Abstract
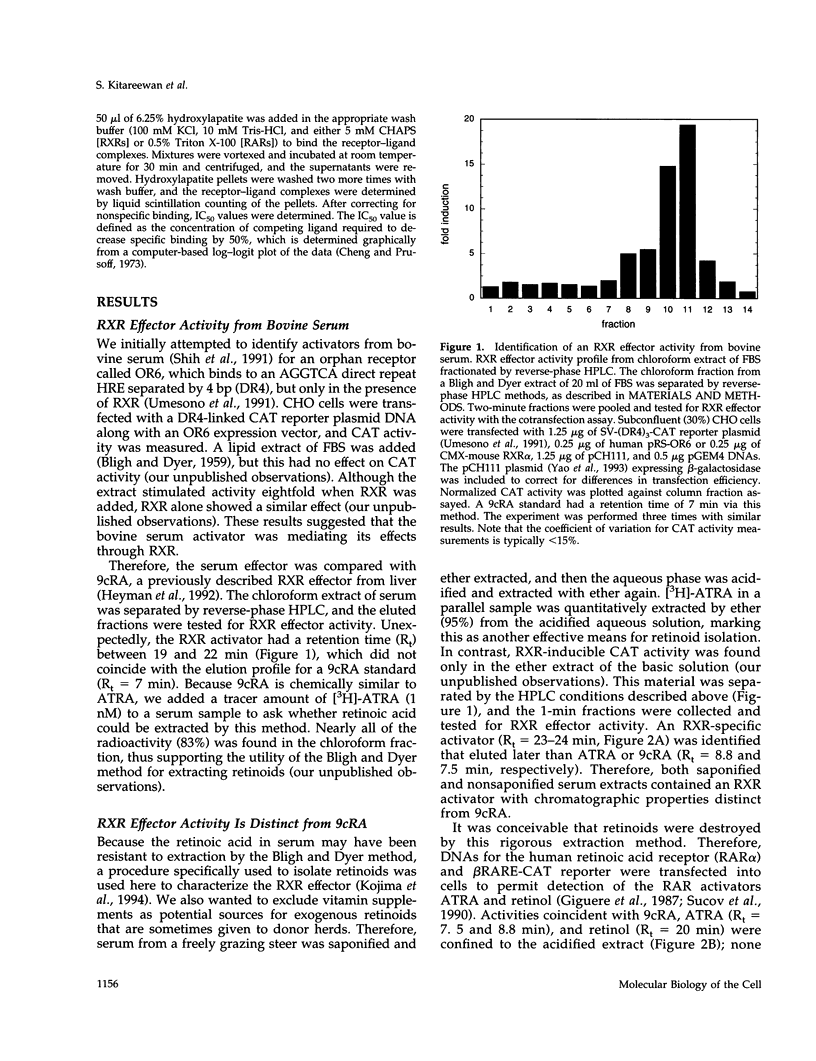
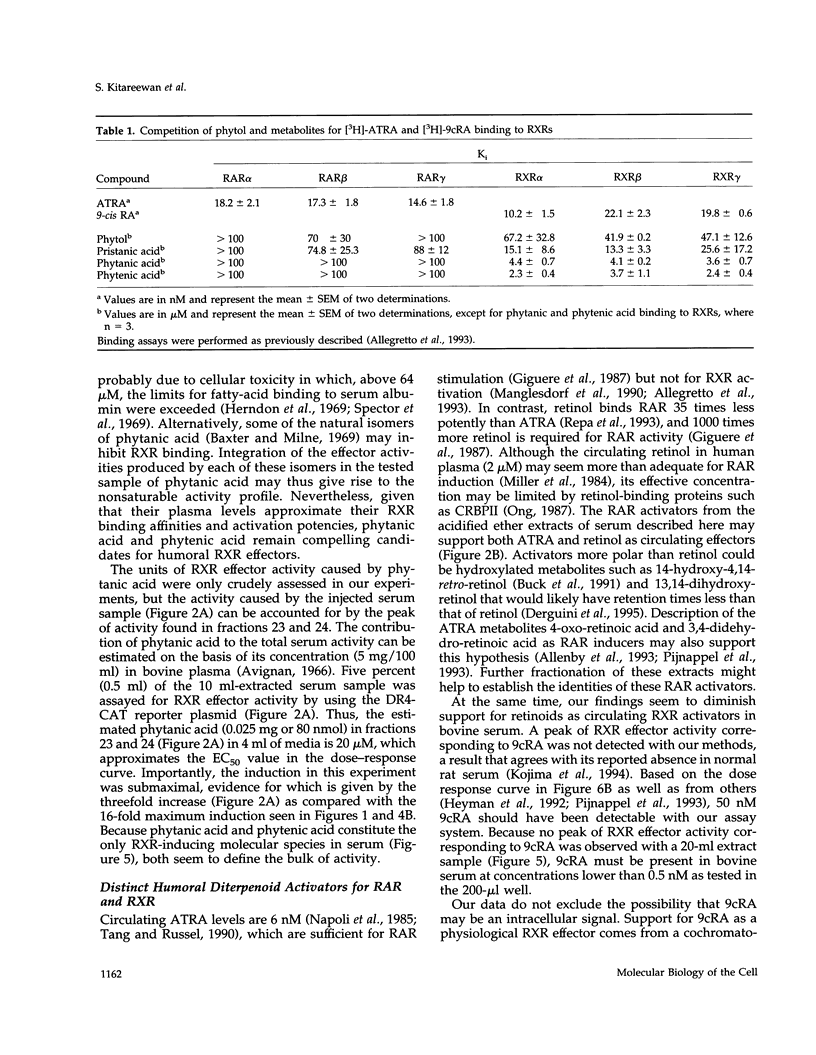
RXR is a nuclear receptor that plays a central role in cell signaling by pairing with a host of other receptors. Previously, 9-cis-retinoic acid (9cRA) was defined as a potent RXR activator. Here we describe a unique RXR effector identified from organic extracts of bovine serum by following RXR-dependent transcriptional activity. Structural analyses of material in active fractions pointed to the saturated diterpenoid phytanic acid, which induced RXR-dependent transcription at concentrations between 4 and 64 microM. Although 200 times more potent than phytanic acid, 9cRA was undetectable in equivalent amounts of extract and cannot be present at a concentration that could account for the activity. Phytanic acid, another phytol metabolite, was synthesized and stimulated RXR with a potency and efficacy similar to phytanic acid. These metabolites specifically displaced [3H]-9cRA from RXR with Ki values of 4 microM, indicating that their transcriptional effects are mediated by direct receptor interactions. Phytol metabolites are compelling candidates for physiological effectors, because their RXR binding affinities and activation potencies match their micromolar circulating concentrations. Given their exclusive dietary origin, these chlorophyll metabolites may represent essential nutrients that coordinate cellular metabolism through RXR-dependent signaling pathways.
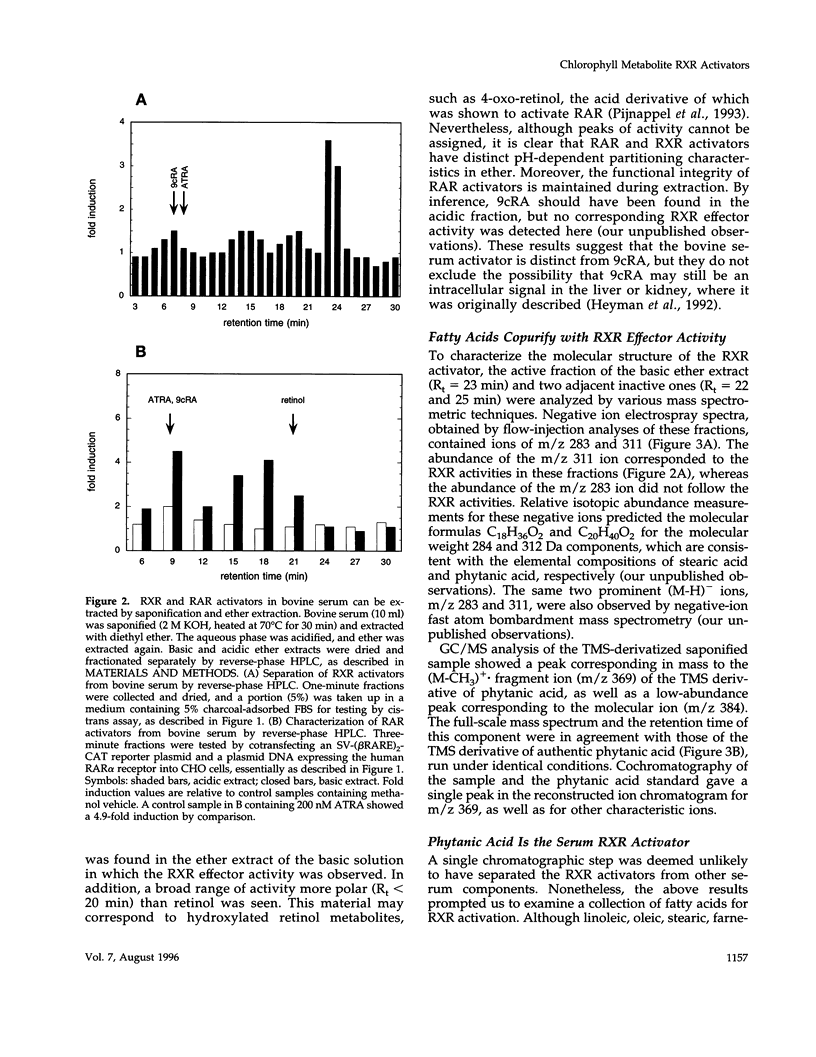
Full text
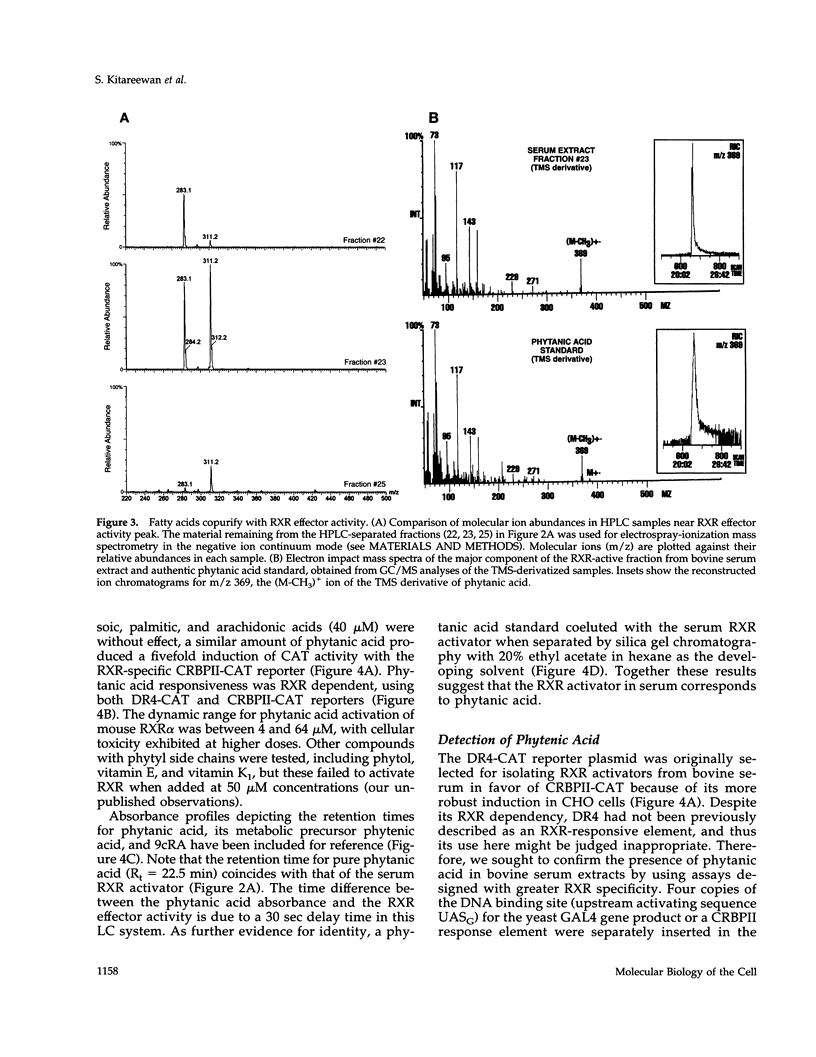
PDF


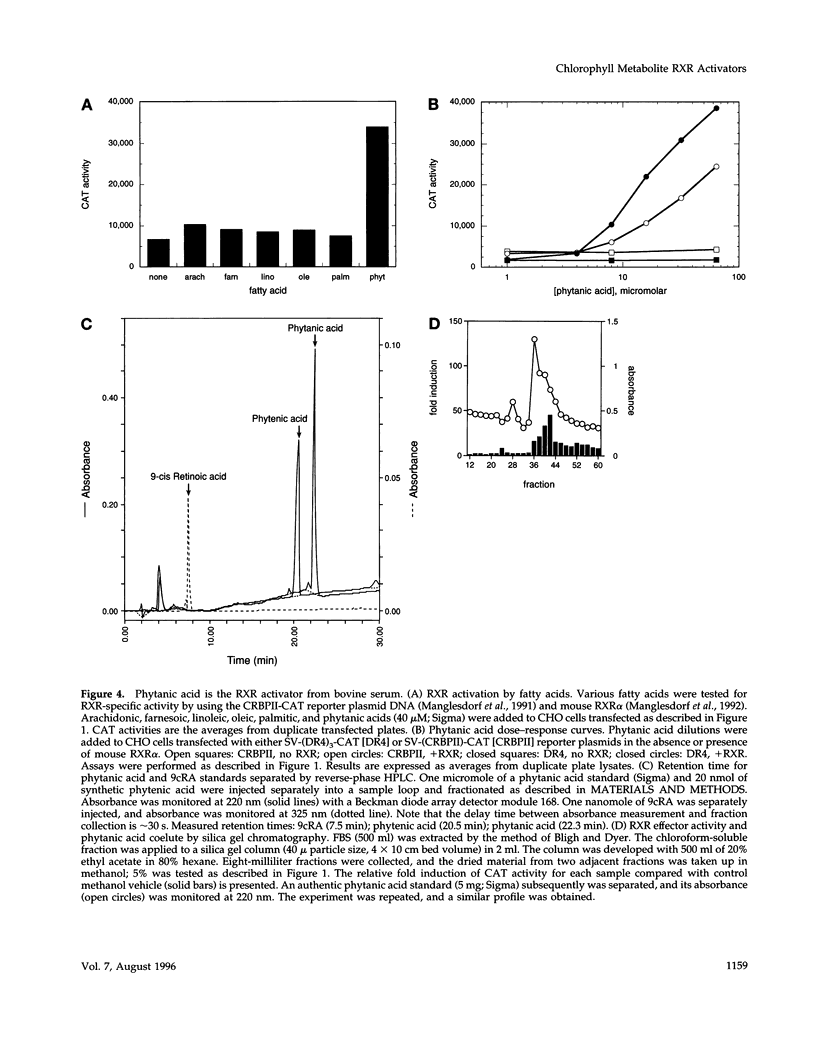




Selected References
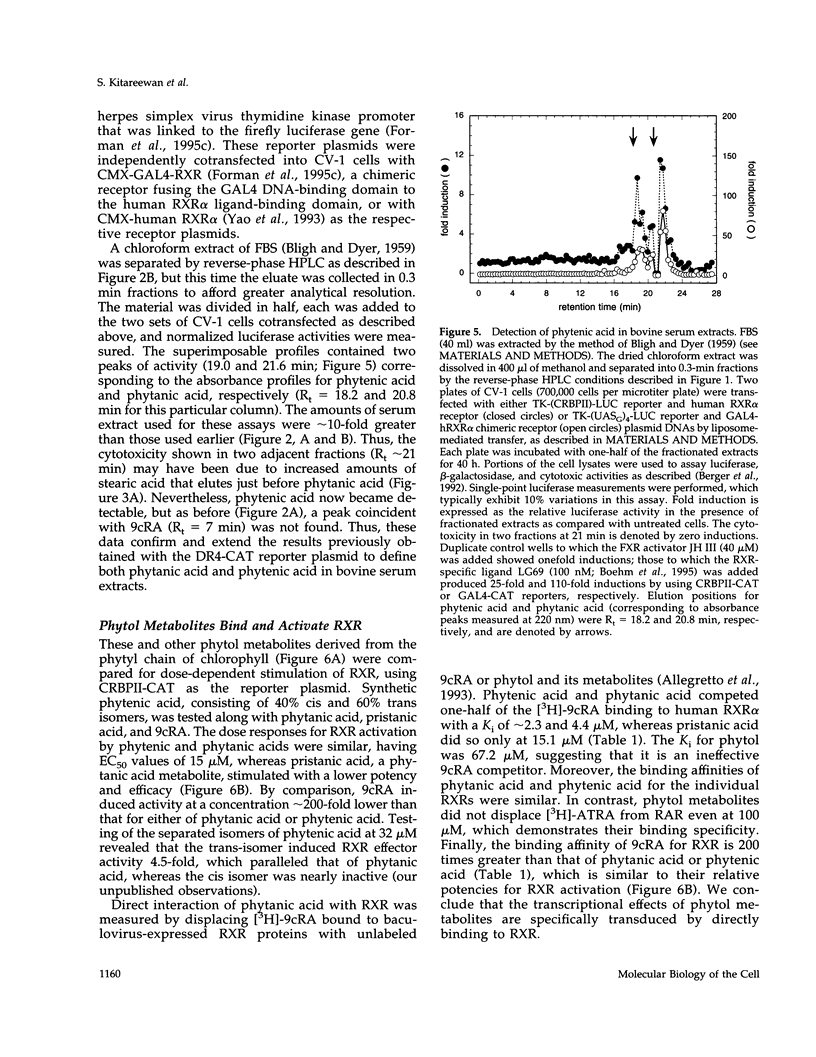
These references are in PubMed. This may not be the complete list of references from this article.
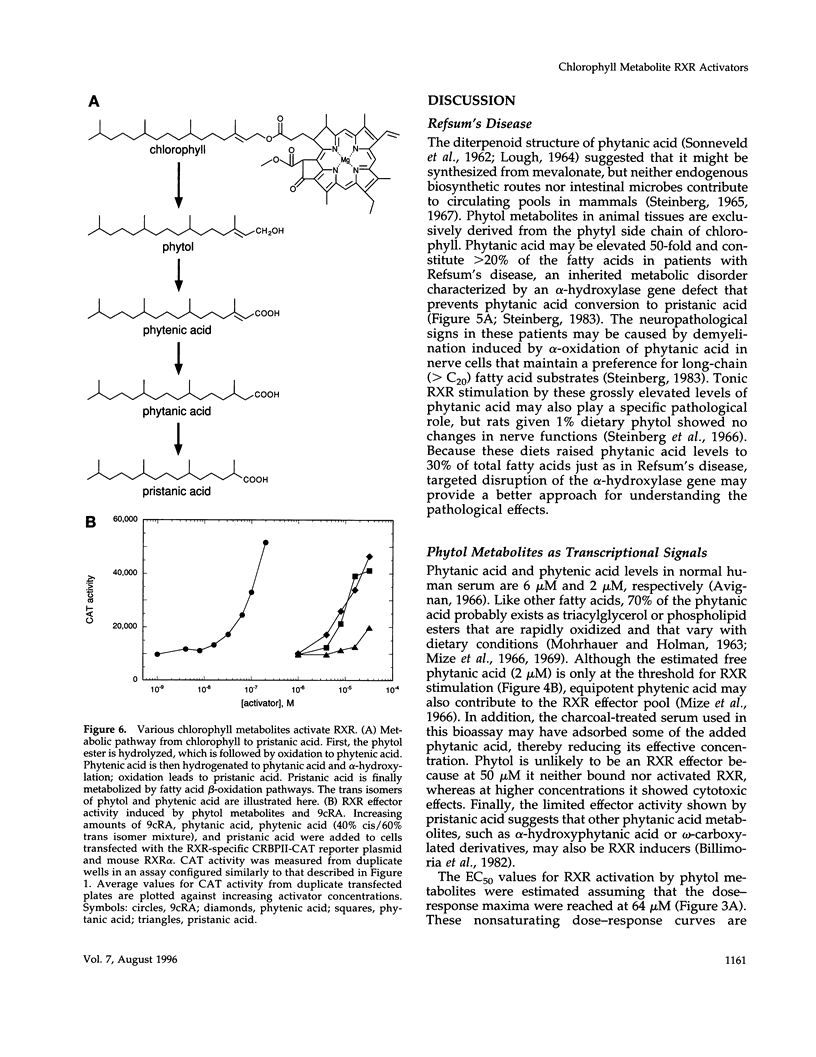
- AAES-JORGENSEN E. Essential fatty acids. Physiol Rev. 1961 Jan;41:1–51. doi: 10.1152/physrev.1961.41.1.1. [DOI] [PubMed] [Google Scholar]
- Allegretto E. A., McClurg M. R., Lazarchik S. B., Clemm D. L., Kerner S. A., Elgort M. G., Boehm M. F., White S. K., Pike J. W., Heyman R. A. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast. Correlation with hormone binding and effects of metabolism. J Biol Chem. 1993 Dec 15;268(35):26625–26633. [PubMed] [Google Scholar]
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banner C. D., Göttlicher M., Widmark E., Sjövall J., Rafter J. J., Gustafsson J. A. A systematic analytical chemistry/cell assay approach to isolate activators of orphan nuclear receptors from biological extracts: characterization of peroxisome proliferator-activated receptor activators in plasma. J Lipid Res. 1993 Sep;34(9):1583–1591. [PubMed] [Google Scholar]
- Baxter J. H., Milne G. W. Phytenic acid: identification of five isomers in chemical and biological products of phytol. Biochim Biophys Acta. 1969 Mar 4;176(2):265–277. doi: 10.1016/0005-2760(69)90185-4. [DOI] [PubMed] [Google Scholar]
- Berger T. S., Parandoosh Z., Perry B. W., Stein R. B. Interaction of glucocorticoid analogues with the human glucocorticoid receptor. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):733–738. doi: 10.1016/0960-0760(92)90414-e. [DOI] [PubMed] [Google Scholar]
- Bergström S. The prostaglandins. Recent Prog Horm Res. 1966;22:153–175. doi: 10.1016/b978-1-4831-9825-5.50007-5. [DOI] [PubMed] [Google Scholar]
- Billimoria J. D., Clemens M. E., Gibberd F. B., Whitelaw M. N. Metabolism of phytanic acid in Refsum's disease. Lancet. 1982 Jan 23;1(8265):194–196. doi: 10.1016/s0140-6736(82)90760-7. [DOI] [PubMed] [Google Scholar]
- Boehm M. F., McClurg M. R., Pathirana C., Mangelsdorf D., White S. K., Hebert J., Winn D., Goldman M. E., Heyman R. A. Synthesis of high specific activity [3H]-9-cis-retinoic acid and its application for identifying retinoids with unusual binding properties. J Med Chem. 1994 Feb 4;37(3):408–414. doi: 10.1021/jm00029a013. [DOI] [PubMed] [Google Scholar]
- Boehm M. F., Zhang L., Zhi L., McClurg M. R., Berger E., Wagoner M., Mais D. E., Suto C. M., Davies J. A., Heyman R. A. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995 Aug 4;38(16):3146–3155. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- Bruenger E., Rilling H. C. Determination of isopentenyl diphosphate and farnesyl diphosphate in tissue samples with a comment on secondary regulation of polyisoprenoid biosynthesis. Anal Biochem. 1988 Sep;173(2):321–327. doi: 10.1016/0003-2697(88)90196-0. [DOI] [PubMed] [Google Scholar]
- Buck J., Derguini F., Levi E., Nakanishi K., Hämmerling U. Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science. 1991 Dec 13;254(5038):1654–1656. doi: 10.1126/science.1749937. [DOI] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Normal reference laboratory values. N Engl J Med. 1980 Jan 3;302(1):37–48. doi: 10.1056/NEJM198001033020108. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Derguini F., Nakanishi K., Hämmerling U., Chua R., Eppinger T., Levi E., Buck J. 13,14-Dihydroxy-retinol, a new bioactive retinol metabolite. J Biol Chem. 1995 Aug 11;270(32):18875–18880. doi: 10.1074/jbc.270.32.18875. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995 Jun 2;81(5):687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Tontonoz P., Chen J., Brun R. P., Spiegelman B. M., Evans R. M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995 Dec 1;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Umesono K., Chen J., Evans R. M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995 May 19;81(4):541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Glass C. K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994 Jun;15(3):391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986 Dec 18;324(6098):615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- Göttlicher M., Widmark E., Li Q., Gustafsson J. A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. Albumin replacement by fatty acids in clonal growth of mammalian cells. Science. 1963 May 17;140(3568):802–803. doi: 10.1126/science.140.3568.802. [DOI] [PubMed] [Google Scholar]
- Harmon M. A., Boehm M. F., Heyman R. A., Mangelsdorf D. J. Activation of mammalian retinoid X receptors by the insect growth regulator methoprene. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6157–6160. doi: 10.1073/pnas.92.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Herndon J. H., Jr, Steinberg D., Uhlendorf B. W., Fales H. M. Refsum's disease: characterization of the enzyme defect in cell culture. J Clin Invest. 1969 Jun;48(6):1017–1032. doi: 10.1172/JCI106058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Forman B. M., Blumberg B., Ong E. S., Borgmeyer U., Mangelsdorf D. J., Umesono K., Evans R. M. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Lenhard J. M., Willson T. M., Patel I., Morris D. C., Lehmann J. M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995 Dec 1;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle M. R., Talbot W. S., Segraves W. A., Bender M. T., Cherbas P., Hogness D. S. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991 Oct 4;67(1):59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Kojima R., Fujimori T., Kiyota N., Toriya Y., Fukuda T., Ohashi T., Sato T., Yoshizawa Y., Takeyama K., Mano H. In vivo isomerization of retinoic acids. Rapid isomer exchange and gene expression. J Biol Chem. 1994 Dec 23;269(51):32700–32707. [PubMed] [Google Scholar]
- Lehmann J. M., Jong L., Fanjul A., Cameron J. F., Lu X. P., Haefner P., Dawson M. I., Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992 Dec 18;258(5090):1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Lough A. K. Blood lipids. 4. The isolation of 3,7,11,15-tetramethylhexadecanoic acid (phytanic acid) from ox-plasma lipids. Biochem J. 1964 Jun;91(3):584–588. doi: 10.1042/bj0910584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough A. K. The phytanic acid content of the lipids of bovine tissues and milk. Lipids. 1977 Jan;12(1):115–119. doi: 10.1007/BF02532982. [DOI] [PubMed] [Google Scholar]
- MOHRHAUER H., HOLMAN R. T. THE EFFECT OF DOSE LEVEL OF ESSENTIAL FATTY ACIDS UPON FATTY ACID COMPOSITION OF THE RAT LIVER. J Lipid Res. 1963 Apr;4:151–159. [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Evans R. M. The RXR heterodimers and orphan receptors. Cell. 1995 Dec 15;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Mize C. E., Avigan J., Baxter J. H., Fales H. M., Steinberg D. Metabolism of phytol-U-14C and phytanic acid-U-14C in the rat. J Lipid Res. 1966 Sep;7(5):692–697. [PubMed] [Google Scholar]
- Mize C. E., Herndon J. H., Jr, Blass J. P., Milne G. W., Follansbee C., Laudat P., Steinberg D. Localization of the oxidative defect in phytanic acid degradation in patients with Refsum's disease. J Clin Invest. 1969 Jun;48(6):1033–1040. doi: 10.1172/JCI106059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli J. L., Pramanik B. C., Williams J. B., Dawson M. I., Hobbs P. D. Quantification of retinoic acid by gas-liquid chromatography-mass spectrometry: total versus all-trans-retinoic acid in human plasma. J Lipid Res. 1985 Mar;26(3):387–392. [PubMed] [Google Scholar]
- O'Malley B. W., Conneely O. M. Orphan receptors: in search of a unifying hypothesis for activation. Mol Endocrinol. 1992 Sep;6(9):1359–1361. doi: 10.1210/mend.6.9.1331771. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W. Did eucaryotic steroid receptors evolve from intracrine gene regulators? Endocrinology. 1989 Sep;125(3):1119–1120. doi: 10.1210/endo-125-3-1119. [DOI] [PubMed] [Google Scholar]
- Ong D. E. Cellular retinoid-binding proteins. Arch Dermatol. 1987 Dec;123(12):1693–1695a. [PubMed] [Google Scholar]
- Perlmann T., Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995 Apr 1;9(7):769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Pijnappel W. W., Hendriks H. F., Folkers G. E., van den Brink C. E., Dekker E. J., Edelenbosch C., van der Saag P. T., Durston A. J. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993 Nov 25;366(6453):340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- Repa J. J., Hanson K. K., Clagett-Dame M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7293–7297. doi: 10.1073/pnas.90.15.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Shih W., Mears T., Bradley D. J., Parandoosh Z., Weinberger C. An adenoviral vector system for functional identification of nuclear receptor ligands. Mol Endocrinol. 1991 Feb;5(2):300–309. doi: 10.1210/mend-5-2-300. [DOI] [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Song C., Kokontis J. M., Hiipakka R. A., Liao S. Ubiquitous receptor: a receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10809–10813. doi: 10.1073/pnas.91.23.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., John K., Fletcher J. E. Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res. 1969 Jan;10(1):56–67. [PubMed] [Google Scholar]
- Steinberg D., Avigan J., Mize C. E., Baxter J. H., Cammermeyer J., Fales H. M., Highet P. F. Effects of dietary phytol and phytanic acid in animals. J Lipid Res. 1966 Sep;7(5):684–691. [PubMed] [Google Scholar]
- Steinberg D., Avigan J., Mize C., Eldjarn L., Try K., Refsum S. Conversion of U-C14-phytol to phytanic acid and its oxidation in heredopathia atactica polyneuritiformis. Biochem Biophys Res Commun. 1965 Jun 9;19(6):783–789. doi: 10.1016/0006-291x(65)90328-1. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Mize C. E., Avigan J., Fales H. M., Eldjarn L., Try K., Stokke O., Refsum S. Studies on the metabolic error in Refsum's disease. J Clin Invest. 1967 Mar;46(3):313–322. doi: 10.1172/JCI105533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G. W., Russell R. M. 13-cis-retinoic acid is an endogenous compound in human serum. J Lipid Res. 1990 Feb;31(2):175–182. [PubMed] [Google Scholar]
- Teboul M., Enmark E., Li Q., Wikström A. C., Pelto-Huikko M., Gustafsson J. A. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2096–2100. doi: 10.1073/pnas.92.6.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995 May 1;9(9):1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yao T. P., Forman B. M., Jiang Z., Cherbas L., Chen J. D., McKeown M., Cherbas P., Evans R. M. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993 Dec 2;366(6454):476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]



