SUMMARY
About 1 to 2 million people die of malaria every year. Anopheline mosquitoes are the obligatory vectors of Plasmodium spp., the causative agent of malaria. For transmission to occur, the parasite has to undergo a complex developmental program in the mosquito, culminating with sporozoite invasion of the salivary glands. Strong circumstantial evidence suggests that sporozoite invasion requires specific interactions and recognition between sporozoite and salivary gland proteins. Here we review recent progress toward the elucidation of invasion mechanisms.
INTRODUCTION
Nearly half of the world population is at risk of contracting malaria and over one million people, mostly African children under the age of 5, die of the disease every year [1]. Resistance of the mosquito and the parasite to agents that kill them and the lack of an effective protective vaccine, all contribute to exacerbate disease burden [2, 3,]
Unlike other major infectious diseases such as AIDS and tuberculosis, malaria parasites need a vector for transmission to occur. When a mosquito takes an infectious blood meal, the ingested gametocytes differentiate into male and female gametes that then mate to generate zygotes. Still in the midgut, zygotes differentiate into motile ookinetes that first traverse the peritrophic matrix and then cross the midgut epithelium. Upon emergence, the parasites lodge themselves between the basal epithelial surface and the basal lamina. This prompts the ookinetes to differentiate into oocysts that upon maturation about 10 days later rupture, each releasing several thousand sporozoites into the open hemolymph circulation of the mosquito [4] (Fig. 1).
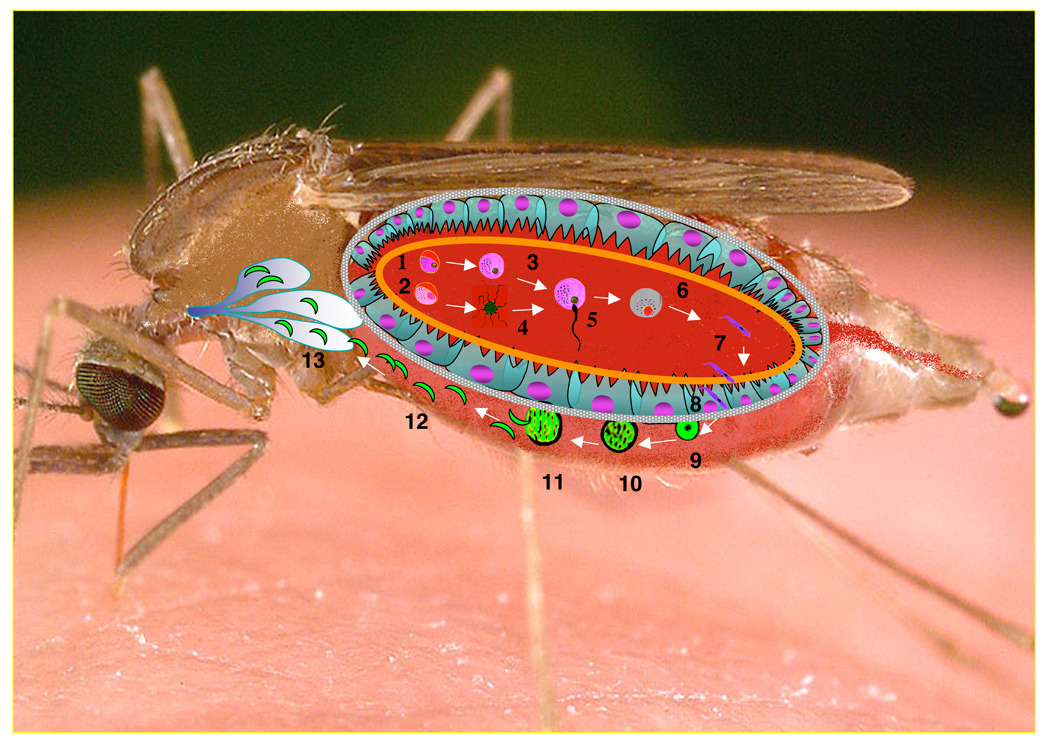
Figure 1. Life cycle of the Plasmodium parasite in its mosquito vector.
Female (1) and male (2) gametocytes differentiate into gametes (3,4). After completion of meiosis, the male gametocyte generates 8 gametes (4) in a process known as “exflagellation”. A male gamete fertilizes a female gamete (5) to generate a zygote (6), which in turn differentiates into a motile ookinete (7). About 24 h later, the mature ookinete first traverses the peritrophic matrix (orange line) and then the midgut epithelium (8), after which it differentiates into a oocyst (9). During the next ~10 days the oocyst grows (10, 11) and when mature, it releases sporozoites into the open hemolymph circulation (12). The circulating sporozoites recognize and invade the salivary glands (13) where they are stored until release at the time when the mosquito bites the next individual.
OVERVIEW OF SALIVARY GLAND INVASION
Mosquito salivary glands are paired organs localized in the thorax. The gland of the female mosquito is formed by two similarly constructed lateral lobes and a shorter and wider medial lobe [5,6]. Two regions can be identified in the lateral lobes, proximal and distal, that are separated by a narrow transitional region. The distal region consists of large secretory cells whose products participate in blood feeding [7]. Detailed ultrastructural studies of sporozoite invasion of the salivary gland were conducted with Aedes aegypti and P. gallinaceum [8, •9] (Fig. 2). Initially sporozoites attach to the filamentous basal lamina via their anterior tip, although in some views the sporozoites can also be found attached along their entire length. As the parasite penetrates the basal lamina, it looses its thick coat and the sporozoite anterior end is found pointing towards the epithelial cell. No information is available as to how the sporozoites traverse the basal lamina. Invasion begins with invagination of the epithelial cell membrane which adopts the shape of the parasite, leading to the formation of a parasitophorous vacuole. Electron microscopy revealed that a specialized junction forms between the apical end of the sporozoite and the forming vacuole [•9]. The parasitophorous vacuole disintegrates during invasion and free parasites accumulate within the cell [10]. Next, the sporozoite invades the apical membrane of its host epithelial cell, resulting into sporozoite release into the gland’s central secretory cavity (Fig. 2). During early stages of infection, sporozoites are found mostly inside the cytoplasm of the secretory cell while very few are found inside the central secretory cavity. At later stages sporozoites are mostly found inside the secretory cavity where they aggregate into large bundles [•9]. In vitro sporozoite invasion of salivary glands has never been reported. It is not clear why this could not be achieved.
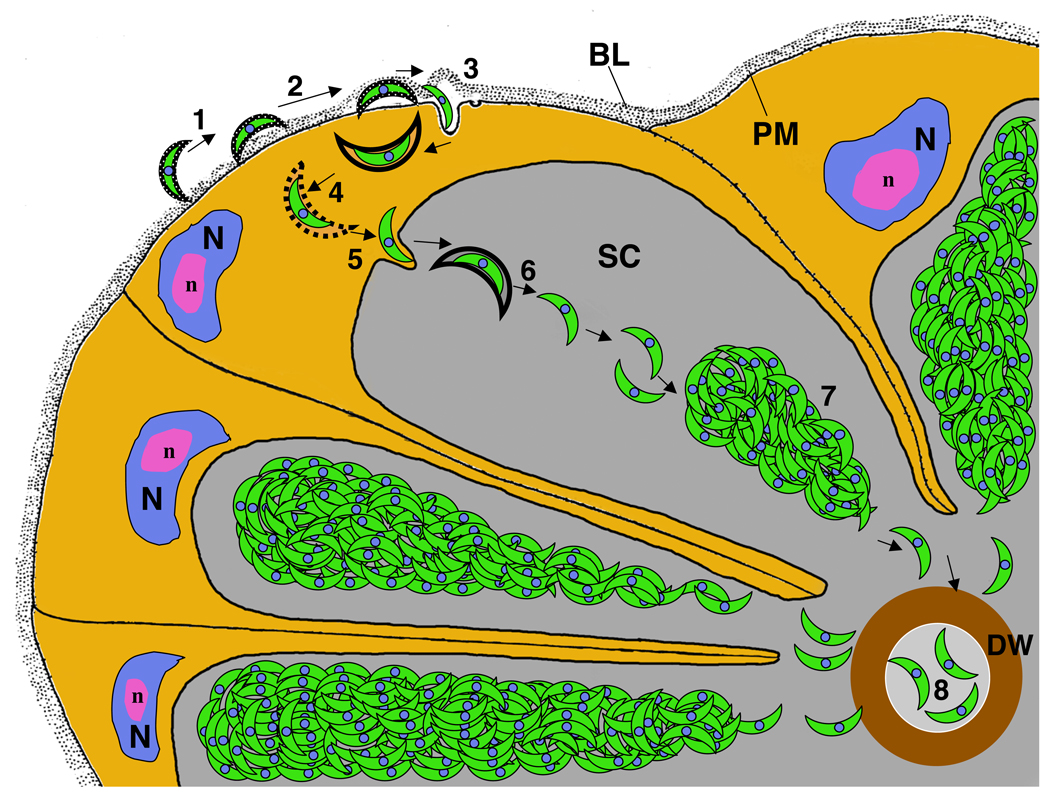
Figure 2. Progression of sporozoite invasion of the salivary gland.
1) The sporozoite attaches to the basal lamina; 2) Sporozoite passage to the space between the basal lamina and the basal epithelial cell plasma membrane, a process associated with the loss of the sporozoite’s thick coat; 3) Penetration of the basal plasma membrane; the sporozoite resides within a vesicle; 4) Release of the sporozoite from the surrounding membrane by an unknown mechanism; 5) Invasion of the apical membrane and entry into the secretory cavity; 6) Sporozoites are released from the surrounding membrane by an unknown mechanism; 7) Sporozoites assemble into bundles within the secretory cavity; and 8) A small number of sporozoites enter the secretory duct by an unknown mechanism. BL: Basal Lamina; DW: Duct wall; N: nucleus; n: nucleolus; PM: Plasma membrane; SC: Secretory cavity. Modified from reference [•5].
SPECIFICITY OF SALIVARY GLAND INVASION
Of all the organs and cell types with which the sporozoites released from the oocysts come in contact, only one is invaded, the salivary glands. This specificity implies that sporozoites actively recognize the salivary glands, most likely via receptor-ligand interactions. Additional evidence for specific interactions was provided by Rosenberg’s classic experiments [••11]. P. knowlesi fully develops in An. dirus mosquitoes, while in An. freeborni development proceeds normally up to release of sporozoites into the hemocoel but the freeborni salivary glands are never infected. Rosenberg demonstrated that the parasite invades An. dirus salivary glands implanted into infected An. freeborni mosquitoes while in the reverse experiment, it failed to infect An. freeborni glands implanted into An. dirus mosquitoes [••11]. These results strongly suggest that P. knowlesi sporozoites are unable to recognize An. freeborni salivary glands. The molecular basis for this specificity remains unknown.
THE SEARCH FOR SALIVARY GLAND RECEPTORS FOR SPOROZOITE INVASION
Several attempts to identify candidate receptors for sporozoite invasion have been reported (Table 1). It was observed that monoclonal antibodies raised against whole salivary glands inhibit sporozoites invasion [12]. A group of high molecular weight proteins (SGS family) of more than 200 kDa were identified in Aedes aegypti. Each SGS gene is encoded by a ~10 kb open reading frame and all SGS proteins possess predicted multipass transmembrane domains near their C-terminal ends. Antibody raised against the aaSGS1 protein inhibits sporozoite invasion [8]. Four SGS orthologues, agSGS2, agSGS3, agSGS4 and agSGS5 are also found in An. gambiae [13]. In a separate set of experiments, monoclonal antibodies raised against An. gambiae salivary glands led to the identification of a 50 kDa protein that forms a 100 kDa homodimer. Antibody against this protein binds to the surface of the salivary glands and inhibits sporozoite invasion by about 75% [•14]. This protein was termed saglin [15]. Independent experiments determined that saglin acts as a receptor for sporozoite invasion via interaction with Plasmodium TRAP [••16] (see below).
TABLE 1.
Candidate salivary gland proteins with a role in sporozoite invasion
THE SEARCH FOR SPOROZOITE LIGANDS FOR SALIVARY GLAND INVASION
A list of candidate ligands is presented in Table 2 (see also [17])
TABLE 2.
Sporozoite proteins with possible role in salivary gland invasion
Circumsporozoite protein (CSP)
CSP is a conserved GPI-anchored membrane protein of ~60 kDa that covers the entire surface of the sporozoite. CSP is shed when the sporozoite glides on a solid surface, creating a CSP protein trail [18]. CSP is essential for both oocyst development and sporozoite differentiation [••19]. It is required for inner membrane deposition and formation of the microtubule network associated with the oocyst’s outer membrane [20].
In addition to oocyst development and sporozoite differentiation, CSP also seems to play a role in sporozoite recognition of the mosquito salivary glands. An anti-CSP monoclonal antibody completely suppressed invasion of P. gallinaceum sporozoites [21]. While this finding could be simply interpreted as antibodies causing steric hindrance for sporozoite access to the salivary gland surface, additional evidence suggests that CSP may play an active role. The recombinant protein binds to salivary glands in preference to other mosquito organs and this binding is inhibited by a highly conserved 15-amino acid motif that includes the 5-amino acid sequence known as region I [22, 23, 24, 25]. Further experiments demonstrated that the 15-amino acid peptide, as well as the whole CSP protein, inhibited sporozoite invasion [26]. However, the 5-amino acid region I peptide by itself had no inhibitory activity [25]. In agreement with these findings, recombinant parasites carrying a deletion of region I showed no impairment of motility or infectivity of the mosquito [27]. A second conserved CSP motif, region II, bears a striking homology to a cell adhesion motif of thrombospondin. Sporozoites carrying a CSP gene with a deleted region II had no motility and were unable to invade the salivary glands [27]. A subsequent study determined that deletion of region II allowed for development of normal number of sporozoites, but in contrast to the previous study, these sporozoites were unable to exit the oocysts [28]. The discrepancy may be explained in part by the fact that the former studies were conducted with hybrid P. berghei parasites that expressed the P. falciparum CSP protein. These sporozoites had a10-fold lower salivary gland infectivity.
MAEBL
MAEBL is a micronemal protein of about 200 kDa that was identified in asexual stages of parasite development. MAEBL has a single transmembrane domain and is structurally related to members of the Plasmodium Duffy binding-like (DBL) family [29]. While MAEBL was initially characterized in asexually reproducing parasites, more recently its expression was described in sporogonic stages in the mosquito [••30, 31]. Targeted disruption of MAEBL revealed that the gene is essential for salivary gland invasion. Gliding motility and infectivity to the vertebrate host were unaffected by MAEBL disruption but mutant sporozoites showed a 20-fold reduction in attachment to the salivary gland surface [••30]. The putative interacting salivary gland molecules have not been identified.
TRAP
Thrombospondin Related Anonymous Protein (TRAP) is expressed during sporozoite differentiation in the oocyst and is stored in micronemes. It is essential for sporozoites gliding and cell invasion [28, ••32]. Upon contact with a target cell, TRAP is released from the micronemes onto the sporozoite’s anterior tip [33]. It is also released on the substrate during gliding locomotion [34]. After invasion of the salivary gland, TRAP is found over the entire surface of P. berghei sporozoites (Ghosh et al., unpublished observations) and in other apicomplexans, the orthologous protein is found over the entire parasite surface. TRAP contains in its extracellular portion two adhesive modules, A-domain of the von Willebrand factor [33] and a thrombospondin type I repeat [36]. Transgenic P. berghei sporozoites with a mutated TRAP A-domain are impaired in salivary gland invasion but not in gliding motility [36, •37]. However, a very small number of P. yoelii TRAP knockout sporozoites were able to bind and invade salivary glands [38]. A detailed mutational analysis revealed that two specific mutations in the A-domain - T126A and D157A - abrogated the sporozoite’s ability to invade the salivary glands [•37].
PCRMP (Cysteine Repeat modular proteins)
The Cysteine Repeat Modular Proteins 1 and 2 are encoded by a small gene family conserved in malaria and other Apicomplexan parasites. P. berghei PCRMP1 is transcribed in developing oocysts and its abundance increases in sporulating oocysts, while PCRMP2 is transcribed in sporulating oocysts and salivary gland sporozoites. Both proteins localize on the sporozoite surface. PCRMP1 and PCRMP2 knockout sporozoites are unable to invade salivary glands, suggesting a role in salivary gland invasion [39]
USO3 and TREP / S6
P. yoelii USO3 (upregulated in oocyst sporozoites) was first identified by the Kappe laboratory [40]. The related TREP protein, first identified via subtractive hybridization experiments [41], has been alternatively named TREP [42] or S6 [43]. The two proteins have related structural features in that they are surface proteins (they possess a transmembrane domain) and have a thrombospondin repeat that presumably functions in protein-protein interactions. Structurally USO3 differs from TREP/ S6 by the lack of an adhesion domain A in the latter protein. USO3 is localized to the apical end of oocyst sporozoites while TREP/S6 is localized to the plasma membrane. Surface localization of both proteins and expression prior to salivary gland invasion are consistent with a role in invasion. Indeed, USO3 knockout of P. yoelii parasites leads to a complete inhibition of salivary gland invasion, while TREP/S6 knockout in P. berghei parasites partially inhibited salivary gland invasion. Knockout of both genes leads to partial loss of motility. It is not known whether either of the proteins directly interacts with mosquito salivary gland proteins, as was shown for TRAP (see below).
A PHAGE DISPLAY SCREEN LEADS TO THE IDENTIFICATION OF A RECEPTOR-LIGAND PAIR REQUIRED FOR SPOROZOITE INVASION OF SALIVARY GLANDS
A screen of a phage library displaying random 12-amino acid peptides led to the identification of a peptide - SM1 (for Salivary gland and Midgut peptide 1) - that binds specifically to the surfaces of the salivary gland and midgut epithelia. Importantly, binding of the peptide resulted in strong inhibition of parasite invasion of these organs [••44]. These results implied that the peptide binds to a surface receptor that the parasite needs to recognize in order for invasion to occur. Discovery of SM1 led to the engineering of the first transgenic mosquito impaired in transmission of the malaria parasite [45]. Recent work identified the molecular nature of the salivary gland receptor and of the sporozoite ligand [••16].
The receptor was identified using a double-derivatized SM1 peptide bearing a biotin residue at one end and an UV-activatable crosslinker in the middle of the peptide. The derivatized peptide was incubated with dissected salivary glands followed by UV irradiation to promote crosslinking with the protein to which the peptide was bound. The glands were then lysed and the peptide (plus the crosslinked protein) was then captured on streptavidin beads. Sequencing of the crosslinked protein identified the putative receptor as the salivary gland surface protein saglin [••16, 15]. Saglin is a 50 kDa protein that has a signal peptide but no transmembrane domain. Saglin occurs on the salivary gland surface, as anti-saglin antibodies administered to mosquitoes inhibit P. berghei [•14] and P. falciparum [••16] sporozoite invasion of salivary glands. However, it is not known whether the protein is associated with the membrane or the basal lamina of the salivary gland, as the available immuno-electron micrographs do not have sufficient resolution to determine its exact location [•14]. Saglin is rich in glutamines and these residues may be involved in protein-protein interactions. Sequencing of the proteins crosslinked to SM1 identified two additional signal peptide-containing salivary gland proteins: gSG1 [46] and gSG1b [47]. Both proteins bear some sequence identity with saglin (unpublished observations) and are specific to female salivary glands. The possible location of the gSG1 and gSG1b proteins on the salivary gland surface has not been verified and it is unclear whether they play a direct role in sporozoite invasion.
Inhibition of sporozoite invasion by SM1 implied that the peptide and a parasite protein compete for binding to a salivary gland receptor, presumably saglin (Fig. 3). Yet, the SM1 amino acid sequence did not match any predicted Plasmodium protein in the database. The hypothesis that SM1 conformation, rather than primary amino acid sequence, resembled a sporozoite protein, led to the production of an anti-SM1 antibody for testing Western blots of sporozoite proteins. These experiments led to the identification of TRAP as a mimotope of SM1 (TRAP is recognized by the anti-SM1 antibody) and raised the hypothesis that TRAP binds to saglin (Fig. 3; [••16]). Further experiments demonstrated that 1) recombinant Plasmodium TRAP A-domain binds to salivary glands and that this binding can be competed by SM1; 2) that recombinant TRAP A-domain binds to recombinant saglin in vitro and that this binding is abrogated by the same A-domain point mutations that prevent sporozoite invasion of salivary glands [•37]; that RNAi knock down of saglin expression strongly inhibits salivary gland invasion (Fig. 3; [••16]). These data strongly argue for an essential role of saglin-TRAP interactions for invasion of the salivary glands. Nevertheless, these experiments need to be interpreted with caution. Sporozoite invasion of the salivary gland is a complex process (Fig. 2) that must depend on the successful completion of a number of other steps. Thus, saglin-TRAP interaction should be considered as only one of many steps required for successful sporozoite invasion of the salivary gland.
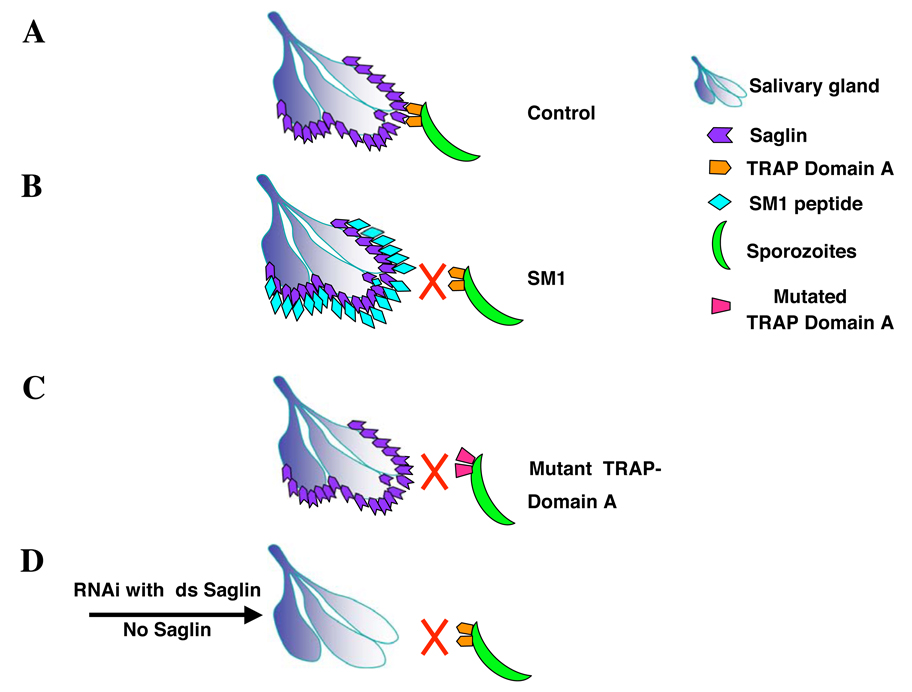
Figure 3. Schematic representation of the interaction of the salivary gland protein saglin with the sporozoite protein TRAP.
A) Binding of Plasmodium TRAP to the salivary gland protein saglin results in productive invasion. B) SM1 binds to saglin occupying this receptor and in this way precludes interaction with the sporozoite TRAP. C) Mutation of the critical TRAP A-domain T126 to A alters its conformation preventing recognition of saglin and sporozoite invasion. D) Down-regulation of saglin expression by RNAi reduces the abundance of the saglin receptor on the salivary gland surface preventing sporozoite invasion. Based on data of reference [••16].
THE FINAL STEPS
The sporozoite invades the salivary gland epithelial cell from the basal side and then exits this cell from the apical side, to reach the lumen of the acinus (Fig. 2). It is not known whether after reaching the epithelial cell cytoplasm, sporozoite migration is directional or how the sporozoite makes its way to the apical membrane. Once in the lumen of the acinus, the sporozoites associate with each other to form bundles (Fig. 2) via an unknown mechanism. A small number of sporozoites can be found in the secretory duct and again, it is not known how they detach from the bundles and how they find their way into the duct lumen. The duct wall of culicine mosquitoes (e.g., Ae. aegypti) contains chitin [6] and sporozoites might secrete a chitinase to make their way into the lumen. However, the duct wall of anopheline mosquitoes is not believed to contain chitin [5] and how the sporozoites find their way into the duct is not known. Most likely this occurs by sporozoite entry via the duct ending, together with the saliva.
CONCLUSIONS
Successful sporozoite invasion of the salivary gland is an essential step for the completion of the Plasmodium cycle in the mosquito. As is apparent from this review, our understanding of this complex, multistep invasion process is rather superficial. Since disruption of any of these many steps is likely to abrogate parasite cycle in the mosquito, further investigation of these processes should prove to be rewarding and should receive high priority. Answers to some of these questions may lead to new means to interfere with parasite transmission.
ACKNOWLEDGEMENTS
We thank Photini Sinnis and an anonymous reviewer for excellent comments that helped improve the manuscript. Work in our laboratory was supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECOMMENDED READING
Papers of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 6 doi: 10.1371/journal.pmed.1000048. e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breman JG, Egan A, Keusch GT. The intolerable burden of malaria: a new look at the numbers. Am J Trop Med Hyg. 2001;64(1–2 Suppl):iv–vii. doi: 10.4269/ajtmh.2001.64.iv. [DOI] [PubMed] [Google Scholar]
- •3. Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342.This paper describes the global distribution of the malaria burden.
- 4.Ghosh A, Edwards MJ, Jacobs-Lorena M. The journey of malaria parasite in the mosquito: Hopes for the new century. Parasitology Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 5.Wright KA. The anatomy of the salivary glands of Anopheles stephensi. Liston Can J Zool. 1969;47:579–588. [Google Scholar]
- 6.Janzen HG, Wright KA. The salivary glands of Aedes aegypti (L.): an electron microscope study. Can J Zool. 1971;49:1343–1346. doi: 10.1139/z71-200. [DOI] [PubMed] [Google Scholar]
- 7.Lehane MJ. The biology of blood sucking insects. Harper CollinsAcademic London. 1991 [Google Scholar]
- 8.Sterling CR, Aikawa M, Vanderberg JP. The passage of Plasmodium berghei sporozoites through the salivary glands of Anopheles stephensi: an electron microscope study. J Parasitol. 1973;59:593–605. [PubMed] [Google Scholar]
- •9. Pimenta PF, Touray M, Miller LH. The journey of malaria sporozoites in the mosquito salivary gland. J Euk Microbiol. 1994;41:608–624. doi: 10.1111/j.1550-7408.1994.tb01523.x.This paper describes the morphological aspects of sporozoite invasion of the mosquito salivary glands.
- 10.Rodriguez MH, Hernández-Hernández Fde L. Insect-malaria parasites interactions: the salivary gland. Insect Biochem Mol Biol. 2004;34:615–624. doi: 10.1016/j.ibmb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- ••11. Rosenberg R. Inability of Plasmodium knowlesi sporozoites to invade Anopheles freeborni salivary glands. Am J Trop Med Hyg. 1985;34:687–691. doi: 10.4269/ajtmh.1985.34.687.This is a classical paper demonstrating species-specificity of mosquito salivary gland invasion.
- 12.Barreau C, Conard J, Fischer E, Lujan HD, Vernick KD. Identification of surface molecules on salivary glands of the mosquito, Aedes aegypti, by a panel of monoclonal antibodies. Insect Bioch Mol Biol. 1999;29:515–526. doi: 10.1016/s0965-1748(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 13.Korochkina S, Barreau C, Pradel G, Jeffery E, Li J, Natarajan R, Shabanowitz J, Hunt D, Frevert U, Vernick KD. A mosquito-specific protein family includes candidate receptors for malaria sporozoite invasion of salivary glands. Cell Microbiol. 2006;8:163–175. doi: 10.1111/j.1462-5822.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- •14. Brennan JD, Kent M, Dhar R, Fujioka H, Kumar N. Anopheles gambiae salivary gland proteins as putative targets for blocking transmission of malaria parasites. Proc Natl Acad Sci USA. 2000;97:13859–13864. doi: 10.1073/pnas.250472597.This paper describes the identification of a salivary gland-specific antibody that inhibits salivary gland invasion.
- 15.Okulate MA, Kalume DE, Reddy R, Kristiansen T, Bhattacharya M, Chaerkady R, Pandey A, Kumar N. Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect Mol Biol. 2007;16:711–722. doi: 10.1111/j.1365-2583.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- ••16. Ghosh AK, Devenport M, Jethwaney D, Kalume DE, Pandey A, Anderson VE, Sultan AA, Kumar N, Jacobs-Lorena M. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the anopheles saglin proteins. PLOS Pathog. 2009;5(1) doi: 10.1371/journal.ppat.1000265. e1000265.This paper describes the first identification of a receptor-ligand pair required for sporozoite invasion of mosquito salivary glands.
- 17.Ménard R. The journey of the malaria sporozoites through its hosts: two parasite proteins lead the way. Microbes Infect. 2000;2:633–642. doi: 10.1016/s1286-4579(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 18.Stewart MJ, Vanderberg JP. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J Protozool. 1991;38:411–421. doi: 10.1111/j.1550-7408.1991.tb01379.x. [DOI] [PubMed] [Google Scholar]
- ••19. Ménard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, Janse CJ, Waters AP, Nussenzweig RS, Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0.By use of gene knockout, this paper demonstrates the essential role of the circumsporozoite protein for sporozoite development in the oocyst.
- 20.Thathy V, Fujioka H, Gantt S, Nussenzweig R, Nussenzweig V, Ménard R. Levels of circumsporozoite protein in the Plasmodium oocyst determine sporozoite morphology. EMBO J. 2002;21:1586–1596. doi: 10.1093/emboj/21.7.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburg A, Touray M, Krettli AU, Miller LH. Plasmodium gallinaceum: antibodies to circumsporozoite protein prevent sporozoites from invading the salivary glands of Aedes aegypti. Exp Parasitol. 1992;75:303–307. doi: 10.1016/0014-4894(92)90215-v. [DOI] [PubMed] [Google Scholar]
- 22.Dame JB, Willams JL, McCutchan TF, Weber JL, Wirtz RA, Hockmeyer WT, Maloy WL, Hynes JD, Schneider I, Roberts DD. Structure of the gene encoding the immunodominant surface antigen on the sporozoites of the human malaria parasite Plasmodium falciparum. Science. 1984;225:593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- 23.Lal AA, de la Cruz VF, Welsh JA, Charoenvit Y, Maloy WL, McCutchan TF. Structure of the gene encoding the circumsporozoite protein of Plasmodium yoelii. A rodent model for examining antimalarial sporozoite vaccines. J Biol Chem. 1987;262:2937–2940. [PubMed] [Google Scholar]
- 24.McCutchan TF, Kissinger JC, Touray MG, Rogers MJ, Li J, Sullivan M, Braga EM, Krettli AU, Miller LH. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc Natl Acad Sci USA. 1996;93:11889–11894. doi: 10.1073/pnas.93.21.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidjanski SP, Vanderberg JP, Sinnis P. Anopheles stephensi salivary glands receptor for region I of the circumsporozoite proteins of Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:33–41. doi: 10.1016/s0166-6851(97)00124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myung JM, Marshall P, Sinnis P. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol Biochem Parasitol. 2004;133:53–59. doi: 10.1016/j.molbiopara.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Tewari R, Spaccapelo R, Bistoni F, Holder AA, Crisanti A. Function of region I and region II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J Biol Chem. 2002;277 doi: 10.1074/jbc.M208453200. 74613-47618. [DOI] [PubMed] [Google Scholar]
- 28.Rogers WO, Malik A, Mellouk S, Nakamura K, Rogers MD, Szarfman A, Gordon DM, Nussler AK, Aikawa M, Hoffman SL. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc Natl Acad Sci USA. 1992a;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kappe SH, Gardner MJ, Brown SM, Ross J, Matuschewski K, Ribeiro JM, Adams JH, Quackenbush J, Cho J, Carucci DJ, Hoffman SL, Nussenzweig V. Exploring the transcriptome of the malaria sporozoite stage. Proc Natl Acad Sci U S A. 2001;98:9895–9900. doi: 10.1073/pnas.171185198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••30. Kariu T, Yuda M, Yano K, Chinzei Y. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J Exp Med. 2002;195:1317–1323. doi: 10.1084/jem.20011876.This paper characterized for the first time MAEBL expression during Plasmodium development in the mosquito and established its essential role in salivary gland invasion.
- 31.Srinivasan P, Abraham EG, Ghosh AK, Valenzuela J, Ribeiro JM, Dimopoulos G, Kafatos FC, Adams JH, Fujioka H, Jacobs-Lorena M. Analysis of the Plasmodium and Anopheles transcriptomes during oocyst differentiation. J Biol Chem. 2004;279:5581–5587. doi: 10.1074/jbc.M307587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••32. Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, Nussenzweig RS, Ménard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5.Using a gene knockout approach, this paper demonstrates that TRAP is not required for sporozoite formation but is essential for sporozoite motility and invasion of the mosquito salivary gland and mammalian liver.
- 33.Gantt S, Persson C, Rose K, Birkett AJ, Abagyan R, Nussenzweig V. Antibodies against thrombospondin-related anonymous protein do not inhibit Plasmodium sporozoite infectivity in vivo. Infect Immun. 2000;68:3667–3673. doi: 10.1128/iai.68.6.3667-3673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kappe S, Bruderer T, Gnatt S, Fujioka H, Nussenzweig V, Ménard R. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J Cell Biol. 1999;147:937–944. doi: 10.1083/jcb.147.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Entzeroth R, Kerckhoff H, König A. Microneme secretion in Coccidia: confocal laser scanning and electron microscope study of Sarcocystis muris in cell culture. Eur J Cell Biol. 1992;59:405–413. [PubMed] [Google Scholar]
- 36.Wengelnik K, Spaccapelo R, Naitza S, Robson KL, Janse CJ, Bistoni F, Waters AP, Crisanti A. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 1999;18:5195–5204. doi: 10.1093/emboj/18.19.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •37. Matuschewski K, Nunes AC, Nussenzweig V, Ménard R. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 2002;21:1597–1606. doi: 10.1093/emboj/21.7.1597.This paper dissects the TRAP domains involved in cell invasion and identify critical residues in A-domain involved in salivary gland invasion.
- 38.Mota MM, Thathy V, Nussenzweig RS, Nussenzweig V. Gene targeting in the rodent malaria parasite Plasmodium yoelii. Mol Biochem Parasitol. 2001;113:271–278. doi: 10.1016/s0166-6851(01)00228-6. [DOI] [PubMed] [Google Scholar]
- 39.Thompson J, Fernandez-Reyes D, Sharling L, Moore SG, Eling WM, Kyes SA, Newbold CI, Kafatos FC, Janse CJ, Waters AP. Plasmodium cysteine repeat modular proteins 1–4: complex proteins with roles throughout the malaria parasite life cycle. Cell Microbiol. 2007;9:1466–1480. doi: 10.1111/j.1462-5822.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 40.Mikolajczak SA, Silva-Rivera H, Peng X, Tarun AS, Camargo N, Jacobs-Lorena V, Daly TM, Bergman LW, de la Vega P, Williams J, et al. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol Cell Biol. 2008;20:6196–6207. doi: 10.1128/MCB.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaiser K, Matuschewski K, Camargo N, Ross J, Kappe SH. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol. 2004;51 doi: 10.1046/j.1365-2958.2003.03909.x. 1221-123. [DOI] [PubMed] [Google Scholar]
- 42.Combe A, Moreira C, Ackerman S, Thiberge S, Templeton TJ, Ménard R. TREP, a novel protein necessary for gliding motility of the malaria sporozoites. Int J Parasitol. 2009;39:489–496. doi: 10.1016/j.ijpara.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Steinbuechel M, Matuschewski K. Role for the Plasmodium sporozoite-specofic transmembrane protein S6 in parasite motility efficient malariatransmission. Cell. Microbiol. 2009;11:279–288. doi: 10.1111/j.1462-5822.2008.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••44. Ghosh AK, Ribolla PE, Jacobs-Loena M. Targeting Plasmodium ligand on mosquito salivary and midgut with a phage display peptide library. Proc Natl Acad Sci USA. 2001;98:13278–13281. doi: 10.1073/pnas.241491198.This paper reports the identification of SM1, a 12-amino acid peptide that binds specifically to the mosquito midgut and salivary glands, while inhibiting parasite invasion of these organs.
- 45.Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 46.Arcà B, Lombardo F, de Lara Capurro M, della Torre A, Dimopoulos G, James AA, Coluzzi M. Trapping cDNAs encoding secreted proteins from the salivary glands of the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 1999;96:1516–1521. doi: 10.1073/pnas.96.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanfrancotti A, Lombardo F, Santolamazza F, Veneri M, Castrignanò T, Coluzzi M, Arcà B. Novel cDNAs encoding salivary proteins from the malaria vector Anopheles gambiae. FEBS Lett. 2002;517:67–71. doi: 10.1016/s0014-5793(02)02578-4. [DOI] [PubMed] [Google Scholar]