of posterior temporal cortices. Such inferences, however, must be interpreted with caution because they can often mislead our understanding of developmental trajectories.65 The earlier cross-sectional studies described above, for example, reported linear decreases in gray matter with age.46,66 If, however, the variance in gray matter volumes was not the same at each age within the age range selected for study, the cross-sectional mean volume likely fell where the cross-sectional variance was the highest, thereby distorting the trajectory of gray matter development that was inferred from the cross-sectional findings.65 Findings from subsequent longitudinal studies in fact indicated that the trajectories of brain development are curvilinear, with preadolescent increases and postadolescent decreases in gray matter volumes,49 and that the patterns of maturation vary across differing subregions.50 Thus, the emergence of these longitudinal data made clear that the earlier, cross-sectional findings were perhaps influenced by cohort effects arising from the age ranges selected for study, as well as by interindividual variation.
SEX DIFFERENCES IN ANATOMICAL MATURATION OF THE BRAIN
Interest in identifying differences in brain development between the sexes has been generated primarily by widely documented differences between males and females in cognitive abilities, including a male advantage for spatial abilities67 and a female advantage for verbal skills.68 Females, however, tend to have smaller bodies, and the scaling relation between body size and brain size (i.e., people with larger bodies tend to have larger heads and brains) accounts, at least in part, for their smaller brains than males.69,70 Thus, differences in overall brain sizes between males and females must be considered, if differences across the sexes in the sizes of brain sub-regions are to be understood and interpreted properly.
In addition to reporting the regional specificity in timing of gray matter development, the first longitudinal study described above also reported evidence for a sex-specific effect in volumes of gray matter, possibly peaking 1 to 2 years earlier in girls than in boys.49 Formal statistical analyses, however, indicated that the shapes of these developmental trajectories did not differ significantly across sexes (Fig. 4; i.e., the interaction of the quadratic age effect with sex was nonsignificant). Moreover, the study did not report overall differences in brain size between males and females, nor did it account for those differences in the comparisons of regional volumes across sexes. A follow-up study of an expanded sample from the same group of investigators compared the trajectories of regional shapes and volumes across sexes.71 This study included 829 scans from 387 subjects ranging in age from 3 to 27 years. After accounting for the effects of differing overall brain size, volumes of frontal gray matter were proportionately greater in females, and volumes of occipital white matter were proportionately greater in males, across all ages studied. The trajectories in temporal and parietal lobes, however, were similar across males and females.
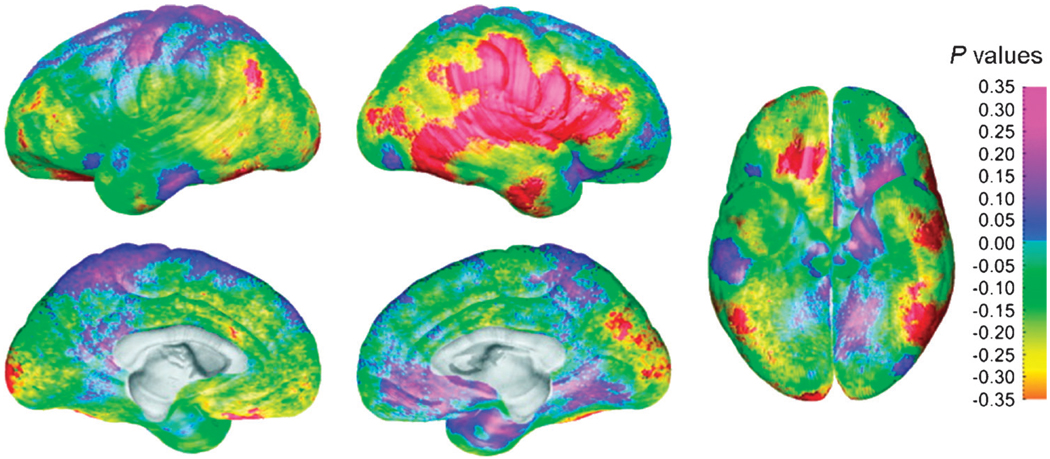
Findings from a cross-sectional study of 176 individuals between 7 and 87 years of age revealed thicker cortices in females in the right inferior parietal and posterior temporal regions.72 These differences remained significant in a subgroup of 36 individuals who were matched for total brain volume and age (Fig. 7), confirming that thicker cortices in temporoparietal regions in females relative to males were independent of differences in brain size. Age-by-sex interactions were not significant in temporoparietal cortices, however, suggesting that thinner cortices are present in males from childhood to adulthood. Given that cortical thinning in these regions during development may arise from either greater synaptic pruning43 or myelination73 or both, and that cortical thinning may thereby contribute to more efficient neural processing,39 thinner cortices in men in these regions may contribute to the superior visuospatial abilities that they seem to have at all ages. These findings differ from those of the NIMH longitudinal study in which sex differences in temporal and parietal gray matter volumes were not observed. Whereas that study used a fully automated method to calculate gray matter thickness, this recent study used “semiautomated” methods in which 35 sulcal and gyral landmarks were manually traced on each image from each individual. A validation study comparing the fully automated method with manual segmentation reported volumetric differences between 10% and 15%.74 Thus, a fully automated method may be sufficiently accurate to detect large effects but is unlikely to be sufficient to detect these smaller effects of age–sex interactions within temporoparietal cortices.
Fig. 7.
Sex differences in gray matter thickness for a subgroup of 36 age- and brain volume–matched subjects. The significance of statistical differences in gray) matter thickness between the male and female subjects is shown according to the color bar on the right (Pearson correlation coefficients). Regions overlaid in red correspond to correlation coefficients that show significant increase in gray matter thickness in the female subjects at a threshold of p= .05. There were no regions where the male subjects had thicker cortex than the females at a threshold of p= .05. Thicker cortices in temporoparietal regions in females relative to males were independent of age and brain size. Reprinted with permission from Oxford University Press.72
To date, the massive increase in sex hormone levels during adolescence that drives pubertal maturation75 and the protracted sculpting of neural connectivity during adolescence76 has not yet been shown to drive the development of sex differences observed in brain images. Rodent studies indicate that exposure to pubertal hormones during adolescence produces changes in brain structure that have long-lasting effects on social behavior.77 Thus, pubertal hormones likely contribute to the dramatic changes in behavior and brain structure in human adolescents as well. In addition, the emergence of sex differences in mood and anxiety disorders during adolescence may relate to sex differences in brain development or to sex differences in pubertal hormones.78 Future longitudinal imaging studies should therefore include measures of sex hormones in an attempt to understand the influences that sex hormones and puberty have on adolescent brain maturation and the sex-based prevalence differences in developmental psychopathologies.
DIFFUSION TENSOR IMAGING OF NORMAL BRAIN DEVELOPMENT
Diffusion tensor imaging (DTI) is an MRI modality that provides information about the direction and integrity of neural fiber tracks in the brain in vivo by characterizing the three-dimensional diffusion of water molecules. Because myelin and cell membranes tend to restrict the diffusion of water, water molecules tend to diffuse along the longitudinal axis of myelinated axons. Thus, by describing mathematically the diffusion of water molecules, investigators can track the direction of bundles of myelinated nerve fibers to study the anatomical connectivity of the brain.79 Color-coded maps are often used in DTI maps to denote the longitudinal axis of nerve fibers.
The increasing directional restriction of the diffusion of water in the brain with advancing age has been a reasonably consistent finding across studies of healthy children (Fig. 8).80–82 This restriction in the diffusion of water increases dramatically immediately before the onset of myelination is visible macroscopically, and it increases more gradually thereafter as myelin is added to axonal sheaths, suggesting that age-related changes in myelination likely produce the increasing constraints on diffusion. Myelination is thought to enhance the speed and fidelity of the transmission of information encoded in action potentials that propagate along neurons, likely contributing to age-related improvements in cognition.
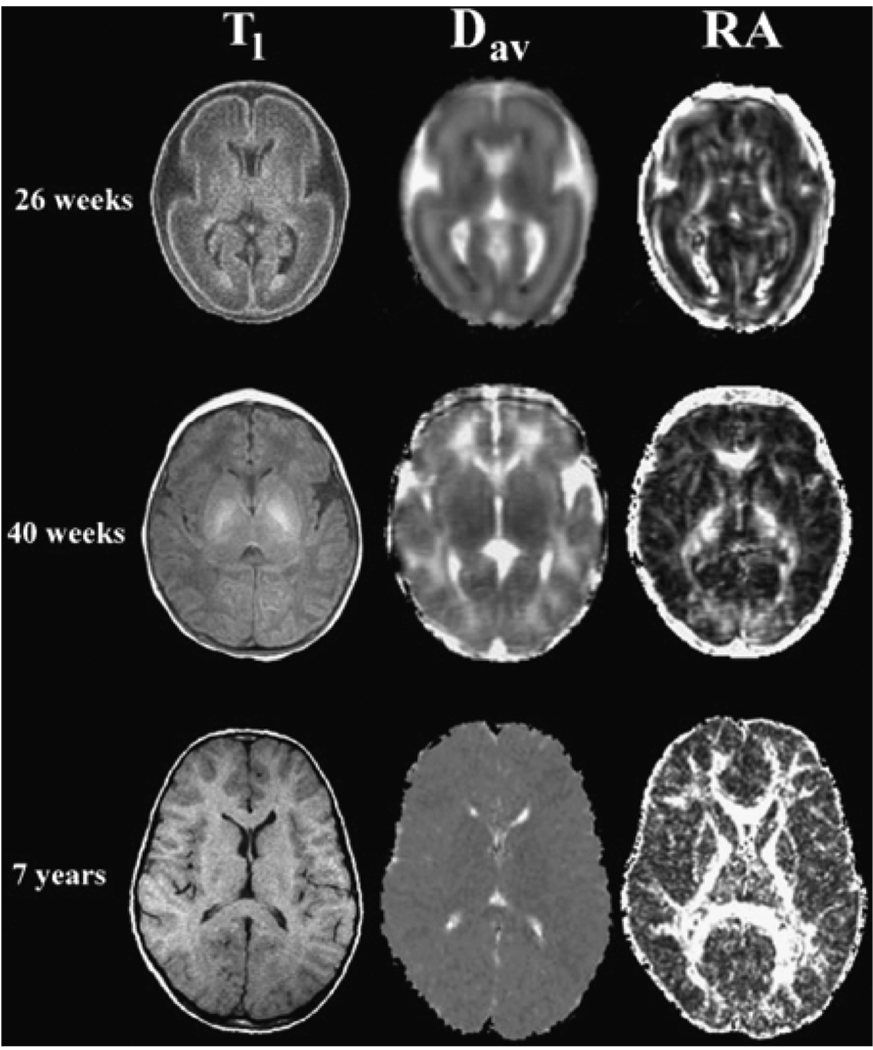
Fig. 8.
The average apparent diffusion and the relative anisotropy (RA) for healthy subjects of differing ages. These are axial slices at the level of the basal ganglia. The top row is from a premature infant of 26 weeks’ GA. The middle row is from a term infant of 40 weeks’ GA, and the bottom row is from a 7-year-old child. The left column consists of T1-weighted images for anatomical reference. The center column consists of Dav parametric maps for which higher diffusion values appear brighter. The right column consists of RA parametric maps for which higher RA values appear brighter. In healthy children, diffusion decreases, and the directional restriction of water diffusion increases, with advancing age. Dav = average apparent diffusion; RA = relative anisotropy; GA = gestational age. NMR Biomed, Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain Va technical review. Copyright © 2002. John Wiley & Sons Limited. Reproduced with permission.
Many DTI studies of infants and young children show that the brain undergoes rapid microstructural changes from birth to 5 years of age.82–85 Findings from studies of older children suggest that the directionality of diffusion in white matter pathways continues to increase from childhood through adolescence.86–89 For example, cross-sectional findings from a study of 34 children and adolescents ranging in age from 6 to 19 years revealed significant associations of increasing diffusion directionality with advancing age in prefrontal regions and in white matter pathways surrounding the basal ganglia.86 Age-related changes in diffusion in the left frontal cortex have been associated with improvements in working memory capacity during childhood,90 and increases in the restriction of diffusion in frontostriatal regions from childhood to adulthood have been associated with improvements in performance on a task requiring the engagement of cognitive control.91 These age-related changes in the organization of white matter and their behavioral correlates suggest that frontostriatal systems myelinate progressively with age, thereby increasing the effective connectivity between frontal and striatal regions and perhaps contributing to the development of the higher-level cognitive functions that these neural systems subserve.92,93 Last, a recent DTI study of 202 individuals ranging in age from 5 to 30 years reported a regional pattern of maturation in which diffusion properties in frontotemporal pathways continue until age 30 years.94 These findings are consistent with findings from anatomical and functional imaging studies suggesting that the development of both frontal and temporal cortices is protracted and extend well into adulthood.50,51
Diffusion tensor imaging studies of normal development are beginning to improve our understanding of abnormal brain development in children with autistic spectrum disorders (ASDs). One cross-sectional study, for example, plotted diffusion indices in children with ASDs against developmental curves that were produced using data points from typically developing individuals.95 These plots revealed a greater restriction of diffusion in white matter in younger children with ASDs, especially within the frontal lobe of the left hemisphere. These DTI findings indicate an early abnormal maturation of frontal white matter, adding to the prior evidence of accelerated brain growth during the first few years of life that suggests the presence of disturbances in synaptogenesis, apoptosis, or myelination (Fig. 1) in children with an ASD.96,97
MAGNETIC RESONANCE SPECTROSCOPY STUDIES OF NORMAL BRAIN DEVELOPMENT
Magnetic resonance spectroscopy (MRS) is an MRI modality that derives signal not only from protons in water but also from protons in molecules such as creatine, N-acetylaspartate (NAA), choline (Cho), and glutamate (Glu).79 Proton MRS studies have reported age-related increases in levels of NAA, which begin at low levels around birth and then increase rapidly during the first 2 years of life, becoming less pronounced thereafter.98,99 In addition, findings from a study of 15 healthy children and adolescents indicated that the ratio of NAA/Cho in cortical gray matter increased with age until 10 years, decreasing thereafter.100 In contrast, the ratios of NAA/ Cho in white matter increased linearly with age. Because NAA is considered to be a marker of neuronal viability,101 the nonlinear increase and subsequent decrease with age in the NAA/Cho ratio within cortical gray matter likely represent the rapid synaptogenesis during childhood and the synaptic pruning during adolescence, which have been reported in animal models.2 Similar to proton MRS, phosphorus (31P) MRS studies, which measure high-energy metabolites in the brain and phospholipids contained in myelin, are also useful for assessing normative changes in brain chemistry during development. Findings from a 31P MRS study of 31 healthy children aged 4 months to 14 years, for example, suggested that precursors of membrane phospholipids are high in concentration before age 2 years and gradually decrease with increasing age, possibly reflecting developmental changes in myelination.102
Findings from MRS studies of adult patients with schizophrenia suggest decreases of NAA concentrations in the dorsolateral prefrontal cortex and hippocampus relative to control values.103–105 Reductions in NAA ratios have been detected in these same brain regions in adolescents with schizophrenia, suggesting a biological continuum between childhood- and adult-onset schizophrenia.106,107 Reduced NAA concentrations in frontal and temporal lobes suggesting the presence of reduced neuronal viability may serve as a biomarker for the presence of disease in children and adults with schizophrenia, consistent with anatomical findings of exaggerated thinning of these cortical regions in this population58 and with functional findings of frontal abnormalities in high-risk individuals with prodromal symptoms.108 This confluence of findings suggests that the pathogenic origins of schizophrenia occur earlier than the ages of the youngest children studied, although the precise developmental timing of these neurometabolic disturbances in schizophrenia remains to be determined.
N-acetylaspartate has also been assessed in the pathophysiology of bipolar disorder (BD). Acute and chronic lithium treatment, for example, seems to decrease NAA concentrations in ventromedial prefrontal cortices in adolescents with BD.109 In contrast, MRS studies of adult patients with BD suggest that lithium treatment increases prefrontal NAA concentrations.110 Whether the neurometabolic and neurochemical responses to lithium do differ across these age groups, or whether the findings represent differing ascertainment biases in children and adults with BD, is unclear and requires longitudinal studies using MRS.
FUNCTIONAL MRI STUDIES OF COGNITIVE DEVELOPMENT
Functional MRI (fMRI) permits exploration of structure-function relations across development, allowing identification of where, when, and how cognitive abilities develop in relation to the maturation of anatomical brain systems. Cognitive processes such as language, executive functioning, and emotion regulation are most likely to elicit differences in patterns of brain activations in children compared with adults because association cortices in the brain that are critically important for these higher-order cognitive functions (especially superior temporal and dorsal prefrontal cortices) are those that undergo neuroanatomical changes well into adolescence and beyond.50
Language development is one of the most widely studied brain functions in healthy children.111– 114 It is a fundamental human trait that begins developing early and rapidly, making language a sensitive index of normal brain development. Findings from studies of language comprehension,115 verbal fluency,111,112 and reading114,116 indicate that healthy individuals show age-related increases and decreases in prefrontal and temporal brain areas when engaging these various linguistic functions.114 Functional MRI findings suggest, for example, that age-related increases in activation of language systems in the left frontal and temporal cortices seem to support the normal acquisition of reading and phonological skills during childhood and adolescence,98 consistent with the protracted anatomical thinning of these cortices during development.39,50
Delineating the trajectory of brain activation association with improved reading skills during the course of typical development can serve as a reference that allows us to identify disruptions in developmental processes that may contribute to reading impairments in children with dyslexia. In addition, these normal developmental trajectories may also be used to identify adaptive, developmentally based compensatory systems that support the acquisition of reading skills in children with dyslexia whose deficits in phonological processing typically persist into adulthood.117 Functional MRI studies of the development of normal language functions, for example, have helped to inform our understanding that dyslexia may be a consequence of disruptions in the development of normal functioning of the left hemisphere parietal- and occipital-temporal brain systems.118,119 These systems support reading abilities, including phonological processing, or the linking of sounds to symbols that ultimately enables the rapid perception of words in unimpaired readers. One fMRI study compared age-related changes associated with phonological processing when reading pseudowords across large samples of children and adolescents with dyslexia and normal reading abilities.120 Individuals with dyslexia relied increasingly more on the left posterior medial occipital-temporal areas with increasing age, whereas normal readers relied on a more anterior occipital-temporal region in the left hemisphere. These findings suggest that when children with dyslexia mature, they rely on an alternative posterior neural system that is involved in recognition memory,121 likely supporting their memorization of words to compensate for their deficits in phonological processing when reading. In contrast, with advancing age, normal readers increasingly rely on an anterior temporo-occipital region that has been termed the visual word form area122,123 and that supports phonological processing in normal adult readers. Although future longitudinal studies on individuals who are dyslexic and unimpaired readers are warranted, these findings suggest that developmental dyslexia may arise from an early functional disruption in the visual word form area that in typical readers supports development of adult-level reading skills.
Another important developmental process investigated extensively using fMRI is the ability to control behaviors that conflict with personal and societal norms.124–126 Both cognitive and emotional maturation requires the development of this capacity for “inhibitory control,” making it one of the most centrally defining characteristics of healthy psychological development. Children must learn to engage inhibitory processes to filter and to organize their thoughts, feelings, and behaviors based on social and emotional cues, especially in the face of competing information or distracting stimuli.127 Findings from fMRI studies of healthy individuals suggest that the maturation of these functions is associated with the development of the prefrontal cortex, along with anatomically connected, subcortical brain regions.124–126,128
Many experimental paradigms have been used to study the development of inhibitory control processes. The Stroop, Simon, flanker, go/no-go, and stop-signal reaction time tasks all require participants to suppress a more automatic behavior in favor of a less automatic one in the face of cognitive conflict that arises from the presentation of competing or distracting stimuli. Inhibitory control is necessary to mobilize attentional resources toward the appropriate stimuli and thereby resolve cognitive conflict to modulate the automatic tendency to respond in one way rather than another. Findings from developmental studies reveal that performance on these tasks improves continuously with age during childhood and does not reach adult levels of performance until at least 12 years of age.124–126 The Stroop task is one of the most commonly studied of these paradigms.129 It requires participants to inhibit word reading in favor of a less automatic behavior, naming the color of ink in which the letters of a color-denoting word are written. When the color that a written word denotes matches the color of the ink in which the letters are printed (e.g., “R-E-D” written in red ink), children perform the task easily, as indexed by their rapid responses and infrequent errors. However, cognitive conflict occurs when the color that a word denotes does not match the color of the printed letters (e.g., “R-E-D” written in blue ink), making the task more difficult, as indicated by slower responses and more frequent errors. Imaging studies of brain activity during color naming of the mismatching compared with the matching stimuli have demonstrated activation in large expanses of anterior cingulate, prefrontal, and parietal cortices, as well as the striatum, in both adults 130 and children.131
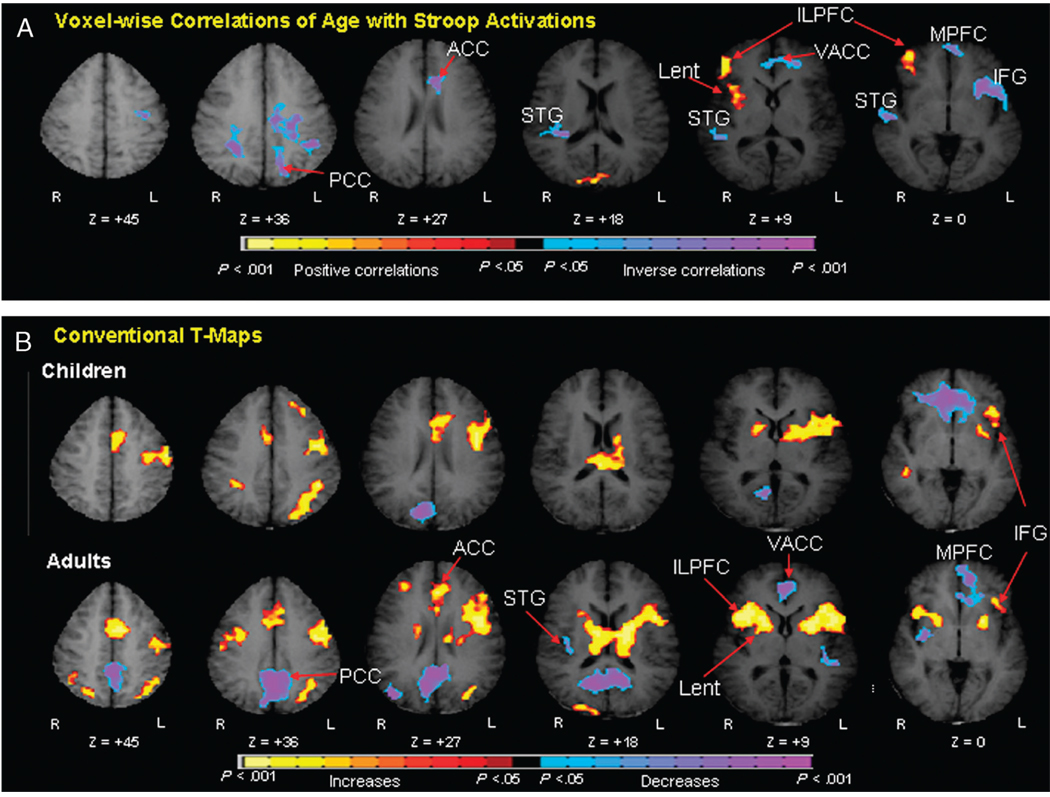
A recent fMRI study, for example, identified age-related differences in the brain activity generated by healthy children and adults during performance of the Stroop task.92 Activation of the inferolateral prefrontal cortex and lenticular nucleus increased with age, as did the speed and accuracy of response on the task, indicating that increasing activity in frontostriatal circuits with age supports the age-related improvement in inhibitory control (Fig. 9). These findings are consistent with those from prior developmental imaging studies showing age-related changes in frontostriatal recruitment during performance of other tasks (e.g., Simon, flanker, and go/no-go) that similarly require the resolution of cognitive conflict.124,125,128 The increasing activation of prefrontal cortices during these inhibitory tasks from childhood to adulthood92,132 likely reflects the development of cognitive control processes that typically begin to emerge during adolescence,133 at a time when anatomical studies suggest that cortical gray matter thins39,50 and when DTI studies suggest that frontostriatal fiber tracts are continuing to myelinate.86 Nevertheless, future longitudinal fMRI studies of inhibitory control processes in healthy individuals are required to ensure that these cross-sectional findings represent true developmental changes in inhibitory control functions.65
Fig. 9.
Age correlates of cognitive control during performance of the Stroop task. A, Voxelwise correlations of age with Stroop activations. These are transaxial slices positioned superiorly to inferiorly (left to right). B, Group composite t-maps for the percent fMRI signal change associated with the naming of colors in incongruent compared with congruent stimuli for children and adults. Increases in signal during the incongruent relative to congruent are coded in yellow, and decreases are coded in purple or blue. Right frontostriatal (ILPFC and Lent) increases in activity associated with incongruent stimuli came online progressively with age. Thus, increasing activity in frontostriatal circuits with age supports the developmental improvements in cognitive control in healthy individuals. PCC = posterior cingulate cortex; ACC = anterior cingulate cortex; VACC = ventral anterior cingulate cortex; STG = superior temporal gyrus; Lnuc = lenticular nucleus; LPFC = lateral prefrontal cortex; MPFC = mesial prefrontal cortex; IFG = inferior frontal gyrus.
The protracted anatomical and functional development of the prefrontal cortices and associated subcortical structures that subserve inhibitory control processes may contribute to the development of a variety of psychiatric disorders in which children have difficulty controlling their thoughts, emotions, and behaviors. These disturbances may release from regulatory control, for example, the various underlying impulses or urges that manifest as either the tics of Tourette’s syndrome (TS), the compulsions of obsessive-compulsive disorder, or the impulsive behaviors that characterize ADHD. These neurodevelopmental disorders, particularly when they occur together, are thought to share a common underlying neural basis involving anatomical disturbances in frontostriatal circuits.134,135 Understanding the normal development of inhibitory control functions mediated by these circuits can therefore inform our understanding of the etiology of TS, obsessive-compulsive disorder, and ADHD.
One fMRI study, for example, compared across individuals with and without TS the correlations of age with frontostriatal activations during performance of the Stroop task.136 Behavioral performance on the task improved with increasing age in patients with TS, just as it did in non-TS controls, reflecting the maturation of neural systems that subserve inhibitory control. In contrast to the normal developmental trajectory of behavioral performance on the task, the imaging findings showed that adults with TS rely on exaggerated activation of frontostriatal regions, which was interpreted as a likely compensatory functional response that produces normal performance on the task, despite deficits in neural plasticity and inhibitory reserve in adults with TS.137 Understanding the normal developmental pattern of Stroop-related activations was required to understand how developmental changes in activation of frontostriatal circuits diverge from normal trajectories in people with TS, and how exaggerated activity likely supports normal behavioral performance, even in the presence of an underlying anatomical hypoplasia or in the presence of impaired neural plasticity.
The prefrontal cortex is thought to modulate activity in subcortical structures,138 including limbic areas that likely give rise to the ability to engage inhibitory control over emotions. Emotional changes during adolescence involve the increasing ability to read a wide variety of social and emotional cues, including facial expressions. Thus, fMRI studies involving the perception of (and attention to) facial expressions have been used to study emotional development and the development of emotional control.139–141 For example, a study comparing brain activation across adolescents and adults revealed that adolescents activated the amygdala and prefrontal regions (orbitofrontal and anterior cingulate cortices ) more than adults when viewing fearful faces.139 When instructed to focus on a nonemotionally salient feature of the face, however, only the adults engaged the orbitofrontal cortex. These findings suggested that adults but not adolescents modulate activity in prefrontal cortices in response to attentional demands, thereby engaging control over the emotionally evocative stimuli. Thus, the maturation of neural systems that subserve emotional control processes is protracted during normal development. The evolving capacity for emotional control likely derives primarily from increasing functional maturation of prefrontal cortices during adolescence.
The developmental trajectories of these processes in individuals with mood disorders likely diverge from normal trajectories, thereby contributing to the development of problems with emotional control. Findings from studies of children and adolescents with BD, for example, indicate that when their attention directed to emotional versus nonemotional aspects of faces, children with BD misjudge neutral faces as more threatening than control children. In addition, their misinterpretations of the emotionally salient stimuli were associated with increased engagement of the amygdala.142 Moreover, increased amygdala activation in adolescents with BD relative to controls in response to emotional stimuli coincides with reduced ventrolateral prefrontal activity.143 These findings suggest that the control of affective responses is impaired in both children and adolescents with BD and therefore emerges early in development. The discovery of this developmental delay in prefrontal functioning in BD underscores the importance of studying the normal developmental trajectories of emotional control processes in healthy individuals and in those who may be at risk for psychopathological findings.
DISCUSSION
Noninvasive imaging techniques now permit investigation of the anatomical and functional maturation of the healthy brain. Understanding healthy developmental trajectories of brain structure and function is of crucial importance for the generation of hypotheses regarding the neural bases of developmentally based psychopathologies. Longitudinal anatomical MRI studies of healthy children have shown that brain maturation typically proceeds in a back-to-front wave, occurring first dorsally, then spreading to temporal cortices, and, finally, moving into prefrontal areas. Cortical thinning during adolescence likely reflects the pruning or elimination of unnecessary or unused synaptic connections that refines and consolidates many cognitive processes during this period. In addition, DTI studies have revealed developmental changes in cortical white matter pathways in prefrontal regions and in pathways surrounding the basal ganglia that presumably reflect increasing myelination of axons during childhood and adolescence and that are thought to increase the speed of neuronal communication and thus to enhance cognitive processing, with increasing age.
Findings from fMRI studies suggest that the later maturation of prefrontal cortices contributes to the protracted development of higher-order cognitive functions such as reading and the inhibitory control over behaviors and emotions during late childhood and adolescence. These cross-sectional findings, however, must be interpreted with caution until replicated in longitudinal fMRI studies which are more costly, time-consuming, and prone to subject attrition over time. Future longitudinal studies comparing the normal and atypical developmental trajectories of cognitive and emotional control processes in individuals with and without psychopathology would aid our understanding of the developmental origins of disturbances in brain maturation that produce psychopathologies during childhood and adolescence. Finally, advances in pediatric neuroimaging require cross-modal imaging studies incorporating the use of fMRI, anatomical MRI, DTI, and MRS in large samples of individuals who are studied over time to improve further our understanding of normal and pathological brain development.
Acknowledgments
This work was supported in part by NIMH grants K02-74677, K01-MH077652, T32 MH16434, and MH068318, by National Institute on Drug Abuse grant DA017820, by a grant from the National Alliance for Research on Schizophrenia and Depression, and by funding from the Sackler Institute for Developmental Psychobiology, Columbia University.
Footnotes
Portions of this article were presented at the 2007 research forum The Future of Neuroimaging: Relevance for Child Psychiatry at the American Academy of Child and Adolescent Psychiatry, Boston, MA, October 2007.
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;l43(suppl 4):S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 3.Black IB, DiCicco-Bloom E, Dreyfus CF. Nerve growth factor and the issue of mitosis in the nervous system. Curr Top Dev Biol. 1990;24:161–192. doi: 10.1016/s0070-2153(08)60087-3. [DOI] [PubMed] [Google Scholar]
- 4.Giedd J. Brain development, IX: human brain growth. Am J Psychiatry. 1999;156(1):4. doi: 10.1176/ajp.156.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Molliver ME, Kostovic I, van der Loos H. The development of synapses in cerebral cortex of the human fetus. Brain Res. 1973;50(2):403–407. doi: 10.1016/0006-8993(73)90741-5. [DOI] [PubMed] [Google Scholar]
- 6.Rakic P. Early developmental events: cell lineages, acquisition of neuronal positions, and areal and laminar development. Neurosci Res Program Bull. 1982;20(4):439–451. [PubMed] [Google Scholar]
- 7.Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the sub ventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol. 2001;172(1):1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- 8.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: boulder committee revisited. Nat Rev Neurosci. 2008;9(2):110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 9.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 10.Huisman TA, Martin E, Kubik-Huch R, Marincek B. Fetal magnetic resonance imaging of the brain: technical considerations and normal brain development. Eur Radiol. 2002;12(8):1941–1951. doi: 10.1007/s00330-001-1209-x. [DOI] [PubMed] [Google Scholar]
- 11.Fogliarini C, Chaumoitre K, Chapon F, et al. Assessment of cortical maturation with prenatal MRI. Part I: normal cortical maturation. Eur Radiol. 2005;15(8):1671–1685. doi: 10.1007/s00330-005-2782-1. [DOI] [PubMed] [Google Scholar]
- 12.Kostovic I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9(2):219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- 13.Kostovic I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4(1):25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- 15.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 16.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12(5):536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 17.Kostovic I, Judas M, Petanjek Z, Simic G. Ontogenesis of goal-directed behavior: anatomo-functional considerations. Int J Psychophysiol. 1995;19(2):85–102. doi: 10.1016/0167-8760(94)00081-o. [DOI] [PubMed] [Google Scholar]
- 18.Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett. 1982;33(3):247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- 19.Becker LE, Armstrong DL, Chan F, Wood MM. Dendritic development in human occipital cortical neurons. Brain Res. 1984;315(1):117–124. doi: 10.1016/0165-3806(84)90083-x. [DOI] [PubMed] [Google Scholar]
- 20.Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271(3):355–386. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- 21.Mrzljak L, Goldman-Rakic PS. Acetylcholinesterase reactivity in the frontal cortex of human and monkey: contribution of AChE-rich pyramidal neurons. J Comp Neurol. 1992;324(2):261–281. doi: 10.1002/cne.903240208. [DOI] [PubMed] [Google Scholar]
- 22.Mrzljak L, Uylings HB, Van Eden CG, Judas M. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog Brain Res. 1990;85:185–222. doi: 10.1016/s0079-6123(08)62681-3. [DOI] [PubMed] [Google Scholar]
- 23.Michel AE, Garey LJ. The development of dendritic spines in the human visual cortex. Hum Neurobiol. 1984;3(4):223–227. [PubMed] [Google Scholar]
- 24.Bourgeois JP. Synaptogenesis, heterochrony and epigenesis in the mammalian neocortex. Acta Paediatr Suppl. 1997;422:27–33. doi: 10.1111/j.1651-2227.1997.tb18340.x. [DOI] [PubMed] [Google Scholar]
- 25.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 27.Huttenlocher PR. Synaptic density in human frontal cortex— developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 28.Huttenlocher PR. Neural Plasticity: The Effects of the Environment on the Development of the Cerebral Cortex. Cambridge: Harvard University Press; 2002. [Google Scholar]
- 29.Bourgeois JA, Nisenbaum J, Drexler KG, Dobbins KM, Hall MJ. A case of subcortical grey matter heterotopia presenting as bipolar disorder. Compr Psychiatry. 1992;33(6):407–410. doi: 10.1016/0010-440x(92)90063-v. [DOI] [PubMed] [Google Scholar]
- 30.Bourgeois J-P, Rakic P. Changing of synaptic density in the primary visual cortex of the rhesus monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50(1):11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
- 32.Zecevic N, Rakic P. Synaptogenesis in monkey somatosensory cortex. Cereb Cortex. 1991;1(6):510–523. doi: 10.1093/cercor/1.6.510. [DOI] [PubMed] [Google Scholar]
- 33.Rakic P. Genesis of Neocortex in Human and Nonhuman Primates. 3rd. Philadelphia: Lippincott Williams & Williams; 2002. [Google Scholar]
- 34.Chugani HT. Development of regional brain glucose metabolism in relation to behavior and plasticity. In: Dawson G, Fischer KW, editors. Human Behavior and the Developing Brain. New York: Guilford; 1994. pp. 153–175. [Google Scholar]
- 35.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs B, Chugani HT, Allada V, et al. Developmental changes in brain metabolism in sedated rhesus macaques and vervet monkeys revealed by positron emission tomography. Cereb Cortex. 1995;5(3):222–233. doi: 10.1093/cercor/5.3.222. [DOI] [PubMed] [Google Scholar]
- 37.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 38.Johnson MH. Development of human brain functions. Biol Psychiatry. 2003;54(12):1312–1316. doi: 10.1016/s0006-3223(03)00426-8. [DOI] [PubMed] [Google Scholar]
- 39.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotrla KJ, Weinberger DR. Developmental neurobiology. In: Sadock BJ, Sadock VA, editors. Comprehensive Textbook of Psychiatry. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 32–40. [Google Scholar]
- 41.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 42.Cao Y, Vikingstad EM, Huttenlocher PR, Towle VL, Levin DN. Functional magnetic resonance studies of the reorganization of the human hand sensorimotor area after unilateral brain injury in the perinatal period. Proc Natl Acad Sci U S A. 1994;91(20):9612–9616. doi: 10.1073/pnas.91.20.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88(5):488–496. [PubMed] [Google Scholar]
- 44.Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- 45.Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 46.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 47.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- 49.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 50.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 52.Levitt P, Reinoso B, Jones L. The critical impact of early cellular environment on neuronal development. Prev Med. 1998;27(2):180–183. doi: 10.1006/pmed.1998.0273. [DOI] [PubMed] [Google Scholar]
- 53.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 54.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu L, Leonard C, Thompson P, et al. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17(5):1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- 57.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98(20):11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47(10):1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 59.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362(9397):1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 60.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 62.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyper-activity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 63.Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burke DM, Mackay DG. Memory, language, and ageing. Philos Trans R Soc Lond B Biol Sci. 1997;352(1363):1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- 66.Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–1 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 67.Jones CM, Braithwaite VA, Healy SD. The evolution of sex differences in spatial ability. Behav Neurosci. 2003;117(3):403–411. doi: 10.1037/0735-7044.117.3.403. [DOI] [PubMed] [Google Scholar]
- 68.Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127(pt 8):1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- 69.Gur RC, Turetsky BI, Matsui M, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riska B, Atchley WR. Genetics of growth predict patterns of brain-size evolution. Science. 1985;229(4714):668–671. doi: 10.1126/science.229.4714.668. [DOI] [PubMed] [Google Scholar]
- 71.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. In: Minkowski A, editor. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- 74.Collins D, Holmes C, Peters T, Evans A. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3(3):190–208. [Google Scholar]
- 75.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinal. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 78.Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- 79.Bansal R, Chung Y, Dong Z, et al. Neuroimaging Methods in the Study of Childhood Psychiatric Disorders. 4th ed. Baltimore: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 80.Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain—a technical review. NMR Biomed. 2002;15(7–8):543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 81.Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: application to the study of the developing brain. J Am Acad Child Adolesc Psychiatry. 2007;46(2):213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- 82.Mukherjee P, Miller JH, Shimony JS, et al. Normal brain maturation during childhood: developmental trends characterized with diffusiontensor MR imaging. Radiology. 2001;221(2):349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 83.McGraw P, Liang L, Provenzale JM. Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol. 2002;179(6):1515–1522. doi: 10.2214/ajr.179.6.1791515. [DOI] [PubMed] [Google Scholar]
- 84.Hermoye L, Saint-Martin C, Cosnard G, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29(2):493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 85.Partridge SC, Mukherjee P, Berman JI, et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22(4):467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- 86.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 87.Ben Bashat D, Ben Sira L, Graif M, et al. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging. 2005;21(5):503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- 88.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 90.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 91.Liston C, Watts R, Tottenham N, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 92.Marsh R, Zhu H, Schultz RT, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73(1):19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 95.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 96.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 97.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 98.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 99.Toft PB, Leth H, Lou HC, Pryds O, Henriksen O. Metabolite concentrations in the developing brain estimated with proton MR spectroscopy. J Magn Reson Imaging. 1994;4(5):674–680. doi: 10.1002/jmri.1880040510. [DOI] [PubMed] [Google Scholar]
- 100.Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J Magn Reson Imaging. 2002;15(2):137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- 101.Lu ZH, Chakraborty G, Ledeen RW, Yahya D, Wu G. N-acetylaspartate synthase is bimodally expressed in microsomes and mitochondria of brain. Brain Res Mol Brain Res. 2004;122(1):71–78. doi: 10.1016/j.molbrainres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Hanaoka S, Takashima S, Morooka K. Study of the maturation of the child’s brain using 31P-MRS. Pediatr Neurol. 1998;18(4):305–310. doi: 10.1016/s0887-8994(97)00201-4. [DOI] [PubMed] [Google Scholar]
- 103.Bertolino A, Nawroz S, Mattay VS, et al. Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1996;153(12):1554–1563. doi: 10.1176/ajp.153.12.1554. [DOI] [PubMed] [Google Scholar]
- 104.Sigmundsson T, Maier M, Toone BK, et al. Frontal lobe N-acetylaspartate correlates with psychopathology in schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr Res. 2003;64(1):63–71. doi: 10.1016/s0920-9964(02)00533-9. [DOI] [PubMed] [Google Scholar]
- 105.Cecil KM, Lenkinski RE, Gur RE, Gur RC. Proton magnetic resonance spectroscopy in the frontal and temporal lobes of neuroleptic naive patients with schizophrenia. Neuropsychopharmacology. 1999;20(2):131–140. doi: 10.1016/S0893-133X(98)00063-3. [DOI] [PubMed] [Google Scholar]
- 106.Bertolino A, Kumra S, Callicott JH, et al. Common pattern of cortical pathology in childhood-onset and adult-onset schizophrenia as identified by proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1998;155:1376–1383. doi: 10.1176/ajp.155.10.1376. [DOI] [PubMed] [Google Scholar]
- 107.O’Neill J, Levitt J, Caplan R, et al. 1H MRSI evidence of metabolic abnormalities in childhood-onset schizophrenia. Neuroimage. 2004;21(4) doi: 10.1016/j.neuroimage.2003.11.005. 1781–1789.r. [DOI] [PubMed] [Google Scholar]
- 108.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62(3):254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel NC, Delbello MP, Cecil KM, Stanford KE, Adler CM, Strakowski SM. Temporal change in N-acetyl-aspartate concentrations in adolescents with bipolar depression treated with lithium. J Child Adolesc Psychopharmacol. 2008;18(2):132–139. doi: 10.1089/cap.2007.0088. [DOI] [PubMed] [Google Scholar]
- 110.Brambilla P, Stanley JA, Nicoletti MA, et al. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J Affect Disord. 2005;86(1):61–67. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 111.Holland SK, Vannest J, Mecoli M, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- 113.Sakai KL. Language acquisition and brain development. Science. 2005;310(5749):815–819. doi: 10.1126/science.1113530. [DOI] [PubMed] [Google Scholar]
- 114.Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- 115.Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60(10):1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- 116.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- 117.Ramus F, Rosen S, Dakin SC, et al. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126(pt 4):841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- 118.Shaywitz BA, Shaywitz SE, Pugh KR, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- 119.Pugh K, Mencl EW, Shaywitz BA, et al. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity in posterior cortex. Psychol Sci. 2000;11:51–59. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- 120.Shaywitz BA, Skudlarski P, Holahan JM, et al. Age-related changes in reading systems of dyslexic children. Ann Neurol. 2007;61(4):363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- 121.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125(pt 5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- 123.Cohen L, Dehaene S, Naccache L, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- 124.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Casey BJ, Trainor R, Orendi JL, et al. A developmental functional MRI study of prefrontal activation during performance of a go/no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 126.Rubia K, Overmeyer S, Taylor E, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24(1):13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 127.Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353(1377):1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 129.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 130.Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45(10):1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 131.Adleman NE, Menon V, Blasey CM, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 132.Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 134.Leckman JF, Peterson BS, Anderson GM, Arnsten AFT, Pauls DL, Cohen DJ. Pathogenesis of Tourette’s syndrome. J Child Psychol Psychiatry. 1997;38:119–142. doi: 10.1111/j.1469-7610.1997.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 135.Plessen KJ, Royal JM, Peterson BS. Neuroimaging of tic disorders with co-existing attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2007;16(suppl 1):60–70. doi: 10.1007/s00787-007-1008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marsh R, Zhu H, Wang Z, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control in Tourette’s syndrome. Am J Psychiatry. 2007;164(6):955–966. doi: 10.1176/appi.ajp.164.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peterson BS, Staib L, Scahill L, et al. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58(5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 138.Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48(4):535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 139.Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 140.Guyer AE, Monk CS, McClure-Tone EB, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thomas KM, Drevets WC, Whalen PJ, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 142.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162(3):244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]