Abstract
Does trauma exposure have a long-term impact on the brain and behavior of healthy individuals? The authors used functional magnetic resonance imaging to assess the impact of proximity to the disaster of September 11, 2001, on amygdala function in 22 healthy adults. More than three years after the terrorist attacks, bilateral amygdala activity in response to viewing fearful faces compared to calm ones was higher in people who were within 1.5 miles of the World Trade Center on 9/11, relative to those who were living more than 200 miles away (all were living in the New York metropolitan area at time of scan). This activity mediated the relationship between group status and current symptoms of posttraumatic stress disorder. In turn, the effect of group status on both amygdala activation (fearful vs. calm faces) and current symptoms was statistically explained by time since worst trauma in lifetime and intensity of worst trauma, as indicated by reported symptoms at time of the trauma. These data are consistent with a model of heightened amygdala reactivity following high-intensity trauma exposure, with relatively slow recovery.
Keywords: amygdala, trauma, stress, neuroplasticity, 9/11
Psychological traumas have been defined as events that threaten death or injury to self or others and that engender intense feelings of fear, helplessness, or horror (e.g., rape, combat, witnessing violence or disaster, or the sudden death of a loved one; American Psychiatric Association, 2000). Trauma exposure is a potent environmental risk factor that predicts immediate and lifetime increases in a diverse array of mental health disorders (Breslau et al., 1998; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995), including anxiety and depression (Brown, 1993; Kendler, Hettema, Butera, Gardner, & Prescott, 2003; McCauley, Kern, Kolodner, Dill, & Schroeder, 1997). Trauma exposure is also a definitional prerequisite for posttraumatic stress disorder (PTSD) and a predisposing factor for further incidence of PTSD if there is a subsequent trauma (Bremner, Southwick, Johnson, Yehuda, & Charney, 1993). In cases where trauma exposure is severe and protracted, rates of mortality and chronic illness far exceed the norm later in life, and mean life expectancy is sharply decreased (McFarlane, 1997).
Trauma exposure is not uncommon. Results from an epidemiological study of 5,877 people within the United States found that more than 50% of women and 60% of men experienced at least one trauma in their lifetime, and more than a quarter of the sample experienced two or more traumas (Kessler et al., 1995). Although only a small percentage of the trauma-exposed population develops PTSD (6% to 9%, Breslau et al., 1998; 8% to 20%, Kessler et al., 1995), this disorder has been the focus of studies of the effects of trauma on humans. The response to trauma in people without a specific clinical disorder has been less well characterized (Bunce, Larsen, & Peterson, 1995; McFarlane, 1997). Thus, although trauma exposure is an established environmental risk factor for a wide range of disorders, the neural mechanisms underlying this overall increase in risk are unclear.
A small number of epidemiological and community-based studies have examined the effects of trauma exposure in individuals without PTSD (Otte et al., 2005: Yehuda, Golier, & Kaufman, 2005; Young & Breslau, 2004a, 2004b; Young, Tolman, Witkowski, & Kaplan, 2004) by using peripheral biological indicators of the stress response (e.g., urinary cortisol, dopamine, epinephrine). From these efforts, the physiological correlates of trauma exposure in healthy (nonclinical) populations are beginning to emerge. For example, trauma-exposed young adults without PTSD were found to have significantly lower urinary catecholamine levels (dopamine and epinephrine) than nontrauma-exposed individuals, whereas a group with lifetime PTSD had higher levels (Young & Breslau, 2004b). Another study (Otte et al., 2005) found significantly higher basal levels of MHPG (3-methoxy-4-hydroxy-phenylglycol, a major metabolite of nonrepinephrine) and greater MHPG reactivity in individuals with childhood trauma as compared to those with no childhood trauma. Although these findings are not conclusive, they are suggestive of long-term effects of trauma exposure on the central nervous system that may be more directly investigated with noninvasive neuroimaging techniques.
There have been a number of neuroimaging studies examining brain function and structure in individuals with PTSD. This research has provided substantial evidence for long-term central nervous system effects of trauma exposure that is accompanied by PTSD. Some of these studies have found enhanced amygdala activation in response to a variety of negatively valenced stimuli (e.g., Rauch et al., 2000; Shin et al., 2004; although, see Lanius et al., 2003; Phan, Britton, Taylor, Fig, & Liberzon, 2006; Sakamoto et al., 2005). The majority of this work has involved reexperiencing paradigms (e.g., Britton, Phan, Fig, Taylor, & Liberzon, 2005; Lanius et al., 2005), which include reinstatement of memories of the traumatic event. The format of these paradigms makes it difficult to assess whether reported results are specific to memory reinstatement or are a reflection of the disorder itself. It has also been argued (Williams et al., 2006) that the use of standardized probes of limbic activity allows for generalization of findings to other populations. As a consequence, a growing number of neuroimaging studies of PTSD have used standardized probes of amygdala activity (e.g., emotional faces, Armony, Corbo, Clement, & Brunet, 2005; Rauch et al., 2000; Shin et al., 2005; Williams et al., 2006; emotional scenes, Hendler at al., 2003; Phan et al., 2006; emotional Stroop task, Shin et al., 2001; emotionally valenced words, Bremner et al., 2003; Protopopescu et al., 2005).
The most frequently used standardized probe of amygdala activity in samples with PTSD has been presentation of emotional faces (Armony et al., 2005; Rauch et al., 2000; Shin et al., 2005; Williams et al., 2006). In general, these studies have reported increased amygdala activity to negative emotional expressions in those with PTSD. Shin and colleagues (2005) found increased activity in the right amygdala and decreased activity in medial prefrontal regions in men with chronic PTSD of long duration. Rauch et al. (2000) and Armony et al. (2005) found increased amygdala activation in samples with PTSD, but did not report significant differences in prefrontal activation. Williams et al. (2006) reported increased activity in ventral amygdala and decreased activity in dorsal amygdala in a mixed sample of men and women with PTSD of relatively short duration. Thus, increased activity in the amygdala is the most consistently observed neural correlate of PTSD as probed with emotional faces, with some evidence that this activation is localized to subregions of the amygdala and is associated with alterations in medial prefrontal activity.
In comparison to the rich body of neuroimaging research on PTSD, there has been very little work examining the neural correlates of trauma exposure in people without a clinical disorder; this is surprising, considering the known impact of negative life events on psychological distress and disorder in the overall population (e.g., Dohrenwend, 2006). One recent structural MRI paper has addressed this question from a developmental perspective (Cohen et al., 2006), showing that retrospective report of accumulated adverse childhood events predicted smaller anterior cingulate and caudate volumes in adulthood. Adverse childhood events with the most impact on adult brain morphology were those more likely to meet criteria as traumas (e.g., death of a parent, witnessing domestic violence, sexual assault). Also, a recent functional neuroimaging study (Sharot, Martorella, Delgado, & Phelps, in press) used a flashbulb memory paradigm that focused on events of September 11th. These results showed significant increases in left amygdala activation in response to evoked memories of the events of September 11th in adults who were in downtown Manhattan that day, relative to those who were in midtown Manhattan, five miles away.
Animal models of stress have highlighted the effects of severe stressors on the amygdala and related structures. Exposure to acute uncontrollable stressors produces extended hyperexcitability of the amygdala in laboratory animals (Adamec, Blundell, & Burton, 2005; Maier & Watkins, 1998), which renders the amygdala and related structures more readily activated independently of the triggering stimulus (Rosen & Schulkin, 1998). This effect has been associated with increased vigilance and fearful responses (e.g., freezing) to ambiguous or mild standardized stressors (Adamec et al., 2005; Rosen & Schulkin, 1998). Exposure to acute stressors increases spine synapse formation in the basolateral amygdala (BLA), which may underlie the associated increases in anxiety-like behavior (Mitra, Jadhav, McEwen, & Chattarji, 2005; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002). Exposure to repeated/chronic stressors produces anxiety-like behavior in response to standardized stressors (e.g., an open field), along with dendritic growth in the BLA, greater increases spine density than seen with acute stressors, and dendritic retraction in the hippocampus (McEwen, 2005; Mitra et al., 2005).
Taken together, these data suggest the hypothesis that the amygdala and closely related structures are persistently more reactive after trauma exposure in healthy adults (i.e., in individuals without a clinical disorder) and that these effects will be observable using mild, standardized stressors (i.e., that they do not require a reexperiencing paradigm). Epidemiological studies indicate that it is the nature of the worst trauma (i.e., the event that precipitates diagnosis and/or the most symptoms) that best predicts long-term negative psychological consequences of trauma exposure. Worst traumas that are more recent (Kessler et al., 1995) and more severe (e.g., violent assault: Breslau et al., 1998; Kessler et al., 1995) are more likely to predict distress and disorder. Selection of a sample with relatively recent, high-intensity, worst-trauma exposure would maximize the possibility of observing the hypothesized increase in amygdala reactivity.
The events of September 11, 2001, and the experiences of the men and women who were in close proximity to that disaster, provide a unique window into the neural correlates of environmental stressor exposure. Notably for this study, proximity to this disaster have been shown to predict psychological morbidity (Blanchard et al., 2004; Galea et al., 2002), making it possible to prospectively titer the effects of the disaster across study groups by studying participants who were near the World Trade Center (WTC) in New York City on September 11, 2001, relative to those who lived far away at that time. We hypothesize that relative increases in amygdala reactivity would be more apparent in the group with closer proximity to the WTC on September 11th. Building on previous assays of amygdala responsiveness (Brieter et al., 1996; Thomas et al., 2001), participants passively viewed blocks of fearful and calm faces while undergoing functional MRI (fMRI).
Methods
Participants
Twenty-two right-handed adults participated in this study; all were living in the New York metropolitan area at time of scan. Recruitment material requested participants who were at different distances from the WTC on September 11th. Eleven participants were within 1.5 miles of the WTC on September 11, 2001 (5 women, aged 30.3 ± 2.5 years [mean ± SE]; range = 19 to 41 years). Eleven lived at least 200 miles from the New York City area on September 11, 2001 (5 women, aged 29.3 ± 1.4 years; range = 23 to 37 years). People who lived in Washington, DC, on September 11th and those who had friends or relatives on aircraft involved in the disaster were excluded. Data were collected between 41 and 48 months after September 11th.
Before imaging, all participants were screened for current or past psychiatric, neurological, or medical illness and trauma exposure, as well as for any contraindications for fMRI. Approximately 30 minutes elapsed between time of interview and time of scan. This investigation was conducted within institutional guidelines established for protection of human subjects. All participants provided informed written consent.
Behavioral Measures
Three different clinical and standardized assessments were used to assess psychiatric symptoms and diagnosis on the day of the MRI visit: (1) the PTSD module of the University of Michigan Composite International Diagnostic Interview (UM-CIDI: Kessler et al., 1994) was used in conjunction with the Life History Calendar methodology developed by Caspi et al. (1996) to assess lifetime incidence of trauma exposure, current PTSD, and PTSD symptoms in lifetime. The UM-CIDI is a fully structured diagnostic interview that allows the assessment of current and lifetime mental disorders in the form of the third revised Diagnostic and Statistical Manual for Mental Disorders (American Psychiatric Association, 1986); (2) the Impact of Events Scale (Horowitz, Wilner, & Alvarez, 1979) is a 15-item scale that was used to assess PTSD stress reactions in the seven days before scanning; (3) the Speilberger State Trait Anxiety Inventory (STAI-S: Spielberger, 1973) is a 20-item scale that was used to assess current state anxiety.
Stimuli and Procedure
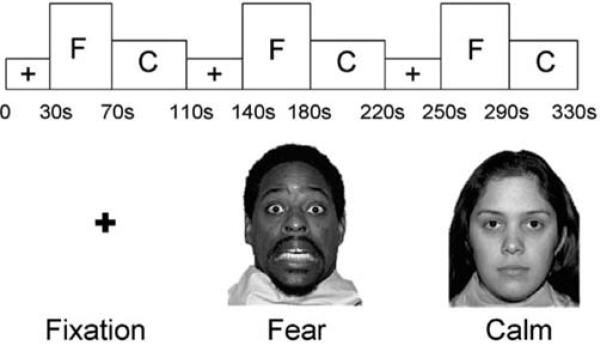
Participants viewed gray-scaled pictures of faces of eight different actors (four female) demonstrating fearful and calm facial expressions (see Figure 1). Images were selected from a standardized picture set (Tottenham et al., in press). Face stimuli were presented on an overhead liquid crystal panel in a pseudorandom sequence of nine blocks of fixation, fearful faces, or calm faces. Participants were instructed, “Please look at the faces and the +'s. You don't have to press any buttons.” There was no reference to the events of September 11th during imaging. Order of presentation was counterbalanced across subjects and across runs using the two following sequences: +FC + FC + FC or + CF + CF + CF (where F indicates a block of fearful faces, C indicates a block of calm faces, and + indicates fixation). In each block of faces, 10 images were presented for 4 seconds each. Fixation blocks were 30 seconds. Total block duration was 330 seconds.
Figure 1.
Fearful and calm faces and a fixation cross were presented in a passive-viewing paradigm that was counterbalanced + FC + FC + FC or + CF + CF + CF.
Imaging Protocols
Subjects were scanned with a General Electric Signa 3-Tesla fMRI scanner (General Electric Medical Systems, Milwaukee, WI) using a quadrature head coil. After a three-plane localizer and a whole-head coronal localizer, a T2-weighted two-dimensional anatomical image with a fast spin-echo (FSE) sequence was acquired: time for repetition (TR) = 4000, time for echo (TE) = 68 ms, flip = 90°, field of view (FOV) = 20, 29 slices, 5-mm slice thickness, 0-mm gap, matrix = 256 × 192, axial-oblique. A three-dimensional spoiled gradient recalled (SPGR) T1-weighted anatomic scan was also acquired (124 axial slices, TR = 25 ms, TE = 5 ms, flip = 20°, FOV = 24 cm, 1.5-mm thickness, 0 mm gap, matrix = 256 × 256 × 160). Functional data was acquired using a spiral in-out sequence (Preston, Thomason, Ochsner, Cooper, & Glover, 2004) and the same spatial prescription as the FSE: TR = 2000 ms, TE = 30 ms, matrix = 64 × 64 mm, 29 slices per volume.
Data Analysis
Preprocessing and statistical analysis of fMRI data was performed by using Statistical Parametric Mapping (SPM2: Wellcome Department of Neurology, London, United Kingdom) implemented on MatLab 7.0. During preprocessing, the first four acquisitions were discarded and functional scans were realigned to the initial image, generating a set of realignment parameters for each run and a mean functional image. The mean functional image was used to coregister functional scans to the FSE anatomical images, which were then coregistered to the SPGR. The resulting parameters were used to realign the functional scans. The SPGR was then transformed to Montreal Neurological Institute (MNI) space, and these parameters were applied to the functional scans. The normalized functional data was smoothed by using a 6-mm full-width/half-maximum kernel.
Individual level analysis was performed by using the general linear model (Friston, Holmes, Price, Buchel, & Worsley, 1999) with a fixed effects model. Contrast images comparing each of the block types were generated for each individual. These individual-level contrasts served as the basis for group-level random effects analyses (Friston et al., 1999). Data were first analyzed by using whole-brain analysis to identify significant areas activated to fearful versus calm faces in all participants. Then, a one-way analysis of variance was performed to compare brain activation across groups (9/11-exposed and control) for fearful versus calm faces. For all analyses, results were reported as significant if they met a voxel-wise threshold of p < .001, with clusters of 20 or more contiguous voxels (Forman, Cohen, Fitzgerald, Eddy, Mintun, & Noll, 1995). Given our strong, directional hypotheses regarding the amygdala, results were reported as significant if p < .01, with clusters of 3 or more voxels (this provides a conservative estimate of statistical significance: Foreman et al., 1995). In addition, small volume corrections from a 5-mm sphere around a priori locations of activation in the amygdala are reported (Worsley, 1996). All activations are reported using MNI coordinates.
In order to test a priori hypotheses regarding activation in the 9/11-exposed group relative to the comparison group, a region-of-interest (ROI) analysis was conducted. ROIs were defined functionally as the voxels found to be reliably activated in the whole-brain analysis of the fearful versus calm contrast at thresholds of p < .01, with cluster extents of three or more contiguous voxels. Because there were no medial prefrontal areas reliably activated in the whole brain analysis of variance, post hoc ROI analyses were limited to the amygdala. The association between the behavioral measures and blood oxygen-level dependent (BOLD) signal change in the amygdala for the fearful-calm contrast in each ROI was examined by using multiple regression analyses.
Results
Behavioral Results
Demographics and trauma
Comparison of demographic and trauma variables across groups revealed no significant differences in age, gender, age at first trauma, number of traumas in lifetime, number of traumas in lifetime with associated shock and/or horror, or history of PTSD (see Table 1). The control group had a significantly longer mean time since their worst trauma in lifetime than the 9/11-exposed group. The 9/11-exposed group also retrospectively reported more symptoms of avoidance and arousal at time of worst trauma in response to probes from the PTSD module of the UM-CIDI. Most, but not all, 9/11-exposed individuals rated the 9/11 disaster as the worst trauma that they had experienced in their lifetime.
Table 1.
Comparison of Demographic, Trauma, and Behavioral Variables Across Study Groups
| Comparison mean (SE) | 9/11-exposed mean (SE) | |
|---|---|---|
| Age at scan (y) | 29.3 (1.4) | 30.5 (2.5) |
| Gender | 5F, 6M | 5F, 6M |
| Age at first trauma | 12.5 (1.6) | 17.3 (2.6) |
| Years since most recent trauma | 6.0 (1.7) | 3.3 (.41)† |
| Years since worst trauma | 11.7 (2.6) | 4.9 (1.3)* |
| Number of traumas in lifetime | 6.6 (2.7) | 4.4 (0.7) |
| Number of traumas in lifetime with shock/horror | 3.4 (0.9) | 3.4 (0.6) |
| Total number of symptoms at worst trauma | 3.2 (1.1) | 7.4 (1.2)* |
| Number intrusion symptoms at worst trauma | 1.2 (0.5) | 2.2 (0.4) |
| Number of avoidance symptoms at worst trauma | 0.8 (0.4) | 2.3 (0.5)* |
| Number of arousal symptoms at worst trauma | 1.2 (0.5) | 2.8 (0.5)* |
| IES | 15.7 (0.5) | 22.3 (2.6)* |
| IES subscale (intrusion) | 7.8 (0.4) | 10.8 (1.2)* |
| IES subscale (avoidance) | 8.1 (0.1) | 11.4 (1.6)* |
| State-Trait Anxiety Inventory | 30.1 (1.1) | 28.5 (2.0) |
| History of posttraumatic stress disorder in lifetime | 0.2 (0.1) | 0.3 (0.1) |
Note. F = female; M = male; IES = Impact of Events Scale. n = 11 in each group, except for trauma variables (where n[comparison] = 10).
p < .05.
p < .10.
Current symptoms and anxiety
Although the 9/11-exposed group did not meet diagnostic criteria for any disorder, they had a higher mean level of current symptoms, as assessed by both subscales of the IES (see Table 1). There were no significant differences between groups on the state measure of the STAI-S.
Imaging Results
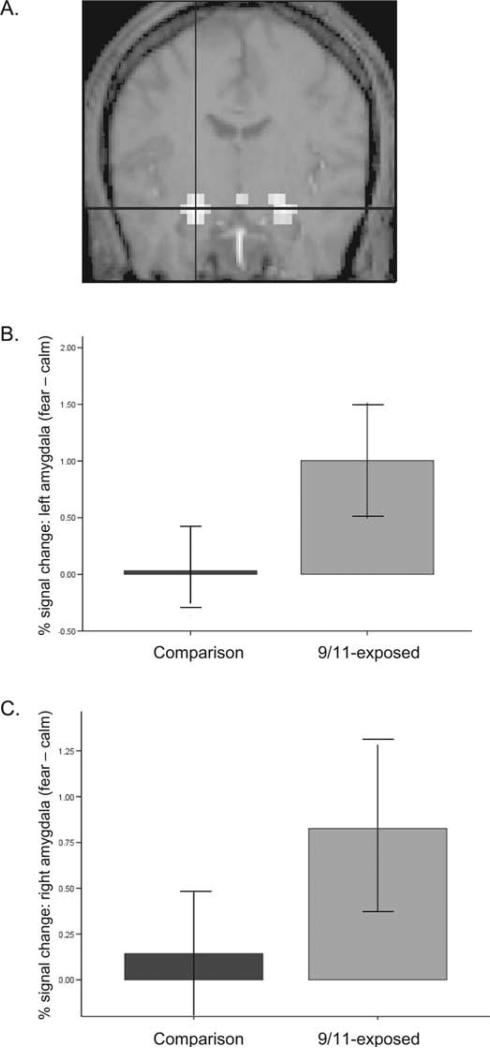
BOLD signal in left and right amygdala was elevated in the group that was closer to the WTC on September 11th in the contrast of fearful versus calm faces (see Figure 2). Voxel-wise analysis of variance results indicated that the 9/11-exposed group had significantly greater activity than controls for fearful versus calm faces in the left amygdala (–25, –9, –20; z = 3.13, p = .001, psvc = .01, 19 contiguous voxels) and in the right amygdala (22, –9, –20; z = 2.97, p = .001, psvc = .01, 5 contiguous voxels).
Figure 2.
Amygdala activity and proximity to the WTC on September 11th, 2001. (A) Fearful emotional faces elicited greater amygdala activity than calm faces in the whole group. Voxel-wise analysis of variance for this contrast indicated that the 9/11-exposed group showed significantly increased bilateral amygdala activation relative to the comparison group. Region of interest analysis showed mean signal change in the (B) left amygdala (p < .01) and (C) right amygdala (p < .05) (fearful vs. calm contrast) was higher in the group that was exposed to the disaster on September 11, 2001 than in the comparison group.
The ROI analysis also showed that the mean signal change in the left, t (22) = –3.3, p = .003 and right amygdala, t (22) = –2.7, p = .01 was higher in the 9/11-exposed group than in the comparison group for the fearful versus calm contrast. Signal change in these regions was not correlated with gender, age, number of traumas in lifetime, age at first trauma, or years since most recent trauma.
Table 2 shows correlations between BOLD signal change in right and left amygdala (fearful-calm contrast) with behavioral variables across the whole group. Signal change in the left amygdala was correlated robustly with current symptoms as reported on the IES, as well as with retrospectively reported symptoms of avoidance on the UM-CIDI. Signal change in the right amygdala was less strongly correlated with overall current symptoms on the IES and with retrospective report of avoidance symptoms at time of worst trauma. One outlier was removed for these analyses (Mahalanobis distance = 12.37: Darlington, 1990). These relationships were not driven by data from individuals with a past history of PTSD; control for history of PTSD strengthened the significance of the relationship between BOLD signal and IES score (left amygdala: ΔR2 = .17, p = .007; right amygdala: ΔR2 = .14, p = .05) and had no effect on the association between signal and symptoms at worst trauma (ΔR2 = .009, not significant). With control of 9/11 group status, there was an association between STAI-S and percent signal change in the left amygdala that was significant (β = .35; p = .05) in this sample, which is in keeping with previous findings (Bishop, Duncan, & Lawrence, 2004).
Table 2.
Correlation of Percent Signal Change in Right and Left Amygdala (Fearful Versus Calm Contrast) With Behavioral Variables (Pearson r), in the Whole Group
| Signal change in left amygdala (r) | Signal change in right amygdala (r) | |
|---|---|---|
| Total number of symptoms at worst trauma | 0.46* | 0.32 |
| Number of intrusion symptoms at worst trauma | 0.35 | 0.32 |
| Number of avoidance symptoms at worst trauma | 0.58** | 0.42† |
| Number of arousal symptoms at worst trauma | 0.27 | 0.13 |
| Impact of Events Scale (IES) | 0.72** | 0.56* |
| IES subscale (intrusion) | 0.67** | 0.41† |
| IES subscale (avoidance) | 0.60** | 0.54* |
| State-Trait Anxiety Inventory | 0.25 | 0.20 |
p < .05.
p < .01.
p < .10.
Mediation by amygdala activation
If increased amygdala reactivity drives the association between trauma exposure and increased vulnerability, then it should statistically mediate the relationship between the two (Baron & Kenny, 1986; Holland, 1986). To determine whether amygdala activation mediated the relationship between 9/11 status and current symptoms as reported on the IES, we performed a standard test of mediation (Baron & Kenny, 1986: please see appendix). Subjects’ 9/11 status predicted higher levels of current symptoms and of amygdala activation, and increased amygdala activation predicted increased current symptoms. When mean signal change in the left amygdala (fear vs. calm contrast) was entered into a linear regression model predicting current symptoms from 9/11 status, the relationship between 9/11 status and symptoms became nonsignficant, indicating mediation of the relationship between 9/11 status and current symptoms by left amygdala activity. Furthermore, this model explained nearly half of the variance in current symptoms in this sample; R2adj = 0.46. Appropriate testing (MacKinnon & Dwyer, 1993; Sobel, 1986) found this mediated effect to be significant, z = 2.80, p < .01. Thus, amygdala reactivity is a reasonable candidate for a potentially causal mechanism associating 9/11 exposure and symptom presentation (Baron & Kenny, 1986). The next analysis identifies the key parameters of exposure to the 9/11 disaster that were associated with persistent amygdala reactivity and increased symptoms in this sample.
Years since worst trauma
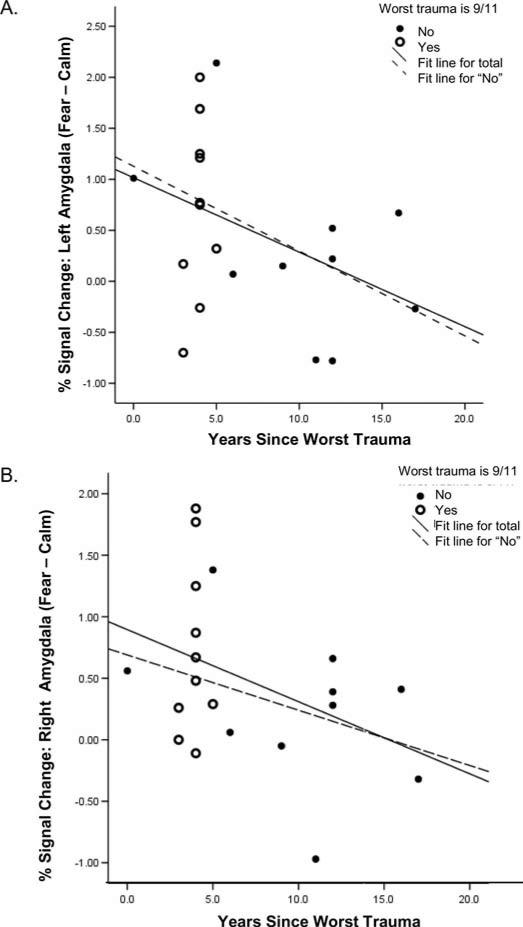
Trauma exposure was prevalent in this sample, which provided the opportunity to examine temporal effects in the psychobiological correlates of trauma exposure, regardless of type of trauma. We previously noted that 9/11-exposed participants had significantly fewer years since worst trauma exposure than those in the comparison group (see Table 1). Of the 21 participants with trauma exposure, 11 identified the 9/11 disaster as their worst trauma in lifetime (eight in the group that was closest to the WTC on 9/11/01 and three in the group that was farther away). The remaining 10 identified other lifetime traumas as their worst trauma (three in the group that was closest to the WTC, seven in the group that was farther away). Overall, years since worst trauma ranged from less than one year to nearly 20 years, with the exception of one person who experienced very early abuse (nearly 40 years before scanning). In the whole group, years since worst trauma predicted reductions in current symptoms (β = –0.51, p = .02), as well as signal in the left (β = –0.46, p = .05) and right (β = –0.45, p = .06) amygdala to fearful versus calm faces (outlier excluded; see Figure 3). Slopes of these relationships are similar for the whole group and for the group that identified non-9/11-related traumas as their worst traumas in lifetime (left amygdala: β = –0.49; right amygdala: β = –0.36), suggesting that this finding may be generalizable to non-9/11 traumas. These analyses included control for age at scan, which was found to serve as a classical suppressor variable in these models (age was not significantly correlated to years since worst trauma or to amygdala activation but the presence of age increased the multiple correlation of the above regression models, suggesting that age was suppressing some of what would otherwise be error variance in the relationship between years since worst trauma and amygdala activation: Darlington, 1990).
Figure 3.
Amygdala activity and time since worst trauma. Partial plots for the whole group controlling for age at scan of (A) mean signal change in left amygdala (fearful vs. calm) versus years since worst trauma in lifetime: β = –0.46, p = .05. (B) mean signal change in right amygdala (fearful vs. calm) versus years since worst trauma in lifetime: β = –0.45, p = .06. Slopes of these relationships for the group with non-9/11 traumas as worst traumas are shown for comparison (see p. 232).
The associations between years since worst trauma and all elements of the mediation model discussed above suggest that 9/11 status may be secondary to this factor in the prediction of current symptoms and amygdala activation (fear vs. calm). In support of this, statistical control of years since worst trauma in the relationship between 9/11 group status and current symptoms rendered the contribution of 9/11 status nonsignificant, suggesting that the contribution of 9/11 status in this model was accounted for by variation in years since worst trauma. Similarly, control of this factor in the relationship between 9/11 status and percent signal change in left amygdala (fear vs. calm) rendered the contribution of 9/11 group status less significant (β = 0.64, p = .003 to β = 0.57, p = .02), indicating that years since worst trauma accounts for part of the explained variance in this relationship.
Trauma intensity
Trauma intensity is difficult to define and quantify, except for its immediate impact on the individual. This relative level of arousal and distress at the time of trauma is an important predictor of long-term vulnerability to mental health disorders (Ozer, Best, Lipsey, & Weiss, 2003). For the purposes of this analysis, we operationally defined intensity by the participant's own report of symptoms at worst trauma. In this sample, the 9/11-exposed participants reported more symptoms of avoidance and arousal at time of worst trauma (see Table 1). Trauma intensity was highly related to current symptoms (β = 0.60; p = .005). Most of the variance in this relationship was explained by years since worst trauma (its inclusion with trauma intensity in a model predicting current symptoms reduced the contribution of trauma intensity to a trend: β = 0.47; p = .07). Together, years since worst trauma and trauma intensity explained a substantial portion of the variance in the relationship between 9/11 group status and current symptoms (R2 = 0.40).
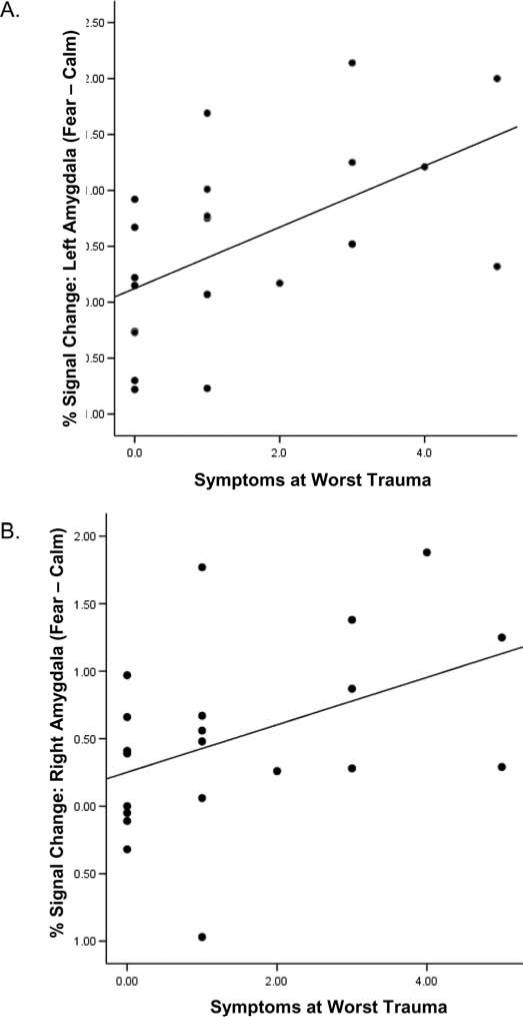
Increased symptoms of avoidance at time of worst trauma also predicted increases in percent signal change in the left and right amygdala to fearful versus calm faces (Table 2; Figure 4). While trauma intensity was correlated with years since worst trauma (r = –0.48; p = .05), it also contributed significantly to the explained variance of percent signal in the left amygdala (fear vs. calm) beyond that already accounted for by years since worst trauma (β = 0.60, p = .02; ΔR2 = 0.23, p = .02). When intensity of worst trauma was entered with years since worst trauma into a model predicting signal change in the left amygdala from 9/11 status, these two variables fully controlled the contribution of 9/11 group status. This suggests that the amount of time since an individual's worst trauma and the intensity of that worst trauma accounted for the observed increases in amygdala reactivity (fearful vs. calm) in participants who were close to the WTC on September 11th, relative to those who were farther away.
Figure 4.
Amygdala activity and trauma intensity. (A) Correlation between the number of retrospectively reported symptoms of avoidance at worst trauma and signal change in left amygdala (fearful vs. calm contrast): r = 0.58, p < .01. (B) Correlation between the number of retrospectively reported symptoms of avoidance at worst trauma and signal change in right amygdala (fearful vs. calm contrast): r = 0.42, p = .06.
Discussion
This fMRI study examined the neural correlates of trauma exposure in a sample of healthy adults. We found that participants who were within 1.5 miles of the WTC on September 11th, 2001, had significantly higher mean levels of activation in bilateral amygdala to fearful versus calm faces relative to those who were living more than 200 miles away, despite having no current diagnoses of PTSD, depression, or anxiety disorder. The 9/11-exposed group also had higher levels of current symptoms and reported more symptoms at time of worst trauma. Signal change in the amygdala (fearful vs. calm contrast) fully mediated the relationship between 9/11-exposure and number of current symptoms. In turn, the effect of group status on both amygdala activation (fearful vs. calm faces) and current symptoms was statistically explained by time since worst trauma and intensity of worst trauma, as indicated by reported symptoms at worst trauma. These data are consistent with a model of heightened amygdala reactivity following high-intensity trauma exposure, with relatively slow recovery. Notably, similar results have recently been identified in a similar population (Sharot et al., in press) using a paradigm that evoked specific memories of the disaster of September 11th. The present study supports and extends this research by suggesting that long-term trauma-related modulation of the amygdala is observable using mild, standardized emotional stimuli (fearful vs. calm faces), indicating that these effects may extend further into everyday life than previously thought.
Animal models of exposure to stress may provide insight into the processes of brain plasticity that underlie these results. Traumatic stressors encountered in the natural environment are likely to involve a mixture of conditioned and unconditioned response (Adamec, Blundell, & Burton, 2006). The most analogous animal models are likely to be those involving relatively severe uncontrollable stressors (for reviews, see Adamec et al., 2006 [unprotected predator stress]; Maier & Watkins, 1998 [inescapable shock]; McEwen, 2005 [acute and chronic restraint stress]). Controllable stressors do not activate the amygdala enough to produce even temporarily increased reactivity of the amygdala; uncontrollable stressors have, however, produced moderately persistent hyperexcitability of the amygdala and related structures in laboratory animals (Adamec et al., 2005; Maier & Watkins, 1998). This hyperexcitability has been associated with increased fearful response to ambiguous or mild stressors (Adamec et al., 2006; Maier & Watkins, 1998).
At the neuronal level, one type of uncontrollable stressor (chronic restraint stress or CRS) has been shown to produce hypertrophy of the dendritic arborization in the BLA and extended amygdala, accompanied by dendritic atrophy and decreases in spine density in medial prefrontal areas and the hippocampus (Mitra et al., 2005; Vyas et al., 2002). In rats, these changes are associated with standard behavioral indicators of anxiety in rodents, including decreased time spent in the open (exposed) arms of an elevated plus maze and reduction in open arm entries (Mitra et al., 2005; Vyas et al., 2002). Acute (single-event) restraint stress produces similar increases in spine density in the BLA and behavioral indicators of anxiety, with no associated changes in prefrontal areas (Mitra et al., 2005). Consistent with these findings, single-event unprotected predator stress produces persistent increases in evoked potentials from neurons in the BLA, which are also associated with increased open arm entry and decreased in exploration in the elevated plus maze (Ademac, Blundell, & Burton, 2005). Thus, a single noxious event of various types can drive alterations in the amygdala that are associated with persistent increases in behavioral reactivity in situations of uncertainty and potential threat. Notably, although CRS-induced changes to prefrontal areas and hippocampus are reversible with an extended stress-free period, hypertrophy of the amygdala and increases in anxiety-related behaviors are more persistent (Vyas, Pillai, & Chattarji, 2004). The effects of predator stress are also reported to be slow to return to baseline (Ademac et al., 2005). This highlights the role of the amygdala in maintaining stress-induced anxiety-like behaviors, as well as the slow recovery of this system over time after exposure to intensive stressors.
The findings of the present study point to the potential durability of human stress-related neural plasticity, even in adulthood, and suggest that such changes may be driven by the recency and intensity of worst adverse event (worst trauma in lifetime). Although accumulated trauma exposure did not add to the statistical prediction any of the outcomes in this analysis, it is a point for future research whether a more general measure of cumulative environment risk would do so. Research has established the relationship between accumulated environmental risk and negative socioemotional outcomes (e.g., Rutter, 1979; Sameroff, Seifer, Baldwin, & Baldwin, 1993). However, increases in cumulative environmental risk (e.g., low socioeconomic status, minority status, compromised social support, mental illness in family) may be confounded with increased likelihood for high-intensity trauma exposure in most community-based samples, and cumulative risk may also exacerbate trauma outcomes (Galea et al., 2002). More basic research is needed on the neural correlates of stressful life events of all types in healthy individuals; this is an exciting new area for investigation in cognitive neuroscience—one that in the past year has begun to show forward movement (e.g., Cohen et al., 2006; Sharot et al., in press; this study: also see recent work on the effects of in-scanner stressor exposure, Dedovic et al., 2005; Wang et al., 2005).
Finally, amygdala hyperexcitability after trauma may provide a tool for examining the neural processes that modulate stress-related neural plasticity. Chief among these potential modulatory processes is emotion regulation. Regulation of emotions is important for social adaptation, and neuroimaging evidence suggests that regulation of negative emotions, in particular, depends on modulation of amygdala response using prefrontal and anterior cingulate control systems (Ochsner & Gross, 2005). For example, amygdala activation is attenuated in response to fearful or angry emotional faces during cognitive evaluation of the type of emotion (Hariri, Bookheimer, & Mazziotta, 2000). The same occurs during cognitive evaluation of threatening emotional scenes and is accompanied by decline in skin conductance (Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003). Different strategies of emotion regulation (e.g., reappraisal vs. suppression: Gross & John, 2003; John & Gross, 2004) are likely to modulate amygdala hyperexcitability and associated behavioral outcomes following trauma exposure. The latter would be a point of interest for intervention.
Limitations
This study uses a retrospective report of trauma exposure. The difficulties with retrospective reporting include revisionist recall, bias because an ensuing disorder is known to have occurred, bias by respondent's depression or cognitive impairment, and normal forgetting (Hardt & Rutter, 2004; Moffitt et al., 2006). Prospective studies of the effects of trauma exposure on the brain would be preferable in many ways. However, this is made difficult because trauma exposure typically occurs unexpectedly. In the absence of a prospective study, it has been argued (Moffitt, Caspi, & Rutter, 2006) that the use of the life history calendar (Caspi et al., 1998) is a highly valid and reliable method for collecting retrospective data on adverse life events. We used this method in the present study to reduce retrospective reporting bias to a minimum.
An additional limitation of this study is that the comparison group in the current sample exhibited a high rate of trauma exposure. This may be due in part to participant self-selection, although high rates of trauma exposure have also been observed in large community-based studies (e.g., Breslau et al., 1998; Young & Breslau, 2004a and 2004b; Young et al., 2004) that used the UM-CIDI measure (Kessler et al., 1994) that was employed in the present study. Because there were few subjects without trauma exposure in this sample, we were unable to test differences between participants with and without trauma exposure. We note, too, that because most of the sample consisted of trauma-exposed individuals without PTSD, this may have inadvertently become a study of resilience; if so, these findings suggest that resilience under stress does not imply lack of physiological consequences following stressor exposure. In addition, specific examination of gender differences was not possible due to the small size of the study, although we found no significant statistical contributions of gender to the results reported here.
A further limitation to this study is that we used a passive viewing paradigm in this “first look” for neural correlates of trauma exposure in adult humans, because passive viewing of emotional faces is one of the most powerful standardized methods for evoking amygdala response (e.g., Whalen et al., 2001) and because tasks that require an active response to emotional stimuli can lead to top-down suppression of the amygdala response (Lange et al., 2003). The limitation of this method is the lack of accompanying behavioral data to determine level of attention to the stimuli. Having established with Sharot et al. that there are robust neural correlates of trauma exposure, the next set of research questions would address the source of the observed differences between groups using more sophisticated paradigms, for example, examination of differences in attention or emotion regulation using more active paradigms.
Finally, in the present research, participants were aware that the study was related to the effects of the 9/11 disaster, and they were interviewed for trauma exposure before scanning. This knowledge, and the screening process, may have had a priming effect on amygdala response. This is an unexplored area and one that requires more research. Because there was trauma exposure in both groups (with the exception of one individual in the comparison group), the trauma inventory might have been expected to have a similar impact across all participants. However, it may be that specific variables related to prescreening interviews (e.g., length and timing of the trauma interview) had significant effects on the BOLD signal response, even years after the trauma. If so, it would suggest even more powerful and subtle long-term effects of previous trauma exposure on the brain than are indicated in the current study.
Conclusions
Close proximity to the WTC on September 11th, 2001, was associated with increased amygdala activation and higher levels of symptoms in healthy adults. Amygdala activation mediated the association between trauma exposure and current symptoms, suggesting a role for amygdala hyperexcitability in the association between trauma exposure and subsequent vulnerability to mental health disorder. Overall, amygdala activation was found to decrease with time since worst trauma, and to increase with the intensity of each individual's worst-ever trauma, as measured by symptoms at time of trauma. Together, these two variables statistically explained the effect of proximity to the 9/11 disaster on both amygdala activation and current symptoms. This finding suggests that there is heightened amygdala activity from high intensity traumas, and that recovery occurs over many years, even in those without a current clinical disorder.
Acknowledgments
We thank Jason Zevin, Chiyoko Kobayashi, Todd Hare, John Eckenrode, and Jason Buhle for helpful discussions throughout the course of this study. Special thanks to Clayton Eccard for valuable assistance with this project and thanks also to Elizabeth Aronstam, Gregor Carrigan, Molly Henderson, Eric Horowitz, Judith Katz, and Michoel Snow. This research was supported by NIH Kirchstein NRSA MH68139-01A2 to BG.
Appendix
Statistical mediation (Baron & Kenny, 1986, p. 1176)
A variable functions as a mediator of the significant relationship between an independent and dependent variable when (a) variation in the levels of the independent variable significantly accounts for variations in the presumed mediator, (b) variations in the presumed mediator significantly account for variations in the dependent variable, and (c) both the independent variable and the presumed mediator are entered simultaneously into a regression model predicting the dependent variable, then the previously significant relationship between the independent variable and the dependent variable is reduced in significance or (in the strongest condition) is no longer significant. MacKinnon & Dwyer (1993) provide a statistical test for significance of this overall process, which was used here.
References
- Adamec RE, Blundell J, Burton P. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neuroscience and Biobehavioral Reviews. 2005;29:1225–1241. doi: 10.1016/j.neubiorev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Blundell J, Burton P. Relationship of predatory attack experience to neural plasticity, pCREB expression and neuroendocrine response. Neuroscience and Biobehavioral Reviews. 2006;30:356–375. doi: 10.1016/j.neubiorev.2005.04.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 2000. [Google Scholar]
- Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. American Journal of Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Lawrence A. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Kuhn E, Rowell D, Hickling EJ, Wittrock D, Rogers R, et al. Studies of the vicarious traumatization of college students by the September 11th attacks: Effects of proximity, exposure and connectedness. Behaviour Research and Therapy. 2004;42:191–295. doi: 10.1016/S0005-7967(03)00118-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick S, Johnson D, Yehuda R, Charney D. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;57:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Brieter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–40. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brown G. Life events and affective disorder: Replications and limitations. Psychosomatic Medicine. 1993;55:248–259. doi: 10.1097/00006842-199305000-00003. [DOI] [PubMed] [Google Scholar]
- Bunce S, Larsen R, Peterson C. Life after trauma: Personality and daily life experiences of traumatized people. Journal of Personality. 1995;63:165–188. doi: 10.1111/j.1467-6494.1995.tb00806.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington HL, et al. The Life History Calendar: A research and clinical assessment method for collecting retrospective event-history data. International Journal of Methods in Psychiatric Research. 1998;6:101–114. [Google Scholar]
- Cohen R, Grieve S, Hoth K, Paul R, Sweet L, Tate D, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Darlington R. Regression and linear models. McGraw-Hill; New York: 1990. [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 2005;30:319–325. [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price C, Buchel C, Worsley KJ. Multisubject fMRI analyses and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, Wlahov D. Psychological sequelae of the September 11 terrorist attacks in New York City. New England Journal of Medicine. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John O. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology, Psychiatry, and Allied Disciplines. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system (2000). Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger D. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, et al. Sensing the invisible: Differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Holland PW. Statistics and causal inference. Journal of the American Statistical Association. 1986;81:945–970. [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosomatic Medicine. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- John O, Gross JJ. Health and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality. 2004;72:1301–1334. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of General Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kessler R, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the National Comorbidity Study. Archives of General Psychiatry. 1995;52:1048–1059. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle K, Zhao S, Nelson CB, Hughes M, Eshelman S, et al. Lifetime and 12-month prevalence of DSM-IIIR psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SC, et al. Task instructions modulate neural responses to fearful facial expressions. Biological Psychiatry. 2003;53:226–232. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Bluhm RL, Densmore M, Boksman K, Neufeld RW, et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: A functional magnetic resonance imaging investigation. Biological Psychiatry. 2005;57:873–884. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, et al. Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biological Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17:144–158. [Google Scholar]
- Maier S, Watkins L. Stressor controllability, anxiety, and serotonin. Cognitive Therapy and Research. 1998;22:595–613. [Google Scholar]
- McCauley J, Kern D, Kolodner K, Dill L, Schroeder A. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. Journal of the American Medical Association. 1997;277:1362–1368. [PubMed] [Google Scholar]
- McEwen B. Glucocorticoids, depression and mood disorders: Structural remodeling in the brain. Metabolism: Clinical and Experimental. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McFarlane A. The prevalence and longitudinal course of PTSD: Implications for neurobiological models of PTSD. In: Yehuda R, McFarlane A, editors. Psychobiology of posttraumatic stress disorder. Vol. 821. New York Academy of Sciences; New York: 1997. pp. 10–24. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Chattarji S. Stress duration modulates the spatioteporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences, USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspectives in Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Gross J. The cognitive control of emotion. Trends in Cognitive Neuroscience. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, et al. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biological Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Ozer E, Best S, Lipsey T, Weiss D. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of Spiral-In/Out and Spiral-out BOLD fMRI at 1.5T and 3T. Neuroimage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rauch S, Whalen P, Shin L, McInerney S, Macklin M, Lasko N. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rosen J, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rutter M. Protective factors in children's responses to stress and disadvantage. In: Kent MW, Rolf JE, editors. Primary Prevention of Psychopathology. Vol. 3. University Press of New England; Hanover, NH: 1979. pp. 49–74. [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: A functional MRI study. Neuroimage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of intelligence from preschool to adolescence: The influence of social and family risk factors. Child Development. 1993;64:80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Sharot T, Martorella EA, Delgado MR, Phelps E. Remembering 9/11: How personal experience modulates the neural circuitry of recollection. Proceedings of the National Academy of Sciences, USA. doi: 10.1073/pnas.0609230103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L, Orr S, Carson M, Rauch S, Macklin M, Lasko N, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Some new results on indirect effects and their standard errors in covariance structure models. In: Tuma N, editor. Sociological methodology. American Sociological Association; Washington, DC: 1986. pp. 159–186. [Google Scholar]
- Spielberger CD. Manual for the state-trait anxiety inventory for children. Consulting Psychologists Press; Palo Alto, CA: 1973. [Google Scholar]
- Thomas K, Drevets W, Whalen P, Eccard C, Dahl R, Ryan N, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. Validity and reliability of the nimStim set of facial expressions. in press. [DOI] [PMC free article] [PubMed]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdalaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. American Journal of Psychiatry. 2005;152:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- Young E, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: An epidemiologic community study. Archives of General Psychiatry. 2004a;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young E, Breslau N. Saliva cortisol in posttraumatic stress disorder: A community epidemiologic study. Biological Psychiatry. 2004b;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Young E, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and Posttraumatic Stress Disorder in a low-income community sample of women. Biological Psychiatry. 2004;55:621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]