Abstract
The authors examined the effects of lifetime trauma exposure on salivary cortisol and mood in a sample of women (N = 37) over 25 days before and after a stressful event. The sample excluded posttraumatic stress disorder (PTSD) and major depression and was divided into three groups: (a) no trauma, (b) prior trauma with no peritraumatic symptoms of acute distress, and (c) prior trauma with peritraumatic symptoms. Because results indicated no significant differences between groups one and two, they were combined for analysis. Women reporting prior trauma with symptoms had lower afternoon cortisol levels across time, with sustained negative mood relative to the comparison group. These data suggest the presence of long-term psychophysiological effects of trauma exposure in healthy women.
The consequences of exposure to psychological trauma have been understudied in the nonclinical human population (McFarlane, 1997), although epidemiological evidence suggests that more than half of the population experiences at least one trauma in their lifetime, and a quarter experiences two or more (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Trauma exposure is a predictor of anxiety and depression (Brown, 1993; Kendler, Hettema, Butera, Gardner, & Prescott, 2003), and a predisposing factor for further incidence of PTSD if there is a subsequent trauma (Bremner, Southwick, Johnson, Yehuda, & Charney, 1993; Breslau, Chilcoat, Kessler, & Davis, 1999). This suggests that there may be lasting psychophysiological consequences of trauma exposure, even in those who do not have a current clinical disorder.
There is a small body of research on the biological effects of trauma exposure in adult samples known to be free of clinical disorder, including two recent neuroimaging studies examining the neural correlates of exposure to the terrorist attacks of September 11, 2001 (Ganzel, Casey, Voss, Glover,&Temple, in press; Sharot, Martorella, Delgado, & Phelps, in press). Using different paradigms, both studies found that increased proximity to the disaster predicted increased amygdala reactivity in healthy adults more than 3 years after the attacks.
Consistent with these results, heightened cardiovascular reactivity to reminders of the Oklahoma City bombing has been observed in survivors of that event more than 6 years afterwards (Tucker et al., 2007). On the other hand, very limited evidence for psychophysiological correlates of trauma exposure in healthy adults has been observed in studies that do not involve group-wide exposure to a disaster-level trauma (Young & Breslau, 2004a, 2004b; Young, Tolman, Witkowski, & Kaplan, 2004). This may be, in part, due to methodological differences across these studies. The definition of trauma employed by Young and colleagues in their large epidemiological studies (Young & Breslau, 2004a, 2004b; Young et al., 2004) did not include peritraumatic arousal (e.g., intense feelings of shock, horror, or helplessness). Conversely, the terrorist attacks on the World Trade Center in New York City and on the Alfred P. Murrah Federal Building in Oklahoma City were likely to be highly arousing to those nearby (e.g., Galea et al., 2002). This highlights the potential importance of peritraumatic arousal in studies of the effects of trauma exposure in resilient populations (i.e., trauma exposed, but without a clinical diagnosis).
The role of peritraumatic arousal in studies of trauma outcomes is also highlighted in a major meta-analysis of predictors of posttraumatic stress disorder (PTSD) and PTSD symptoms (Ozer, Best, Lipsey, & Weiss, 2003), which reports that peritraumatic emotional response is the strongest predictor of PTSD and PTSD symptoms (stronger than history of prior trauma, prior psychological adjustment, family history of disorder, trauma severity, and posttrauma social support). This suggests that the psychobiological sequelae of trauma exposure may be less dependent on the occurrence of a threatening event than on the individual's level of arousal in response to that event. If so, peritraumatic arousal may be a more salient predictor of persistent change in other indicators of stressor exposure, such as cortisol production and mood state (Blazer & Hybels, 2004; Steptoe, Wardle, & Marmot, 2005), where few effects of trauma have been observed in resilient samples.
To investigate this point, we compared salivary cortisol data, mood data from daily diaries, and survey information (lifetime mental health and weekly anxiety assessment) collected from a sample of women with varying levels of trauma exposure, which had been screened to exclude PTSD and major depression. Following the work of Ozer and colleagues (Ozer et al., 2003; Ozer & Weiss, 2004), we divided the sample by prior history of trauma exposure: (a) no trauma, (b) trauma exposure with no peritraumatic symptoms of acute stress, and (c) any trauma exposure with peritraumatic symptoms. We chose to study women because they are understudied in the trauma literature (Young et al., 2004).
Because it has been argued that hypothalamic-pituitary-adrenal (HPA) axis-behavior associations are most likely to manifest under stressful circumstances of high ecological validity (Granger, Weisz, & Kauneckis, 1994; Heim, Ehlert, & Hellhammer, 2000), this study used the graduate entrance examination paradigm successfully employed by Bolger and Eckenrode (1991) in a prospective assessment of the normative human stress response to challenge (see Methods section below). The examination stressor was used here to help differentiate HPA axis function and mood over time between women with different levels of trauma exposure.
We focused on two specific questions. First, do salivary cortisol levels differ over time across our study groups? Second, does mood over time differ across groups?
METHOD
These data were collected as part of a 25-day prospective study of the effects of prior traumatic experience on HPA-axis function during a stressful experience (the Medical College Admissions Test; MCAT). Longitudinal data collection started on day 20 before the MCAT examination and continued for 4 days afterwards. Prior to data collection, all participants were interviewed to assess demographics and lifetime mental health. Longitudinal data collection included four waves of salivary cortisol collection across the 25-day study period. This study was approved by a university human subjects review committee and all participants provided informed consent for both the interview and salivary cortisol collection.
Participants
The 37 participants in this study were female premedical students who were in the process of studying for and taking the MCAT. They were recruited at information sessions for the MCAT examination, at registration for the MCAT, and at MCAT preparatory classes. Excluded were those who did not complete study requirements (e.g., postponed the MCAT) as well as those with significant medical illness, past or present history of psychotic symptoms or bipolar disorder, PTSD, major depression, substance abuse or dependency, endocrine malfunction, use of psychotropic medication, previous experience taking the MCAT, or age greater than 30 years. In their daily diaries, participants recorded number of drinks per day of alcohol and caffeine for that day. Oral contraceptive and substance use (including alcohol and caffeine) was documented at intake and included in analyses. No participant smoked more than 5 cigarettes per week. Due to issues with the survey instrument, data on timing of menses was only collected for a subset of women (n = 23).
Measures
Diagnostic and behavioral assessments
The University of Michigan Composite International Diagnostic Interview (UM-CIDI: Kessler et al., 1994) is a fully structured diagnostic interview that allows the assessment of lifetime mental disorders according to the Diagnostic and Statistical Manual for Mental Disorders, third edition revised (DSM-III-R; American Psychiatric Association, 1987). The UM-CIDI was developed for the National Comorbidity Survey (1990–1992; N = 8600). The UM-CIDI modules used in this study included demographics, social support, a mental health screener, depression, irritable depression, suicidality, agoraphobia, social phobia, panic disorder, bipolar disorder, general anxiety disorder, posttraumatic stress disorder, childhood separation anxiety, pharmacoepidemiology, and life events. In this study, the number of affective and anxiety diagnoses prior to first trauma was used as an indicator of pretrauma individual differences in stress reactivity.
The Brief Symptom Inventory 18 (BSI 18; Derogatis, 2000) is self-report symptom inventory designed to measure symptom change and intensity during the past week across three dimensions: (a) anxiety, (b) depression, and (c) somatization. Dimension and global scores correlate highly with the Symptom Check List-90-R (SCL-90-R) based on a large community sample (Derogatis, 2000). Internal consistency is acceptable for all dimensions. Estimates of test-retest reliability are obtained from the parent scale, the Brief Symptom Inventory, and are within the acceptable range (Derogatis, 2000).
Participants also kept a daily dairy of mood. They were introduced to the diary format (Eckenrode & Bolger, 1997) in the baseline session and asked to fill out subsequent versions on paper, beginning three weeks pretest and continuing through four days posttest. Positive and negative mood were recorded every evening before bed using a positive and negative emotionality scale (PANAS; Watson, Clark, & Tellengen, 1988). Each of these is a ten-item scale assessing current ranking of mood state, with a high score representing high activation. These scales are based on the full range of adjectives for each mood state. These adjectives have been shown to have excellent factor homogeneity, retest reliabilities, and internal consistencies (Watson et al., 1988).
Cortisol measurement
There were four waves of salivary cortisol collection: baseline (preexam days 21 and 20, pooled); preexam day 3, preexam day 1, and postexam (postexam days 2 and 3, pooled). In the sampling procedure for salivary cortisol, the participant expelled saliva via a wide straw into a cryotube, then sealed the tube and refrigerated it at home refrigerator temperatures. Participants were instructed to collect their saliva samples one half hour after normal waking for the morning samples and at 4 p.m. for the afternoon samples. The morning guidelines were established to capture realistic ambulatory levels, although we recognized that they would result in a range of sampling times. Participants were asked to refrain from eating or drinking 15 minutes before the sample and to avoid caffeine or vigorous exercise for an hour prior to sampling. The participants were asked to record time of saliva sampling, with the added request that each participant note the actual times of sampling even if they were not able to sample exactly at the specified times.
All materials were mailed to the researcher (unfrozen salivary cortisol samples are expected to be stable under these conditions: Clements & Parker, 1998). The sealed saliva samples were then frozen and shipped to Salimetrics Laboratories (Salimetrics Laboratories, State College, PA), where they were assayed in duplicate for salivary cortisol using a high-sensitive enzyme immunoassay 510k. All samples were tested in duplicate and the averages of the duplicates were used in all analyses. Samples with duplicate results that varied by more than 10% were subject to repeat testing. The test uses 25 μL of saliva, range of sensitivity from .007 to 1.8 μg/dL, and average intra- and interassay coefficients of variation were less than 5% and 10% respectively. All samples for a single subject were run on the same assay plate.
Examination of the cortisol data revealed that the distributions were positively skewed. Prior to analysis, natural log transforms were used to establish approximately normal distributions. All cortisol analyses used the log-transformed values. To facilitate interpretation, nontransformed data are used in the figures and tables.
Trauma exposure and group status
The participants received screening for exposure to potentially traumatic events using the PTSD module of the UM-CIDI. Report of peritraumatic symptoms of acute distress was determined by an affirmative answer to queries regarding: (a) feelings of being very frightened or terrified, helpless, shocked or horrified, or emotionally numb during the event; and (b) the presence of upsetting memories or dreams, feelings of emotional distance or depression, trouble sleeping, trouble concentrating, and feeling jumpy or easily startled. The sample was initially divided into three groups: (a) those who reported no traumatic events in lifetime (no trauma), (b) those who reported no peritraumatic symptoms of acute stress at the time of trauma exposure (no symptoms), and (c) those who reported trauma exposure with at least one peritraumatic symptom (symptoms).
RESULTS
Participant Characteristics and Traumatic Experiences
The 37 women in the study had a mean age of 21.3 years (SD = 0.7) and they were predominantly single (98% never married). The group as a whole was 77.3% White, 9.1% Asian, 0% Latina, 4.5% African American, and 9.1% mixed/other ethnic backgrounds. There were no significant differences between the two groups in terms of age, self-reported physical health, ethnicity, educational level, socioeconomic status (SES), substance use, or use of oral contraceptives. Of the subset providing data on timing of menses (n = 23), there was no significant difference across groups in luteal versus follicular phase at baseline.
There was no reported current consumption of any psychoactive substances except caffeine and alcohol. There were no significant differences across groups in amount of caffeine and alcohol consumed. There was no significant difference between groups in alcohol consumed on any evening prior to cortisol sampling days.
Five of the 37 women reported no traumas in their lifetime. Fifteen of the women reported experiencing traumas that did not engender peritraumatic symptoms indicating acute distress. The remaining 17 women in the sample reported having at least one instance of trauma in lifetime accompanied by at least one symptom, as defined above. As previously stated, the sample was initially divided into three groups: (a) no traumatic events, (b) those who reported no peritraumatic symptoms at time of trauma exposure, and (c) those who reported trauma exposure with at least one symptom. However, we found no significant differences in demographics or behavioral findings (e.g., age, marital status, SES, and physical health, alcohol and caffeine use) between the no trauma group and the group reporting trauma exposure, but not symptoms. Analyses (random intercept models, as described in the Results section) were conducted as two-group models including the no trauma group (no trauma or trauma with no symptoms vs. trauma with symptoms) and compared to two-group models with the no trauma group removed (trauma with symptoms vs. trauma with no symptoms) for each set of outcomes as they varied over time. There were no significant differences in the results. For parsimony, all results below are reported for a two-group model that compares the no-symptom group (no trauma or trauma with no symptoms: n = 20) with the group that experienced trauma with symptoms (n = 17).
There was no significant difference between the two groups in mean age at first traumatic event, years since worst trauma, or years since most recent trauma. There was a significant difference between groups in number of traumatic events in lifetime, t(35) = −2.60, p < .05, with the symptom group having the higher mean number of traumas. Further investigation revealed that there was no difference between groups in mean number of prepubertal traumas. The group with symptoms had a significantly higher mean number of postpuberal trauma exposure (M = 3.5, SD = 2.5) than the no-symptom group (M = 1.5, SD = 1.7), t(35) = −2.90, p < .01. There were also significant differences across groups in the type of trauma exposure these women experienced (see Table 1). In particular, the women in the group reporting peritraumatic symptoms also reported exposure to significantly higher numbers of traumas involving other people (e.g., unexpected death or life-threatening injury of a loved one). Examination of the significance of number of traumas and type of traumas in the prediction of cortisol levels and mood was carried out as a set of post hoc analyses, below.
Table 1.
Types of Trauma Exposure Across Groups—Total Count per Group With Means and Standard Deviation
| No symptoms n = 20 |
Symptoms n = 17 |
|||||
|---|---|---|---|---|---|---|
| Type of trauma | Total | M | SD | Total | M | SD |
| Traumas to others | ||||||
| Unexpected death: Loved one | 2 | 0.10 | 0.31 | 15 | 0.89** | 1.11 |
| Life threatening illness/injury: Loved one | 8 | 0.45 | 0.69 | 19 | 1.12* | 0.99 |
| Traumatic experience: Loved one | 1 | 0.05 | 0.22 | 8 | 0.47 | 1.01 |
| Witness serious injury/death | 6 | 0.30 | 1.13 | 3 | 0.18 | 0.39 |
| Saw atrocities/badly damaged bodies | 0 | 0 | 0 | 6 | 0.35** | 0.61 |
| Number of traumas to others | 17 | 0.90 | 1.48 | 51 | 3.00*** | 2.09 |
| Traumas to self | ||||||
| Life-threatening illness or injury | 3 | 0.15 | 0.49 | 4 | 0.23 | 0.44 |
| Motor vehicle accident, other accident | 2 | 0.10 | 0.31 | 5 | 0.29 | 0.47 |
| Man-made or natural disaster | 7 | 0.35 | 0.93 | 3 | 0.18 | 0.53 |
| Rape | 1 | 0.05 | 0.22 | 0 | 0 | 0 |
| Nonrape sexual assault | 2 | 0.10 | 0.45 | 5 | 0.17 | 0.59 |
| Other assault | 1 | 0.05 | 0.22 | 3 | 0.18 | 0.73 |
| Living with ongoing terrorism/war | 4 | 0.20 | 0.70 | 5 | 0.29 | 0.59 |
| Stalking: Serious danger | 2 | 0.10 | 0.45 | 3 | 0.18 | 0.53 |
| Number of traumas to self | 22 | 1.22 | 1.48 | 28 | 1.65 | 2.00 |
| Undisclosed/other | 4 | 0.20 | 0.52 | 2 | 0.12 | 0.33 |
p < .05.
p < .01.
p < .001.
There were no lifetime or current diagnoses of irritable depression, bipolar disorder, or separation disorder in either group. There were no significant differences between the two groups in incidence of lifetime or current general anxiety disorder, lifetime or current social phobia, or lifetime or current agoraphobia. There were also no reported differences between groups in received social support, social conflict, or social network size.
Trauma Exposure and HPA-Axis Function
Means of morning and afternoon cortisol levels and cortisol change scores (morning cortisol levels minus afternoon cortisol levels) for the two groups are shown in Table 2. Mean morning sampling time was 8:31 p.m. (SD = 0.97 hours); mean afternoon sampling time was 4:46 p.m. (SD = 1.30). Because each person was repeatedly measured over time of day and day of sampling, the data has a multilevel structure and required the use of a random intercept model (Singer & Willet, 2003), with subject as the random effect. This allowed us to test how group status (no symptom vs. symptoms) was associated with intraindividual changes in morning and afternoon cortisol levels, over days of sampling (baseline, 3 days prior to exam, one day prior to exam, 3 days after exam). This analytic approach allowed us to effectively capture within- and between-individual cortisol level changes during the day and over the course of the study, while controlling for interindividual differences in sampling times (Singer & Willet, 2003). This mixed models approach allowed us to include time-invariant variables as covariates in the model; SES (parental income) and use of oral contraceptives were introduced as covariates in all analyses.
Table 2.
Mean and Standard Deviation for Morning and Afternoon Cortisol, Positive and Negative Mood by Time of Sampling and Group
| No symptoms n = 20 |
Symptoms n = 17 |
|||
|---|---|---|---|---|
| Variable | M | SD | M | SD |
| Salivary cortisol (μg/dL) | ||||
| a.m. baseline | 0.49 | 0.19 | 0.49 | 0.21 |
| a.m.–3 days | 0.58 | 0.41 | 0.61 | 0.29 |
| a.m.–1 day | 0.48 | 0.25 | 0.53 | 0.31 |
| a.m. + 3 days | 0.58 | 0.28 | 0.50 | 0.26 |
| p.m. baseline* | 0.15 | 0.07 | 0.13 | 0.05 |
| p.m.–3 days | 0.15 | 0.08 | 0.13 | 0.08 |
| p.m.–1 day | 0.17 | 0.15 | 0.12 | 0.06 |
| p.m. + 3 days | 0.16 | 0.07 | 0.12 | 0.04 |
| a.m.–p.m./baseline | 0.34 | 0.18 | 0.36 | 0.22 |
| a.m.–p.m./–3 days | 0.43 | 0.37 | 0.48 | 0.27 |
| a.m.–p.m./–1 day | 0.31 | 0.22 | 0.41 | 0.33 |
| a.m.–p.m./+3 days | 0.42 | 0.30 | 0.39 | 0.23 |
| Mood | ||||
| Positive/week 1 | 2.0 | 0.7 | 1.6 | 0.7 |
| Positive/week 2 | 2.1 | 1.0 | 1.7 | 0.9 |
| Positive/week 3 | 1.7 | 0.5 | 1.5 | 0.6 |
| Positive/week 4 | 2.6 | 1.5 | 2.1 | 1.5 |
| Negative/week 1 | 2.9 | 1.2 | 3.0 | 0.8 |
| Negative/week 2 | 2.7 | 1.1 | 2.9 | 0.8 |
| Negative/week 3 | 3.4 | 1.1 | 3.2 | 1.1 |
| Negative/week 4* | 1.8 | 0.8 | 2.7 | 1.3 |
| Anxiety | ||||
| Week 1 | 3.2 | 2.5 | 3.9 | 3.3 |
| Week 2 | 4.6 | 3.9 | 3.6 | 3.0 |
| Week 3 | 4.1 | 3.8 | 4.3 | 3.6 |
| Week 4 | 6.0 | 6.5 | 5.2 | 3.1 |
There was significant difference between groups at all time points: p < .05.
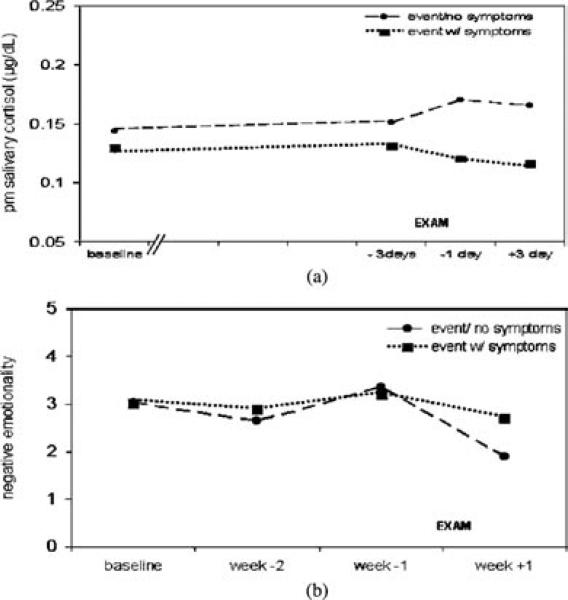
With cortisol level as the dependent variable, there was a significant interaction between time of day and group status on cortisol level, F(1, 253.7) = 5.90, p < .05, indicating that the effect of p < .05 group status on cortisol level depended on the time of day. Post hoc tests to clarify the form of this interaction indicate that the trauma with symptoms group had lower cortisol levels in the afternoon, but not the morning (p < .05), relative to the comparison group. Notably, the three-way interaction between group status, time of day, and day of sampling was not significant, indicating that there were no significant Group × Day of sampling interactions in cortisol levels in the morning or in the afternoon over the course of the study. In these data, afternoon cortisol levels were significantly lower on all sampling days in the group reporting trauma exposure with symptoms (see Figure 1a).
Figure 1.
(A) Mean afternoon salivary cortisol levels (μg/dL) were significantly lower across the study period among women who had experienced trauma with peritraumatic symptoms of acute stress (n = 17) relative to women who had not (n = 20). (B) After the examination stressor was over, negative=mood persisted among women who have experienced trauma with peritraumatic symptoms of acute stress (n = 17) relative to the comparison group (n = 20).
DAILY DIARY OF MOOD
Analysis of the influence of group status on positive and negative mood repeatedly measured over time again required the use of a random intercept model (Singer & Willet, 2003), with subject as the random effect. This allowed us to test how group status (no symptom vs. symptoms) was associated with intraindividual changes in mood (positive and negative), over the four weeks of the study. Again, socioeconomic status was introduced as a time-invariant covariate in the model.
With mood as the dependent variable, there was a significant three-way interaction between group status, mood valence, and day of sampling, F(1, 233) = 7.81, p < .01. Pairwise comparisons indicated that there was a significant difference between groups in negative mood (p < .01) only during the final sampling period (after the MCATs were over). The interaction is seen in Figure 1b as the symptom group maintaining high levels of negative mood after the examination, whereas the comparison group shows recovery. No correlations between mood (either positive or negative) and cortisol level were found at any time point.
Mental Health
Again, the use of a random intercept model with subject as the random effect allowed us to test how group status (no symptom vs. symptoms) was associated with intraindividual changes in mental health symptoms (anxiety, depression, somatic complaints), over the 4 weeks of the study. Socioeconomic status was included as a covariate. With mental health symptoms as the dependent variable, there were no significant three-way interactions. There was one significant two-way interaction between type of mental health symptoms and week of assessment, F(2, 402) = 3.02, p < .05, which took the form of an increase in reported anxiety in the whole group after the examination. This does not survive Bonferroni correction for multiple comparisons if both categories of self-report (mood and mental health) are considered; hence, these data do not support a finding of significant changes in mental health symptoms over time.
Number of Traumas in Lifetime and Traumas to Others
The analyses above identified a significantly higher rate of exposure to traumas to others among women reporting peritraumatic symptoms. However, when the sum of traumas to others was included in a random intercept model including time of sampling (a.m., p.m.), day of sample, and SES in predicting cortisol level, this factor also did not contribute significantly to variation in cortisol level. Likewise, the symptom group reported a significantly higher mean number of postpubertal traumas. When this factor was included in a random intercept model including time of sampling (a.m., p.m.), day of sample, and SES in predicting cortisol level, there was no effect of number of traumas on cortisol level and only a trend of an effect for number of postpubertal traumas on cortisol level, F(1, 253.7) = 3.63, p = .08. Thus, women with a higher number of traumas and traumas to others were more likely to report having at least one peritraumatic symptom, but the presence of peritraumatic symptoms continued to be the more powerful predictor of cortisol level.
DISCUSSION
Salivary cortisol levels and mood varied significantly as a function of lifetime trauma exposure in this sample of healthy young women. This is consistent with the hypothesis that there are significant psychophysiological consequences of trauma exposure in women who do not have PTSD or major depression. These effects were observed in those who reported higher levels of peritraumatic arousal at time of trauma; women reporting at least one prior trauma with associated symptoms of peritraumatic distress had significantly lower afternoon cortisol levels across all sampling times than did the comparison group (women with either no trauma exposure or trauma exposure with no symptoms). Women in this group also maintained higher mean levels of negative mood after experiencing an examination stressor, suggesting that the central emotional processing systems that drive mood may also be affected by prior trauma exposure. Women with peritraumatic symptoms also reported more social loss traumas than their peers, although the best predictor of cortisol levels continued to be the presence of peritraumatic symptoms.
Major epidemiological studies of trauma exposure in women have failed to find evidence of effects of prior trauma exposure on cortisol levels in women who did not have a clinical disorder (Young & Breslau, 2004a, 2004b; Young et al., 2004). The present study suggests that selection for the subsample of women who report exposure to highly arousing traumas may reveal differences in HPA-axis functioning that have not otherwise been observable among healthy, trauma-exposed women.
Because this study used a retrospective report of trauma exposure and symptoms, there are alternate possibilities for how peritraumatic arousal, number of trauma, and cortisol levels may have interacted to produce these data. One possibility is that women who experienced repeated traumas had a greater chance of having at least one trauma that was so severe that it engendered high levels of arousal, with subsequent alterations in psychophysiology. In keeping with this view, social loss traumas are reported to be highly salient predictors of PTSD, particularly in women (Breslau et al., 1998). Lowered cortisol (for a review, see Yehuda, 2001) and sustained negative mood might follow as symptoms of partial PTSD (Breslau, Lucia, & Davis, 2004; Ozer & Weiss, 2004).
This interpretation may also be reversed; the women in the symptom group may have had preexisting low cortisol and/or sustained negative mood, which rendered them more likely to report symptoms of peritraumatic arousal. Preexisting low cortisol has been suggested as a vulnerability factor for future occurrence of PTSD (Yehuda, McFarlane, & Shalev, 1998). In this model, catecholamine increases associated with trauma exposure are exaggerated because there is a lack of regulatory feedback from cortisol (Yehuda et al., 1998), which lead to inappropriate memory consolidation (Pitman, 1989). The majority of research in this area suggests that prior trauma exposure is the catalyst for these initial decreases in cortisol levels (for a review, see Delhanty & Nugent, 2006). The supporting evidence is most often from studies of high-impact initial traumas (Delhanty & Nugent, 2006; Yehuda et al., 1998), which again raises the question of peritraumatic arousal as a potentially key factor in this initial process.
We note other difficulties with retrospective reporting of trauma exposure, including bias because an ensuing disorder is known to have occurred, bias by respondent's depression or other mood state, cognitive impairment, and normal forgetting (Hardt & Rutter, 2004; Moffitt et al., 2006). In the absence of a prospective study, it has been argued (Moffitt, Caspi, & Rutter, 2006) that the use of the life history calendar (Caspi et al., 1998) is a highly valid and reliable method for collecting retrospective data on adverse life events. We employed this method in the present study to reduce retrospective reporting bias to a minimum. In addition, there were no differences in overall mental health, or current level of anxiety, depression, somatic symptoms, or positive or negative mood between groups at baseline, when participants provided their retrospective self-report of symptoms. Thus, multiple indicators suggest that the two groups did not differ by depression or anxiety level, or general mood state at the time of self-report of trauma exposure and symptoms, suggesting that these factors were not driving retrospective differences in reporting.
It is also of note that there was no correlation between negative mood and cortisol level at any time point. This was unexpected; however, recent functional magnetic resonance imaging (fMRI) studies of amygdala activation during in-scanner stressor exposure report no association between amygdala activation and cortisol levels (Dedovic et al., 2005; Wang et al., 2005). If replicated, this suggests less association between limbic processing of negative emotionality and cortisol production than has been previously thought to be the case (e.g., Stratakis & Chrousos, 1995).
Limitations
These data were obtained from a sample of female premedical students. Findings of interest require replication, preferably with a large, ethnically and economically diverse sample of both genders. The current sample also exhibits high rates of trauma exposure similar to those observed in large, community-based studies (e.g., Breslau et al., 1998; Young & Breslau, 2004a, 2004b; Young et al., 2004) that assess trauma exposure using versions of the same instrument for trauma assessment employed here (the UM-CIDI: Kessler et al., 1994). Because of the small number of subjects without trauma exposure in this sample, we were unable to adequately test differences between those with and without trauma exposure. Further methodological issues include the incomplete sampling of participants with respect to timing of menses and the lack of monitoring of subjects’ compliance in the appropriate timing of saliva sampling using compliance devices. Participants were specifically asked to record actual time of sampling whether or not it was precisely the same as the sampling guidelines. The variation in sampling times reported here suggests compliance on this point.
Clinical Implications
We found consistently lower afternoon cortisol levels and sustained negative mood in women who reported having one or more peritraumatic symptoms of distress at time of trauma, relative to the comparison group. This might suggest that exposure to high-arousal traumas is associated with a subclinical variant of PTSD (partial PTSD; Breslau et al., 2004; Ozer & Weiss, 2004) that operates by similar biological mechanisms as PTSD. Investigation of such characteristics is of clinical note because of the pathogenic nature of trauma exposure (Brown, 1993; Kendler et al., 2003). Thus, basic research on the psychophysiological correlates of trauma exposure in otherwise healthy individuals potentially inform the etiology of a wide range of disorders.
Acknowledgments
The development of this article was supported by predoctoral (F31 MH63544) and postdoctoral (F32 MH68139) National Research Service Awards from the National Institute of Mental Health. In addition, this research was supported the College of Human Ecology, the Family Life Development Center, and the Laboratory for Developmental Cognitive Neuroscience at Cornell University, and the Sacker Institute for Developmental Psychobiology at the Weill Medical College of Cornell University. We are very grateful to the women who participated in this study. We also wish to acknowledge the help of Margaret Altemus, Douglas Granger, Sarah Watamura, and the Human Development Writing Group in reading and commenting on drafts of this paper.
Contributor Information
Eric Horowitz, Psychology Department, Cornell University, Ithaca, NY.
Elise Temple, Department of Human Development, Cornell University, Ithaca, NY.
REFERENCES
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Author; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Author; Washington, DC: 2000. [Google Scholar]
- Blazer DG, Hybels CF. What symptoms of depression predict mortality in community-dwelling elders? Journal of the American Geriatrics Society. 2004;52:2052–2056. doi: 10.1111/j.1532-5415.2004.52564.x. [DOI] [PubMed] [Google Scholar]
- Bolger N, Eckenrode JE. Social relationships, personality, and anxiety during a stressful event. Journal of Personality and Social Psychology. 1991;61:440–449. doi: 10.1037//0022-3514.61.3.440. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick S, Johnson D, Yehuda R, Charney D. Childhood physical abuse and combat-related post-traumatic stress disorder in Vietnam veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat H, Kessler R, Davis G. Previous exposure to trauma and PTSD effects of subsequent trauma: Results from the Detroit Area Survey of Trauma. American Journal of Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community—The 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;57:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Breslau N, Lucia VC, Davis GC. Partial PTSD versus full PTSD: An empirical investigation of associated impairment. Psychological Medicine. 2004;34:1205–1214. doi: 10.1017/s0033291704002594. [DOI] [PubMed] [Google Scholar]
- Brown G. Life events and affective disorder: Replications and limitations. Psychosomatic Medicine. 1993;55:248–259. doi: 10.1097/00006842-199305000-00003. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington HL, et al. The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. International Journal of Methods in Psychiatric Research. 1998;6:101–114. [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 2005;30:319–335. [PMC free article] [PubMed] [Google Scholar]
- Delahanty DL, Nugent N. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. In: Yehuda R, editor. Psychobiology of posttraumatic stress disorder: A decade of progress. Annals of the New York Academy of Sciences. Vol. 1071. New York Academy of Sciences; New York: 2006. pp. 27–40. [DOI] [PubMed] [Google Scholar]
- Derogatis . Brief Symptom Inventory 18: Administration, scoring, and procedures manual. National Computer Systems, Inc; Minneapolis, MN: 2000. [Google Scholar]
- Eckenrode J, Bolger N. Daily and within-day event measurement. In: Cohen S, Kessler R, Gordon L, editors. Measuring stress: A guide for health and social scientists. Oxford University Press; New York: 1997. pp. 80–101. [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, et al. Psychological sequelae of the September 11 terrorist attacks in New York City. The New England Journal of Medicine. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- Ganzel B, Casey BJ, Voss HU, Glover G, Temple E. The aftermath of 9/11: Effect of intensity and recency of trauma on outcome. Emotion. 2007;7:227–238. doi: 10.1037/1528-3542.7.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D, Weisz J, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. Journal of Abnormal Psychology. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology, Psychiatry, and Allied Disciplines. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocorticalism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of General Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kessler R, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the National Comorbidity Study. Archives of General Psychiatry. 1995;52:1048–1059. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle K, Zhao S, Nelson CB, Hughes M, Eshelman S, et al. Lifetime and 12-month prevalence of DSM-IIIR psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- McEwen B. Effects of adverse experiences for brain structure and function. Biological Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McFarlane A. The prevalence and longitudinal course of PTSD: Implications for neurobiological models of PTSD. In: Yehuda R, McFarlane A, editors. Psychobiology of posttraumatic stress disorder. Vol. 821. New York Academy of Sciences; New York: 1997. pp. 10–24. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspectives in Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Ozer E, Best S, Lipsey T, Weiss D. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Ozer E, Weiss D. Who develops posttraumatic stress disorder? Current Directions in Psychological Science. 2004;13:169–172. [Google Scholar]
- Pitman R. Posttraumatic stress disorder, hormones, and memory. Biological Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Sharot T, Martorella EA, Delgado MR, Phelps E. Remembering 9/11: How personal experience modulates the neural circuitry of recollection. Proceedings of the National Academy of Sciences USA. 2007;104:389–394. doi: 10.1073/pnas.0609230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis. Oxford University Press; New York: 2003. [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis C, Chrousos G. Neuroendocrinology and pathophysiology of the stress system. In: Chrousos G, McCarty R, Pacak K, Cizza G, Sternberg E, Gold P, et al., editors. Stress: Basic mechanisms and clinical implications. Vol. 771. The New York Academy of Sciences; New York: 1995. pp. 1–18. [DOI] [PubMed] [Google Scholar]
- Tucker PM, Pfefferbaum B, North CS, Kent A, Burgin C, Parker DE, et al. Physiologic reactivity despite emotional resilience several years after direct exposure to terrorism. American Journal of Psychiatry. 2007;164:230–235. doi: 10.1176/ajp.2007.164.2.230. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. Journal of Clinical Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev A. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biological Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Young E, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: An epidemiologic community study. Archives of General Psychiatry. 2004a;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young E, Breslau N. Saliva cortisol in posttraumatic stress disorder: A community epidemiologic study. Biological Psychiatry. 2004b;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Young E, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biological Psychiatry. 2004;55:621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]