Abstract
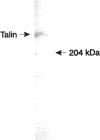
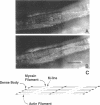
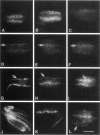
In cultured cells, the 230-kDa protein talin is found at discrete plasma membrane foci known as focal adhesions, sites that anchor the intracellular actin cytoskeleton to the extracellular matrix. The regulated assembly of focal adhesions influences the direction of cell migrations or the reorientation of cell shapes. Biochemical studies of talin have shown that it binds to the proteins integrin, vinculin, and actin in vitro. To understand the function of talin in vivo and to correlate its in vitro and in vivo biochemical properties, various genetic approaches have been adopted. With the intention of using genetics in the study of talin, we identified a homologue to mouse talin in a genetic model system, the nematode Caenorhabditis elegans. C. elegans talin is 39% identical and 59% similar to mouse talin. In wild-type adult C. elegans, talin colocalizes with integrin, vinculin, and alpha-actinin in the focal adhesion-like structures found in the body-wall muscle. By examining the organization of talin in two different C. elegans mutant strains that do not make either beta-integrin or vinculin, we were able to determine that talin does not require vinculin for its initial organization at the membrane, but that it depends critically on the presence of integrin for its initial assembly at membrane foci.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstead R. J., Kleiman L., Waterston R. H. Cloning, sequencing, and mapping of an alpha-actinin gene from the nematode Caenorhabditis elegans. Cell Motil Cytoskeleton. 1991;20(1):69–78. doi: 10.1002/cm.970200108. [DOI] [PubMed] [Google Scholar]
- Barstead R. J., Waterston R. H. The basal component of the nematode dense-body is vinculin. J Biol Chem. 1989 Jun 15;264(17):10177–10185. [PubMed] [Google Scholar]
- Barstead R. J., Waterston R. H. Vinculin is essential for muscle function in the nematode. J Cell Biol. 1991 Aug;114(4):715–724. doi: 10.1083/jcb.114.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle M. C., Miller D. E., Bertagnolli M. E., Locke S. J. Activation-dependent redistribution of the adhesion plaque protein, talin, in intact human platelets. J Cell Biol. 1989 Dec;109(6 Pt 2):3333–3346. doi: 10.1083/jcb.109.6.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle M. C. The adhesion plaque protein, talin, is phosphorylated in vivo in chicken embryo fibroblasts exposed to a tumor-promoting phorbol ester. Cell Regul. 1990 Jan;1(2):227–236. doi: 10.1091/mbc.1.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands R., de Boer A., Feltkamp C. A., Roos E. Disintegration of adhesion plaques in chicken embryo fibroblasts upon Rous sarcoma virus-induced transformation: different dissociation rates for talin and vinculin. Exp Cell Res. 1990 Jan;186(1):138–148. doi: 10.1016/0014-4827(90)90220-5. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Integrin, a transmembrane glycoprotein complex mediating cell-substratum adhesion. J Cell Sci Suppl. 1987;8:231–250. doi: 10.1242/jcs.1987.supplement_8.13. [DOI] [PubMed] [Google Scholar]
- Burn P., Burger M. M. The cytoskeletal protein vinculin contains transformation-sensitive, covalently bound lipid. Science. 1987 Jan 23;235(4787):476–479. doi: 10.1126/science.3099391. [DOI] [PubMed] [Google Scholar]
- Burn P., Kupfer A., Singer S. J. Dynamic membrane-cytoskeletal interactions: specific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 1983;3(5-6):405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- Burridge K., Mangeat P. An interaction between vinculin and talin. Nature. 1984 Apr 19;308(5961):744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Appeddu P. A., Parsons J. T., Hildebrand J. D., Schaller M. D., Guan J. L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995 Jul 14;270(28):16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Phosphorylation of talin at tyrosine in Rous sarcoma virus-transformed cells. Mol Cell Biol. 1987 Jan;7(1):371–378. doi: 10.1128/mcb.7.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale J. A., Izzard C. S. Accumulation of talin in nodes at the edge of the lamellipodium and separate incorporation into adhesion plaques at focal contacts in fibroblasts. J Cell Biol. 1991 Jun;113(6):1351–1359. doi: 10.1083/jcb.113.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J. L., Thiery J. P. Spatial and temporal distribution of vinculin and talin in migrating avian neural crest cells and their derivatives. Development. 1990 Mar;108(3):421–433. doi: 10.1242/dev.108.3.421. [DOI] [PubMed] [Google Scholar]
- Epstein H. F., Casey D. L., Ortiz I. Myosin and paramyosin of Caenorhabditis elegans embryos assemble into nascent structures distinct from thick filaments and multi-filament assemblages. J Cell Biol. 1993 Aug;122(4):845–858. doi: 10.1083/jcb.122.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Ashman L. K. The use of alkaline phosphatase-conjugated anti-immunoglobulin with immunoblots for determining the specificity of monoclonal antibodies to protein mixtures. Methods Enzymol. 1986;121:497–509. doi: 10.1016/0076-6879(86)21050-2. [DOI] [PubMed] [Google Scholar]
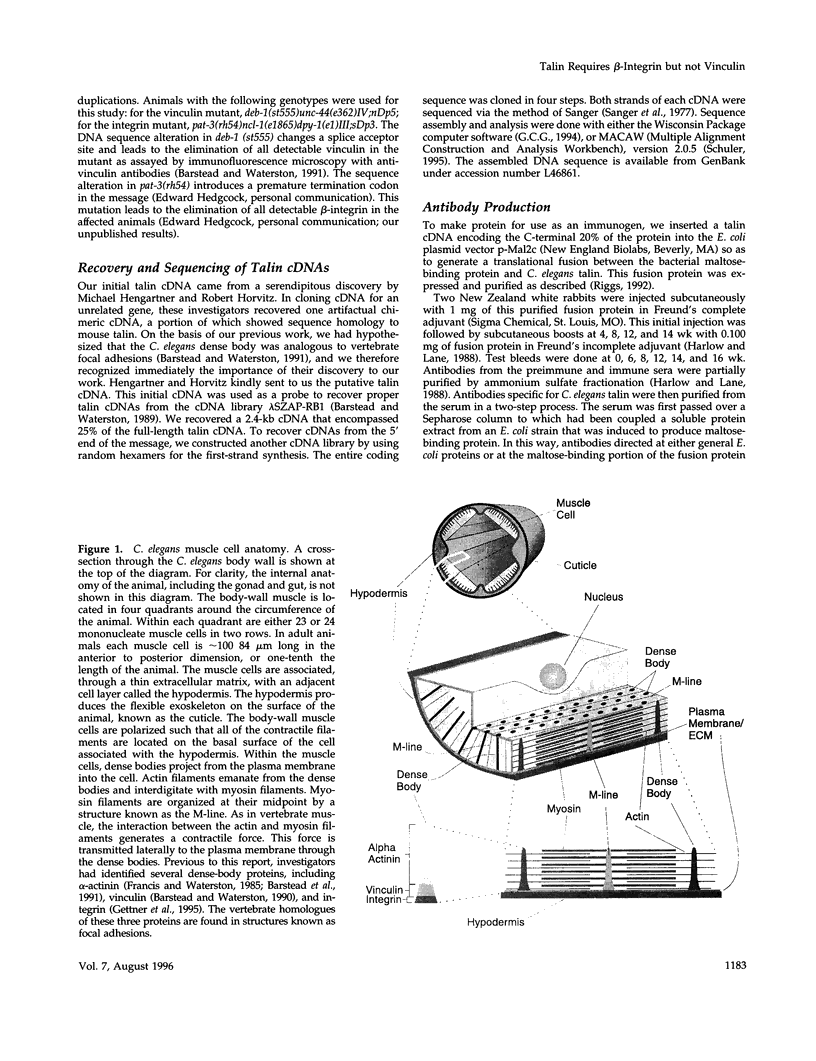
- Francis G. R., Waterston R. H. Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J Cell Biol. 1985 Oct;101(4):1532–1549. doi: 10.1083/jcb.101.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B. Microheterogeneity of avian and mammalian vinculin distinctive subcellular distribution of different isovinculins. J Mol Biol. 1982 Aug 25;159(4):685–701. doi: 10.1016/0022-2836(82)90108-5. [DOI] [PubMed] [Google Scholar]
- Gettner S. N., Kenyon C., Reichardt L. F. Characterization of beta pat-3 heterodimers, a family of essential integrin receptors in C. elegans. J Cell Biol. 1995 May;129(4):1127–1141. doi: 10.1083/jcb.129.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. P., Wood C., Ohanian V., Jackson P., Patel B., Rees D. J., Hynes R. O., Critchley D. R. The cytoskeletal protein talin contains at least two distinct vinculin binding domains. J Cell Biol. 1993 Jul;122(2):337–347. doi: 10.1083/jcb.122.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann J., Grob M., Burger M. M. The cytoskeletal protein talin is O-glycosylated. J Biol Chem. 1992 Jul 15;267(20):14424–14428. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hresko M. C., Williams B. D., Waterston R. H. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J Cell Biol. 1994 Feb;124(4):491–506. doi: 10.1083/jcb.124.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. P., Craig S. W. An intramolecular association between the head and tail domains of vinculin modulates talin binding. J Biol Chem. 1994 Apr 29;269(17):12611–12619. [PubMed] [Google Scholar]
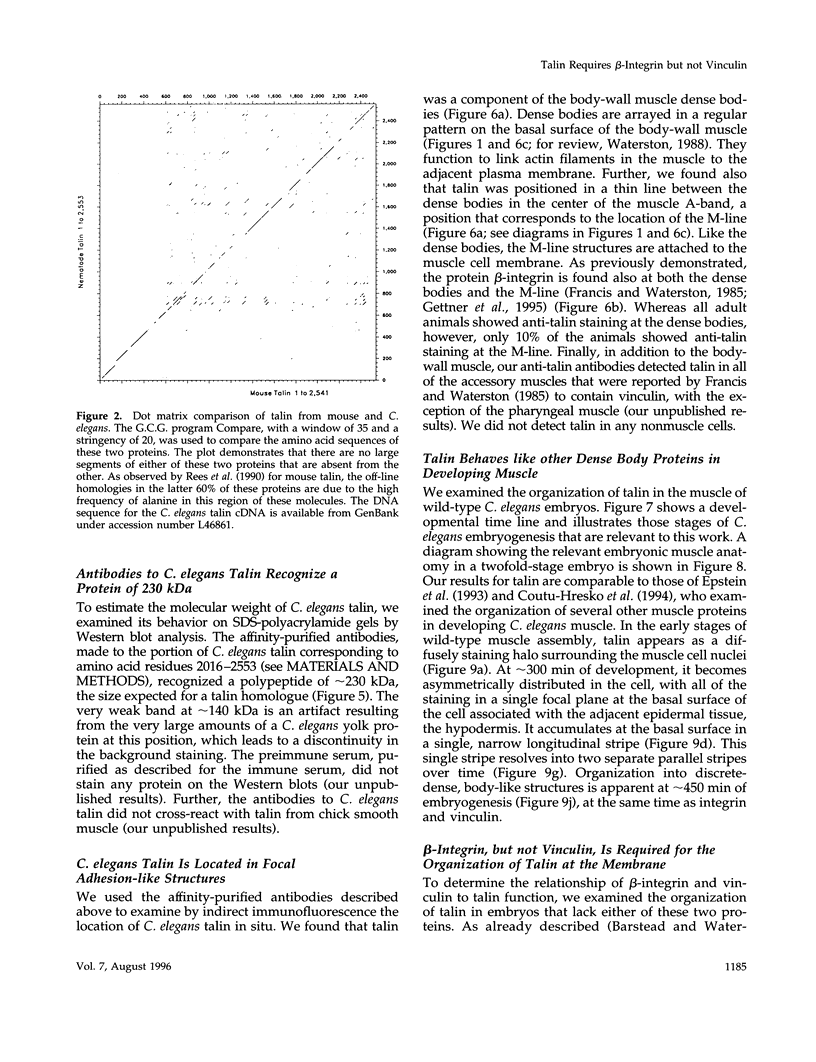
- Johnson R. P., Craig S. W. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995 Jan 19;373(6511):261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S., Piekenbrock T., Goldmann W. H., Bärmann M., Isenberg G. Talin binds to actin and promotes filament nucleation. FEBS Lett. 1991 Jun 24;284(2):187–191. doi: 10.1016/0014-5793(91)80681-r. [DOI] [PubMed] [Google Scholar]
- Kellie S., Wigglesworth N. M. The cytoskeletal protein vinculin is acylated by myristic acid. FEBS Lett. 1987 Mar 23;213(2):428–432. doi: 10.1016/0014-5793(87)81536-3. [DOI] [PubMed] [Google Scholar]
- Kondo K., Makovec B., Waterston R. H., Hodgkin J. Genetic and molecular analysis of eight tRNA(Trp) amber suppressors in Caenorhabditis elegans. J Mol Biol. 1990 Sep 5;215(1):7–19. doi: 10.1016/S0022-2836(05)80090-7. [DOI] [PubMed] [Google Scholar]
- Kreitmeier M., Gerisch G., Heizer C., Müller-Taubenberger A. A talin homologue of Dictyostelium rapidly assembles at the leading edge of cells in response to chemoattractant. J Cell Biol. 1995 Apr;129(1):179–188. doi: 10.1083/jcb.129.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Burn P., Singer S. J. The PMA-induced specific association of LFA-1 and talin in intact cloned T helper cells. J Mol Cell Immunol. 1990;4(6):317–325. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Wulfkuhle J. D., Otto J. J. Vinculin binding site mapped on talin with an anti-idiotypic antibody. J Biol Chem. 1992 Aug 15;267(23):16355–16358. [PubMed] [Google Scholar]
- Menkel A. R., Kroemker M., Bubeck P., Ronsiek M., Nikolai G., Jockusch B. M. Characterization of an F-actin-binding domain in the cytoskeletal protein vinculin. J Cell Biol. 1994 Sep;126(5):1231–1240. doi: 10.1083/jcb.126.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
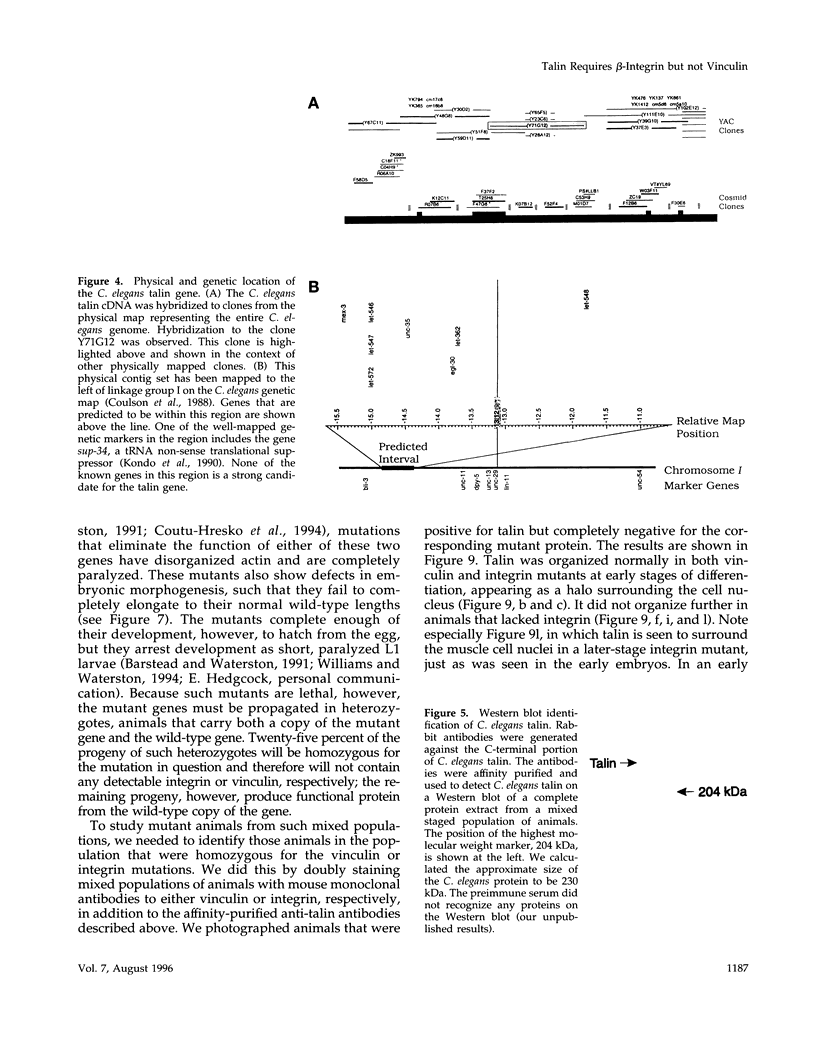
- Moerman D. G., Benian G. M., Barstead R. J., Schriefer L. A., Waterston R. H. Identification and intracellular localization of the unc-22 gene product of Caenorhabditis elegans. Genes Dev. 1988 Jan;2(1):93–105. doi: 10.1101/gad.2.1.93. [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Kelly T., Dai M. Z., Dai H. N., Chen W. T. Dynamic cytoskeleton-integrin associations induced by cell binding to immobilized fibronectin. J Cell Biol. 1989 Dec;109(6 Pt 2):3455–3464. doi: 10.1083/jcb.109.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma M., Matsumura S., Fukazawa T. Direct interactions between talin and actin. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1217–1223. doi: 10.1016/0006-291x(90)90815-5. [DOI] [PubMed] [Google Scholar]
- Niggli V., Gimona M. Evidence for a ternary interaction between alpha-actinin, (meta)vinculin and acidic-phospholipid bilayers. Eur J Biochem. 1993 May 1;213(3):1009–1015. doi: 10.1111/j.1432-1033.1993.tb17848.x. [DOI] [PubMed] [Google Scholar]
- Nuckolls G. H., Turner C. E., Burridge K. Functional studies of the domains of talin. J Cell Biol. 1990 May;110(5):1635–1644. doi: 10.1083/jcb.110.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. Talin is phosphorylated on tyrosine in chicken embryo fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5507–5511. doi: 10.1073/pnas.83.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qwarnström E. E., MacFarlane S. A., Page R. C., Dower S. K. Interleukin 1 beta induces rapid phosphorylation and redistribution of talin: a possible mechanism for modulation of fibroblast focal adhesion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1232–1236. doi: 10.1073/pnas.88.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
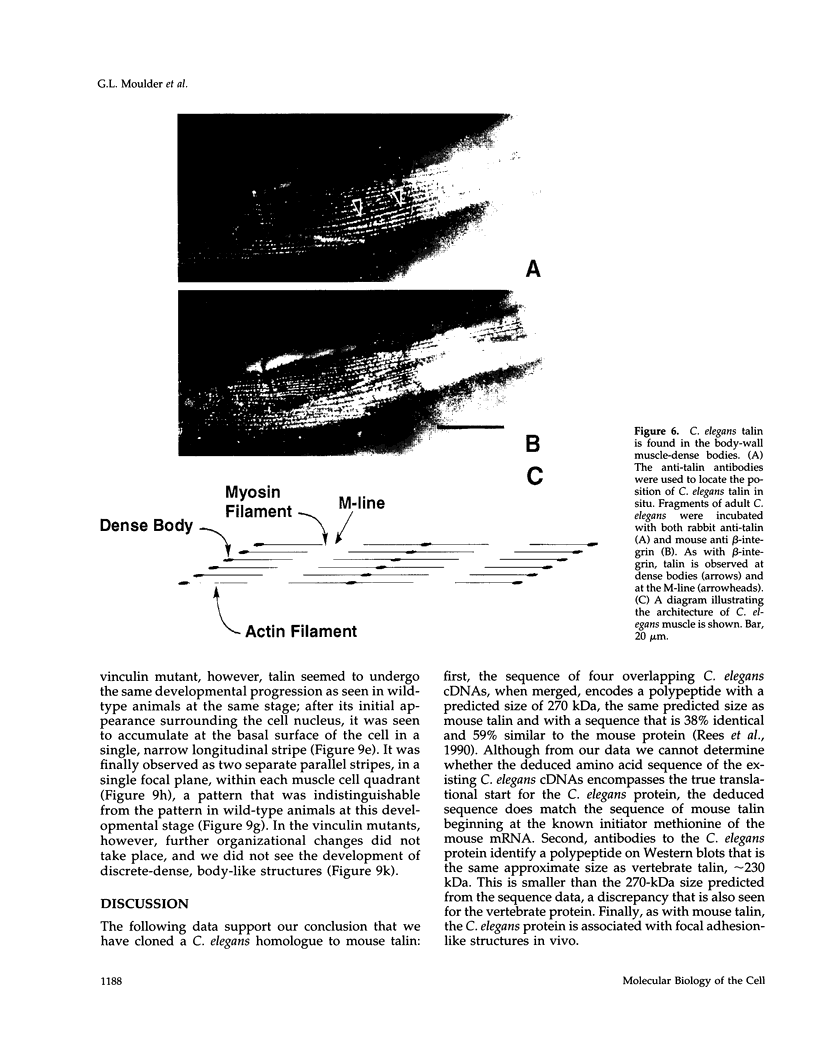
- Rees D. J., Ades S. E., Singer S. J., Hynes R. O. Sequence and domain structure of talin. Nature. 1990 Oct 18;347(6294):685–689. doi: 10.1038/347685a0. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Ultrastructural organization of obliquely striated muscle fibers in Ascaris lumbricoides. J Cell Biol. 1965 Jun;25(3):495–515. doi: 10.1083/jcb.25.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley P., Horwitz A., Buck C., Duggan K., Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989 Mar;4(3):325–333. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. E., Pavalko F. M., Burridge K. The role of phosphorylation and limited proteolytic cleavage of talin and vinculin in the disruption of focal adhesion integrity. J Biol Chem. 1989 Jul 15;264(20):11938–11944. [PubMed] [Google Scholar]
- Volberg T., Sabanay H., Geiger B. Spatial and temporal relationships between vinculin and talin in the developing chicken gizzard smooth muscle. Differentiation. 1986;32(1):34–43. doi: 10.1111/j.1432-0436.1986.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Wachsstock D. H., Wilkins J. A., Lin S. Specific interaction of vinculin with alpha-actinin. Biochem Biophys Res Commun. 1987 Jul 31;146(2):554–560. doi: 10.1016/0006-291x(87)90564-x. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Waterston R. H. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J Cell Biol. 1994 Feb;124(4):475–490. doi: 10.1083/jcb.124.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]