Abstract
Two distinct classes of nociceptive primary afferents, peptidergic and non-peptidergic, respond similarly to acute noxious stimulation; however the peptidergic afferents are more likely to play a role in inflammatory pain, while the non-peptidergic afferents may be more characteristically involved in neuropathic pain. Using multiple immunofluorescence, we determined the proportions of neurons in the rat L4 dorsal root ganglion (DRG) that co-express AMPA or NMDA glutamate receptors and markers for the peptidergic and non-peptidergic classes of primary afferents, substance P and P2X3, respectively. The fraction of DRG neurons immunostained for the NR1 subunit of the NMDA receptor (40%) was significantly higher than that of DRG neurons immunostained for the GluR2/3 (27%) or the GluR4 (34%) subunits of the AMPA receptor. Of all DRG neurons double-immunostained for glutamate receptor subunits and either marker for peptidergic and non-peptidergic afferents, a significantly larger proportion expressed GluR4 than GluR2/3 or NR1 and in a significantly larger proportion of P2X3- than SP-positive DRG neurons. These observations support the idea that nociceptors, involved primarily in the mediation of neuropathic pain, may be presynaptically modulated by GluR4-containing AMPA receptors.
Keywords: Glutamate receptor, Dorsal root ganglion, Substance P, P2X3, Rat
Introduction
The involvement of glutamate receptors in the mediation of nociception is documented by a large body of literature (for a review, see Bleakman et al. 2006). Besides being expressed in the membrane of spinal neurons postsynaptic to nociceptive afferents, subunits of both ionotropic and metabotropic glutamate receptors are synthesized by dorsal root ganglion (DRG) neurons and transported both peripherally and centrally (Willis and Coggeshall 2004). Peripheral terminals of primary afferents in the skin (Nunzi et al. 2004; Brumovsky et al. 2007) and palatine mucosa (Nunzi et al. 2004; Brumovsky et al. 2007) express vesicular glutamate transporters, suggesting that they release glutamate. Conversely, activation of the same peripheral terminals by glutamate or its agonists can induce aversive behavior (Carlton et al. 1995), suggesting that they also express glutamate receptors. Moreover, activation of glutamate receptors expressed in primary afferent terminals can decrease their release of glutamate (Lee et al. 2002; Bardoni et al. 2004) and/or facilitate their release of the neuropeptide substance P (SP, (Liu et al. 1997; Marvizón et al. 1997; Malcangio et al. 1998).
In our previous work we used immunohistochemistry to show that central terminals of DRG neurons in the superficial laminae of the dorsal horn express subunits of ionotropic and metabotropic glutamate receptors (Jia et al. 1999; Hwang et al. 2001a, 2001b; Lu et al. 2002, 2003). To provide further insight into the role that presynaptic glutamate receptors may play in pain modulation, we also identified the subunits of the kainate glutamate receptor that are expressed by nociceptive primary afferents (Lucifora et al. 2006). As a direct continuation of this study, we here aimed at determining whether α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) or N-methyl-d-aspartic acid (NMDA) receptors are preferentially expressed in either one or both of the two major classes of nociceptors, i.e. peptidergic (identified by immunostaining for SP) and non-peptidergic (identified by immunostaining for the P2X3 subunit of the purinergic receptor).
Materials and Methods
All animals were treated according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina. Nine Sprague-Dawley rats (200–300 g, Charles River, Raleigh, NC) were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and perfused through the heart with 100 ml normal saline, containing 500U heparin sodium followed by 4% paraformaldehyde (PF) in phosphate buffer (PB, 0.1M, pH 7.4) at room temperature. L4 DRG were removed and post-fixed in 4% PF for 2 hrs at 4 °C. The DRG were cryoprotected in 30% sucrose in PB overnight at 4 °C, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA), and sectioned on a cryostat at 15 µm, thaw-mounted on slides, and stored at −20 °C.
For immunohistochemistry, all incubations were carried out on a shaker at room temperature. Sections were permeabilized with 50% ethanol, rinsed with phosphate-buffered saline (PBS, 0.01M, pH 7.2), blocked in 10% normal donkey serum (NDS) for 30 min, and incubated overnight in a mixture containing one antibody against a glutamate receptor subunit and either an anti-P2X3 or an anti-SP antibody (Table 1). Following rinses with PBS and blocking with 2% NDS, the sections were incubated in Cy3- or FITC-conjugated secondary antibodies raised in donkey (1:200 in PBS; Jackson ImmunoResearch, West Grove, PA) for 2 hrs, then rinsed and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). For optimal staining for NR1 (Table 1), we used tyramide signal amplification (TSA; Perkin Elmer, Boston, MA): Sections were pretreated with 1% H2O2 followed by blocking buffer (5% NDS/0.1% Triton-X100/0.1 M PBS), and then incubated overnight in a mixture of anti-NR1 and anti-P2X3 or anti-SP antibodies in blocking buffer. After brief rinses with PBS and blocking buffer, sections were incubated for 2 hrs in biotinylated donkey anti-rabbit and FITC-conjugated donkey anti-guinea pig antibodies (1:200; Jackson Immunoresearch). After several rinses, the sections were incubated with streptavidin-horseradish peroxidase (1:100 in amplification diluent) for 30 min, and then with Cy3-tyramide (1:100 in amplification diluent) for 8 min.
Table 1.
Primary antibodies used in the study
| Antibody | Epitope (AA) | Host | Source | Cat. # | Lot | Dilution |
|---|---|---|---|---|---|---|
| GluR2/3 | 871–883 | Rabbit | Chemicon | AB1506 | 0507005717 | 1:100 |
| GluR4 | 889–902 | Rabbit | Chemicon | AB1508 | 21081551 | 1:250 |
| NR1 | 909–938 | Rabbit | Millipore | AB9864 | 0704057185 | 1:10,000 |
| P2X3 | 383–397 | Guinea pig | Neuromics | GP10108 | 400457 | 1:250 |
| SP | 1–11 | Guinea pig | Neuromics | GP14103 | 20046 | 1:250 |
All antibodies used in this study are well characterized and are in common use in our laboratory (Lu et al. 2002, 2003; Bae et al. 2004; Lucifora et al. 2006; Kim et al. 2008). As a matter of routine control, sections were processed according to the above protocols, except that primary or secondary antibodies were omitted, or blocking peptides were added; omission of primary or secondary antibodies or preadsorption with blocking peptides completely abolished specific staining.
Dual-color images from DRG sections were acquired with a Retiga EX cooled CCD camera (QImaging, Burnaby, Canada) attached to a Leitz DMR fluorescent microscope (Leitz, Wetzlar, Germany) at 10X, and saved as TIFF files using OpenLab software (Improvision, Lexington, MA). Brightness and contrast were adjusted with Photoshop CS2 (Adobe Systems, San Jose, CA); all enhancements were applied to the entire image. To quantify colocalization of GluR2/3, GluR4, and NR1 with SP or P2X3 in DRG neurons, NP were counted in 4–5 sections per ganglion at 90 µm intervals from each of 6 ganglia from 3 rats for each double staining by an investigator blinded to the source material. Neuronal profiles were identified by their characteristic cellular morphology and clearly visible nucleus. In every section, NP were counted in one gray-scale image for each of the two fluorescent channels. For each channel, the cut-off brightness level (labeling density threshold) was determined by averaging the integral brightness of three neuronal profiles per image that were judged to be minimally positive using Image J 1.38x software (NIH, Bethesda, MD); all profiles whose mean labeling density exceeded this threshold were counted as positive. To avoid counting the same NP more than once, we used a modified version of the physical dissector principle (Coggeshall 1992; Carlton and Hargett 2002): each positive NP was labeled with a dot, color-coded after the respective channel, as they were counted. Immuno-negative NP with clearly visible nuclei were identified using sub-threshold (“background”) labeling on the green channel and labeled with white dots; in some cases, these were verified in images of the same sections taken with DIC optics. To determine colocalization, the two gray-scale images containing the colored dots were overimposed and the fraction of NP in each of the four categories [green, red, yellow (green+red, double-labeled), and white (unlabeled)] in each section were normalized to the total number of NP and expressed as a percentage of total counted NP (Marvizón et al. 2002). Data were analyzed with one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test, using SPSS 11.5x software (SPSS, Chicago, IL) and graphed using Microsoft Excel.
Results
The percentages of immunostained DRG neuronal profiles (NP) and the patterns of colocalization varied according to the antibodies employed (Fig. 1, 2). Among the glutamate receptor subunits studied, the sparsest number of glutamate receptor-positive NP was immunostained with an antibody against GluR2/3 (26.7±0.9 of all NP; mean % ± S.E.M.); a larger proportion of NP were immunostained for either GluR4 (33.9±1.0) or NR1 (39.3±1.1). Of the markers for peptidergic and non-peptidergic primary afferent neurons, the proportion of NP immunostained for P2X3 (22.1±0.8%) was higher than that for SP (13.1±0.4%).
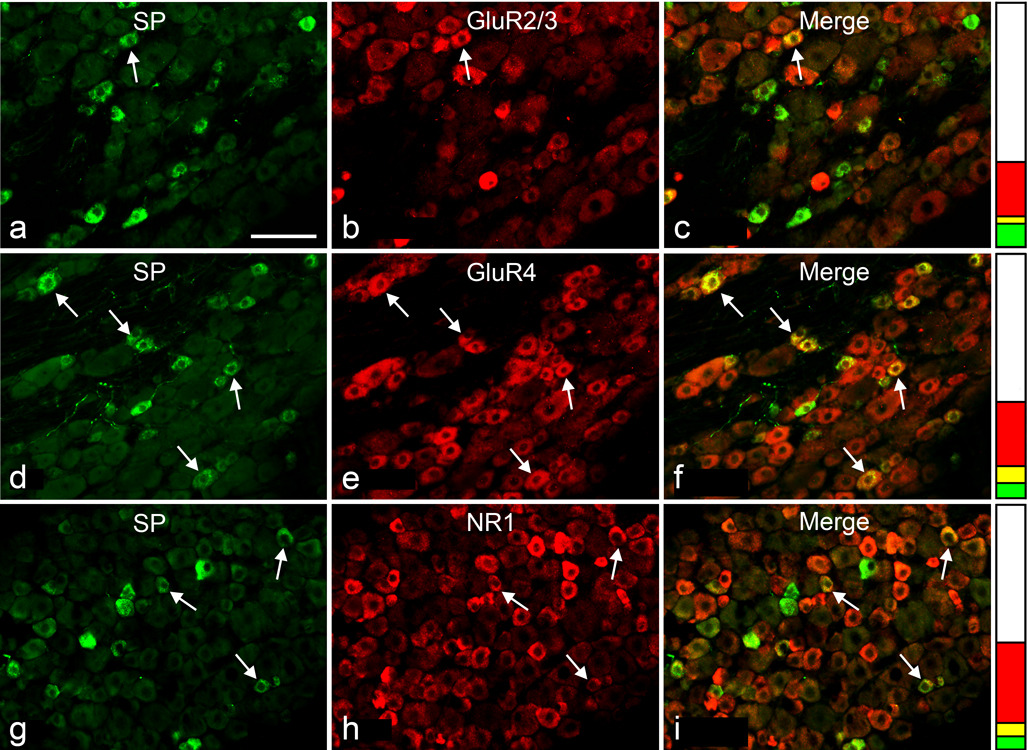
Fig. 1.
Double immunofluorescence staining for SP (a, d, g) and either GluR2/3 (b), GluR4 (e) or NR1 (h) in sections of L4 DRG; examples of double-stained neuronal profiles (arrows) appear yellow in the merged images (c, f, i). Stacked columns next to images in each row represent the neuronal profiles single-stained for SP (green), single-stained for glutamate receptors (red), double-stained (yellow), and unstained (white), as a fraction of all profiles for each double immunostaining. Scale bar, 100 µm
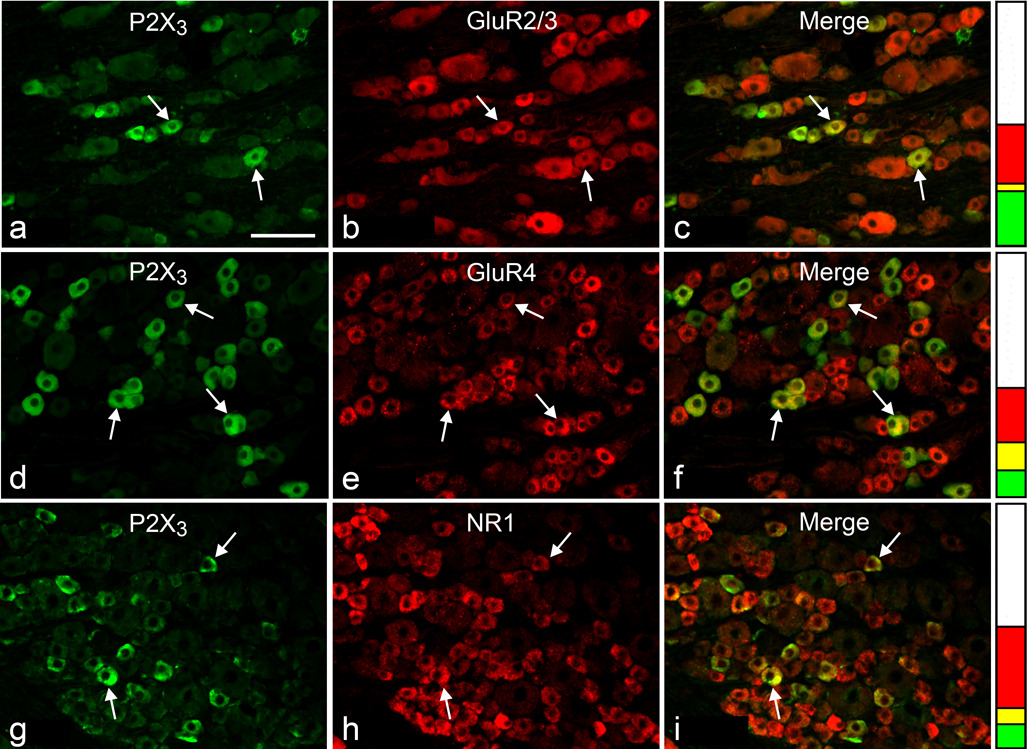
Fig. 2.
Double immunofluorescence staining for P2X3 (a, d, g) and either GluR2/3 (b), GluR4 (e) or NR1 (h) in sections of L4 DRG; examples of double-stained neuronal profiles (arrows) appear yellow in the merged images (c, f, i). Stacked columns next to images in each row represent the neuronal profiles single-stained for P2X3 (green), single-stained for the respective glutamate receptor (red), double-stained (yellow), and unstained (white), as a fraction of all profiles for each double immunostaining. Scale bar, 100 µm
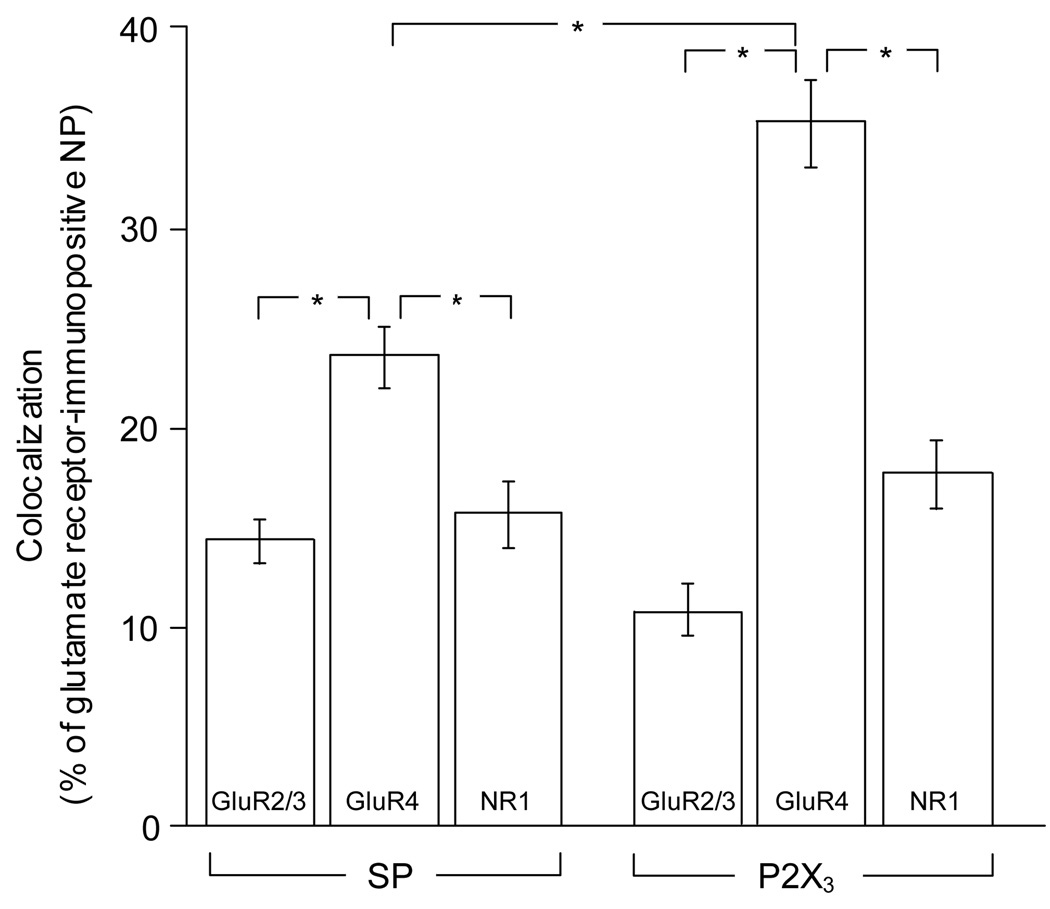
The proportions of double-labeled NP for each glutamate receptor subunit and either SP or P2X3 are presented in Fig. 3. Since inter-animal variability of these proportions was insignificant, results from different animals and ganglia were pooled together. More SP-expressing NP were immunopositive for GluR4 (56.1±3.3) or NR1 (48.6±2.5) than for GluR2/3 (28.7±2.4). Similarly, more P2X3-expressing NP were immunopositive for GluR4 (53.0±2.8) or NR1 (41.9±3.5) than for GluR2/3 (11.7±1.3). The results also showed that: a) the proportion of glutamate receptor-expressing NP that also express either marker for peptidergic and non-peptidergic primary afferents was significantly higher for GluR4 (SP=23.6±1.4; P2X3=35.2±2.1) than for either NR1 (SP=15.7±1.5; P2X3=17.7±1.6) or GluR2/3 (SP=14.4±1.0; P2X3=10.6±1.1), and b) the proportion of GluR4-positive NP that express P2X3 was significantly higher than that of GluR4- positive NP that express SP (Fig. 3).
Fig. 3.
Graphs show percent (mean ± S.E.M.) of SP-positive (indicated at the bottom) or P2X3-positive DRG neuronal profiles that immunostain for glutamate receptor subunits (indicated within the columns), as a fraction of all glutamate receptor-expressing neuronal profiles (NP) for each double immunostaining. *p<0.001, one-way ANOVA followed by Tukey’s post hoc test
Discussion
This study shows that i) a significantly larger proportion of glutamate receptor-positive primary afferents express GluR4 than GluR2/3 or NR1, and ii) GluR4 is expressed in a significantly larger number of P2X3- than SP-positive DRG neurons.
Nociceptive afferents have been classified into two largely distinct groups, peptidergic and non-peptidergic, based not only on their expression of neuropeptides, but also on their dependence on different growth factors for survival during postnatal development (Molliver et al. 1997) and topographic segregation of their termination, both centrally in different laminae of the dorsal horn, and peripherally in the skin (Hunt and Rossi 1985; Zylka et al. 2005). Functional differences include lower threshold and shorter duration of action potentials and shorter TTX-resistant currents and bigger heat currents (Stucky and Lewin 1999) and stronger tendency to sprout extensively after dorsal rhizotomy for the peptidergic nociceptors (Bennett et al. 1996). Moreover, even though both classes may respond similarly to acute noxious stimulation (Malmberg et al. 1997), the peptidergic afferents are more likely to play a role in inflammatory pain (Hunt and Mantyh 2001), while the non-peptidergic afferents may be more characteristically involved in neuropathic pain (Julius and Basbaum 2001).
The most commonly used histochemical markers for peptidergic and non-peptidergic nociceptive afferents are the expression of SP (Lawson et al. 1997; Nichols et al. 1999) and the propensity for binding the isolectin B4 of Griffonia Simplicifolia (IB4, (Silverman and Kruger 1990), respectively. However, since IB4 has been reported to bind more than one-half of the peptidergic afferents (Wang et al. 1994; Bergman et al. 1999; Kashiba et al. 2001), the classification of nociceptors based on the use of IB4 may be flawed (Hwang et al. 2005; Price and Flores 2007), and segregating peptidergic from non-peptidergic nociceptors remains controversial (Woolf and Ma 2007). Conversely, the colocalization of SP with P2X3 is negligible (only 3% of the P2X3-immunopositive DRG neurons were found to be SP-positive, (Vulchanova et al. 1998), making P2X3 a marker of choice for a class of nociceptors that is distinct from those that could be identified by immunostaining for SP (Lucifora et al. 2006).
Depending on the ganglion level, species, and, perhaps more importantly, different criteria for determining immunopositivity, counts of SP-expressing neurons and of P2X3- expressing neurons may vary, up to about 20% (Hokfelt et al. 1975; Battaglia and Rustioni 1988), and 35–65% (Bradbury et al. 1998; Vulchanova et al. 1998; Novakovic et al. 1999; Fukuoka et al. 2002), respectively, of the total DRG neuronal population. Counts in the present study were within the lower ranges reported previously for all antigens, including glutamate receptors (see also below).
All AMPA receptor subunits may be synthesized by DRG neurons (Chambille and Rampin 2002). Microscopic evidence for central transport has been provided mainly for the GluR2/3 and GluR4 subunits (Lu et al. 2002), suggesting a selective expression of Ca2+-permeable homomeric or heteromeric complexes of AMPA receptors in primary afferents to the spinal cord. Presynaptic GluR4 is mainly expressed in terminals in lamina I and II of the dorsal horn, while presynaptic GluR3 is mainly expressed in terminals of primary afferents to deeper laminae that receive low-threshold mechanoreceptor input (Lu et al. 2002). Based on this pattern of termination, it is more likely that nociceptors express GluR4-containing presynaptic glutamate receptors. However, the anatomical definition of nociceptors as primarily small DRG neurons with central terminations in the superficial laminae of the dorsal horn may need to be revised (Light and Perl, 2003).
That NR1-expressing afferents include nociceptors is suggested by their pattern of termination in the spinal cord (Lu et al. 2003). After having been reported in central terminals of C fibers (Liu et al. 1994), presynaptic NMDA receptors were assigned a tentative role in the facilitation of release of SP from these terminals (Liu et al. 1997). The present observations suggest that, if indeed activation of presynaptic NMDA receptors facilitates SP release, this may occur in a fraction of central afferents that express the NMDA receptor. In the present work, we applied more stringent criteria of what is considered immunopositive, which lead to a more conservative estimate of NR1 expression in DRG neurons (40%) than the previously reported 84–90% (Wang et al. 1999; Marvizón et al. 2002). In an earlier study, we found that virtually all DRG neurons stain for NR1 but noted that the staining displayed a gradient of immunoreactivity making it difficult to sort out “positive” from “negative” cell bodies in DRG (Lu et al. 2003). In the same study, we also suggested that NR1-expressing primary afferents are mostly non-peptidergic since we saw no colocalization with CGRP. Since all DRG neurons possess high levels of mRNA for NR1 (Sato et al. 1993), but apparently express varying levels of the protein, the accuracy of any estimates obtained with immunohistochemistry will be limited by the inherent arbitrariness in choosing the cutoff labeling density. The use of TSA and higher cutoff density for counting in the present study may have accounted for the discrepancies between the current and our previous results.
In a previous report, we demonstrated that the kainate receptor (predominantly the GluR5 subunit) is expressed by a significantly larger fraction of P2X3- than SP-positive DRG neurons (Lucifora et al. 2006). In the present work, we found that a significantly larger proportion of both classes of nociceptive afferents express GluR4 than GluR2/3 or NR1. This result is in agreement with a possible selectivity of GluR4 expression in nociceptive afferents (Lu et al. 2002). Furthermore, the results also show that, like in the case of GluR5, GluR4 is expressed in a significantly larger number of P2X3- than SP-positive DRG neurons. Although both classes of primary afferents considered here may contribute to the sensation of acute pain, peptidergic afferents may play a role in inflammatory pain while afferents expressing P2X3 receptors are more likely to be involved in neuropathic pain (Malmberg et al. 1997; Hunt and Mantyh 2001). Thus, the results of this study support the idea that nociceptive primary afferents, particularly those involved in the mediation of neuropathic pain, may be presynaptically modulated by GluR4-containing AMPA receptors.
Acknowledgments
We thank Dr. A. Rustioni for support of this research and Dr. Lyndon M. Foster (Millipore Corporation, Temecula, CA) for expert technical assistance with the glutamate receptor antibodies.
Funding: Financial support for his work was provided by The National Institutes of Health, USA, through research grants NINDS12440 and AR053721.
References
- Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol. 2004;478:62–71. doi: 10.1002/cne.20272. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci. 2004;24:2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol. 1988;277:302–312. doi: 10.1002/cne.902770210. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur J Neurosci. 1996;8:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Bergman E, Carlsson K, Liljeborg A, Manders E, Hokfelt T, Ulfhake B. Neuropeptides, nitric oxide synthase and GAP-43 in B4-binding and RT97 immunoreactive primary sensory neurons: normal distribution pattern and changes after peripheral nerve transection and aging. Brain Res. 1999;832:63–83. doi: 10.1016/s0006-8993(99)01469-9. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Watanabe M, Hökfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Stereological analysis of Ca(2+)/calmodulin-dependent protein kinase II alpha -containing dorsal root ganglion neurons in the rat:colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Chambille I, Rampin O. AMPA glutamatergic receptor-immunoreactive subunits are expressed in lumbosacral neurons of the spinal cord and neurons of the dorsal root and pelvic ganglia controlling pelvic functions in the rat. Brain Res. 2002;933:66–80. doi: 10.1016/s0006-8993(02)02309-0. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975;100:235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Rossi J. Peptide- and non-peptide-containing unmyelinated primary afferents: the parallel processing of nociceptive information. Philos Trans R Soc Lond B Biol Sci. 1985;308:283–289. doi: 10.1098/rstb.1985.0028. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG. The majority of bladder sensory afferents to the rat lumbosacral spinal cord are both IB4- and CGRP-positive. Brain Res. 2005;1062:86–91. doi: 10.1016/j.brainres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Pagliardini S, Rustioni A, Valtschanoff JG. Presynaptic kainite receptors in primary afferents to the superficial laminae of the rat spinal cord. J Comp Neurol. 2001a;436:275–289. [PubMed] [Google Scholar]
- Hwang SJ, Rustioni A, Valtschanoff JG. Kainate receptors in primary afferents to the rat gracile nucleus. Neurosci Lett. 2001b;312:137–140. doi: 10.1016/s0304-3940(01)02204-2. [DOI] [PubMed] [Google Scholar]
- Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol. 1999;410:627–642. [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Difference in binding by isolectin B4 to trkA and c-ret mRNA-expressing neurons in rat sensory ganglia. Brain Res Mol Brain Res. 2001;95:18–26. doi: 10.1016/s0169-328x(01)00224-8. [DOI] [PubMed] [Google Scholar]
- Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC. Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. J Comp Neurol. 2008;506:627–639. doi: 10.1002/cne.21544. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol. 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35:135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Unmyelinated afferent fibers are not only for pain anymore. J Comp Neurol. 2003;461:137–139. doi: 10.1002/cne.10691. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci U S A. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CR, Hwang SJ, Phend KD, Rustioni A, Valtschanoff JG. Primary afferent terminals in spinal cord express presynaptic AMPA receptors. J Neurosci. 2002;22:9522–9529. doi: 10.1523/JNEUROSCI.22-21-09522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CR, Hwang SJ, Phend KD, Rustioni A, Valtschanoff JG. Primary afferent terminals that express presynaptic NR1 in rats are mainly from myelinated, mechanosensitive fibers. J Comp Neurol. 2003;460:191–202. doi: 10.1002/cne.10632. [DOI] [PubMed] [Google Scholar]
- Lucifora S, Willcockson HH, Lu CR, Darstein M, Phend KD, Valtschanoff JG, Rustioni A. Presynaptic low- and high-affinity kainate receptors in nociceptive spinal afferents. Pain. 2006;120:97–105. doi: 10.1016/j.pain.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Fernandes K, Tomlinson DR. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. Br J Pharmacol. 1998;125:1625–1626. doi: 10.1038/sj.bjp.0702260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Marvizón JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizón JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-d-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Pisarek A, Mugnaini E. Merkel cells, corpuscular nerve endings and free nerve endings in the mouse palatine mucosa express three subtypes of vesicular glutamate transporters. J Neurocytol. 2004;33:359–376. doi: 10.1023/B:NEUR.0000044196.45602.92. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Rivero-Melian C, Robertson B, Grant G. Transganglionic transport and binding of the isolectin B4 from Griffonia simplicifolia I in rat primary sensory neurons. Neuroscience. 1994;62:539–551. doi: 10.1016/0306-4522(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang RX, Wang R, Qiao JT. Decreased expression of N-methyl-d-aspartate (NMDA) receptors in rat dorsal root ganglion following complete Freund's adjuvant-induced inflammation: an immunocytochemical study for NMDA NR1 subunit. Neurosci Lett. 1999;265:195–198. doi: 10.1016/s0304-3940(99)00246-3. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. New York: Plenum; 2004. [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]