Abstract
We used noninvasive MRI and voxel-based morphometry (VBM) to detect changes in brain structure in three adult Japanese macaques trained to use a rake to retrieve food rewards. Monkeys, who were naive to any previous tool use, were scanned repeatedly in a 4-T scanner over 6 weeks, comprising 2 weeks of habituation followed by 2 weeks of intensive daily training and a 2-week posttraining period. VBM analysis revealed significant increases in gray matter with rake performance across the three monkeys. The effects were most significant (P < 0.05 corrected for multiple comparisons across the whole brain) in the right superior temporal sulcus, right second somatosensory area, and right intraparietal sulcus, with less significant effects (P < 0.001 uncorrected) in these same regions of the left hemisphere. Bilateral increases were also observed in the white matter of the cerebellar hemisphere in lobule 5. In two of the monkeys who exhibited rapid learning of the rake task, gray matter volume in peak voxels increased by up to 17% during the intensive training period; the earliest changes were seen after 1 week of intensive training, and they generally peaked when performance on the task plateaued. In the third monkey, who was slower to learn the task, peak voxels showed no systematic changes. Thus, VBM can detect significant brain changes in individual trained monkeys exposed to tool-use training for the first time. This approach could open up a means of investigating the underlying neurobiology of motor learning and other higher brain functions in individual animals.
Keywords: intraparietal sulcus, second somatosensory area, superior temporal sulcus, voxel-based morphometry
The brain exhibits use-dependent structural flexibility, which is far greater than realized previously and which is detectable even at a macroscopic level and in adulthood. Structural MRI studies of the human brain have demonstrated differences in the hippocampus of experienced London taxi drivers (1), a relationship between musical proficiency and the volume of motor and auditory cortex (2), enlarged prefrontal and parietal areas in mathematicians (3), and increased inferior parietal gray matter density in adolescents with enriched vocabulary knowledge (4). There is also an extensive literature on the effect of experience-driven plasticity in animals (see refs. 5–7).
In humans, rapid changes in gray matter after the acquisition of a new motor skill were demonstrated by Draganski et al. (8): after 3 months of learning to juggle, gray matter increases were observed in the extrastriate motion area and the posterior intraparietal sulcus. These changes were detected with voxel-based morphometry (VBM) after pooling data from a large group of human subjects. The neurobiological underpinnings of structural brain changes associated with the acquisition of new skills remain unknown and could involve a wide variety of different neuronal mechanisms, including angiogenesis and even neurogenesis (9). Ultimately, invasive experiments in an animal model will be needed to investigate which mechanisms are responsible for structural brain changes associated with higher cognitive abilities and how, for example, these changes can be induced by the learning of a novel and demanding motor skill. A necessary first step would be the noninvasive demonstration of structural changes in specific brain areas that are associated with learning a skilled task by a nonhuman primate, with some insights into the time course of these changes. These are the main objectives of the present study.
We have focused on tool use by macaque monkeys. Tool use is defined as the manipulation of an object to change the position or form of another object (10). Although a variety of animals use tools, tool use is best developed in humans and some nonhuman primates. Macaque monkeys rarely use tools in the wild (11), but they are able to master tool use within a few weeks of training (12). Because normal adult monkeys have no experience of tool use but can be trained to use tools over a short period, it should be possible to use structural MRI to detect significant brain changes that occur during training in individual animals. This offers a significant advantage over pooling large datasets from different individuals, because the time course and strategy for learning a skilled motor task may vary from one individual to another (13, 14).
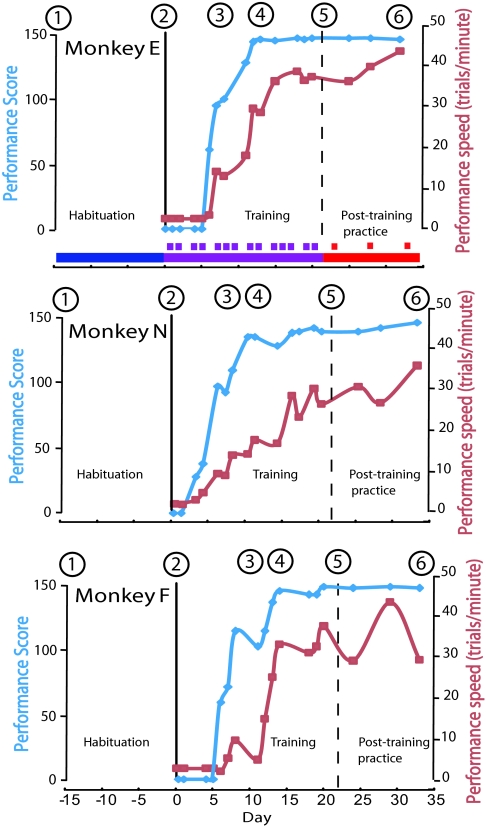
Here, we collected structural MRI images from three adult Japanese macaques before, during, and after they had acquired the new skill of using a rake to retrieve food (12). The monkeys were naive to the use of tools and had not been used in any previous experimental procedure. Fig. 1 shows the study design: after an initial habituation period (blue bar, days −14 to 0), each monkey received intensive daily training on the rake task (purple squares, days 1–21). They were trained to pick up the rake and swing it horizontally, placing the head of the rake behind a small food reward placed on a table out of the monkey's reach. The monkey then used the rake to pull the reward to within its reach for retrieval. Performance on the rake task improved rapidly during the training period, and then it reached a plateau. Brief testing sessions carried out during the 2-week posttraining period (red squares, days 22 to 33) confirmed that the monkeys had retained the newly acquired skill. By using the technique of VBM (15), we were able to detect changes in gray and white matter in the brains of individual monkeys, and we related these changes to the monkeys' performance as they acquired the skill of using the rake.
Fig. 1.
Schedule of the experiment and monkey performance on the rake task. Over an initial 2-week period, each monkey was habituated to the training room and contact with experimenters and was trained to reach out and take food rewards (blue bar, days −14 to 0). Thereafter, the intensive training period on the rake task began (purple bar, day 1, black vertical line). Training sessions were given throughout this period (purple squares), which finished around day 21 (vertical dashed line). The training period was followed by a posttraining period (red bar, days 22–33), during which monkeys were given a brief (30-min) testing session on 3 days (red squares). During each training and testing session, there was a timed test period of 50 trials used to quantify task performance (blue trace). Each trial was assigned a score based on whether the food was retrieved on the first attempt (score 3), second attempt (score 2), or after multiple attempts (score 1). Six MRI scanning sessions (sessions 1–6) were carried out on each monkey. Sessions 1 and 2 were at the beginning and end, respectively, of the habituation period; session 3 was after monkeys began to master the task; and sessions 4 and 5 were in the middle and at the end, respectively, of the training period. Scan 6 was at the end of posttraining period. Blue trace indicates each of three monkeys' performance scores on the 50 trial test period during training and in posttraining testing. Red line indicates each monkey's performance speed (no. of completed trials per minute of test period).
Results
Performance on the Tool-Use Task.
All three monkeys (monkeys E, N, and F) learned to use the rake within the 14-day training period. Monkeys E and F used their right hand to rake and their left hand to retrieve the food reward. Monkey N used both hands to both rake and retrieve the food. Their performance on the rake task was scored by documenting how many attempts were needed to retrieve a total of 50 successive food rewards presented to the monkey: when the food was retrieved on the first attempt, a score of 3 was given; a score of 2 was given if two attempts were needed; and a score of 1 was given if multiple attempts were required. The maximum possible score was thus 150 (3 × 50 trials). The monkeys' performance scores are plotted in Fig. 1 (blue line), in which each day is referenced to the beginning of the intensive training period (day 1). We also documented performance speed, which was given by the number of successful trials completed within a fixed time (1 min; Fig. 1, red).
All three monkeys achieved performance scores of 130 or above during the timed test period after 8–10 training days. Performance score values generally reached a plateau earlier in training than did performance speed, showing that the monkeys performed successful trials with increasing speed as training proceeded. Monkey N began using the rake in only the second training session, and both monkeys N and E showed a fast and continuous improvement in both performance score and speed; their scores plateaued after 10 and 12 days, respectively. Monkey F showed a slower and much more erratic pattern of learning the task than the other two monkeys (Fig. 1). His performance score did not plateau until day 14 (Fig. 1, blue line), and his trials per minute measure (Fig. 1, red trace) did not show much improvement until after the third scanning session.
By using digital video, we demonstrated that rake velocity increased significantly during training in monkeys N and E (P ≤ 0.001 and P ≤ 0.05, respectively), but in monkey F there were no significant increases over the entire training period (Fig. S1). Analysis within SPSS showed that in the training period, there was a significant positive correlation between performance score and rake velocity in both monkeys E and N (r = 0.69, P ≤ 0.01 and r = 0.70, P ≤ 0.01, respectively), but no correlation was found for monkey F (r = −0.33, P = 0.27). To assess features of the monkey's motor performance that were not directly related to tool use, we also measured the mean velocity of the movements made by the nonraking hand to collect food rewards after raking movements. This parameter showed no significant change in monkeys E and N during the training and posttraining period, whereas there was a significant change in monkey F (P ≤ 0.01; Fig. S1). The monkeys had extensive practice on the rake task. We estimated that over the training period, 27,000 successful trials were carried out by monkey E, 21,300 by monkey N, and 21,800 by monkey F. In comparison, monkeys carried out relatively few trials in the posttraining testing period (2,800, 2,300, and 2,400 in monkeys E, N, and F, respectively). In Fig. 1, training sessions are indicated by purple squares, and testing sessions are indicated by red squares.
Changes in Cortical Gray Matter Detected by MRI.
Structural MRI scans were obtained by using a 4-T Varian Unity Inova scanner. Six scanning sessions were carried out in each monkey (Fig. 1, numbered circles); monkeys were deeply anesthetized during scanning (see Methods). Two MRI sessions were in the habituation period and three during the intensive training period (at the beginning, in midtraining, and at the end of training); a final scan was carried out at the end of the posttraining testing period. To confirm the reproducibility of the results, three separate scans were carried out during each session (yielding 18 scans per monkey in all; see Methods). We found that the T1/T2* image provided the best contrast between gray and white matter (16, 17) (see Methods); images from the three different monkeys were spatially normalized by using a template based on gray matter MR images from 17 Japanese macaques and were used for the VBM analysis with SPM5 (15) (Wellcome Trust Centre for Neuroimaging, UCL Institute of Neurology, London; see Methods).
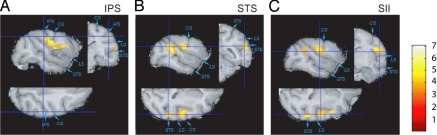
VBM analysis with SPM5 revealed significant increases in gray matter with performance score across the three monkeys (Fig. 2). SPM5 uses voxel-by-voxel t tests based on the general linear model. The performance score (Fig. 1, blue) used was that achieved by the monkey in the training session closest to the relevant MRI session (Fig. 1). Significant gray matter increases with performance score (P < 0.05 after correction for multiple comparisons across the whole brain at the voxel level) were found in the right superior temporal sulcus (STS) and right secondary somatosensory area (SII) in the upper bank of the lateral sulcus (Table 1). When the statistical threshold was lowered to P < 0.001, uncorrected for multiple comparisons, it became clear that the cluster of contiguous significant voxels in the right SII (Fig. 2C) expanded to include tissue around the intraparietal sulcus (IPS), and particularly anterior intraparietal area (AIP; Fig. 2A). Additionally, gray matter increases with performance were found in the left STS, left IPS, and left SII, which illustrates that the effects were clearly bilateral rather than right-lateralized (Table 1 and Fig. S2).
Fig. 2.
Gray matter increases with improvements in rake task performance. Areas where gray matter increased with performance score on the rake task (P < 0.001 uncorrected) in the group analysis (n = 3 monkeys), superimposed on a normalized T1/T2* scan (see Methods and Table 1 legend for further details). The color scale indicates the t score. (A) Increases in gray matter in right IPS, including AIP (right, x = 19; y = −19; z = 9; Z score = 3.89). (B) Increases in right STS (x = 23; y = −23; z = −1; Z score = 5.53). (C) Increases in right SII (x = 19; y = −14; z = 5; Z score = 5.20). CS, central sulcus; LS, lateral sulcus.
Table 1.
Areas of gray and white matter change in the group and individual analyses
| Region | Coordinates |
Group |
Individual Z scores monkey |
|||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Cluster size | Z score | E | F | N | |
| Gray matter region | ||||||||
| STS, right | 23 | −23 | −1 | 73* | 5.53* | 5.75* | N.S. | 4.88* |
| STS, left | −23 | −23 | 0 | 25 | 4.24† | 5.30* | N.S. | N.S. |
| SII, right | 19 | −14 | 5 | 133* | 5.20* | 3.00† | N.S. | 5.69* |
| IPS, right | 19 | −19 | 9 | 3.89† | 2.31 | N.S. | 3.20† | |
| SII, left | −18 | −15 | 5 | 105 | 3.78† | N.S. | N.S. | 4.28† |
| IPS, left | −19 | −20 | 10 | 4 | 4.19† | 3.97† | N.S. | 2.37† |
| White matter region | ||||||||
| Cerebellum, right | 10 | −27 | −14 | 38* | 5.75* | 5.25* | N.S. | 5.29* |
| Cerebullum, left | −12 | −26 | −13 | 98* | 6.15* | 5.04* | N.S. | 5.66* |
Table 1 indicates the regions of significant gray and white matter change with performance, identified in a group analysis across monkeys. Regions were first identified by using a statistical threshold of P < 0.05 after correction for multiple comparisons across the whole brain at the voxel level (indicated by *). We then lowered the threshold to P < 0.001 uncorrected (indicated by †) across the group to provide a fuller picture of the results; see Methods for details. Coordinates and number of contiguous voxels (cluster size) at P < 0.001 (uncorrected) are reported across all three monkeys. Within these regions, we report the Z scores for each monkey individually after lowering the threshold P < 0.05 uncorrected. N.S. indicates not significant even when the statistical threshold was lowered to P < 0.05 uncorrected.
*Significant at the voxel level after correction for multiple comparisons across the whole-brain level at P < 0.05.
†Significant at P < 0.001 uncorrected.
As shown in Table 1, the changes in the group analysis were primarily driven by monkeys E and N, and there were no significant changes for monkey F. The results of the VBM analysis with performance for individual monkeys are presented in Fig. S2, as are the results of VBM analysis using average rake velocity and food retrieval velocity (Fig. S2).
Time Course and Extent of Change in Peak Voxels.
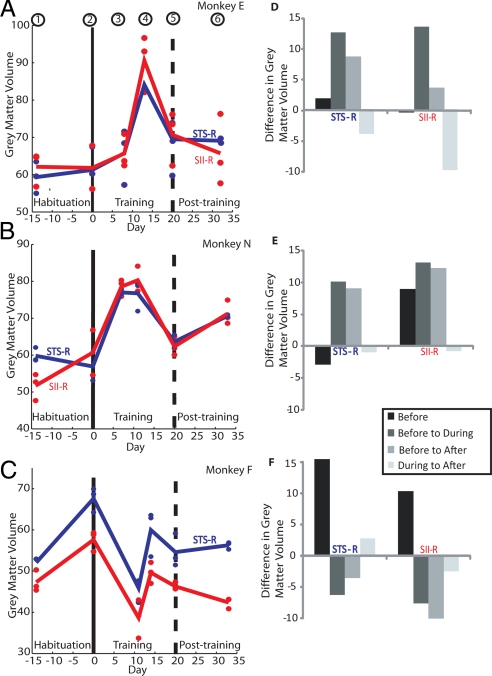
To illustrate in each monkey the extent and time course of the gray matter changes associated with intensive tool-use training, we plotted the mean gray matter volume for each time point for voxels within the right STS (blue) and right SII (red) regions that showed the most significant change in gray matter (Table 1). To be conservative, voxels were selected that showed little or no increase in the habituation period (sessions 1–2; Fig. 3 A–C). Corresponding plots for all other regions listed in Table 1 can be found in the Fig. S3. Fig. 3 A and B shows that in both monkeys E and N, there were increases in gray matter volume during the training period (scanning sessions 3–5) above those found in the habituation period (sessions 1 and 2). In monkey N, increases were seen after the seventh day of the training period. Interestingly, the increases in monkey N (Fig. 3B) occurred more rapidly than in monkey E (Fig. 3A), who first started using the rake 3 days later than monkey N (Fig. 1).
Fig. 3.
Time course and extent of gray matter change in peak voxels. (A–C) Gray matter volume for each time point in monkeys E (A), N (B), and F (C) for voxels in right STS (blue) and right SII (red), the cortical areas with most significant effects (Table 1). Circles indicate gray matter volume from each of the three scans acquired at each scanning session. Lines are drawn between mean values for each session (sessions 1–6). Only two scans were available for monkey N for session 2. (D–F) Changes in gray matter volume across scans in each monkey. “Before” indicates mean session 1 − mean session 2; “before to during,” mean (sessions 1 and 2) − mean (sessions 3, 4, and 5); “before to after,” mean (sessions 1 and 2) − mean session 6; and “during to after,” mean (sessions 3, 4, and 5) − mean session 6. In monkeys E (D) and N (E), there were increases in gray matter volume during the training period relative to habituation of up to 14% (monkey E) and 13% (monkey N). Across monkeys E and N, Z scores were 7.2 (STS) and 6.7 (SII). Gray matter after training (sessions 5 and 6) remained higher than before training [sessions 1 and 2; Z scores were 5.8 (STS) and 5.9 (SII)] but fell slightly relative to training [sessions 3 and 4; Z scores 4.7 (STS) and 4.3 (SII)]. This pattern of effects is consistent with the time course of learning rather than time in the experiment per se. In monkey F (F), gray matter volume decreased in the right STS and SII voxels during training.
The percentage changes in gray matter volume are plotted in Fig. 3 D–F and show that, compared with the habituation period, gray matter volume increased during the training period in STS right and SII right by 13% and 14% in monkey E, and by 10% and 13% in monkey N. Post hoc tests on the results from monkeys E and N showed that, compared with the habituation period, gray matter volume was significantly increased during the training and posttraining periods. They also showed that gray matter volume in the posttraining period was significantly reduced compared with that in the intensive training period (see Fig. 3 legend). Thus, although increases were generally sustained after the posttraining period, the overall pattern suggested a decrease after the monkey's performance on the task had plateaued. By contrast, no training-related increases were observed in monkey F (Fig. 3 E and F). On the contrary, the gray matter volume in both the STS and SII voxels decreased between scanning sessions 2 and 3, which coincided with the period in which this monkey was showing rather slow and erratic performance on the task (Fig. 1).
Overall, global change in gray matter, estimated by comparing total gray matter volume from scans in sessions 1 with those in session 6, showed only minor changes (E, −1.1%; N, 2%; and F, −2.6%).
Changes in White Matter Detected by MRI.
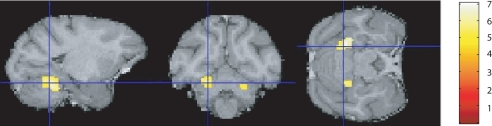
The same VBM analysis was used to investigate changes in the segmented white matter. This revealed that rake learning was associated with significant increases in white matter volume bilaterally in the cerebellar white matter beneath lobule 5 in both hemispheres (Fig. 4, Fig. S4, and Table 1). These changes involved a larger number of voxels on the left side than on the right (Table 1). Significant changes in white matter were not observed in the cerebrum.
Fig. 4.
Areas of white matter increase. Regions where white matter increased with performance score are shown superimposed on a T1/T2* image; there were bilateral white matter increases in cerebellar hemisphere, including lobule 5 (right, x = 10; y = −27; z = −14; Z score = 5.75; left, x = −12; y = −26; z = −13; Z score = 6.15). Color scale is the same as in Fig. 2.
Discussion
A number of structural MRI studies have reported learning- or experience-related structural plasticity in the adult human brain. To understand the neurobiological underpinnings of this plasticity, we need to develop an animal model of the structural brain changes during acquisition of a learned skill. The ideal task would be one of direct relevance to advanced human motor behavior. It should also be a task that the animal can acquire reasonably rapidly but of which it has had no previous experience, thereby maximizing the chance of inducing detectable changes in brain structure. The use of a tool, which, although by no means restricted to primates, is most highly developed in this species (18), satisfies all these criteria. Here, we have demonstrated that as macaque monkeys learn to use a tool, there are large changes in the brain that are detectable in individual animals. This is a previously undescribed development because all of the human studies in which skill-induced gray matter changes have been detected have required pooling of images from large groups of volunteers (n = 12–69; refs. 8, 19, and 20). Learning a new skill may involve different strategies, time course, and brain networks in different individuals (13, 14), so the potential to detect changes in individuals as they acquire the skill may allow us to understand neural mechanisms underpinning individual patterns of learning.
At the group level, our analysis showed significant gray matter increases with the monkeys' performance score over the time course of the experiment (Figs. 1 and 2 and Table 1). The areas affected by tool-use learning included STS, SII, and IPS, a network in keeping with earlier human and monkey studies of tool use (see below). The group results were driven largely by the images from monkeys E and N, which showed consistent changes in these cortical areas.
The longitudinal nature of this study also allowed us to define the time course of the gray matter increases. The STS and SII peak voxel data in monkeys E and N showed gray matter volume changes ranging from 6% to 14% over the intensive training period (Fig. 3 A and B). Changes of up to 17% were seen in other voxels (Fig. S3). The size of these increases contrasts with the much smaller global change in gray matter of only ±1–2%. In peak voxels, the gray matter increases occurred earliest in the monkey (N) that was the quickest to learn (Fig. 3B) and more gradually in the slower-learning monkey (E; Fig. 3A). In both monkeys, there was a decrease in gray matter volume after the monkey's performance on the task had plateaued; i.e., from scanning session 4 onward. Interestingly, no significant changes were observed in the third monkey (F; Fig. 3 C and F and Table 1). This monkey learned the rake task slowly, and its performance on it was erratic (Fig. 1) and, unlike the other two, it showed no significant increase in mean rake velocity over the training period (Fig. S1).
The gray matter changes detected in this study in monkeys E and N are large in comparison with the 3% changes reported in a recent study in which human volunteers learned to juggle (8); in that study, changes were detectable only at the group level (n = 12 volunteers). In contrast with the human participants in the juggling study, who would have had prior experience with a wide range of skilled motor tasks, our animals were completely naive to tool use, and this may explain the large changes during training. Thus, tool use in nonhuman primates could provide an ideal model to investigate the neurobiological mechanisms that drive learning-related changes in MRI images. In keeping with our findings, a recent study showed significant differences in brain size between New World capuchin monkeys that were habitual tool users and those that did not use tools (21).
These changes are unlikely to reflect the increased level of environmental enrichment caused by introducing monkeys into a new setting with daily interactions with the experimenters (cf. ref. 19). Any such changes would have been present throughout the experiment, rather than following the same time course as the learning, with the strongest increase during the intensive training period (Fig. 3 D and E) and negligible changes during the habituation and posttraining testing, when the monkeys performed only around 10% of trials relative to the intensive training period. Indeed, future studies should concentrate on gray matter changes during this intensive learning period.
Our conclusion is that the gray matter increases were related to tool use learning. Consistent with this conclusion, two of the cortical areas (STS and IPS) that showed significant gray matter changes in our monkeys correspond to the areas of the human brain that are activated in fMRI studies of tool use. These fMRI studies have demonstrated that tool use involves a functionally specialized network (22, 23) that includes the left posterior temporal cortex (24), the dorsal and ventral premotor areas (24, 25), and the IPS (26, 27). The posterior IPS also showed gray matter increases in the group study of human volunteers learning to juggle (2).
With respect to the function of the identified areas, the left posterior temporal cortex is involved in tool recognition, in which human subjects were required to name (28), observe, and answer questions about tools (24). In the monkey, STS neurons are active during observation of object manipulation (29). The second somatosensory area (SII) is known to be involved in tactile-based learning (30) and tactile attention (31), although in human activation studies it is most frequently cited as being involved in the processing of pain. SII may also have a role in retrieval of food (32) and in establishing the body schema (33) into which the rake is readily incorporated by monkeys during training (34, 35), but its role in tool use learning clearly merits further investigation.
The IPS region is also important in human tool use and has been shown to be activated in object grasping and manipulation (26) and tool-use pantomime (36). The IPS (including AIP) plays a critical role in visuomotor control of grasp in macaques (37) and has been directly implicated in tool use in macaques, with increased regional blood flow during tool use (38). Head and Holmes first showed that the tool becomes incorporated into the body representation in the human parietal cortex (39). Likewise, in trained monkeys, bimodal neurons in the bank of the IPS, which usually respond to visual stimuli near the hand, adapt their visual receptive field to include the entire rake (34). During this training, levels of expressions of BDNF, and of its receptor trkB, and NT-3 were increased in the IPS (40). In parallel, plastic changes induced by training on the rake task were observed in the connections of the IPS, revealing a novel projection from the higher-order visual area in the temporoparietal junction to the IPS (7). Gray matter in primary sensory (visual, somatosensory) and motor areas involved in the execution of the task appeared unchanged during and after training (Fig. 2 and Fig. S2).
Taken together, prior evidence supports the view that the areas showing significant gray matter changes are related to skill improvement on the rake task shown by the monkeys, and they reflect novel integration of information processing within those areas. There are a number of possible mechanisms that could underlie the observed gray matter changes. Animal studies support the view that increases in gray matter are due to the formation of new connections mediated by dendrite and spine growth, leading to strengthening of existing connections (41, 42). In line with the animal studies, humans with a higher IQ show a greater number of dendrites and an increased length of dendrites (43). Learning the rake task can induce expression of cortical neurotrophins (6) and the formation of new connections (7), and trained animals have a greater number of synaptic terminals and increased dendritic spine density on cortical neurons (5). Neurogenesis in the adult brain is still very controversial, with studies still suggesting that neurogenesis in humans is restricted to the developmental period (44). Spontaneous neurogenesis has been claimed to exist in the naive adult macaque brain (45), and this might be enhanced by intensive training on a novel task. It has also been suggested that astrocytes show experience-dependant changes (5).
We also used VBM to search for changes in white matter. Large effects were detected bilaterally in the white matter underlying the cerebellar hemisphere in the region of lobule 5 (Fig. 4). The cerebellum is well known to be involved in the acquisition of new motor skills (46–48) and is thought to store an internal model of a new tool (47). It is bilaterally active in monkeys during performance of the rake task (38). Although learning-induced changes in cerebellar white matter have not yet been reported, such changes have been reported in other brain structures (49, 50). White matter changes might reflect an increase in axon diameter or an increase in myelination, perhaps reflecting an increase in oligodendrocytes (51). Angiogenesis supporting any of these changes might also contribute. Interestingly, the most extensive changes were seen on the left side (Fig. 4), contralateral to the largest gray matter changes in the cerebral cortex (Fig. 2).
In conclusion, we have advanced the possibility of investigating the neurobiological mechanisms underpinning the learning of an advanced motor skill by demonstrating: first, that such learning is associated with large changes in a number of cortical areas; second, that such changes are detectable in the brains of individual monkeys; and finally, that the time course of these effects is in the order of 1–2 weeks.
Methods
Monkeys.
The three adult male Japanese macaques (Macaca fuscata) used in this study weighed 4.1 kg (monkey N), 5.1 kg (monkey E), and 5.6 kg (monkey F). This study was approved by the local Animal Experiment Committee and was conducted in accordance with the Guidelines for Conducting Animal Experiments of the RIKEN Brain Science Institute.
Habituation and Training.
Habituation period.
During the initial 2-week habituation period (days −14 to 0; Fig. 1, blue bar), monkeys were transported from their home cage to the training room in a chair. The habituation sessions (60 min long) were carried out 5 days a week. Monkeys were restrained at the waist and the neck but were able to move the head freely; they were trained to reach out and grasp food rewards.
Intensive training period.
This period began after habituation (days 1 to 21; Fig. 1, purple bar). A total of 13–14 days of training sessions were held over the 21-day period (Fig. 1, purple squares). The remaining days were either scan days or weekends. Each session lasted 90 min. The monkey's goal was to use a light rake to retrieve small food rewards (usually 1-cm cubed pieces of apple or sweet potato). In the first training stage, monkeys were introduced to a spatula-like tool that helped shape their use of the rake. Food was placed on the circular head, with only the shaft within reach of the monkey. The monkey was required to pull the tool toward itself to retrieve the food. After the monkey learned to use this device (usually on day 2 of training), it was replaced with the rake. Food was placed directly behind the head of the rake so that the monkey could continue to apply a pulling motion to the rake to retrieve the reward (Movie S1). To encourage the monkey to swing the rake horizontally (so as to place the head of the rake behind the food reward), more and more trials were conducted with the food placed slightly to the side of the rake (Movie S2). As the monkey became more proficient, the food was placed further away, and by the end of the training period, the monkey could retrieve food placed anywhere on the table (Movie S3)
Posttraining period.
During the 2-week period that followed the intensive training period (Fig. 1, red bar, days 22 to 33), three testing sessions (red squares), each 30 min long, were carried out to confirm that monkeys had retained their newly acquired skill on the rake task.
Behavioral Scoring and Kinematic Analysis.
Performance on the rake task was assessed in a timed test period of 50 successive trials, generally carried out 1 hour into each training session (15 min into each posttraining testing session). Each trial was assigned a score. Score 3: food was retrieved on the first attempt; score 2: food was retrieved on the second attempt; score 1: food was retrieved only after multiple attempts; score 0: a zero was given if the monkey was unable to retrieve the food within a defined time-out period of 30 s. Digital video was recorded for analysis of the kinematics of the monkeys' performance (Movie S1, Movie S2, and Movie S3).
MRI Scans.
The scanning schedule (Fig. 1, numbered circles) lasted 47 days (14-day habituation period, 20-day intensive training period, and 13-day posttraining period). There were six scanning sessions per monkey. Three separate scans were carried out during each session, yielding 18 scans for each monkey. This procedure ensured that any differences between sessions did not correlate with systematic signal differences related to the inhomogeneity of the signal.
VBM and Statistical Analysis.
Preprocessing and analysis of MRI scans were carried out in SPM5 (Wellcome Trust Centre for Neuroimaging) running under Matlab (MathWorks). VBM uses voxel-by-voxel statistical comparison for the identification of regional differences in the context of Gaussian random fields (15). VBM is optimized when images are segmented into gray matter, white matter, and CSF. To average data across different brains, spatial normalization of the brains to a common template is required. We therefore needed to create a gray and white matter template of the Japanese macaque brain (see SI Methods). All of the normalized images were smoothed by convolving with an isotropic Gaussian kernel of 3 mm. This was justified by the small voxel size, relatively small size of the brain, and number of animals investigated (Fig. S5). The gray matter segmentation for each scan and each monkey was used to calculate any changes in global gray matter volume between the first and last scanning sessions (sessions 1 and 6).
The design matrix for the group analysis modeled 18 conditions (six scanning sessions × three monkeys). We performed a t test for a parametric effect of performance using a contrast (mixture) of session-specific parameter estimates (weighted according to the corresponding performance at each session; see ref. 52, p. 201). The t test identifies whether the linear contrast of coefficients (describing the relation between gray matter and performance) was significantly different from zero. The performance scores were those achieved during the training session immediately before each session (Fig. 1). The scores of the first two sessions were set to zero because the monkeys could not perform the task at this stage of the experiment. The performance score was mean-centered within each monkey to preclude testing for between-monkey differences in gray matter volume that are not related to performance. See SI Methods, Table S1, and Fig. S6 for details of statistical thresholds used.
To illustrate the time course of the structural changes in the regions identified as highly significant in the above analysis, we extracted the gray matter volume from voxels in each of the identified regions. These data were also used to calculate the percent changes in gray matter volume in the different stages of training (see Fig. 3 legend). Post hoc statistical tests were carried out to compare changes in gray matter volume in monkeys E and N across the habituation, training, and posttraining periods.
Supplementary Material
Acknowledgments.
We thank Prof. Karl Friston, Dr. Alexander Kraskov, Dr. Joe Devlin, Yuki Kurihara, and Dr. Ferath Kherif for expert technical advice and help. This work was funded by grants from the Medical Research Council, Wellcome Trust, and the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909751106/DCSupplemental.
References
- 1.Maguire EA, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Aydin K, et al. Increased gray matter density in the parietal cortex of mathematicians: A voxel-based morphometry study. AJNR Am J Neuroradiol. 2007;28:1859–1864. doi: 10.3174/ajnr.A0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci. 2007;27:1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markham JA, Greenough WT. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishibashi H, et al. Tool-use learning induces BDNF expression in a selective portion of monkey anterior parietal cortex. Brain Res Mol Brain Res. 2002;102:110–112. doi: 10.1016/s0169-328x(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 7.Hihara S, et al. Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool-use training in adult monkeys. Neuropsychologia. 2006;44:2636–2646. doi: 10.1016/j.neuropsychologia.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Draganski B, et al. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 9.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 10.Beck BB. Animal Tool Behavior: The Use and Manufacture of Tools by Animals. New York: Garland STPM; 1980. [Google Scholar]
- 11.Ducoing AM, Thierry B. Tool-use learning in Tonkean macaques (Macaca tonkeana) Anim Cogn. 2005;8:103–113. doi: 10.1007/s10071-004-0240-0. [DOI] [PubMed] [Google Scholar]
- 12.Ishibashi H, Hihara S, Iriki A. Acquisition and development of monkey tool-use: Behavioral and kinematic analyses. Can J Physiol Pharmacol. 2000;78:958–966. [PubMed] [Google Scholar]
- 13.Philip BA, Wu Y, Donoghue JP, Sanes JN. Performance differences in visually and internally guided continuous manual tracking movements. Exp Brain Res. 2008;190:475–491. doi: 10.1007/s00221-008-1489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humle T. Ant dipping in chimpanzees: An example of how microecological variables, tool use, and culture reflect the cognitive abilities of chimpanzees. In: Matsuzawa T, Tomonaga M, Tanaka M, editors. Cognitive Development in Chimpanzees. Tokyo: Springer; 2006. pp. 452–475. [Google Scholar]
- 15.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 16.Van de Moortele PF, et al. T(1) weighted brain images at 7 Tesla unbiased for proton density, T(2)* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization. NeuroImage. 2009;46:432–446. doi: 10.1016/j.neuroimage.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mugler JP, III, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 18.van Schaik CP, Deaner RO, Merrill MY. The conditions for tool use in primates: Implications for the evolution of material culture. J Hum Evol. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- 19.Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning–revisited. PLoS ONE. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilg R, et al. Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips KA, Sobieski CA, Thompson CR, Sherwood CC. Brain structures differ among tool using and non-tool using capuchin monkeys. SFN Abstract. 2008 79.8/OO30. Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=c022edee-ebf9-4dbb-b7cf-e68fc8b0905b&cKey=12baac91-805b-4026-aa04-cf463b8f707b. [Google Scholar]
- 22.Johnson-Frey SH. The neural bases of complex tool use in humans. Trends Cogn Sci. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- 24.Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. NeuroImage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- 25.Grafton ST, Fadiga L, Arbib MA, Rizzolatti G. Premotor cortex activation during observation and naming of familiar tools. NeuroImage. 1997;6:231–236. doi: 10.1006/nimg.1997.0293. [DOI] [PubMed] [Google Scholar]
- 26.Binkofski F, et al. A fronto-parietal circuit for object manipulation in man: Evidence from an fMRI-study. Eur J Neurosci. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- 27.Inoue K, et al. Activation in the ipsilateral posterior parietal cortex during tool use: A PET study. NeuroImage. 2001;14:1469–1475. doi: 10.1006/nimg.2001.0942. [DOI] [PubMed] [Google Scholar]
- 28.Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- 29.Lorincz EN, et al. Do monkeys understand actions and minds of others? Studies of single cells and eye movements. In: Dehaene S, Duhamel JR, Hauser MD, Rizzolatti G, editors. From Monkey Brain to Human Brain. Boston: MIT Press; 2005. pp. 189–210. [Google Scholar]
- 30.Murray EA, Mishkin M. Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res. 1984;11:67–83. doi: 10.1016/0166-4328(84)90009-3. [DOI] [PubMed] [Google Scholar]
- 31.Burton H, et al. Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: A positron emission tomography study. Cereb Cortex. 1999;9:662–674. doi: 10.1093/cercor/9.7.662. [DOI] [PubMed] [Google Scholar]
- 32.Taoka M, Tanaka M, Ojima H, Iriki A. Electrophysiological study of neurons representing the hand and mouth in the secondary somatosensory cortex of the macaque monkey during a simple feeding task. Dent Jpn (Tokyo) 2007;43:23–27. [Google Scholar]
- 33.Lin YY, Forss N. Functional characterization of human second somatosensory cortex by magnetoencephalography. Behav Brain Res. 2002;135:141–145. doi: 10.1016/s0166-4328(02)00143-2. [DOI] [PubMed] [Google Scholar]
- 34.Maravita A, Iriki A. Tools for the body (schema) Trends Cogn Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- 36.Moll J, et al. Functional MRI correlates of real and imagined tool-use pantomimes. Neurology. 2000;54:1331–1336. doi: 10.1212/wnl.54.6.1331. [DOI] [PubMed] [Google Scholar]
- 37.Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- 38.Obayashi S, et al. Functional brain mapping of monkey tool use. NeuroImage. 2001;14:853–861. doi: 10.1006/nimg.2001.0878. [DOI] [PubMed] [Google Scholar]
- 39.Head H, Holmes GM. Sensory disturbances from cerebral lesions. Brain. 1911;34:102–254. [Google Scholar]
- 40.Ishibashi H, et al. Tool-use learning selectively induces expression of brain-derived neurotrophic factor, its receptor trkB, and neurotrophin 3 in the intraparietal multisensory cortex of monkeys. Brain Res Cogn Brain Res. 2002;14:3–9. doi: 10.1016/s0926-6410(02)00056-3. [DOI] [PubMed] [Google Scholar]
- 41.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 42.Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H. Principles of long-term dynamics of dendritic spines. J Neurosci. 2008;28:13592–13608. doi: 10.1523/JNEUROSCI.0603-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobs B, Schall M, Scheibel AB. A quantitative dendritic analysis of Wernicke's area in humans. II. Gender, hemispheric, and environmental factors. J Comp Neurol. 1993;327:97–111. doi: 10.1002/cne.903270108. [DOI] [PubMed] [Google Scholar]
- 44.Bhardwaj RD, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 46.Friston KJ, Frith CD, Passingham RE, Liddle PF, Frackowiak RS. Motor practice and neurophysiological adaptation in the cerebellum: A positron tomography study. Proc Biol Sci. 1992;248:223–228. doi: 10.1098/rspb.1992.0065. [DOI] [PubMed] [Google Scholar]
- 47.Imamizu H, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 48.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: A diffusion tensor imaging study. Neurosci Lett. 2002;321:57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 50.Bengtsson SL, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 51.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friston KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.