Abstract
Lanthanide complexes (Eu3+, Gd3+ and Yb3+) of two different 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid tetraamide derivatives containing two (2) and four (3) O-benzyl-l-serine amide substituents were synthesized and their chemical exchange saturation transfer (CEST) and relaxometric properties were examined in the presence and absence of human serum albumin (HSA). Both Eu2 and Eu3 display a significant CEST effect from a single slowly exchanging Eu3+-bound water molecule, making these PARACEST complexes potentially useful as vascular MRI agents. Yb2 also showed a detectable CEST effect from both the Yb3+-bound water protons and the exchangeable NH amide protons, making it potentially useful as a vascular pH sensor. Fluorescence displacement studies using reporter molecules indicate that both Gd2 and Gd3 displace dansylsarcosine from site II of HSA with inhibition constants of 32 and 96 µM, respectively, but neither complex significantly displaces warfarin from site I. Water proton relaxation enhancements of 135 and 171% were observed upon binding of Gd2 and Gd3 to HSA, respectively, at 298 K and pH 7.4.
Keywords: Chemical exchange saturation transfer agents, MRI contrast, Albumin binding, Paramagnetic relaxation
Introduction
Magnetic resonance imaging (MRI) is one of the most powerful and versatile tools in biomedicine, providing not only high-resolution anatomical images but also functional data such as blood flow, tissue perfusion, and distribution of metabolites. MRI detects mostly tissue water and fat and does not require injection of exogenous agents to produce image contrast. Nevertheless, paramagnetic contrast agents (CAs) are often used to selectively alter tissue contrast by altering the relaxation rate of tissue water. Current diagnostic CAs for MRI are largely based on paramagnetic gadolinium complexes [1, 2] that highlight only those tissue regions where an agent accumulates for a period of time that is abnormal compared with that for well-vascularized tissue. Accumulation of a Gd3+-based agent results in a shortening of the bulk water spin-lattice relaxation time (T1) and hence brightening of the image in that region. Although current nonspecific, extracellular Gd3+-based agents are widely accepted and used clinically, interest in “smart” or “responsive” agents that can provide additional physiological or metabolic information to aid in a clinical diagnosis is growing.
Recently a new class of CA based upon a chemical exchange saturation transfer (CEST) mechanism have been proposed and demonstrated. Unlike other image-darkening agents that function by shortening the transverse relaxation time (T2) of water protons, CEST agents act by decreasing the water signal intensity via chemical exchange of saturated spins. This approach was first demonstrated by Ward and Balaban [3] using low molecular weight diamagnetic molecules containing exchangeable OH or NH groups. They showed that magnetic resonance contrast can be switched on/off by applying a saturating irradiation pulse and, since chemical exchange between such groups and bulk water is pH dependent, such systems can potentially be used to image tissue pH [3, 4]. One disadvantage of such diamagnetic agents is that the chemical shift difference between exchanging OH and NH protons and bulk water is typically quite small, often less than 5 ppm. This makes it difficult to selectively activate the CEST agent in tissues where the bulk water signal can be rather broad. Nevertheless, Geoffeney et al. [5], Snoussi et al. [6], and Zhou et al. [7] have shown that CEST can be amplified by using endogenous and exogenous macromolecules with large numbers of exchanging sites or endogenous proteins or peptides. We recently reported that the weakly paramagnetic Eu1 (Structure 1) has a Eu3+-bound water resonance near 50 ppm that can be used as an RF antenna to initiate CEST. Protons on this bound water molecule also have a much shorter exchange lifetime compared with diamagnetic NH or OH protons, a feature that can potentially enhance CEST contrast at equivalent agent concentrations [8, 9]. Since that early report, several other papers have appeared on responsive paramagnetic CEST agents capable of sensing physiological indices such as pH [10–12], lactate [13], glucose [14], and temperature [4, 15].
Structure 1.
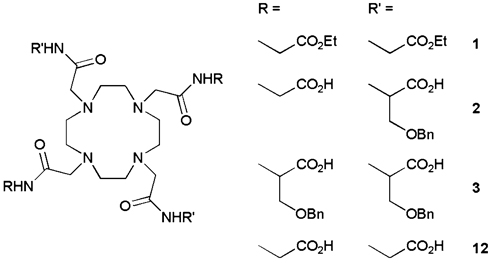
Just as one might amplify the effect of a Gd3+-based T1 agent by conjugation to a higher molecular weight macromolecule such as albumin, polylysine, or a dendrimer, one could also conjugate multiple PARACEST agents to a polymer backbone to decrease the “effective” concentration of the agent. Since the CEST efficiency of such agents is highly dependent upon the rate of water exchange, one question of interest is whether interactions between a PARACEST agent and protein surface residues for example might either enhance or quench the CEST effect. To test this hypothesis, we prepared and characterized PARACEST agents containing two (Ln2) or four (Ln3) O-benzyl functionalities, groups known to impart a high binding affinity for albumin [16, 17] (Structure 1). The albumin-binding properties of these agents were measured using fluorescence spectroscopy and their CEST behavior in the presence and absence of albumin was measured to determine whether water or proton exchange was altered upon binding of the agent to albumin.
Materials and methods
General remarks
All reagents and solvents were purchased from commercially available sources and used as received. 1H, CEST and 13C spectra were recorded with a JEOL Eclipse 270 spectrometer or a Varian INOVA-400 spectrometer operating at 270 and 67.5 MHz or 400 and 100 MHz, respectively. Water proton relaxation measurements were made using an inversion-recovery pulse sequence with a MRS-6 NMR analyzer (Institut “Jožef Stefan,” Ljubljana, Slovenia) operating at 20 MHz. IR spectra were recorded using a PerkinElmer 1600 Fourier transform IR spectrophotometer. Melting points were determined with a Fisher–Johns melting point apparatus and are uncorrected.
1,7-Dibenzyloxycarbonyl-1,4,7,10-tetraazacyclododecane was prepared according to previously published methods [18].
2-(2-Bromoacetamido)-3-(benzyloxy)propanoic acid
A solution of bromoacetyl bromide (12.9 g; 64.0 mmol) in CH2Cl2 (20 mL) was added dropwise to a solution of 2-amino-3-(benzyloxy)propanoic acid (5.0 g, 25.6 mmol) and potassium carbonate (14.2 g, 102.4 mmol) in aqueous 2 N NaOH (12 mL) at 273 K. The reaction mixture was stirred at 273 K for 10 min before warming it to room temperature and stirring for a further 2 h. The two layers were separated, and the pH of the aqueous layer was adjusted to between 1 and 2 with 20% hydrochloric acid. The aqueous phase was then extracted with CH2Cl2 (200 mL). The organic extracts were washed with water (2 × 100 mL), dried (Na2SO4), and the solvents were removed under reduced pressure to afford 2-(2-bromoacetamido)-3-(benzyloxy)propanoic acid (4) as a colorless gum (5.70 g, 70%). 1H NMR (400 MHz, CDCl3, δ): 10.38 (1H, s br, COOH), 7.26 (5H, m, Ar), 4.68 (1H, m, NCHCO), 4.53 (1H, d aa′ system, 2JH–H = 12 Hz, OCH2Ar), 4.47 (1H, d aa′ system, 2JH–H = 12 Hz,OCH2Ar), 4.04–3.44 (4H, m, CHCH2O, COCH2Br). 13C NMR (100 MHz, CDCl3, δ): 173.6 (NCO2), 166.9 (NHC=O), 137.3 (Ar), 128.8 (Ar), 128.3 (Ar), 128.0 (Ar), 73.6 (OCH2Ar), 69.1 (CHCH2O), 53.5 (CH), 28.7 (COCH2Br); νmax (cm−1): 3,379 (br, OH), 2,969, 2,938, 2,866, 1,733 (C=O); 1,652 (C=O), 1,538, 1,454, 1,396, 1,361, 1,206, 1,105, 1,048. m/z (ESMS, ESI–): 316 (100%, [M – H]−); an appropriate bromine isotope pattern was observed.
tert-Butyl 2-(2-bromoacetamido)-3-(benzyloxy)propanoate
To a solution of the acid 4 (5.5 g, 17.5 mmol) in tetrahydrofuran (THF; 150 mL) cooled in an ice bath was slowly added tert-butyl trichloroacetimidate (9.6 g, 43.8 mmol) in THF (50 mL). After addition was complete, BF3·OEt2 (0.66 mL, 5.3 mmol) was also added very slowly. The reaction mixture was allowed to warm to room temperature and stirred for 12 h. The resulting solution was diluted with diethyl ether (200 mL) and washed with saturated aqueous NaHCO3 (2 × 60 mL), water (2 × 80 mL), and dried (Na2SO4). The solvents were removed in vacuo and the residue suspended in CH2Cl2 (30 mL). The suspension was cooled to 273 K and allowed to stand for 1 h. The solids were removed by filtration and the solvents were removed in vacuo. The oily residue was purified by column chromatography over silica gel eluting with 5% CH3OH in CH2Cl2 to afford tert-butyl 2-(2-bromoacetamido)-3-(benzyloxy)propanoate (5) as a colorless oil (3.7 g, 56%). Rf = 0.71 (SiO2, 5% CH3 OH in CH2Cl2). 1H NMR (400 MHz, CDCl3, δ): 7.25 (5H, m, Ar), 4.54 (1H, m, NHCHCH2), 4.51 (1H, d aa′ system, 2JH–H = 12 Hz, OCH2Ar), 4.44 (1H, d aa′ system, 2JH–H = 12 Hz, OCH2Ar), 3.90–3.47 (4H, m, CHCH2O, COCH2Br), 1.41 (9H, s, C(CH3)3). 13C NMR (100 MHz, CDCl3, δ): 169.2 (CO2), 166.1 (NHC=O), 138.1 (Ar), 129.0 (Ar), 128.4 (Ar), 128.3 (Ar), 83.2 [C(CH3)3], 73.9 (OCH2Ar), 70.2 (CHCH2O), 54.4 (NHCHCH2), 29.4 (COCH2Br), 28.6 [C(CH3)3]. νmax (cm−1): 2,974, 2,920, 2,862, 1,734 (C=O), 1,652 (C=O), 1,539, 1,520, 1,506, 1,455, 1,393, 1,367, 1,242, 1,149, 1,104, 1,032. m/z (ESMS, ESI–): 370 (100%, [M – H]−); an appropriate bromine isotope pattern was observed.
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetra-(2-(methylene benzyloxy ether)-tert-butyl acetate) acetamide
The bromoacetamide 5 (1.4 g, 4.1 mmol), cyclen (170 mg, 0.96 mmol), and N,N-diisopropylethylamine (0.9 g, 7.0 mmol) were dissolved in CH3CN (30 mL). The resulting solution was heated with stirring at 328 K for 3 days. The reaction mixture was then cooled to room temperature and the solvents removed in vacuo. The oily residue was taken up in CHCl3 (200 mL) and washed with water (2 × 75 mL). The organic layer was dried (Na2SO4) and solvents were removed under reduced pressure. The oily residue was then titrated with diethyl ether to afford 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetra-(2-(methylene benzyloxy ether)-tert-butyl acetate) acetamide (6) as a colorless gum (1.1 g, 93%). 1H NMR (270 MHz, CDCl3, δ): 7.25 (24H, m, Ar and NH), 4.65 (4H, m, CH), 4.54 (4H, q aa′ system, 2JH–H = 12 Hz, CH2Ar), 4.52 (4H, q aa′ system, 2JH–H = 12 Hz, CH2Ar), 3.85 (8H, m, CHCH2), 3.70 (8H, s br, NCH2CO), 3.50-2.80 (16H, m br, NCH2 ring), 1.20 [36H, s, C(CH3)]. 13C NMR (68 MHz, CDCl3, δ): 170.0 (C=O), 169.2 (C=O), 137.7 (Ar), 128.5 (Ar), 128.0 (Ar), 127.9 (Ar), 73.31 [C(CH3)3], 69.2, (OCH2Ar), 57.1 (CHCH2), 53.0 (NCH2 ring), 52.2 (CH), 51.1 (NCH2O), 14.2 [C(CH3)3]. m/z (ESMS ESI+): 1,224 (100%, [M + H]+).
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetra-[2-(methylene benzyloxy ether)-acetic acid] acetamide
The tert-butyl ester 6 (1.0 g, 0.72 mmol) was dissolved in 50% trifluoroacetic acid (TFA) in CH2Cl2 (10 mL). The resulting solution was stirred at room temperature for 5 h. The solvents were removed under vacuum and dichloromethane (20 mL) was added to the residue. The solvents were again removed under reduced pressure and this procedure was repeated a total of three times. After drying under high vacuum, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetra-[2-(methylene benzyloxy ether)-acetic acid] acetamide (3) was obtained as a colorless gum (0.55 g, 69%). 1H NMR (270 MHz, CD3OD, δ): 7.28 (5H, m, Ar), 4.63 (4H, m, CH), 4.59 (4H, q aa′ system, 2JH–H = 12 Hz, CH2Ar), 4.47 (4H, q aa′ system, 2JH–H = 12 Hz, CH2Ar), 3.82 (8H, m, CHCH2), 3.72 (8H, s, OCH2N), 3.32-3.21 (16H, m br, NCH2 ring). 13C NMR (68 MHz, CD3OD, δ): 172.0 (C=O), 169.2 (C=O), 137.9 (Ar), 128.2 (Ar), 127.8 (Ar), 127.7 (Ar), 73.0 (CHCH2), 69.4 (OCH2Ar), 53.3 (CH2 ring), 50.8 (CHCH2), 50.7 (NCH2CO). m/z (ESMS, ESI+): 1,113 (100% [M + H]+), 1,135 (20% [M + Na]+).
tert-Butyl 2-(2-chloroacetamido)acetate
Glycine tert-butyl ester hydrochloride (6.3 g, 37.8 mmol) and potassium carbonate (15.7 g, 113.5 mmol) were dissolved in water (200 mL) and CH2Cl2 (200 mL) was added. The biphasic reaction mixture was cooled to 273 K and a solution of chloroacetyl chloride (8.5 g, 75.6 mmol) in CH2Cl2 (40 mL) was added dropwise. The reaction was stirred at 273 K for 30 min and then allowed to warm to room temperature and was stirred for 1 h. The two layers were separated and the organic layer was washed with 5% aqueous citric acid (2 × 40 mL) and water (2 × 40 mL) before drying (Na2SO4). The solvents were removed in vacuo to afford tert-butyl 2-(2-chloroacetamido)acetate (7) as a colorless solid (7.8 g, 99%). Melting point 340.5-341 K. 1H NMR (400 MHz, CDCl3, δ): 7.17 (1H, s br, NH), 4.11 (2H, s, CH2Cl), 4.00 (2H, d, 2JH–H = 4.0 Hz, NHCH2), 1.51 [9H, s, C(CH3)3]. 13C NMR (100 MHz, CDCl3, δ): 168.2 (CO2), 166.0 (NHCO), 82.5 [C(CH3)3], 42.2 (NHCH2CO), 42.0 (ClCH2), 27.8 [C(CH3)3]. νmax (cm−1): 3,245 (NH), 3,087, 2,975, 1,734 (C=O), 1,652 (C=O), 1,557, 1,540, 1,409, 1,365, 1,228, 1,160, 1,039. m/z (ESMS, ESI–): 206 (100%, [M – H]−); an appropriate chlorine isotope pattern was observed. Anal. Found (Calcd for C8H14ClNO3): C, 46.1 (46.3); H, 6.8 (6.8); N, 6.7 (6.8).
1,7-Bis(benzyloxycarbonyl)-4,10-bis(tert-butylacetamidoacetate)-1,4,7,10-tetraazacyclododecane
The chloroacetamide 7 (3.0 g, 14.4 mmol) and potassium carbonate (3.0 g, 21.7 mmol) were added to a solution of the diprotected cyclen 8 (3.2 g, 7.2 mmol) in CH3CN (80 mL). The reaction mixture was heated with stirring at 333 K for 3 days. After cooling to room temperature, the reaction mixture was filtered and the solvents were removed under reduced pressure. The oily residue was dissolved in diethyl ether (200 mL) and washed with water (2 × 50 mL), and dried (Na2SO4). The solvents were removed under vacuum and the oily residue was purified by column chromatography over silica gel eluting with 5% CH3OH and 0.3% NH4OH in CH2Cl2 to afford 1,7-bis (benzyloxycarbonyl)-4,10-bis(tert-butyl-acetamidoacetate)-1,4,7,10-tetraazacyclododecane (9) as a colorless solid (4.1 g, 73%). Rf = 0.33 (SiO2, 5% CH3OH, and 0.3% NH4OH in CH2Cl2). Melting point 334-335.5 K. 1H NMR (270 MHz, CDCl3, δ): 7.29 (10H, m, Ar), 5.06 (4H, s, OCH2Ar), 3.78 (4H, s br, NHCH2CO), 3.42 (8H, s br, NCH2 ring), 3.17 (4H, s br, NCH2CO), 2.80 (8H, s br, NCH2 ring), 1.40 [18H, s, C(CH3)3]. 13C NMR (68 MHz, CDCl3, δ): 171.3 (NHC=O), 168.9 (NCO2), 156.9 (NHC=O), 136.6 (Ar), 128.6 (Ar), 128.4 (Ar), 128.3 (Ar), 81.8 [C(CH3)3], 67.4 (OCH2Ar), 58.4 (NCH2 ring), 55.1 (NCH2CO), 48.9 (NCH2 ring), 41.6 (NHCH2CO), 28.1 (C(CH3)3). νmax (cm−1): 3,288 (NH), 2,974, 2,922, 2,811, 1,740 (C=O), 1,687 (C=O), 1,650 (C=O), 1,521, 1,456, 1,416, 1,366, 1,220, 1,151. m/z (MALDI/TOF): 783 (100% [M + H]+). Anal. Found (Calcd for C40H58N6O10·0.2HCl): C, 60.6 (60.8); H, 7.3 (7.4); N, 10.4 (10.6).
1,7-Bis(tert-butyl acetamidoacetate)-1,4,7, 10-tetraazacyclododecane
To a solution of the dicarbamate 9 (8.2 g, 10.5 mmol) in absolute ethanol (80 mL) was added 10% Pd on carbon (1.0 g). The mixture was placed on a Parr hydrogenation apparatus and shaken under a hydrogen pressure of 40 psi for 3 days. The reaction mixture was then filtered and the solvents were removed from the filtrate under reduced pressure toafford 1,7-bis(tert-butyl acetamidoacetate)-1,4,7,10-tetraazacyclododecane (10) as a colorless oil (5.2 g, 96%). 1H NMR (270 MHz, CDCl3, δ): 7.77 (2H, s br, NH), 3.83 (4H, d, 2JH–H = 4 Hz, NHCH2CO), 3.17 (4H, s, NCH2CO), 2.69-2.64 (16H, m br, NCH2 ring), 1.30 [18H, s, C(CH3)3]. 13C NMR (68 MHz, CDCl3, δ): 171.6 (CO2), 169.6 (CONH), 82.0 [C(CH3)3], 60.5 (NHCH2CO), 53.0 (NCH2 ring), 46.5 (NCH2 ring), 41.7 (NCH2CO), 28.1 [C(CH3)3] νmax (cm−1): 3,288 (NH), 2,974, 2,915, 1,740 (C=O), 1,689 (C=O), 1,524, 1,454, 1,416, 1,366, 1,263, 1,152, 730. m/z (MALDI/TOF): 515 (100% [M + H]+).
1,7-Bis[2-(methylene benzyloxy ether)-tert-butyl acetate] acetamide-4,10-bis(tert-butylacetamidoacetate)-1,4,7,10-tetraazacyclododecane
Hünig’s base (3.53 g, 27.30 mmol) and the bromoacetamide 5 (3.4 g, 9.1 mmol) were added to a solution of the diamide 10 (2.3 g, 4.6 mmol) in CH3CN (60 mL). The reaction mixture was heated with stirring at 333 K for 3 days. After cooling to room temperature, the solvents were removed under reduced pressure and the oily residue was taken up into diethyl ether (150 mL). The solution was filtered and the solvents were removed from the filtrate to afford 1,7-bis[2-(methylene benzyloxy ether)-tert-butyl acetate] acetamide-4,10-bis(tert-butyl-acetamidoacetate)-1,4,7,10-tetraazacyclododecane (11) as a glassy solid, which was used for the next step without further purification. Melting point 330-333 K. 1H NMR (400 MHz, CDCl3, δ): 8.32 (2H, s br, NH), 7.45 (2H, s br, NH), 7.18 (10H, m, Ar), 4.49 (2H, m, NCHCO2), 4.44 (2H, d aa′ system, 2JH–H = 12 Hz, OCH2Ar), 4.36 (2H, d aa′ system, 2JH–H = 12 Hz, OCH2Ar), 3.77 (8H, m, CHCH2O, NCH2CO2), 3.65 (4H, s br, NCH2CO), 3.43 (4H, s br, NCH2CO), 2.45–3.20 (16H, br, NCH2 ring), 1.34 [18H, s, C(CH3)3], 1.32 [18H, s, C(CH3)3]. 13C NMR (100 MHz, CDCl3, δ): 193.2 (CO2), 188.0 (CO2), 169.6 (NHC=O), 169.4 (NHC=O), 138.0 (Ar), 129.0 (Ar), 128.4 (Ar), 128.3 (Ar), 82.9 [C(CH3)3], 73.9 (OCH2Ar), 70.3 (CHCH2O), 62.5 (NCHCH2), 57.1 (NCH2CO), 53.7 (br, NCH2 ring), 42.3 (NCH2CO2), 28.6 [C(CH3)3], 28.5 [C(CH3)3]. νmax (cm−1): 2,974, 2,922, 2,828, 2,812, 1,739 (C=O), 1,670 (C=O), 1,517, 1,454, 1,392, 1,366, 1,222, 1,152, 1,104. m/z (MALDI/TOF): 1,097 (100%, [M + H+]).
1,7-Bis[2-(methylene benzyloxy ether)-acetic acid] acetamide-4,10-bis(acetamidoacetic acid)-1,4,7,10-tetraazacyclododecane
The tert-butyl ester 11 (1.0 g; 0.9 mmol) was dissolved in CH2Cl2 (5 mL) and TFA (8 mL) was added. The reaction mixture was stirred at 298 K for 12 h and the solvents were removed under reduced pressure. The resulting oily residue was washed with diethyl ether (3 × 50 mL) and dried under vacuum. The solid residue was dissolved in water (18 mL) and purified by preparative reversed-phase high-performance liquid chromatography (HPLC) over a Phenomenex Luna C-18(2) (250 mm × 50 mm) column on a Waters δ-prep HPLC system. Absorbance was monitored at 205 and 254 nm. The system was eluted with water (0.1% TFA) for 5 min and then with a linear gradient to 50% MeCN (0.1% TFA) and 50% water (0.1% TFA) after 15 min and maintained isocratically for a further 10 min, at a flow rate of 100 mL min−1. 1,7-Bis[2-(methylene benzyloxy ether)-acetic acid] acetamide-4,10-bis(acetamidoacetic acid)-1,4,7,10-tetraazacyclododecane (2) was obtained as a colorless solid (0.44 g, 68%). HPLC Rt = 17.8 min. Melting point 397-399 K. 1H NMR (400 MHz, ND3OD, δ): 7.15 (10H, m, Ar), 4.39 (2H, m, NCHCO2), 4.38 (2H, d aa′ system, 2JH–H = 12 Hz, OCH2Ar), 4.32 (2H, d aa′ system, 2JH–H = 12 Hz, OCH2Ar), 4.11–3.34 (16H, m, CHCH2O, NCH2CO2, NCH2CO), 2.40–3.34 (16H, m br, NCH2 ring). 13C NMR (100 MHz, CDCl3, δ): 172.9 (CO2), 163.2 (NHC=O), 162.8 (NHC=O), 137.1 (Ar), 128.8 (Ar), 128.5 (Ar), 128.4 (Ar), 73.0 (OCH2Ar), 68.5 [CHCH2O], 55.1 (NCH2CO), 54.5 (NCHCH2), 53.2 (NCH2CO), 50.0 (br, NCH2 ring), 41.1 (NCH2CO2). νmax (cm−1): 3,318 (OH), 2,973, 2,839, 1,731 (C=O), 1,688 (C=O), 1,538, 1,505, 1,455, 1,194, 1,134. m/z (MALDI/TOF): 873 (100% [M + H]+). Anal. Found (Calcd for C40H56N8O14·3CF3CO2H·H2O): C, 45.3 (44.8); H, 4.9 (5.0); N, 9.0 (9.1).
General procedure for the preparation of lanthanide(III) complexes
An aqueous lanthanide chloride solution (0.21 M, 614 µL) was added to a solution of ligand 3 (0.14 g, 0.13 mmol) in 50:50 water/methanol (15 mL, pH 6.0). The resulting solution was stirred for 3 days at 333 K and the pH was maintained between 6 and 7 throughout by addition of 1 N KOH. The absence of free Ln3+ (Eu or Gd) was verified by colorimetric assay using xylenol orange (1 M AcONa/AcOH buffer, pH 5.3). The reaction mixture was allowed to cool to room temperature and the solvents were removed by freeze-drying. Eu2 m/z (ESMS, ESI+): 1,022 (100% [M – 2H]+); an appropriate isotope pattern was observed. Gd2 m/z (ESMS, ESI+): 1,030 (100% [M – 2H]+); an appropriate isotope pattern was observed. Yb2 m/z (ESMS, ESI+): 1,044 (100% [M – 2H]+); an appropriate isotope pattern was observed. Eu3: m/z (ESMS, ESI+): 1,263 (100%, [M – 2H]+); an appropriate isotope pattern was observed. Gd3 m/z (ESMS, ESI+): 632 (100% [M – H]2+); an appropriate isotope pattern was observed.
CEST measurements
A quantitative description of the NMR behavior of two or more exchanging pools of nuclei is described by the Bloch equations modified for exchange [19]. For the simplest situation of two exchanging pools A and B, it has been shown that the maximum reduction in the water signal intensity occurs when (1) the nuclei in pool B are completely saturated so that and (2) there is no direct excitation of pool A nuclei by the B1 irradiation that excites B nuclei. Condition 1 is met when B1 is sufficiently large. Condition 2 is met when the relative difference in resonance frequency of the two pools (Δω) is very large so that Δω/ω1 ≫ 1. Under these conditions, the steady-state solution of the Bloch equations gives
| (1) |
where c is the concentration of the PARACEST agent and q is the number of bound water molecules per PARACEST complex; τa is the residence lifetime in pool A, which is related, through the relative concentration of the two pools, to τM, the residence lifetime in pool B; and T1a is spinlattice relaxation time of bulk water.
CEST images were acquired using a 200-MHz (1H) Varian Inova scanner. Spin-echo images of two phantoms each containing 20 mM solutions of Eu2, one with and one without 5% albumin, were acquired using a 6-s presaturation pulse applied off-resonance (−54 ppm) and on-resonance (54 ppm), respectively, for each phantom. The percentage CEST enhancement map, E, was computed from these images, where E = 100 × (Soff − Son)/Soff, using MATLAB image processing software.
Results and discussion
Synthesis
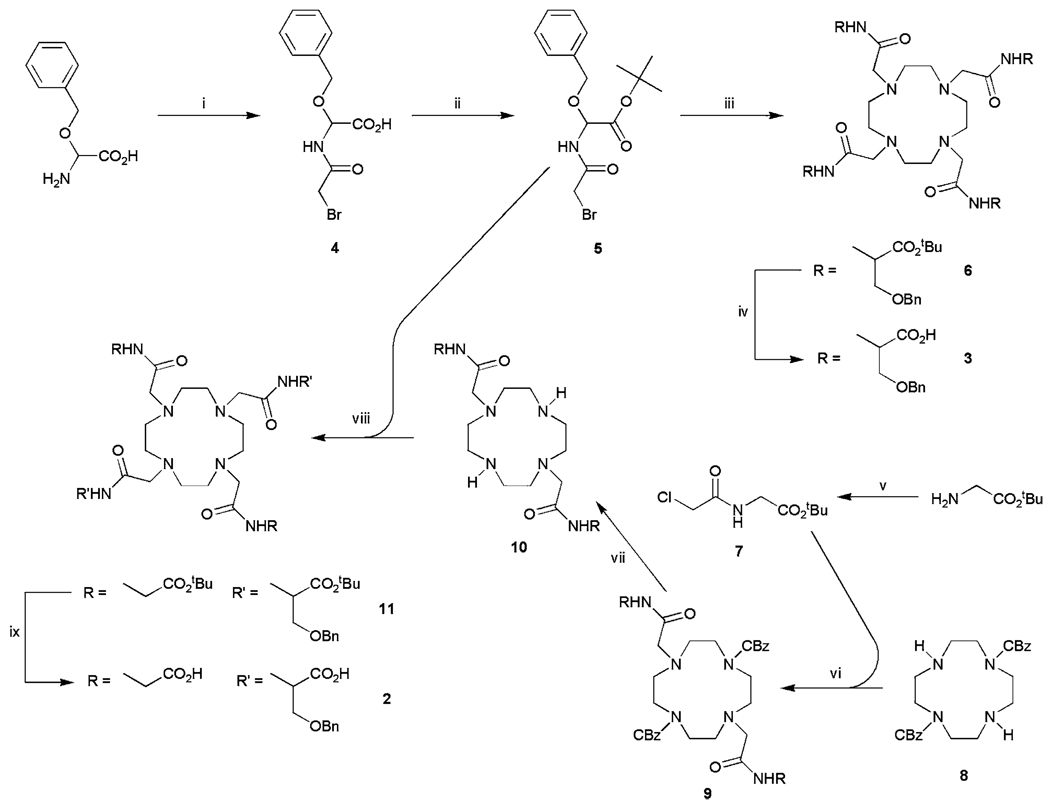
The synthetic routes to ligands 2 and 3 are outlined in Scheme 1. O-Benzyl-l-serine was neutralized with sodium hydroxide and reacted with bromoacetyl bromide in a biphasic reaction using potassium carbonate as a base. The carboxylate of the resulting bromoacetamide 4 was protected as a tert-butyl ester using tert-butyl trichloroace-timidate and BF3·OEt. Although the yields for preparing derivatives of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) where the O-benzyl substituent is in the α-position of the acetate side arms was reportedly low [20, 21], alkylation of cyclen with 4 equiv of 5 in acetonitrile using Hünig’s base at 328 K afforded the protected ligand 6 in 93% yield. Ligand 6 was then deprotected using TFA to afford 3 in 25% overall yield from O-benzyl serine.
Scheme 1.
The synthetic route to ligands 2 and 3. Reagents and conditions: i BrCH2COBr/K2CO3/NaOH/273 K; ii tert-butyl trichloroacetimidate/BF3·OEt/tetrahydrofuran/273 K; iii cyclen/iPr2NEt/MeCN/328 K; iv trifluoroacetic acid (TFA); v ClCH2COCl/K2CO3/H2O/CH2Cl2/273 K; vi K2CO3/MeCN/333 K; vii H2/Pd on C; viii iPr2NEt/MeCN/333 K; ix TFA
The trans-O-benzyl serine substituted ligand 2 was prepared by first preparing bisbenzylcarbamate-protected cyclen from a well-established procedure using benzyl chloroformate [18]. The tert-butyl ester of glycine was reacted with chloroacetyl chloride in a biphasic reaction using potassium carbonate as a base to afford the chloroacetamide 7. This was reacted with 8 using standard alkylating conditions to give 9 and the carbamate protecting groups were then removed by catalytic hydrogenolysis over palladium on carbon to afford the bisamide 10 in 96% yield. 10 was then alkylated with 5 in acetonitrile using Hünig’s base to give the tetra-tert-butyl ester 11. Subsequent deprotection using TFA afforded 2 in 48% overall yield from compound 8.
Lanthanide complexes of 2 and 3 were prepared by mixing equimolar quantities of the ligand and the appropriate lanthanide chloride in a water/methanol solution at 333 K. The isolated yields of the complexes of 3 were lower than usual (approximately 30%), the result of the poor solubility of ligand 3 in water. However, the resulting lanthanide complexes of 3 were found to be more soluble in water than the previously reported LnDOTA–(BOM)4 complexes (where BOM is benzyloxymethyl ether) [20, 21].
Binding studies with human serum albumin
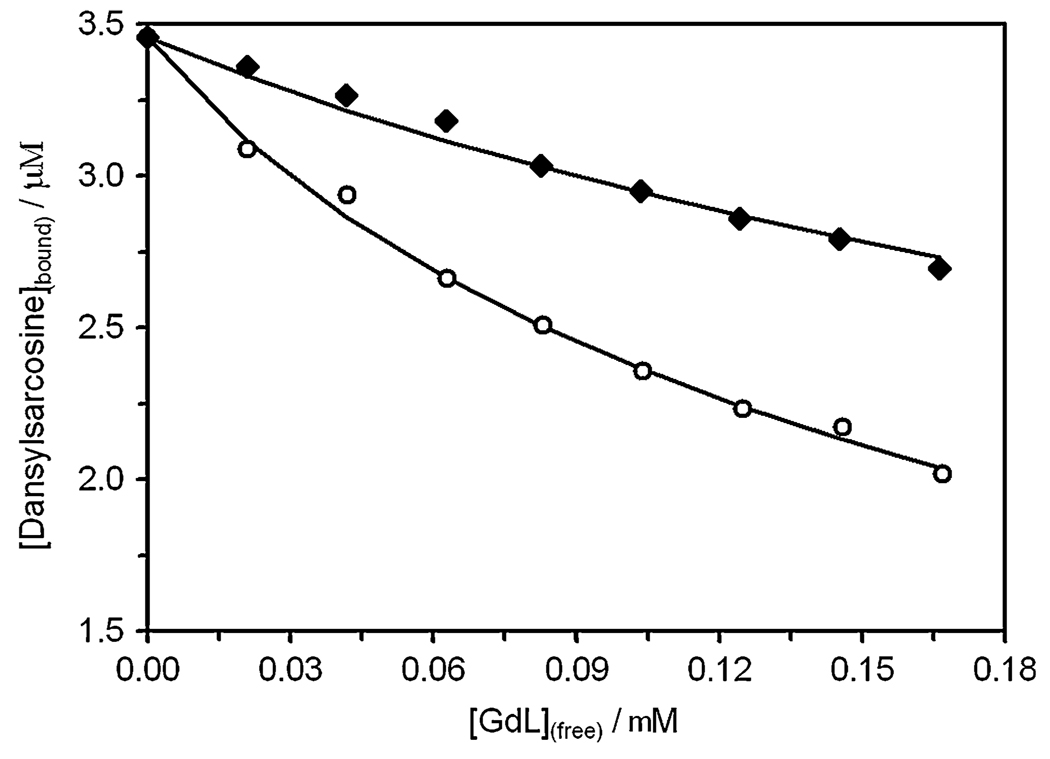
Human serum albumin (HSA) is known to bind a variety of hydrophobic drugs in one of two major binding domains, referred to as site I and site II [22, 23]. A variety of fluorescence methods have been developed to evaluate the binding sites and association constants of drugs with HSA. Site I is the primary binding site for drugs like warfarin and various phenylbutazone analogs, whereas diazepam and ibuprofen bind primarily at site II [24]. Warfarin itself has fluorescence properties (λem = 320 nm) that allow it to be used to assay binding at site I, whereas dansylsarcosine (λem = 360 nm) is frequently used as a fluorescent probe for site II [25]. Neither Gd2 nor Gd3 fluoresces at those wavelengths. Consequently, the ability of Gd2 and Gd3 to displace either of the fluorescent probes, warfarin or dansylsarcosine, from HSA was investigated by monitoring the changes in fluorescence with added Gd2 or Gd3. Full details of this procedure have previously been reported by Caravan et al. [25]. The fluorescence emission intensity of these probes is higher when they are bound to HSA and consequently their emission intensity decreases when they are displaced from their normal binding sites by other compounds. The emission intensity of dansylsarcosine was found to fall after addition of either Gd2 or Gd3 to a solution containing an equal concentration of dansylsar-cosine (5 µM) and HSA (5 µM) at 298 K and pH 7.4 (Fig. 1). In contrast, little or no change in fluorescence intensity was observed when either Gd2 or Gd3 was added to a sample of HSA (5 µM) containing 1 equiv of warfarin (5 µM). This indicates that Gd2 and Gd3 both bind at a site II subdomain of HSA but have no, or only weak, binding affinity for site I. The data obtained for the displacement of dansylsarcosine from HSA by both Gd2 and Gd3 (Fig. 1) were fitted to an inhibition binding model (Eq. 2, Eq. 3).
| (2) |
where
| (3) |
Fig. 1.
The inhibition of dansylsarcosine binding to human serum albumin (HSA) by Gd2 (circles) and Gd3 (diamonds)
Here [FP]bound and [FP]t are concentrations of the fluorescent probe bound to HSA and the total concentration of fluorescent probe, respectively, [HSA]t is the total concentration of HSA and [GdL]free is the concentration of unbound gadolinium complex. KA is the association constant of the fluoresecent probe with HSA, is the apparent dissociation constant of the fluorescent probe from HSA in the presence of Gd2 and Gd3, and 1/KI is the site-specific association constant of the gadolinium complexes with HSA. The value of KA for HSA and dansylsarcosine in phosphate-buffered saline (PBS) (1.45 × 106 M−1) was taken from the literature [26]. Fitting the titration curves obtained by displacement of dansylsarcosine with Gd2 and Gd3 (Fig. 1) to Eq. 2 and Eq. 3 gave 1/KI values of 3.14 and 1.04 × 104 M−1 for the binding of Gd2 and Gd3 at site II, respectively. These association constants are considerably higher (40-fold) than those determined for the binding of GdEOB-DTPA is (where EOB-DTPA (2-ethoxybenzyl-diethylenetriamine pentaacetic acid) with HSA [16], a lipophilic hepatobiliary targeted CA, and are comparable with that of the high-affinity HSA binding blood pool agent MS-325 [25].
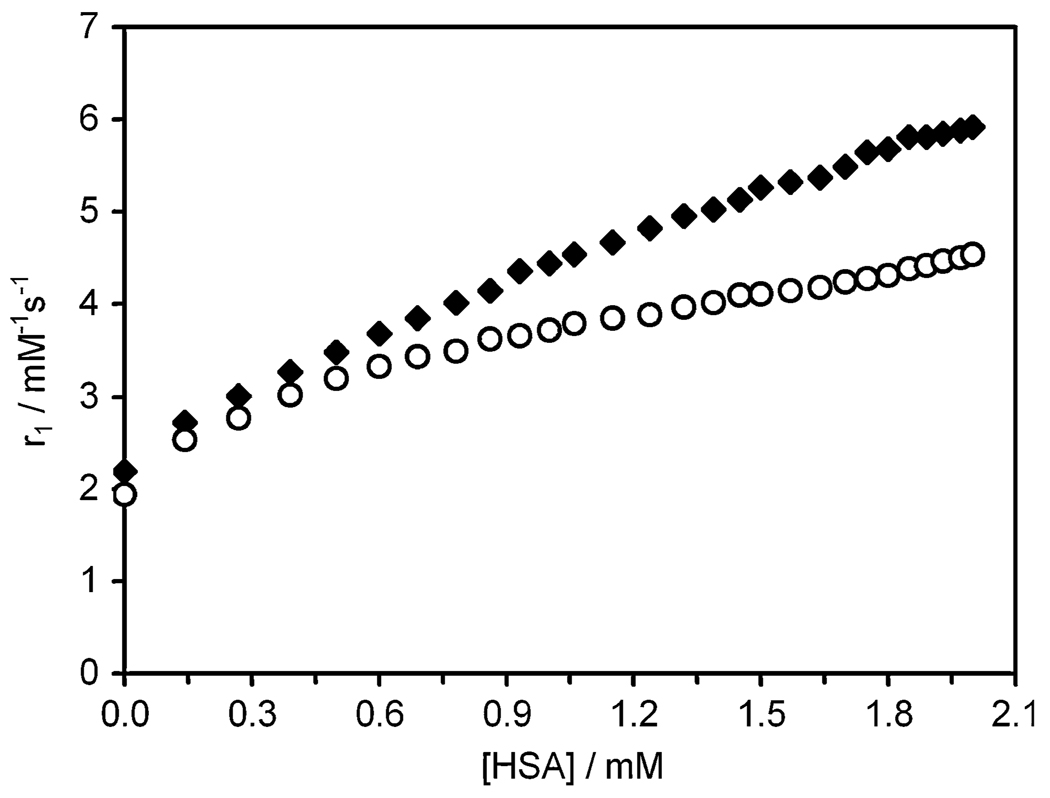
The binding of Gd2 and Gd3 to HSA was further verified by water proton relaxation titrations. The relaxivities of Gd2 (1.9 mM−1 s−1) and Gd3 (2.2 mM−1 s−1) (20 MHz, 298 K) resemble values reported for outer-sphere-only complexes (q = 0) [27] more closely than those of gadolinium complexes containing a single, inner-sphere coordinated water molecule (q = 1) [28]. However, these values are also consistent with complexes that have a single, inner-sphere water molecule that is in slow water exchange with bulk water (also validated in CEST spectra; see later). Upon addition of HSA, the relaxivities of Gd2 and Gd3 gradually increased with increasing HSA concentration to values approaching 4.6 and 5.9 mM−1 s−1 at 2 mM HSA, respectively, consistent with binding of the complexes to HSA (Fig. 2). This increase in relaxivity is small compared with that for other HSA-binding systems that exhibit more rapid water exchange [21, 25, 29], also indicating the water exchange kinetics in both Gd2 and Gd3 are slow.
Fig. 2.
Binding of the complexes Gd2 (circles) and Gd3 (diamonds) to HSA has the effect of increasing the longitudinal relaxivity (r1) of each complex. Titrations were performed at 20 MHz, 298 K, in N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid buffer, pH 7.4
CEST studies
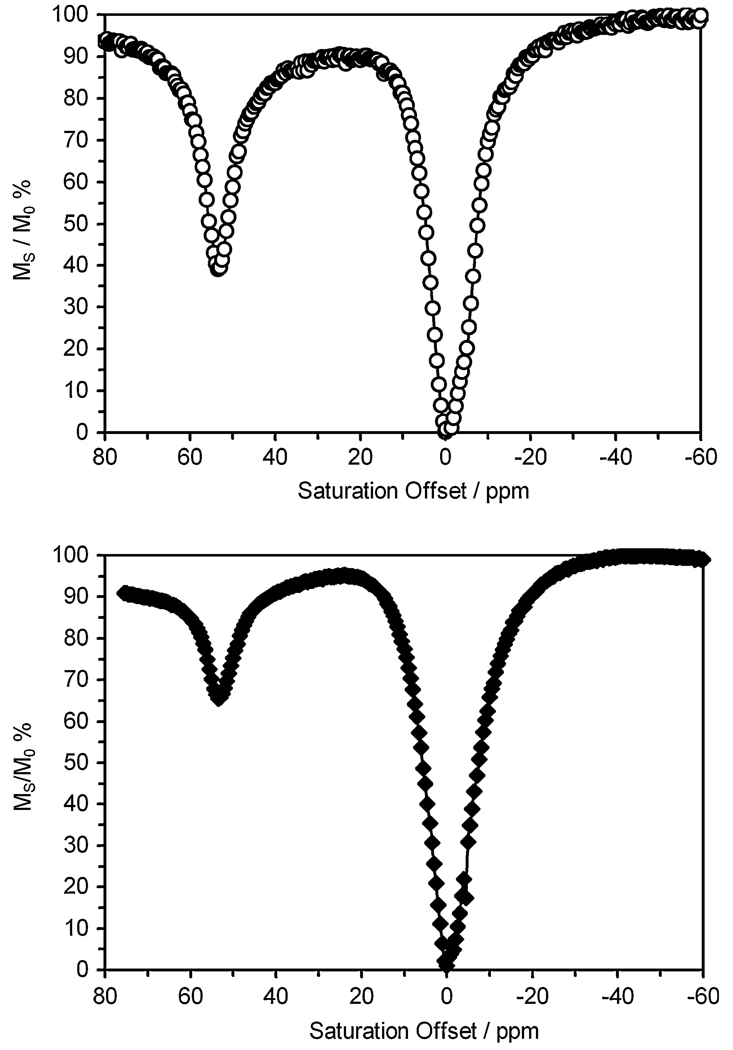
PARACEST agents are commonly characterized by selectively presaturating an aqueous sample of the agent in incremental steps over a range of frequencies and plotting the remaining steady-state bulk water signal, Ms/M0, versus saturation frequency. This was originally referred to as a Z-spectrum [30] and more recently as a CEST spectrum [31]. Figure 3 shows plots of Ms/M0 versus saturation frequency for 40 mM Eu2 and 20 mM Eu3 both collected in pure water as solvent at 298 K. The peak at 0 ppm represents direct saturation of bulk water, while the peak centered near 54 ppm reflects the chemical exchange between a Eu3+-bound water molecule and bulk solvent. The hyperfine shift and water exchange properties of EuDOTA tetraamide complexes typically have properties favorable for CEST with a peak arising from the slowly exchanging water molecule near 50 ppm. An approximately 35% decrease in bulk water signal intensity was observed for the Eu3 sample, while the more highly concentrated Eu2 sample resulted in a 64% decrease in bulk water signal intensity under identical conditions. Eu2 proved to be more soluble in water (approximately 3 M) than Eu3.
Fig. 3.
Chemical exchange saturation transfer (CEST) spectra, plotting the solvent water signal intensity (expressed as a percentage of its initial intensity) against presaturation frequency, for a 40 mM aqueous solution of Eu2 (top) and a 20 mM aqueous solution of Eu3 (bottom) at 298 K, irradiation time 2 s, B1 = 26 µT
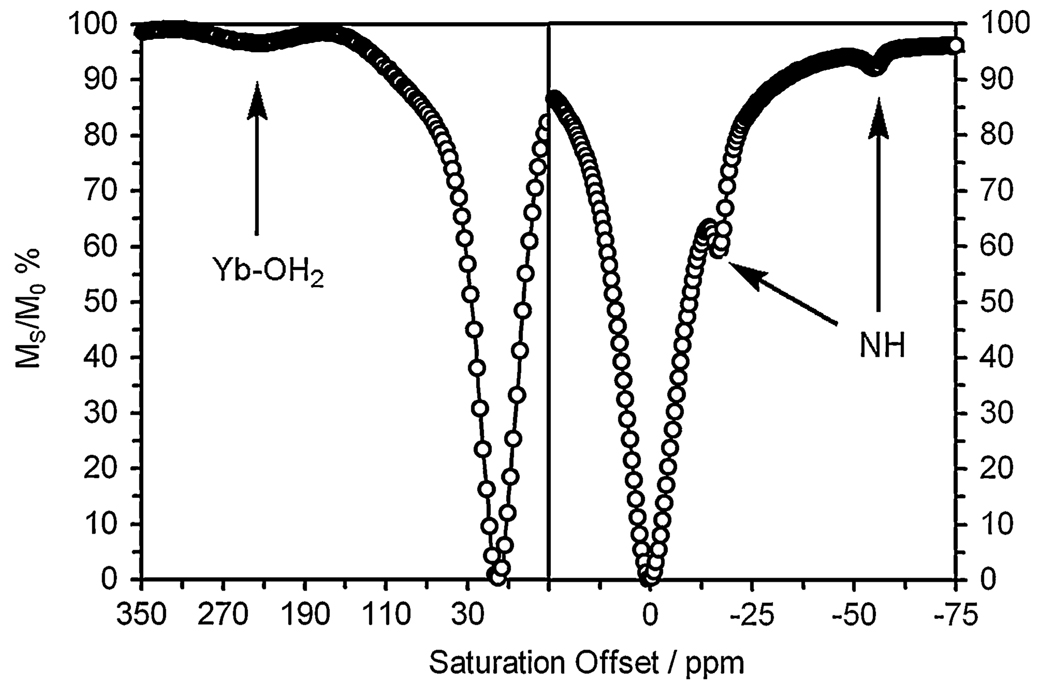
In contrast to these EuDOTA tetraamide complexes, a water-exchange CEST peak is typically not seen for the corresponding Yb3+ complexes. This is believed to be the result of water exchange kinetics that lie outside the permissible range for CEST, viz., water exchange is too fast [32, 33]. Nonetheless, a CEST effect arising from the amide protons of these ytterbium complexes can usually be observed [4, 12] and so the CEST spectrum of Yb2 at 25 mM was recorded. Two different irradiation powers and durations were employed in the acquisition of these CEST spectra. Two peaks were detected near −18 and −56 ppm in the CEST spectrum when a relatively high-power (26-µT) presaturation pulse was applied for 1 s. These were assigned to the exchanging –NH amide protons in the complex; the peak at −18 ppm is at a shift similar to those reported for other Yb3+ complexes by Aime et al. [11] and Zhang et al. [12]. The peak at −56 ppm is unusually highly shifted; the largest shift previously reported was that observed by Aime et al. [13] and was −29 ppm. When a lower-power (6.5-µT) presaturation pulse was applied for 4 s, a broad (relatively fast exchanging) but detectable CEST peak was observed near 230 ppm. This peak can only be assigned to an exchanging inner-sphere water molecule on the Yb3+. Although a Yb3+-bound water resonance has not been detected previously by 1H NMR, presumably owing to rapid water exchange, the chemical shift position for a Yb3+-bound water molecule for ligand systems such as these was previously predicted to be near 200 ppm on the basis of chemical shift comparisons with other ligand resonances [32]. The low irradiation power and long duration used to record this spectrum were critical to the observation of this bound water molecule because at higher powers the rapid exchange kinetics and off-resonance direct saturation led to a coalescence of the bulk and bound water peaks, obscuring the CEST peak from the bound water. Although the CEST effect from the Yb3+-bound water molecule in Yb2 is relatively weak, this system does offer the possibility of using the CEST ratio (H2O vs. NH) [10] as a direct indicator of sample pH (Fig. 4).
Fig. 4.
CEST spectra of a 25 mM solution of Yb2 recorded at 500 MHz, pH 7.4, and 298 K. On the left is shown the downfield region of the spectrum recorded with an irradiation time of 4 s, B1 = 6.5 µT; a small CEST peak arising from the coordinated water molecule is clearly visible. On the right is shown the upfield region for an irradiation time of 1 s, B1 = 26 µT; CEST peaks arising from the amide NH protons can be seen
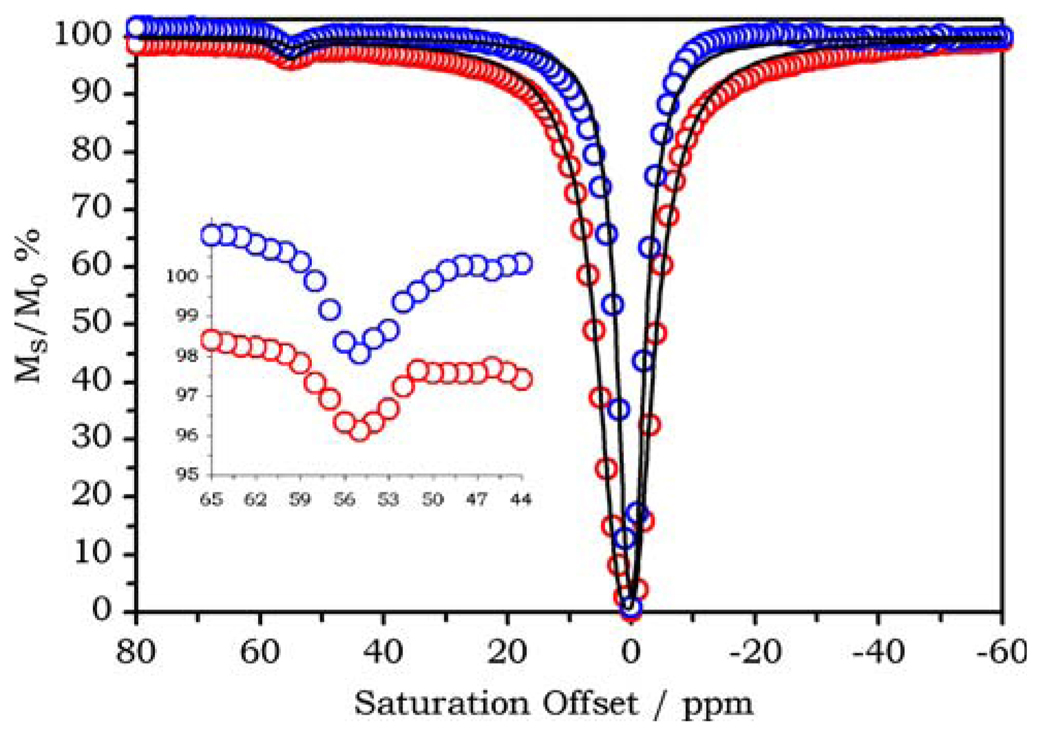
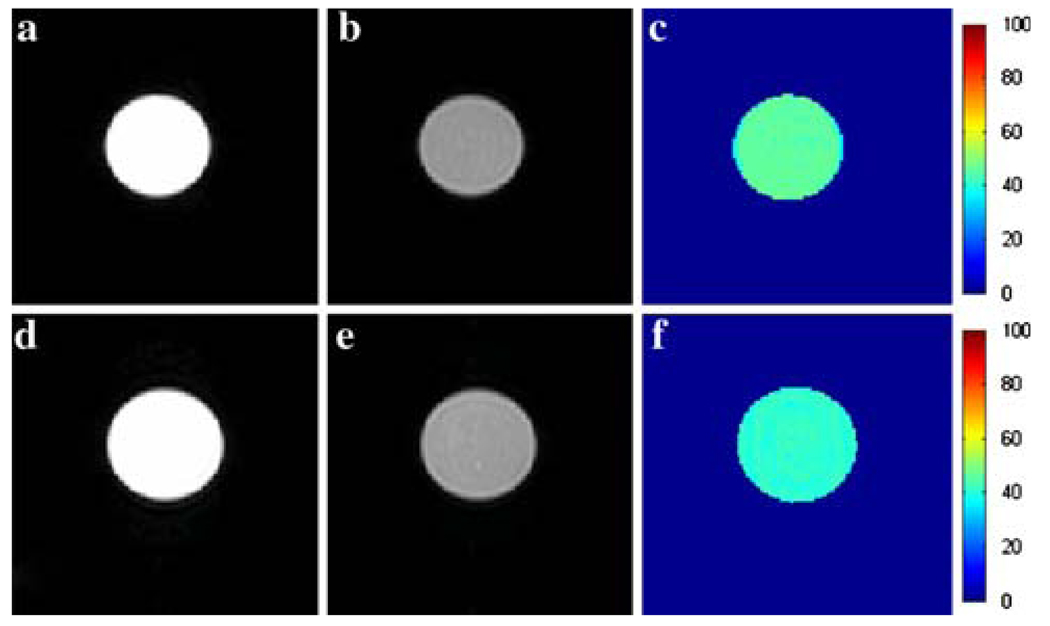
Many of the gadolinium complexes designed to bind to serum albumin have been reported to exhibit a change in their water exchange kinetics upon binding to the protein [17, 25, 34]. Usually the result is a deceleration of water exchange. Given the sensitivity of CEST to changes in water exchange rates [19, 35] it is critical that binding of the PARACEST agents Ln2 and Ln3 to HSA does not result in adverse water exchange kinetics. Accordingly, the CEST spectra of Eu2, Yb2, Tm2, and Eu3 were recorded in PBS (pH 7.4) in the presence and absence of HSA. The change in the CEST properties of the chelate upon binding to HSA were only minimal as exemplified by the spectra of Eu2 with and without HSA (Fig. 5). The CEST spectra were recorded under conditions designed to ensure that essentially all of the Eu2 was bound to HSA; the concentrations of both protein and PARACEST agent were 0.75 mM in a PBS buffer and for comparative purposes a second CEST spectrum was acquired in the absence of HSA. The two spectra resemble one another closely, although the width of the direct saturation peak at 0 ppm is greater in the presence of the protein, a result of proton-exchange events occurring between the protein and bulk water in addition to an increase in sample viscosity that shortens T2. The similarity in the CEST peaks arising from the coordinated water molecule in the presence and absence of HSA indicates that the water exchange kinetics of the complex are not negatively impacted by the protein binding event. Color-coded CEST images of phantoms collected in the absence and presence of HSA (Fig. 6) demonstrate nicely that the images are relatively insensitive to the presence of protein.
Fig. 5.
The CEST spectra of 0.75 mM Eu2 in phosphate-buffered saline (PBS) recorded in the absence (blue) and presence (red) of 0.75 mM HSA. B0 = 400 MHz, B1 = 19 µT, irradiation time 6 s, 298 K
Fig. 6.
Images of 20 mM solutions of Eu2 in the absence and presence of 5% HSA. Left (a, d), center (b, e), and right (c, f) columns represent the off-resonance (−54 ppm), on-resonance (54 ppm), and the percentage CEST enhancement maps, respectively. Samples in the top row contained 20 mM Eu2 in PBS, while samples in the bottom row contained in addition 5% HSA (Buminate®). Each image was collected using a standard spin-echo sequence with a 6-s presaturation pulse applied at the indicated offset
The CEST spectra of Eu2 shown in Fig. 5 were fitted to the Bloch equations modified for CEST [19]. This fitting procedure is hampered by the necessarily low concentration of PARACEST agent that results in small CEST effects, and the presence of the protein, which broadens the direct saturation peak. The latter is the most problematic during the fitting procedure because account must be taken for the additional OH, NH, and water molecules that are associated with the protein. The CEST spectrum of Eu2 (Fig. 5, blue spectrum) was fitted to a two-pool (bulk and coordinated water) and three-pool (bulk and coordinated water and amide protons) model; the water residence lifetime (τM) obtained from this fitting was 1.0 ± 0.1 ms. This corresponds to a much slower rate of water exchange than was reported for the parent complex Eu12 (τM = 0.38 ms), consistent with the hypothesis put forward by Aime et al. [36] that bulkier and more hydrophobic amide substituents lead to slower water exchange rates. In fitting the CEST spectrum of Eu2 bound to HSA (Fig. 5, red spectrum) two approaches were taken. For model 1, no steps were taken to account for the presence of exchanging protons associated with the protein and allowed extremely short bulk water T2 values to account for the increased linewidth of the bulk solvent. For model 2, a large number of exchanging protons that could exchange with bulk water but not the agent were added; the relaxation time, shift, and magnitude of this “protein” pool were allowed considerable freedom during the fitting procedure. The fitting of this spectrum is, as a result, highly qualitative; however, irrespective of the approach taken in fitting the data, the water residence lifetime (τM) of Eu2 was found to decrease by a factor of approximately 2 upon binding to HSA. The water residence lifetime (τM) of Eu2 bound to HSA was estimated at 0.44 ms from model 1 and 0.66 ms from model 2. This acceleration of the water exchange rate is in marked contrast to the deceleration in water exchange rate observed in many other systems [17, 25, 34] but is not without precedent; complexes entrapped in the protein apoferritin have been reported to undergo acceleration of water exchange [37, 38].
Conclusions
Water exchange in Gd3+-based CAs plays a part in determining the relaxation efficiency or relaxivity of a complex, particularly when the complex experiences slower rotation such as when it is bound to a macromolecule. The bound-water lifetime in octadentate complexes of Gd3+, such as those formed with simple polyamino–polycarboxylate ligands like DTPA or DOTA, is typically 200–300 ns, but even this can limit the relaxivity of such complexes when they are targeted to a biological macromolecule. The optimal bound-water lifetime has been estimated at 20–30 ns. CEST-based CAs are also sensitive to water exchange even in the absence of a macromolecular binding. If water exchange is too fast, the chemical shift of the exchanging water molecule averages with that of bulk water and CEST cannot be initiated, but if water exchange is too slow, the presaturated spins fully relax before CEST can occur. Hence, there is an optimal water exchange rate for any CEST agent and that optimal rate can be further influenced by the chemical environment of the agent. For example, acidic or basic environments have been shown to catalyze proton exchange between a slowly exchanging Ln3+-bound water molecule and bulk water; this effect would be likely to alter the CEST properties of the agent [28], [41]. It has been reported that water exchange in Gd3+-based CAs slows when such complexes are bound to albumin. For example, the bound-water lifetime in GdDTPA(BOM)3, which also binds to HSA through benzyloxyether groups, increases by approximately 50% when this complex binds noncovalently to HSA [16]. The effect is even more dramatic in GdPCTP (where PCTP is 3,6,10,16-tetraazabicyclo[10.3.1]hexadeca-1(16),12,14-triene-N′,N″,N′″-trimethylenephosphonic acid) [13], where the bound-water lifetime increases from approximately 8 to 290 ns (approximately 30-fold slower) when this q = 1 complex binds to HSA [40]. Such a substantial change in water exchange would have a profound influence on the CEST properties of similar albumin-binding PARACEST agents. In the case of Eu2, the bound-water lifetime determined from fitting CEST spectra indicates that water exchange accelerates (by a factor of approximately 2) when this complex binds with HSA. Given the slow water exchange rate in this complex when it is not bound to HSA, the change in water exchange rate was found to have little influence on the CEST properties of the agent upon binding to HSA. The slow water exchange kinetics of these complexes mean that a CEST effect arising from the protons of a water molecule coordinated to Yb3+ can be observed for the first time.
In summary, fluorescence displacement experiments showed that both Gd2 and Gd3 bind reversibly at site II of HSA and this results in a slowing of water exchange by about two fold. The relatively small increase in water proton relaxivity that was observed upon addition of HSA to either Gd2 or Gd3 is also consistent with slow water exchange systems both in the absence and in the presence of HSA. The fact that HSA has a high binding affinity for these PARACEST agents and that CEST is not quenched by protein binding make them potentially useful as vasculature imaging agents although improvements in sensitivity may be required prior to in vivo application.
Acknowledgements
This research was supported in part by grants from the National Institutes of Health (CA-115531, DK-058398, EB-04285, and RR-02584), the Department of Defense Breast Cancer Research Program (Idea grant W81XWH-05-1-0223), and the Robert A. Welch Foundation (AT-584).
Contributor Information
M. Meser Ali, Department of Chemistry, University of Texas at Dallas, P.O. Box 830688, Richardson, TX 75083-0688, USA.
Mark Woods, Department of Chemistry, University of Texas at Dallas, P.O. Box 830688, Richardson, TX 75083-0688, USA; Macrocyclics, Inc, 2110 Research Row, Suite 425, Dallas, TX 75235, USA.
Eul Hyun Suh, Advanced Imaging Research Center, University of Texas Southwestern Medical Center, 2201 Inwood Road, Dallas, TX 75390-8568, USA, e-mail: dean.sherry@utsouthwestern.edu.
Zoltan Kovacs, Advanced Imaging Research Center, University of Texas Southwestern Medical Center, 2201 Inwood Road, Dallas, TX 75390-8568, USA, e-mail: dean.sherry@utsouthwestern.edu.
Gyula Tircsó, Department of Chemistry, University of Texas at Dallas, P.O. Box 830688, Richardson, TX 75083-0688, USA.
Piyu Zhao, Department of Chemistry, University of Texas at Dallas, P.O. Box 830688, Richardson, TX 75083-0688, USA.
Vikram D. Kodibagkar, Department of Radiology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390, USA
A. Dean Sherry, Department of Chemistry, University of Texas at Dallas, P.O. Box 830688, Richardson, TX 75083-0688, USA; Advanced Imaging Research Center, University of Texas Southwestern Medical Center, 2201 Inwood Road, Dallas, TX 75390-8568, USA, e-mail: dean.sherry@utsouthwestern.edu.
References
- 1.Merbach AE, Toth E. The chemistry of contrast agents in medical magnetic resonance imaging. Chichester: Wiley; 2001. [Google Scholar]
- 2.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 3.Ward KM, Balaban RS. Magn Reson Med. 2000;44:799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Terreno E, Castelli Daniela D, Cravotto G, Milone L, Aime S. Invest Radiol. 2004;39:235–243. doi: 10.1097/01.rli.0000116607.26372.d0. [DOI] [PubMed] [Google Scholar]
- 5.Goffeney N, Bulte JWM, Duyn J, Bryant LH, Jr, van Zijl PCM. J Am Chem Soc. 2001;123:8628–8629. doi: 10.1021/ja0158455. [DOI] [PubMed] [Google Scholar]
- 6.Snoussi K, Bulte JWM, Gueron M, van Zijl PCM. Magn Reson Med. 2003;49:998–1005. doi: 10.1002/mrm.10463. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Payen J-F, Wilson DA, Traystman RJ, van Zijl PCM. Nat Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Winter P, Wu K, Sherry AD. J Am Chem Soc. 2001;123:1517–1518. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Wu K, Sherry AD. J Am Chem Soc. 2002;124:4226–4227. doi: 10.1021/ja017133k. [DOI] [PubMed] [Google Scholar]
- 10.Aime S, Barge A, Castelli DD, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. Magn Reson Med. 2002;47:639–648. doi: 10.1002/mrm.10106. [DOI] [PubMed] [Google Scholar]
- 11.Aime S, Castelli DD, Terreno E. Angew Chem Int Ed Engl. 2002;41:4334–4336. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Michaudet L, Burgess S, Sherry AD. Angew Chem Int Ed Engl. 2002;41:1919–1921. [PubMed] [Google Scholar]
- 13.Aime S, Delli Castelli D, Fedeli F, Terreno E. J Am Chem Soc. 2002;124:9364–9365. doi: 10.1021/ja0264044. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Trokowski R, Sherry AD. J Am Chem Soc. 2003;125:15288–15289. doi: 10.1021/ja038345f. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Malloy C, Sherry AD. J Am Chem Soc. 2005;127:17572–17573. doi: 10.1021/ja053799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aime S, Chiaussa M, Digilio G, Gianolio E, Terreno E. J Biol Inorg Chem. 1999;4:766–774. doi: 10.1007/s007750050349. [DOI] [PubMed] [Google Scholar]
- 17.Aime S, Botta M, Fasano M, Crich SG, Terreno E. J Biol Inorg Chem. 1996;1:312–319. [Google Scholar]
- 18.Kovacs Z, Sherry AD. J Chem Soc Chem Commun. 1995:185–186. [Google Scholar]
- 19.Woessner DE, Zhang S, Merritt ME, Sherry AD. Magn Reson Med. 2005;53:790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 20.Aime S, Botta M, Ermondi G, Fedeli F, Uggeri F. Inorg Chem. 1992;31:1100–1103. [Google Scholar]
- 21.Hovland R, Aasen AJ, Klaveness J. Org Biomol Chem. 2003;1:1707–1710. doi: 10.1039/b301944f. [DOI] [PubMed] [Google Scholar]
- 22.Sudlow G, Birkett DJ, Wade DN. Mol Pharm. 1976;12:1052–1061. [PubMed] [Google Scholar]
- 23.Sudlow G, Birkett DJ, Wade DN. Mol Pharm. 1975;11:824–832. [PubMed] [Google Scholar]
- 24.Peters TJ. All about albumin: biochemistry, genetics and medicinal applications. San Diego: Academic; 1996. [Google Scholar]
- 25.Caravan P, Cloutier NJ, Greenfield MT, McDermid SA, Dunham SU, Bulte JWM, Amedio JC, Jr, Looby RJ, Supkowski RM, Horrocks WD, Jr, McMurry TJ, Lauffer RB. J Am Chem Soc. 2002;124:3152–3162. doi: 10.1021/ja017168k. [DOI] [PubMed] [Google Scholar]
- 26.Sakai T, Yamasaki K, Sako T, Kragh-Hansen U, Suenaga A, Otagiri M. Pharm Res. 2001;18:520–524. doi: 10.1023/a:1011014629551. [DOI] [PubMed] [Google Scholar]
- 27.Geraldes CFGC, Urbano AM, Alpoim MC, Sherry AD, Kuan KT, Rajagopalan R, Maton F, Muller RN. Magn Reson Imaging. 1995;13:401–420. doi: 10.1016/0730-725x(94)00117-l. [DOI] [PubMed] [Google Scholar]
- 28.Aime S, Barge A, Bruce JI, Botta M, Howard JAK, Moloney JM, Parker D, de Sousa AS, Woods M. J Am Chem Soc. 1999;121:5762–5771. [Google Scholar]
- 29.Woods M, Zhang S, Von Howard E, Sherry AD. Chem Eur J. 2003;9:4634–4640. doi: 10.1002/chem.200305159. [DOI] [PubMed] [Google Scholar]
- 30.Grad J, Bryant RG. J Magn Reson. 1990;90:1–8. [Google Scholar]
- 31.Ward KM, Aletras AH, Balaban RS. J Magn Reson. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Sherry AD. J Solid State Chem. 2003;171:38–43. [Google Scholar]
- 33.Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD. Acc Chem Res. 2003;36:783–790. doi: 10.1021/ar020228m. [DOI] [PubMed] [Google Scholar]
- 34.Aime S, Gianolio E, Longo D, Pagliarin R, Lovazzano C, Sisti M. Chem Biol Chem. 2005;6:818–820. doi: 10.1002/cbic.200400364. [DOI] [PubMed] [Google Scholar]
- 35.Woods M, Woessner DE, Sherry AD. Chem Soc Rev. 2006;35:500–511. doi: 10.1039/b509907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aime S, Barge A, Batsanov AS, Botta M, Castelli DD, Fedeli F, Mortillaro A, Parker D, Puschmann H. Chem Commun. 2002:1120–1121. doi: 10.1039/b202862j. [DOI] [PubMed] [Google Scholar]
- 37.Aime S, Frullano L, Geninatti Crich S. Angew Chem Int Ed Engl. 2002;41:1017–1019. doi: 10.1002/1521-3773(20020315)41:6<1017::aid-anie1017>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Vasalatiy O, Zhao P, Zhang S, Aime S, Sherry AD. Contrast Media Mol Imaging. 2006;1:10–14. doi: 10.1002/cmmi.93. [DOI] [PubMed] [Google Scholar]
- 40.Aime S, Botta M, Crich SG, Giovenzana GB, Pagliarin R, Piccinini M, Sisti M, Terreno E. J Biol Inorg Chem. 1997;2:470–479. [Google Scholar]
- 41.Kálmán FK, Woods M, Caravan P, Jurek P, Spiller M, Tircsó G, Király R, Brücher R, Sherry AD. Inorg Chem. 2007 doi: 10.1021/ic0702926. [DOI] [PMC free article] [PubMed] [Google Scholar]