Abstract
Reactive oxygen species (ROS) activate hepatic stellate cells and enhance fibrogenesis. This study determine the role of NAD(P)H oxidase deficiency in the development of hepatocellular necrosis, inflammation and apoptosis in relation to fibrosis produced by chronic CCl4 administration. Wild-type (WT) mice or mice with deficiency of the gp91phox subunit of NAD(P)H complex (gp91phox−/−) were subjected to biweekly CCl4 injections over 8 weeks, while controls were given isovolumetric injections of olive oil. Serum aspartate aminotransferase (AST) was higher after CCl4 administration in gp91phox−/− than in WT mice, correlating with increased necrosis on liver histology. By contrast more hepatocyte apoptosis was found after CCl4 in the WT than in the gp91phox−/− mice, which was associated with changes in components of the mitochondrial pathway of apoptosis, namely an increase in the pro-apoptotic BAX protein in the WT, but not in the gp91phox−/− mice and also a lower cytosolic cytochrome c in the gp91phox−/− mice. There were fewer stellate cells and less fibrosis after CCl4 in the gp91phox−/− as compared to the WT mice. The increase in α1(I) collagen mRNA however was greater after CCl4 in the gp91phox−/− mice. Matrix metalloproteinase-2 (MMP-2) and MMP-9 mRNA increased more in the gp91phox−/− than in WT mice after CCl4. Tissue inhibitor of metalloproteinase 1 (TIMP-1) and TIMP-2 increased after CCl4 only in the gp91phox−/− mice.
Conclusions
Decreased hepatic fibrosis after chronic CCl4 administration in mice with NAD(P)H oxidase deficiency occurs in the setting of greater necrosis and inflammation but decreased apoptosis.
Keywords: Stellate cells, collagen, matrix metalloproteinases, necrosis, apoptosis
Reactive oxygen species (ROS) activate stellate cells and stimulate fibrogenesis (1). Lipid peroxidation products stimulate α1(I) collagen expression and collagen synthesis by stellate cells in culture (2,3). NAD(P)H oxidase, which generates superoxide ion (O2 •-) from oxygen (O2), is a principal source of ROS. Platelet derived growth factor (PDGF) induced proliferation of hepatic stellate cells, which is mediated by ROS produced by NAD(P)H oxidase, was abrogated by inhibition of NAD(P)H with diphenylene iodonium (DPI) pretreatment (4). Furthermore, hepatic fibrosis produced in vivo by dimethylnitrosamine was eliminated by daily DPI administration (4). Liver cell death due to necrosis or apoptosis results in inflammatory responses and in fibrogenesis (5, 6). Mice with deficiency of the p47phox subunit of NAD(P)H oxidase complex developed less hepatic necrosis and fibrosis after bile duct ligation than wild-type (WT) mice (7). In another study, using a pan-caspase inhibitor, hepatocyte apoptosis, was found to be critical for the development of liver inflammation and fibrosis after bile duct ligation (8). In prior studies, we found that mice with NAD(P)H oxidase deficiency, due to lack of the gp91phox−/− subunit of NAD(P)H complex (gp91phox−/−), developed less hepatic fibrosis after chronic CCl4 administration than WT mice (9), but the means of cell death were not assessed. In a more recent study, gp91phox−/− and WT mice fed a methionine and choline deficient diet for 8 weeks developed similar amounts of necroinflammatory change and fibrosis (10).
The purpose of this study was to determine how NAD(P)H oxidase deficiency decreases hepatic fibrosis produced by chronic CCl4 administration by examining the roles of hepatocellular necrosis, inflammation and apoptosis.
MATERIALS & METHODS
Animals and Materials
Male wild-type (WT) C57BL/6J and gp91phox−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animals received humane care in compliance with the guidelines from the Animal Care and Use Committee of The Johns Hopkins University. Sirius Red was obtained from Polysciences, Inc, Warrington, PA. Carbon tetrachloride (CCl4) and goat anti-mouse α-smooth muscle actin Cy3 conjugate antibody were purchased from Sigma Chemical Co., St. Louis, MO. Caspase 3 and Caspase 8 fluorometric assay kits were obtained from BioVision (Mountain View, CA)
Animal treatment
Mice at 4–6 weeks of age weighing 20–30g were kept in a temperature-controlled room with an alternating 12-h dark and light cycle. Eight WT and 8 gp91phox−/− mice were given intraperitoneal (i.p) injections of CCl4 biweekly as 5 µl of a 20% solution of CCl4 in olive oil per g body weight (1.0 ml/kg of CCl4). The 8 control WT and 8 gp91phox−/− mice received the same isovolumetric dose of olive oil as i.p. injections. The animals were sacrificed at one week and at 8 weeks after the start of the injections. At the time of sacrifice, blood was obtained from the aorta for measurements of aminotransferases and the samples were stored at −20°C. The liver was removed, rinsed with phosphate buffered saline (PBS), and divided into four portions: (a) fixed in 10% buffered formaldehyde formalin and embedded in paraffin; (b) snap frozen at −70°C for sectioning and immunohistochemistry; (c) homogenized in appropriate buffer(s) and aliquots of the homogenates or the separated cytosols after centrifugation frozen at −70°C for biochemical assays; and (d) placed in RNA STAT-60 solution (from Tel-Test, Inc, Friendswood, TX) and stored in −70°C for RNA isolation.
Liver histology and morphometric collagen determination
The liver sections imbedded in paraffin were cut (5µ) and stained with hematoxylin-eosin (H&E), Masson’s trichrome, or sirius red. The extent of necrosis and inflammation was evaluated on blinded slides by M.S.T. from our Department of Pathology. Fibrosis was determined histologically by measuring the intensity of fibrosis in four to six (x 100) digital images captured from slides of each mouse stained with sirius red. The total fibrosis density score was determined by dividing the image intensity by the image area as described previously (11).
Quantitation of stellate cells
Immunofluorescent staining for α-smooth muscle actin (α -SMA) was done in deparaffinized liver sections. The slides were washed in deionized water for 1 min and in PBS for 5 min, followed by blocking using PBS-5% FBS. The slides were incubated with Cy3 conjugated monoclonal α -SMA antibody (Sigma, 1:500 in PBS-5% FBS) for 1 h at room temperature and subsequently at 4°C overnight. After washing with PBS 4 times for 15 min, the slides were mounted and 8 areas per slide captured by fluorescent microscopy (magnification x100). Stellate cells were counted in 8 fields per slide for each mouse.
Serum Aminotransferases
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by the spectrophotometric method of Bergmeyer et al. (12).
Mitochondrial ATP
Liver mitochondria were isolated by differential centrifugation from liver homogenates as described previously (13). ATP was determined with a Bioluminescent Assay Kit obtained from Sigma Chemical Company. Mitochondrial protein was determined by the method of Lowry et al (14)
Liver malondialdehyde
Liver slices were homogenized with cold 1.15% KCl and malondialdehyde was determined using thiobarbituric acid by the method of Uchiyama and Mihara (15).
Determination of messenger RNA by real time quantitative polymerase chain reaction RT-qPCR
The 7900 HT (Applied Biosystems, Foster City, CA) and the SDS 2.2.1 software was used to perform RT-qPCR at the The Johns Hopkins DNA Analysis Facility. Total cellular RNA from a portion of liver was placed in RNA STAT 60 reagent and following their protocol, RNA was purified and isolated. The concentration of the isolated RNA was determined from the optical density at 260nm and its purity from the 260nm/280nm OD ratio. The isolated RNA was stored at −80° C. RT-qPCR for α1(I) collagen mRNA and transforming growth factor-β (TGF-β )were performed using sequence-specific probes from TaqMan gene expression assays of Applied Biosystems (Foster City, CA). Probes for mouse TGF-β 1, α1(I) collagen, β–actin (as endogenous control), mouse matrix metalloproteinase (MMP) -2 (MMP-2), and MMP-9 were obtained from Applied Biosystems. Superscript III first strand synthesis from Invitrogen (Carlsbad, CA) was used to synthesize first strand cDNA from the purified RNA. Gene-transcript levels of the above probes were compared to β-actin, the housekeeping endogenous control. Variation in the amount of the transcripts was corrected by the level of expression of the β-actin gene in each individual sample.
Western Blot Analysis
Liver sections were homogenized in 50 mM Tris-HCl buffer pH 7.6 containing 150 mM NaCl, 10 mM CaCl2, 0.25% Triton-X, 0.1 µM phenylmethanesulfonyl fluoride (PMSF), 10 µM leupetin, 10 µM pepstatin, 0.1 mM iodoacetamide and 25 µg aprotonin and then centrifuged at 9,000g for 10 min at 4°C. The cytosol protein in the supernatant was initially stored at −80°C. The proteins were separated on mini-SDS gels at 100 V for 1 h and electrotransferred to nitrocellulose transblot membranes (BioRad, Hercules, CA). The membranes were washed in PBS, pH 7.6, containing 0.1% Tween 20 (PBS-T), blocked with 5% (W/V) dry nonfat milk in PBS-T for 1 h, rinsed with PBS-T and then incubated with either rabbit anti-mouse antibodies to Fas, Bax, BclXs/l, Bcl-2, cytochrome c, MMP-2, MMP-9, TIMP-1, TIMP-2 or β actin, obtained from Santa Cruz Biotechnology, Inc, Santa Cruz, CA. After repeated washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000 dilution; Amersham Biosciences, Piscataway, NJ) at room temperature for 1 h. The membranes were then washed again and visualized by enhanced chemiluminescence reaction (ECL Plus; Amersham Biosciences). Densitometry was determined using Image J v 1.30 obtained from NIH.
Terminal Deoxynucleotidyl Transferase-Mediated Nick-End Labeling Assay
Terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) assay was performed on paraffin embedded liver slices with the cell death detection kit from Roche Applied Science (Nutley, NJ). Fluorescence microscope using the FITC filter revealed the apoptotic bodies which were counted.
Apoptosis in stellate cells
Apoptosis in stellate cells was determined in ultrathin liver slices by formamide-induced DNA denaturation with detection of single stranded DNA (ssDNA) described by Frankfurt and Krishan (16), with mouse monoclonal antibodies to ssDNA (Millipore, Temecula, CA) and anti-mouse IgG (whole molecule) FITC as secondary antibody (Sigma). The immunostained slices were evaluated and photographed with laser confocal microscopy.
Caspase activities
The activities of caspase 3 and caspase 8 were determined in liver homogenates by measuring proteolytic cleavage of the specific fluorogenic substrates, DEVD-AFC and IETD-AFC (AFC: 7-amino-4-trifluoromethyl coumarin) respectively BioVision). The results are expressed as relative units per milligram of protein.
Statistical Analysis
In most measurements, the mean and the standard error of the mean were calculated. The data was analyzed with the Student’s t test or by two way analysis of variance (ANOVA) when comparing means of more than two groups.
RESULTS
Liver injury and fibrosis
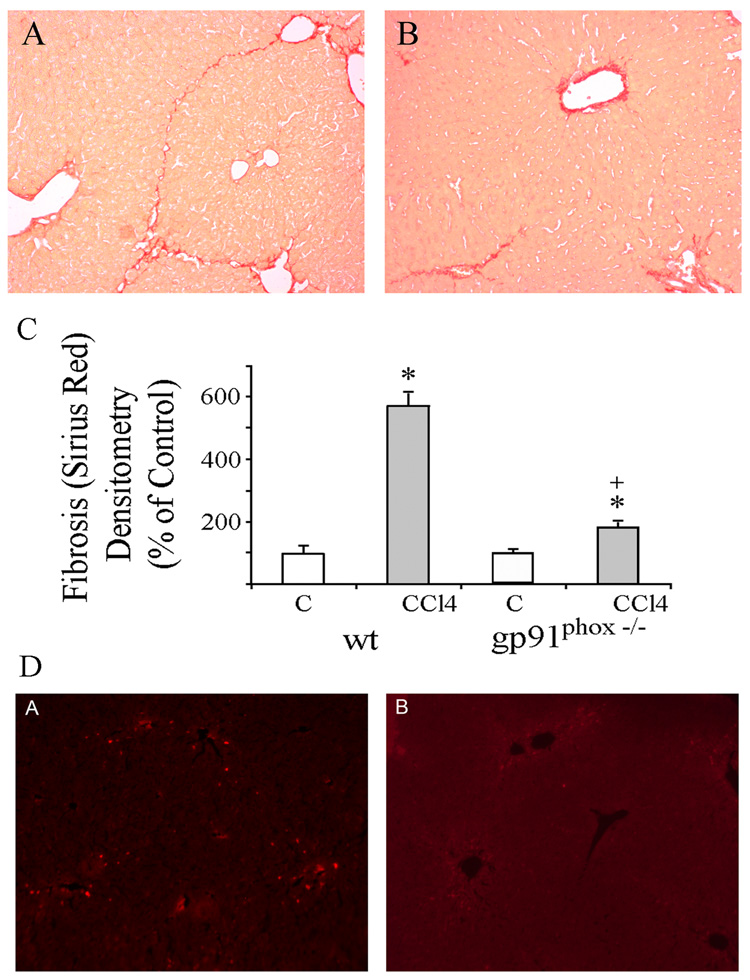
The morphological changes of liver injury and fibrosis caused by CCl4 were visualized in sections stained by H&E (not shown) and sirius red. The changes include necrosis, inflammation with macrophages, lymphocytes and fibrosis. Fatty infiltration was minimal. The grades of necroinflammatory changes were higher after CCl4 in the gp91phox−/− mice as compared to the WT mice (p<0.05) (Fig. 1). Liver fibrosis was more evident in the WT than in the gp91phox−/− mice (Fig.2A–B). The area of hepatic fibrosis detected by Sirius red staining and densitometric analysis was significantly higher in the WT mice after CCl4 as compared to the other mice groups (p<0.01) (Fig. 2C) The number of stellate cells, identified by α-smooth muscle actin staining, was lower per x200 field after CCl4 in the gp91phox−/− mice than in WT mice (Fig 2D). The values were 33.9 ± 2.8 in gp91phox−/− as compared to 50.0 ± 4.8 in the WT mice (p<0.05).
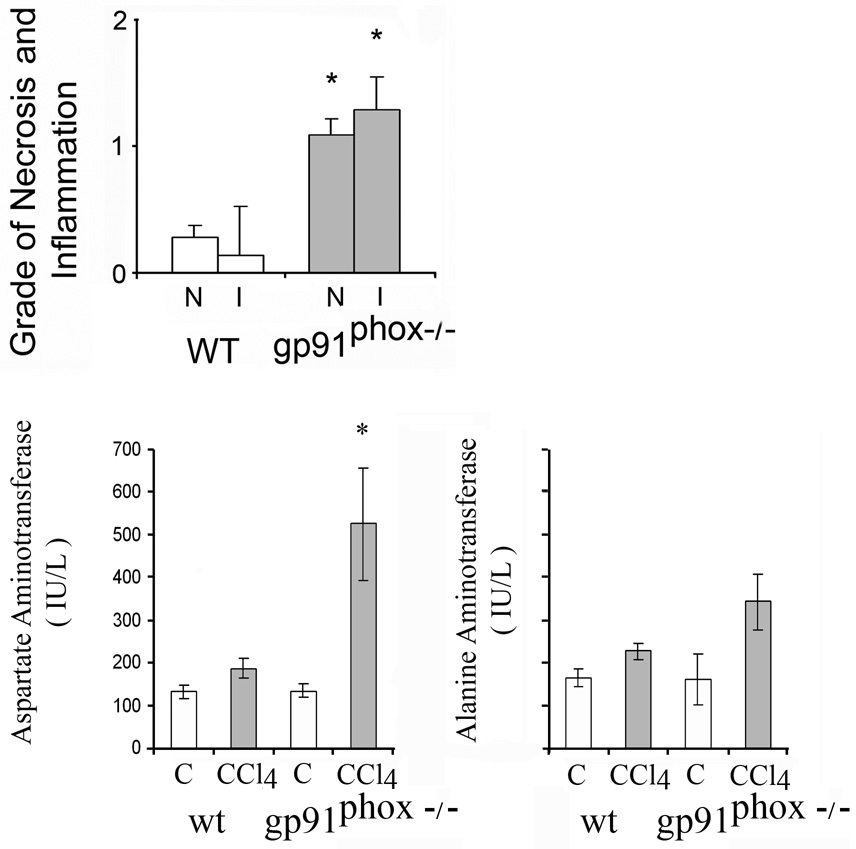
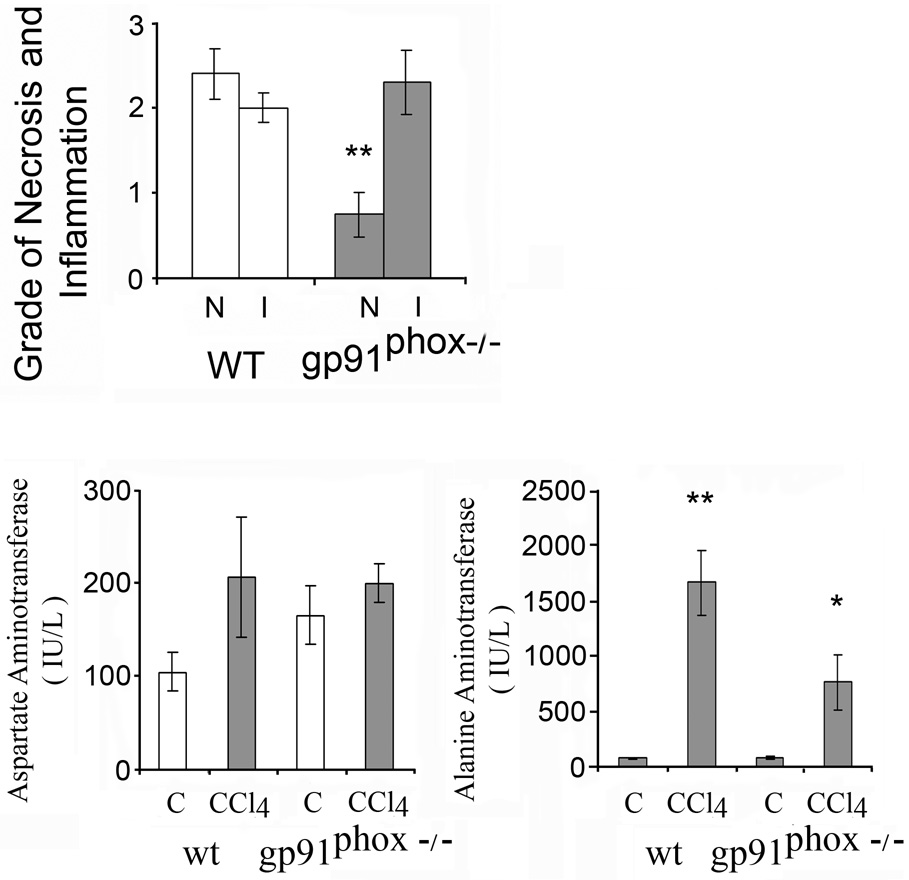
Fig. 1.
Necroinflammatory changes and serum aminotransferases after 8 weeks of chronic carbon tetrachloride (CCl4) administration in wild type (WT) and gp91phox−/− mice. Liver cell necrosis (N) was evaluated on a scale of 1–4 as follows: 1, occasional; 2, mild scattered; 3, confluent zonal and 4, extensive. Grades for hepatic inflammation (I) were: 1, scattered; 2, mild; 3, moderate and 4, marked. The histological changes were assessed in x 20 magnification fields of slides stained with hematoxylin-eosin (H&E). The values are expressed as means ± SE of 8 determinations per group. *p<0.05 vs. WT for necroinflammatory changes. *p<0.05 vs control for serum aminotransferases.
Fig.2.
Histology with sirius red collagen staining of liver sections from (A) WT and (B) gp91phox−/− mice after 8 weeks of chronic CCl4 administration. (original magnification × 100). C. Relative densitometry of fibrosis in WT and gp91phox−/− mice after chronic CCl4 administration. The degree of fibrosis was measured by sirius red staining and densitometry of the various groups. The density of fibrosis was determined as intensity of sirius red staining divided by the area of the captured field. A total of 24 fields were captured from livers of each group of mice. The values are expressed as means ± SE. * p< 0.01 vs. respective control. + p<0.01 vs WT + CCl4. D. Immunofluorescent detection of activated stellate cells in (A) WT and (B) gp91phox−/− mice after chronic CCl4 administration. The liver slices were incubated with Cy3 monoclonal α-smooth muscle actin antibody (original magnification × 100).
Serum aminotransferases were elevated after CCl4 administration (Fig.1). The increase of AST was higher in gp91phox−/− than in WT mice (p<0.05). By contrast, ALT was not significantly different in the gp91phox−/− than in the WT mice.
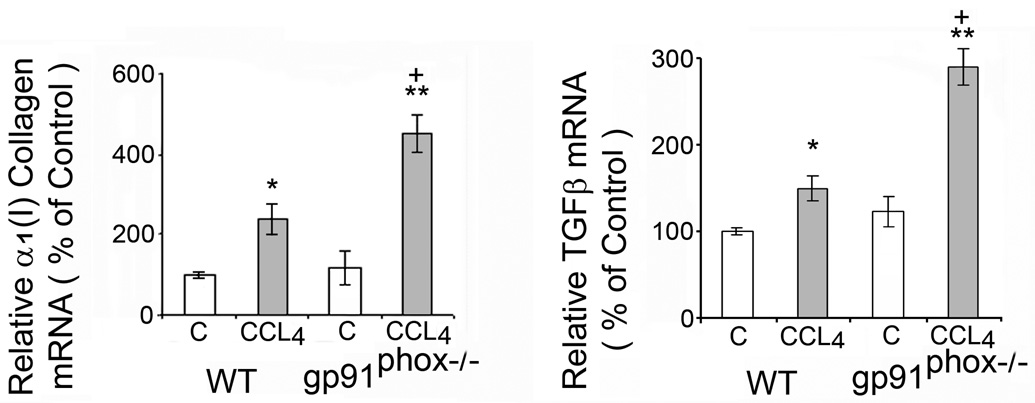
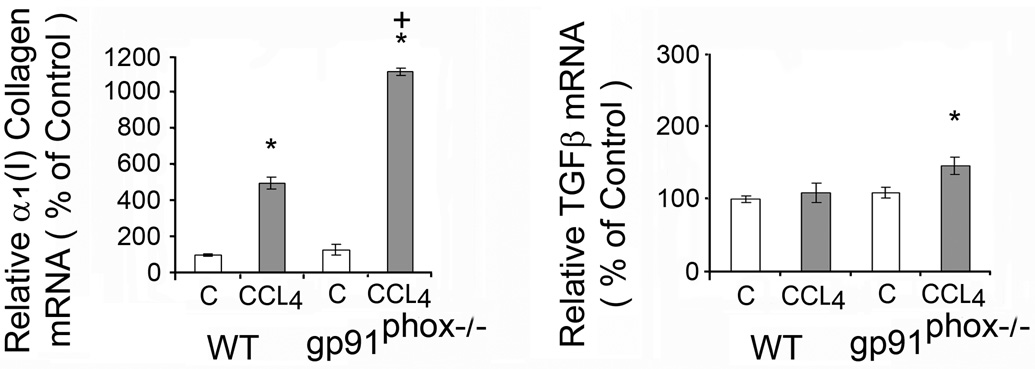
The increase in α1(I) collagen mRNA after CCl4 administration was greater in the gp91phox−/− than in the WT mice (p<0.05) (Fig. 3). The increase in TGFβ mRNA was also greater in the gp91phox−/− than in the WT mice (p<0.05) (Fig. 3). However, TGFβ protein on Western blot analysis was not significantly changed by CCl4 in either group of mice (data not shown).
Fig.3.
Liver α1(I) collagen mRNA and transforming growth factor β (TGFβ) mRNA in WT and gp91phox−/− mice after chronic CCl4 administration. The mRNAs were determined by real time quantitative PCR. The relative amounts of mRNA were normalized against β-actin in the same samples. The values are expressed as means ± SE of 8 determinations per group. * p<0.05 vs. control. **p<0.01 vs. control. + p<0.05 vs. WT + CCl4.
Liver malondialdehyde was markedly increased in the WT mice after CCl4 administration from a control value of 9.4 ± 1.4 to 19.1 ± 1.5 µmoles/g liver (p<0.01). In the gp91phox−/− animals, liver malondialdehyde increased from 5.8 ± 1.8 to 10.3 ± 0.2 (p<0.05).
Apoptosis
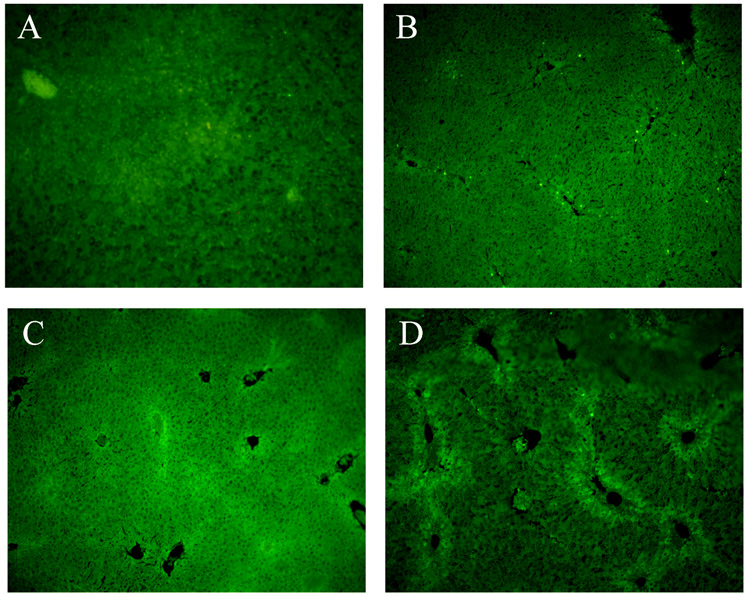
The number of apoptotic hepatocytes was highest in the WT mice after CCl4 administration (Fig. 4B). The values from 40–100 fields (x100) examined were 1.63 ± 0.42 and 1.67 ± 0.43 apoptotic cells per field for WT and gp91phox−/− controls respectively and 69.2 ± 4.05 and 14.93 ± 2.17 for WT and gp91phox−/− after CCl4 administration (p<0.01).
Fig.4.
Apoptosis determined by TUNEL staining of liver sections from WT and gp91phox−/− mice after chronic CCl4 administration. (A) WT-control; (B) WT + CCl4; (C) gp91phox−/− control; (D) gp91phox−/− + CCl4. Original magnification × 100.
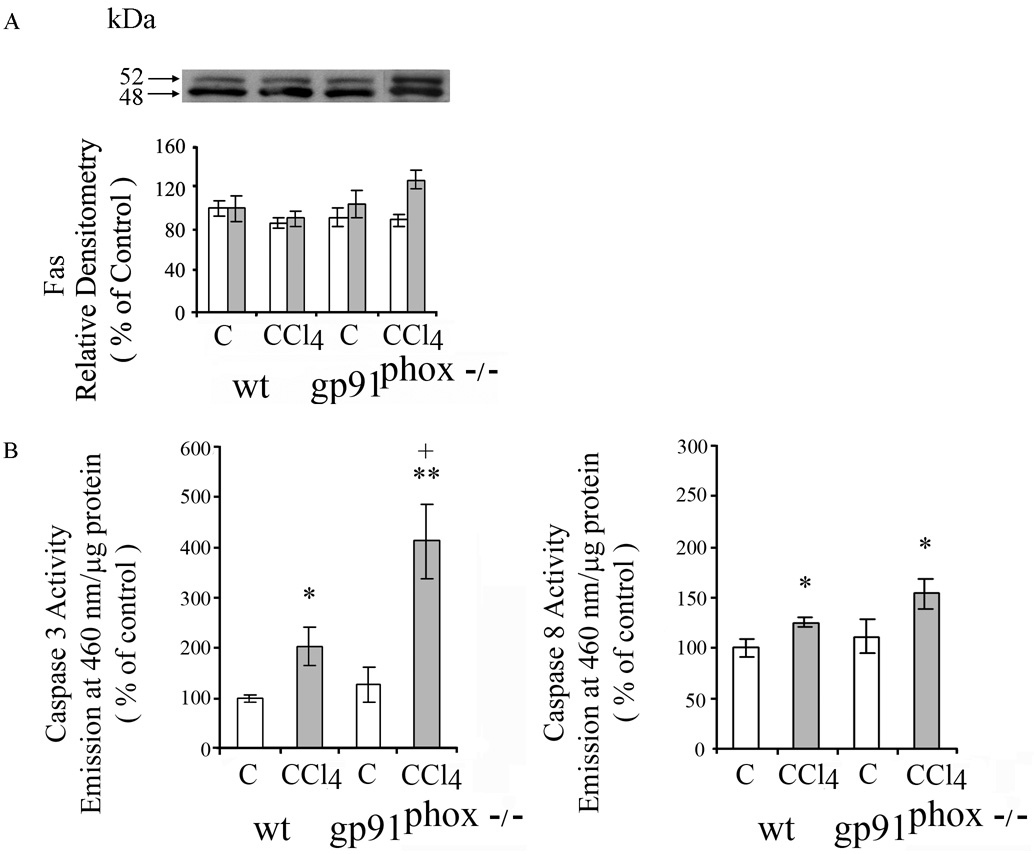
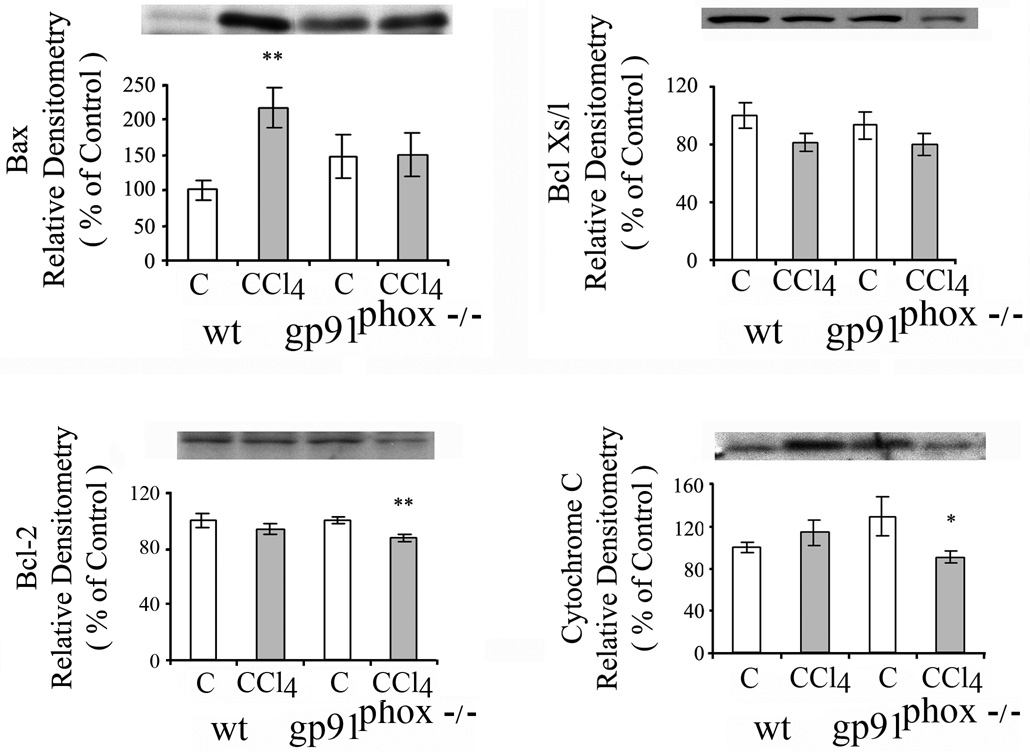
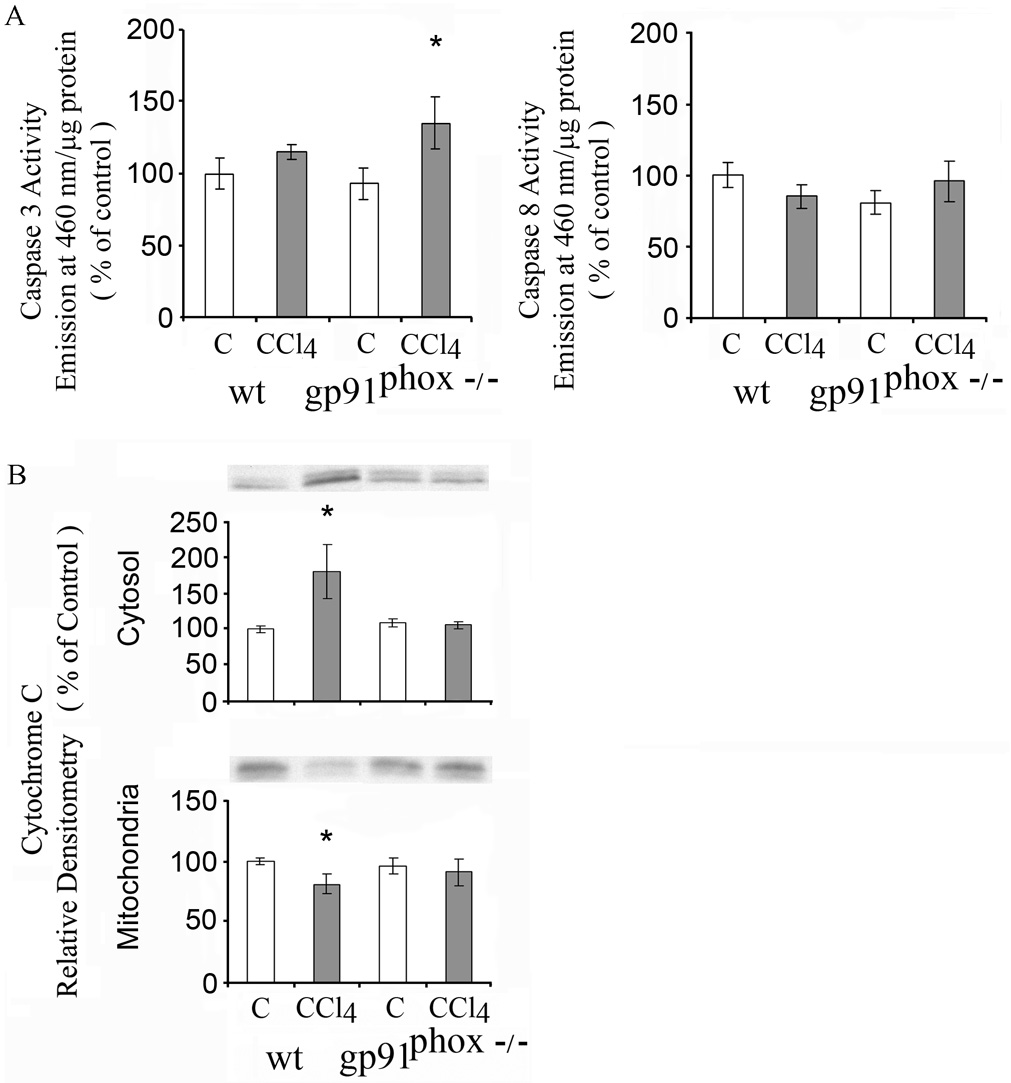
Fas (CD95/APO-1) receptor mediates apoptosis principally via the extrinsic death receptor pathway. Two Fas receptor proteins were detected by western blot in livers of the mice. CCl4 did not result in significant changes in Fas receptor proteins in the WT or gp91phox−/− mice (Fig.5A). Activated caspases 3 and 8 are essential in the extrinsic death receptor pathway of apoptosis. Caspase 3 was increased to a greater extent in the gp91phox−/− mice than the WT mice after CCl4 administration (p<0.05) (Fig. 5B), while the increase in Caspase 8 was similar after CCl4 in the gp91phox−/− and WT mice. Components of the mitochondrial pathway of apoptosis that respond to intracellular stress signals were examined. Pro-apoptotic BAX protein was increased after CCl4 in the WT, but not in the gp91phox−/− mice (p<0.01) (Fig.6), while BclX S/L was not affected by CCl4 administration in either the gp91phox−/− or WT mice (Fig.6). The anti-apoptotic protein Bcl-2 decreased after CCl4 in the gp91phox−/− (p<0.01) but not in the WT mice (Fig.6). Cytosolic cytochrome c, which is released from the mitochondria during apoptosis and is regulated by both pro- and anti-apoptotic members of the Bcl-2 family of proteins, was decreased after CCl4 in the gp91phox−/− CCl4 group ( p<0.05), but not in the WT mice (Fig. 6).
Fig. 5.
Effects of chronic CCl4 on in WT and gp91phox−/− mice on A. FAS and B. Activities of caspase 3 and 8. FAS was determined by western blot and quantitated by densitometry. White bars represent 52 kDA and shaded bars 48 kDa Fas. All values are expressed as means ± SE of 8 measurements per group.
* p< 0.05 vs. respective control. **p<0.01 vs.respective control; +p<0.05 vs. WT + CCl4.
Fig. 6.
Effects of chronic CCl4 administration on Bax, Bcl Xs/l Bcl-2 and cytosolic cytochome c in WT and gp91phox−/− mice. The determinations were done by western blot. The values are expressed as means ± SE of relative densitometry of 8 measurements per group. * p< 0.05 vs. control. **P<0.01 vs. control.
Stellate cell apoptosis, evaluated by the presence of ssDNA under confocal microscopy, was not detected in the gp91phox−/− or the WT mice after CCl4 (data not shown).
Matrix metalloproteinases
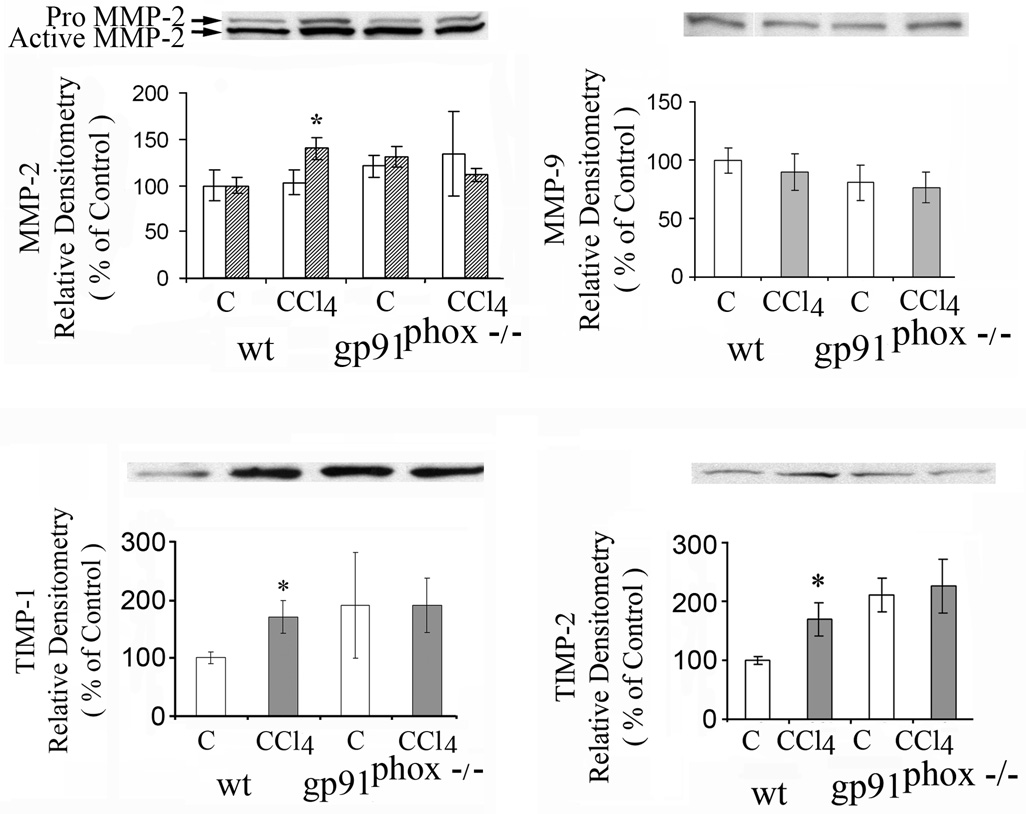
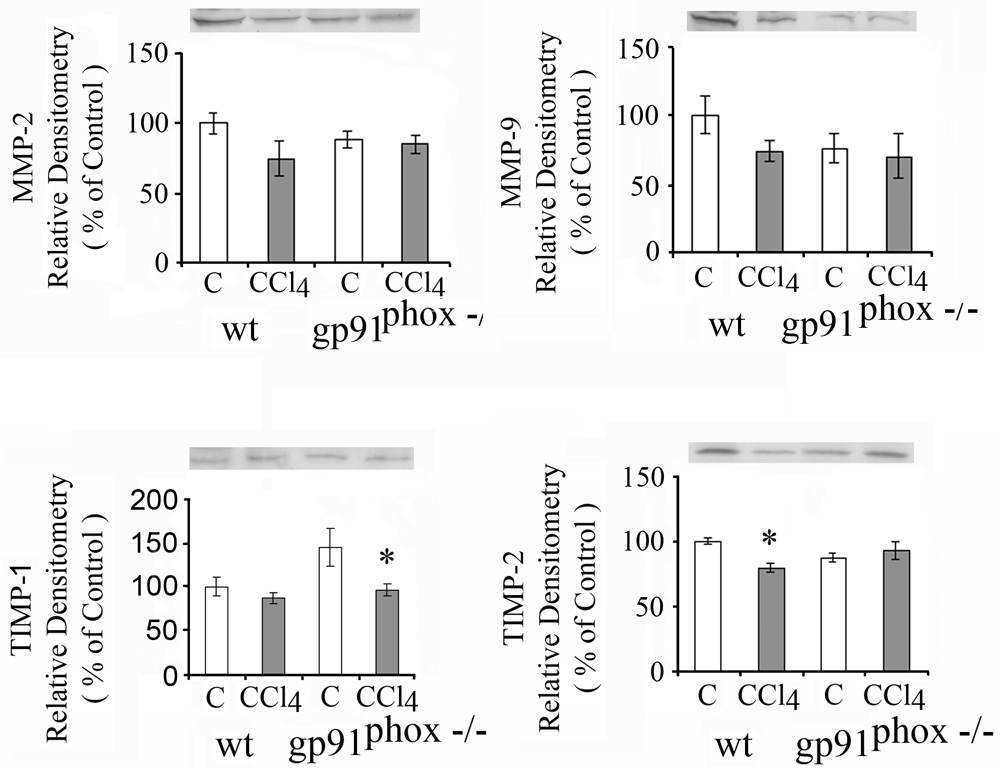
Liver matrix metalloproteinase-2 (MMP-2) was increased after CCl4 in the WT mice (p<0.05) but not in the gp91phox−/− mice (Fig.7), while matrix metalloproteinase 9 (MMP-9) was not changed by CCl4 in either group of mice (Fig.7). Increases in MMP-2 mRNA and in MMP-9 mRNA were greater after CCl4 in the gp91phox−/− than in the WT mice. The increase in MMP-2 mRNA after CCl4 was 30 fold in the gp91phox−/− mice as compared to 7 fold in the WT mice (p<0.01), while the increase in MMP-9 mRNA was 111 fold in the gp91phox−/− mice as compared to 5 fold in the WT mice (p<0.01) (data not shown).
Fig. 7.
Effects of chronic CCl4 administration on matrix metalloproteinase 2 (MMP-2), 9 (MMP-9), tissue inhibitor of metalloproteinase 1 (TIMP-1) and TIMP-2 in WT and gp91phox−/− mice determined by western blot. *p< 0.05 vs. respective control.
Tissue inhibitor of metalloproteinase 1 (TIMP-1) (Fig. 7) and TIMP-2 were lower in the control WT than in the control gp91phox−/− mice (Fig.10). After CCl4, both TIMP-1 and TIMP-2 increased only in the WT mice (p<0.05) to levels similar to those found in the control or CCl4 treated gp91phox−/− mice (Fig. 7).
Fig. 10.
Liver α1(I) collagen mRNA and transforming growth factor β (TGFβ) mRNA in WT and gp91phox−/− mice after one week of CCl4 administration. The mRNAs were determined by real time quantitative PCR. The relative amounts of mRNA were normalized against β-actin in the same samples. The values are expressed as means ± SE. * p< 0.05 vs. respective control. +p<0.05 vs. WT + CCl4.
Effects of administration of CCl4 for one week
The effects of acute CCl4 on hepatic injury and fibrosis were determined in a separate experiment. After one week CCl4 administration the grade of necrosis, but not of inflammatory changes, was higher in the WT mice than in gp91phox−/− mice (p<0.05) (Fig.8). Liver fibrosis detected by sirius red staining was minimal and similar in the WT than in the gp91phox−/− mice (not shown). Also the number of stellate cells, identified by α-smooth muscle actin staining, was similar per x200 field after CCl4 in the gp91phox−/− mice than in WT mice. The values were 9.8 ± 1.6 and 9.3 ± 0.7 in gp91phox−/− and the WT mice respectively. The increase of serum ALT was higher in WT (p<0.01) than in gp91phox mice (p<0.05) (Fig. 8). By contrast, AST was not significantly different in the gp91phox−/− than in the WT mice.
Fig. 8.
Necroinflammatory changes and serum aminotransferases after 1 week of CCl4 administration in wild type (WT) and gp91phox−/− mice. Liver cell necrosis (N) was evaluated on a scale of 1–4 as follows: 1, occasional; 2, mild scattered; 3, confluent zonal and 4, extensive. Grades for hepatic inflammation (I) were: 1, scattered; 2, mild; 3, moderate and 4, marked. The histological changes were assessed in x 20 magnification fields of slides stained with hematoxylin-eosin (H&E). The values are expressed as means ± SE of 8 determinations per group. **p<0.01 vs. WT for necroinflammatory changes. *p<0.05 and **p<0.01 vs. control for serum aminotransferases.
The number of apoptotic hepatocytes was similar in the WT than in gp91phox−/− mice after CCl4 administration. The values from 32 fields (x100) examined were 1.2 ± 0.5 and 1.2 ± 0.4 apoptotic cells per field for WT and gp91phox−/− controls respectively and 12.4 ± 1.7 and 8.9 ± 1.5 for WT and gp91phox−/− after CCl4 administration. Caspase 3 was increased in the gp91phox−/− mice but not the WT mice, while caspase 8 was unchanged in both groups of mice (Fig. 9A) Cytosolic cytochrome c was increased, while mitochondrial cytochrome c was decreased, after CCl4 in the WT mice (p<0.05), while no significant changes occurred in the gp91phox−/− (Fig. 9B). There was a mean 31% decrease in mitochondrial ATP after CCl4 in the WT mice, and an opposite 34% increase in the gp91phox−/− , however these changes were not statistically significant. The values for 8 mice in each group were 16.4 ± 3.9 and 11.3 ± 1.7 nmoles/mg protein for WT mice, compared to 9.5 ± 1.8 and 12.7 ± 2.6 nmoles/mg protein for gp91phox−/− before and after CCl4 respectively.
Fig. 9.
Effects of one week of chronic CCl4 administration on A. Activities of caspase 3 and 8 and (B) on cytosolic and mitochondrial cytochrome c in WT and gp91phox−/− mice. Cytochrome c was determined by western blot and quantitated by densitometry. The values are expressed as means ± SE of of 8 measurements per group. * p< 0.05 vs. respective control.
The increase in α1(I) collagen mRNA after CCl4 administration was greater in the gp91phox−/− than in the WT mice (p<0.05) (Fig.10 ) and TGFβ mRNA was increased in the gp91phox−/− but not in the WT mice (p<0.05) (Fig.10). MMP-2 mRNA and in MMP-9 mRNA increased after CCl4 in the gp91phox−/− but not in the WT mice. TIMP-1 mRNA increased to as greater extent in the gp91phox−/− than in the WT mice (Table 1). MMP-2 and MMP-9 proteins were not changed significantly after 1 week of CCl4 in either the WT or the gp91phox−/− mice (Fig.11). TIMP-1 protein decreased after CCl4 in the gp91phox−/− mice but not in the WT mice, while TIMP2 decreased in the WT mice remaining unchanged in the gp91phox−/− mice (Fig. 11).
Table 1.
Effects of carbon tetrachloride (CCl4) administration for one week on matrix metalloproteinase-2 (MMP-2) mRNA, matrix metalloproteinase-8 (MMP-9) mRNA and tissue inhibitor of metalloproteinase 1 (TIMP-1) mRNA
| mRNA | WT | gp91phox−/− | ||
|---|---|---|---|---|
| Control | CCl4 | Control | CCl4 | |
| MMP-2 | 100 ± 6.2 | 131 ± 3.7 | 107 ± 9.0 | 244 ± 45.1** |
| MMP-9 | 100 ± 8.3 | 148 ± 30.0 | 111 ± 9.6 | 198 ± 29.2* |
| TIMP-1 | 100 ± 12.1 | 33917 ± 77.6 | 124 ± 22.9 | 46469 ± 909**+ |
The mRNAs were determined by real time quantitative PCR. The relative amounts of mRNA were normalized against β-actin in the same samples and expressed as a % of WT control for each determination. The values are expressed as means ± SE of 8 determinations per group.
p<0.05 vs. control.
p<0.01 vs. control.
p<0.05 vs. WT + CCl4.
Fig. 11.
Effects of one week of CCl4 administration on matrix metalloproteinase 2 (MMP-2), 9 (MMP-9), tissue inhibitor of metalloproteinase 1 (TIMP-1) and TIMP-2 in WT and gp91phox−/− mice determined by western blot. * p< 0.05 vs. respective control.
DISCUSSION
The development of less hepatic fibrosis after chronic CCl4 administration in the gp91phox−/− mice than in WT mice in this study was associated with greater hepatocellular necrosis and inflammation but with decreased hepatocyte apoptosis. The higher serum AST in the gp91phox−/− mice, which correlated with the greater hepatocellular necrosis and inflammation, is consistent with enhanced mitochondrial injury, since approximately 80% of the AST is found in mitochondria, while ALT is found in the cytosol (17). Our results of greater hepatocellular necrosis and inflammation in the gp91phox−/− mice differ from the findings of other studies using different means to produce liver injury and/or fibrosis. Steatosis, inflammation and necrosis were observed in WT, but not in NAD(P)H oxidase deficient mice given chronic ethanol administration (18), however the effect on fibrosis was not assessed in that study because no significant fibrosis occurred. Our study also shows that the short term administration of CCl4 ,which does not result in significant increase in fibrosis, results in greater hepatocellular necrosis and higher serum ALT in the WT than in the gp91phox−/− mice.
The development of less hepatic fibrosis after bile duct ligation in mice with deficiency of the p47phox subunit of NAD(P)H oxidase complex than in WT mice was associated with lower elevations of serum AST, and less necrosis around the biliary tracts (7). Hepatocyte apoptosis, which was not determined in the above study, has since been demonstrated to be critical for the development of liver inflammation and fibrosis after bile duct ligation (8). In another study, gp91phox−/− and WT mice fed a methionine and choline deficient diet developed similar amounts of necroinflammatory change and fibrosis (10). The mechanism for the decreased apoptosis after CCl4 in the gp91phox−/− mice is most likely due to a decrease in the mitochondrial pathway of apoptosis. The pro-apoptotic protein BAX protein, which was increased in the WT mice, remained unchanged in the gp91phox−/− mice, while cytosolic cytochrome c, which is released from the mitochondria during apoptosis was decreased after CCl4 in the gp91phox−/− mice. Of note is that one week after CCl4 administration there was a marked increase in cytosolic cytochrome c in association with a decrease in mitochondrial cytochrome c in the WT but not in the gp91phox−/− mice CCl4. These changes are most likely a reflection of the more severe hepatocellular injury in the WT mice. The increase in cytosolic cytochrome probably predisposes to increased apoptosis in the WT mice, although no differences in apoptosis were observed between WT and gp91phox−/− mice at one week. The Fas (CD95/APO-1) receptor which mediates apoptosis caused by a variety of insults (19), principally via the extrinsic death receptor pathway, was not changed significantly by chronic CCl4 administration in either the WT or the gp91phox−/− mice. Activated caspases 3 and 8, are also essential in the extrinsic death receptor pathway of apoptosis. Caspase 3 was increased to as greater extent in the gp91phox−/− mice than in the WT mice after CCl4 administration, while the increases in Caspase 8 were similar after CCl4 in both groups of mice. An increase in caspase 3 in the gp91phox−/− but not the WT mice was already evident after one week of CCl4 administration. The significance of the greater increase in caspase 3 in the gp91phox−/− mice than in the WT mice, in relation to the lesser increase in apoptosis after CCl4 in the gp91phox−/− mice, is unknown.
The decrease in apoptosis in the gp91phox−/− mice as compared to the WT mice after chronic CCl4 is most likely due to decreased ROS generation. Previous studies have shown that TGFβ induced apoptosis of fetal rat hepatocytes requires ROS generation, mitochondrial permeability transition with cytochome c release, and caspase activation (20). Furthermore, blockage of the TGFβ induced increase in ROS by DPI, which inhibits NAD(P)H oxidase and other flavoproteins, abrogated the effect of TGFβ on increasing apoptosis (18). Also, relating to the role of NAD(P)H oxidase, it has been observed that neutrophils exposed to stress stimuli from patients with chronic granulomatous disease, which are deficient in NAD(P)H oxidase, show decreased apoptosis as compared to neutrophis from normal subjects (21). Furthermore, apoptosis induced acutely by bile salt in cultured rat hepatocytes was blunted by inhibition of NAD(P)H oxidase (22). Increased apoptosis of hepatocytes with phagocytosis of apoptotic bodies by Kuppfer and stellate cells has been linked to fibrogenesis (6). On the other hand, apoptosis of stellate cells was shown to contribute to the resolution of fibrosis after discontinuation of chronic CCl4 administration in rats (23). In our study, apoptosis was not demonstrated in stellate cells from either gp91phox−/− or WT mice after CCl4 administration.
As relates to the mechanism for the decreased hepatic fibrosis after chronic CCl4 administration in the gp91phox−/− as compared to the WT mice, changes in both collagen formation and degradation were considered. The greater increase in the α1(I) collagen mRNA in the gp91phox−/− mice than in the WT mice, found after 1 and 8 weeks of CCl4 administration, indicates that NAD(P)H deficiency does not decrease collagen formation.. Furthermore, TGFβ mRNA was also higher in the gp91phox−/− mice than in the WT mice. This observation agrees with prior findings indicating that TGFβ transcription is negatively regulated by NAD(P)H oxidase mediated oxygen radicals (24). In our study, however, the greater increase in TGFβ mRNA in the gp91phox−/− mice than in the WT mice was not accompanied by changes in TGFβ protein. Post-translational changes, such as feedback inhibition by procollagen propeptides, may be a factor for the lower accumulation of collagen in the gp91phox−/− mice after CCl4 in the setting of increased α1(I) collagen mRNA. However, while this was shown in cultured stellate cells from normal rats, it was not found in stellate cells isolated from fibrotic livers (25). It is well established that ROS activate stellate cells and stimulate fibrogenesis (1). In our study the lesser accumulation in malondialdehyde, a product of lipid peroxidation, after CCl4 in the gp91phox−/− was associated with lower collagen deposition, indicating lower but not absent ROS formation from other sources most likely the mitochondria. It is well known that chronic CCl4 administration impairs the mitochondrial electron transport chain and that this is associated with increased formation of ROS (26–28). There is evidence in this study that increased collagen degradation contributes to the lesser accumulation of hepatic fibrosis in gp91phox−/− mice. Increased collagen formation from CCl4 administration (29, 30) and from other insults (31) is associated with an increase in collagen degradation. Indeed, in this study, the increases in MMP-2 mRNA and MMP-9 mRNA were greater after one and 8 weeks of CCl4 in the gp91phox−/− than in the WT mice. However, this was not accompanied by higher MMP-2 and MMP-9 protein levels. More importantly, TIMP-1 and TIMP-2 proteins increased after chronic CCl4 administration in the WT mice but not in the gp91phox−/− mice. TIMPs inhibit secretion and activation of metalloproteinases, hence inhibiting collagen degradation (32). In conclusion, this study shows decreased hepatic fibrosis after chronic CCl4 administration in NAD(P)H oxidase deficient mice as compared to WT mice in association with greater hepatocellular necrosis and inflammation but decreased hepatocyte apoptosis. The lower hepatic fibrosis was found in the setting of a lower number of stellate cells, a greater increase in α1(I) collagen mRNA, but a lack of an increase in TIMP in the NAD(P)H deficient mice as compared to the WT mice, suggesting that NAD(P)H deficient mice have enhanced degradation of the formed collagen.
Acknowledgment
Financial support
This work was supported by Grant AA000626 from the United States Public Health Service. G.A. is a postdoctoral fellow on National Research Service Award 2 T32 AA07467 from the National Institute of Alcohol Abuse and Alcoholism (NIH). The authors acknowledge assistance from The Hopkins Digestive Diseases Basic Research Development Center (NIH grant 2464388) in the performance of this study.
Abbreviations
- ROS
reactive oxygen species
- CCl4
carbon tetrachloride
- WT
wild type
- gp91phox−/−
glycoprotein subunit 91 NADPH oxidase deficient mice
- AST
aspartate aminotransferase
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor metalloproteinase
- PDGF
platelet derived growth factor
- DPI
diphenylene iodonium
- PBS
phosphate buffered saline
- FBS
fetal bovine serum
- H&E
hematoxylin-eosin
- α-SMA̤
α -smooth muscle actin
- ALT
alanine aminotransferase
- RT-qPCR
real time quantitative polymerase chain reaction
- TGF-β
transforming growth factor-β̣
- PMSF
phenylmethanesulfonyl fluoride
- TUNEL
terminal deoxynucleotidyl transferase-mediated in situ end labeling
Contributor Information
Ghazaleh Aram, Email: ghazaleharam@hotmail.com.
James J. Potter, Email: jpotter@jhmi.edu.
Xiaopu Liu, Email: xliu32@jhmi.edu.
Michael S. Torbenson, Email: mtorben@jhmi.edu.
Esteban Mezey, Email: emezey@jhmi.edu.
REFERENCES
- 1.Nieto N, Friedman SL, Greenwel P, Cederbaum AI. CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells. Hepatology. 1999;30:987–996. doi: 10.1002/hep.510300433. [DOI] [PubMed] [Google Scholar]
- 2.Chojkier M, Houglum K, Solis-Herruzo J, Brenner DA. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. A role for lipid peroxidation? J Biol Chem. 1989;264:16957–16962. [PubMed] [Google Scholar]
- 3.Maher JJ, Tzagarakis C, Gimenez A. Malondialdehyde stimulates collagen production by hepatic lipocytes only upon activation in primary culture. Alcohol Alcohol. 1994;29:605–610. [PubMed] [Google Scholar]
- 4.Adachi T, Togashi H, Suzuki A, Kasai S, Ito I, Sugahara K, et al. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272–1281. doi: 10.1002/hep.20719. [DOI] [PubMed] [Google Scholar]
- 5.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 6.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: A tale of two deaths? Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canbay A, Feldstein A, Baskin-Bey E, Bonk SF, Gores GJ. The caspase inhibitor IND-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 9.Novitskiy G, Potter JJ, Wang L, Mezey E. Influences of reactive oxygen species and nitric oxide on hepatic fibrogenesis. Liver Int. 2006;26:1248–1257. doi: 10.1111/j.1478-3231.2006.01364.x. [DOI] [PubMed] [Google Scholar]
- 10.dela Pena A, Leclercq IA, Williams J, Farrell GC. NADPH oxidase is not an essential mediator of oxidative stress or liver injury in murine MCD diet-induced steatohepatitis. J Hepatol. 2007;46:304–313. doi: 10.1016/j.jhep.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Potter JJ, Rennie-Tankersley L, Mezey E. Influence of leptin in the development of hepatic fibrosis produced in mice by Schistosoma mansoni infection and by chronic carbon tetrachloride administration. J Hepatol. 2003;38:281–288. doi: 10.1016/s0168-8278(02)00414-2. [DOI] [PubMed] [Google Scholar]
- 12.Bergmeyer HU, Scheibe P, Wahlefeld AW. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin Chem. 1978;24:58–73. [PubMed] [Google Scholar]
- 13.Reed WD, Mezey E. Effects of chronic ethanol feeding on enzymes of rat brain and liver mitochondria. Life Sci. 1972;11:847–857. doi: 10.1016/0024-3205(72)90169-5. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Uchiyama M, Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 16.Frankfurt OS, Krishan A. Identification of apoptotic cells by formamide-induced DNA denaturation in condensed chromatin. J Histochem Cytochem. 2001;49:369–378. doi: 10.1177/002215540104900311. [DOI] [PubMed] [Google Scholar]
- 17.Rej R. Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin Chem. 1978;24:1971–1979. [PubMed] [Google Scholar]
- 18.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, et al. NADPH oxidase–derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera B, Murillo MM, Alvarez-Barrientos A, Beltran J, Fernandez M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor- β in fetal rat hepatocytes. Free Radic Biol Med. 2004;36:16–26. doi: 10.1016/j.freeradbiomed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Gardai S, Whitlock BB, Helgason C, Ambruso D, Fadok V, Bratton D, et al. Activation of SHIP by NADPH oxidase-stimulated Lyn leads to enhanced apoptosis in neutrophils. J Biol Chem. 2002;277:5236–5246. doi: 10.1074/jbc.M110005200. [DOI] [PubMed] [Google Scholar]
- 22.Reinehr R, Becker S, Keitel V, Eberle A, Grether-Beck S, Haussinger D. Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology. 2005;129:2009–2031. doi: 10.1053/j.gastro.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf G, Hannken T, Schroeder R, Zahner G, Ziyadeh FN, Stahl RAK. Antioxidant treatment induces transcription and expression of transforming growth factor β in cultured renal proximal tubular cells. FEBS Lett. 2001;488:154–159. doi: 10.1016/s0014-5793(00)02403-0. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda H, Wu GY, Wu CH. Lipocytes from fibrotic rat liver have an impaired feedback response to. procollagen propeptides. Am J Physiol Gastrointest. Liver Physiol. 1993;264:G157–G162. doi: 10.1152/ajpgi.1993.264.1.G157. [DOI] [PubMed] [Google Scholar]
- 26.Krahenbuhl L, Ledermann M, Lang C, Krahenbuhl S. Relationship between hepatic mitochondrial functions in vivo and in vitro in rats after carbon tetrachloride-induced liver cirrhosis. J. Hepatol. 2000;33:216–1223. doi: 10.1016/s0168-8278(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 27.Wang CY, Ma FL, Liu JT, Tian JW, Fu FH. Protective effect of salvianic acid A on acute liver injury induced by carbon tetrachloride in rats. Biol Pharm Bull. 2007;30:44–47. doi: 10.1248/bpb.30.44. [DOI] [PubMed] [Google Scholar]
- 28.Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez-Munoz R, Diaz-Munoz M, Suarez-Cuenca JA, Trejo-Solis C, Lopez V, Sanchez-Sevilla L, et al. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic actrivity and stimulating hepatocyte cell proliferation in rats. Hepatology. 2001;34:677–687. doi: 10.1053/jhep.2001.27949. [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Hovell CJ, Pawley S, Hutchings MI, Arthur MJP, Iredale JP, et al. Expression of matrix metalloproteinase-2 and -14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004;24:492–501. doi: 10.1111/j.1478-3231.2004.0946.x. [DOI] [PubMed] [Google Scholar]
- 31.Mezey E, Potter JJ, Slusser RJ, Abdi W. Changes in hepatic collagen metabolism in rats produced by chronic ethanol feeding. Lab Invest. 1977;36:206–214. [PubMed] [Google Scholar]
- 32.Benyon RC, Arthur MJP. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373–384. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]