Abstract
The present study tests the hypothesis that local bioavailability of IGF-I is capable of regulating muscle protein balance and that muscle-directed IGF-I can selectively maintain muscle mass during bacterial infection. Initial studies in C57BL/6 mice demonstrated that increasing or decreasing bioavailable IGF-I within muscle by local administration of either Leu24 Ala31 IGF-I or IGF binding protein (IGFBP)-1, respectively, produced proportional changes in surrogate markers (e.g., phosphorylation of 4E–BP1 and S6K1) of protein synthesis. We next examined the ability of a sustained local administration of IGF-I to prevent sepsis-induced muscle atrophy over a 5-day period. At the time of cecal ligation and puncture or sham surgery, mice had a time-release pellet containing IGF-I implanted next to the gastrocnemius and a placebo pellet placed in the contralateral limb. Data indicated IGF-I released locally only affected the adjacent muscle and was not released into the circulation. Gastrocnemius from septic mice containing the placebo pellet was atrophied and had a reduced IGF-I protein content. In contrast, locally-directed IGF-I increased IGF-I protein within adjacent muscle to basal control levels. This change was associated with a proportional increase in muscle weight and protein, as well as increased phosphorylation of 4E–BP1 and the redistribution of eIF4E from the inactive eIF4E· 4EBP1 complex to the active eIF4E· eIF4G complex. Local IGF-I also prevented the sepsis-induced increase in atrogin-1 mRNA in the exposed muscle. Finally, local IGF-I prevented the sepsis-induced increase in muscle IL-6 mRNA. Thus, muscle-directed IGF-I attenuates the sepsis-induced atrophic response apparently by increasing muscle protein synthesis and potentially decreasing proteolysis. Collectively, our data suggest agents which increase the bioavailability of IGF-I within muscle per se might be effective in ameliorating the sepsis-induced loss of muscle mass without having undesirable effects on metabolic processes in distant organs.
Keywords: IGF-I, IGFBP-1, IL-6, 4E–BP1, atrogin-1
INTRODUCTION
Unremitting bacterial infection increases nitrogen excretion resulting from an imbalance between rates of whole-body protein synthesis and degradation [1]. Although sepsis-induced changes in protein balance can be detected in several tissues, much of the urinary loss of nitrogen and increased protein turnover is attributable to the erosion of muscle mass [1,2]. The mechanism by which prolonged sepsis leads to muscle atrophy involves both a decreased rate of protein synthesis and increased the rate of proteolysis [1,3,4]. Such changes adversely affect morbidity and mortality in severely catabolic individuals [5,6], so limiting muscle loss in an attempt to preserve muscle function should improve the recovery process.
The mechanism by which sepsis alters muscle protein balance is undoubtedly multifactorial being attributable to increases in various catabolic agents (e.g., inflammatory cytokines, excess glucocorticoids) and decreases in either the concentration or tissue responsiveness to various anabolic agents (e.g., growth factors) and nutrients (e.g., leucine) [1,7]. Of these, the concentration of insulin-like growth factor (IGF)-I in either the muscle per se or the general circulation occupies a central regulatory role in the accretion of muscle mass during development and promotes a hypertrophic-like response in cultured myocytes [8]. The systemic administration of IGF-I, which increases the peptide level in the general circulation, is capable of increasing muscle weight and protein synthesis, and therefore the hormone clearly functions in an endocrine manner [9]. Furthermore, muscle hypertrophy is also seen in transgenic mice which specifically overexpress IGF-I in skeletal muscle [10]. Importantly, the over-expression of IGF-I in muscle per se does not increase IGF-I in the circulation and does not lead to a generalized increase in body weight, thereby confirming IGF-I is also capable of intrinsic metabolic regulation via autocrine/paracrine signaling. Moreover, the local infusion of IGF-I in adult mice also increases muscle mass independent of a general stimulation of somatic growth [11]. The relative intrinsic importance of locally produced IGF-I on muscle protein balance is further evidenced by the hypertrophy seen in overloaded skeletal muscle of hypophysectomized rats. Under such a low-growth condition, where the circulating concentration of IGF-I is extremely low due to the lack of growth hormone, the autocrine/paracrine effect of this growth factor is evidenced by the correlation in muscle IGF-I content and increased muscle mass [12]. However, at this time it is unclear whether it is the local IGF-I content within muscle or the concentration of IGF-I in the blood which is the primary determinant of muscle protein balance during infection because of the difficulty in isolating these different growth factor pools [13]. Therefore, the purpose of the present study was to test the hypothesis that the local bioavailability of IGF-I is capable of regulating in vivo muscle protein balance, and that increasing the local IGF-I content within skeletal muscle over a sustained period of time can prevent sepsis-induced atrophy.
MATERIALS AND METHODS
Animals and experimental protocol
All experiments used specific pathogen-free, male C57BL/6 mice (9–10 wk of age, 22–25 g; Jackson Laboratories, Bar Harbor, ME) which were housed under constant environmental conditions and maintained on standard rodent chow and water ad libitum for at least 1 wk prior to the start of studies. In the first study, freely fed control mice were injected intraperitoneally with either IGF binding protein (IGFBP)-1 (5 µg/ g body weight [BW]; Calbiochem, La Jolla, CA) or the same volume (0.5 ml) of vehicle (0.9% saline). A separate group of mice in this study was injected with IGFBP-1 (1 µg/muscle) into the right gastrocnemius and vehicle injected into the contralateral left gastrocnemius. IGFBP-1 or saline were administered by multiple intramuscular injections using a 30-g needle to deliver a total of 50 µl per muscle. The amount of IGFBP-1 needed to achieve a maximal response was based on preliminary studies (data not shown) and published data [14,15]. The second study was comparable to the first, except Leu24 Ala31 IGF-I was administered to mice instead of IGFBP-1. For this study, mice were fasted for 5 hours to increase the endogenous production of IGFBP-1 and enhance the opportunity to demonstrate an effect of the IGF-I analog. Leu24 Ala31 IGF-I is an analog of native IGF-I which displaces IGF-I and -II from IGF binding proteins because of its high affinity for IGFBPs but itself does not bind to the IGF-I receptor [16]. As a result, Leu24 Ala31 IGF-I increases the “free” or bioavailable IGF-I. The Leu24 Ala31 IGF-I was administered by either intraperitoneal (2 µg/g body weight) or intramuscular (0.5 µg/muscle) injection, as described above. The doses of Leu24 Ala31 IGF-I used were based on preliminary studies showing maximal effectiveness (data not shown). Mice were anesthetized with ketamine/xylazine and 20 min after administration of either IGFBP-1 or Leu24 Ala31 IGF-I, the gastrocnemius from both legs excised. This time point was selected based on preliminary studies and published reports indicating a maximal stimulation of muscle protein synthesis and translation initiation [4,17]. Tissues from this and subsequent studies were weighed, frozen in liquid nitrogen, and stored at −70 C until analyzed.
In the third study, polymicrobial peritonitis was induced by cecal ligation and puncture (CLP) [18]. Briefly, an abdominal midline incision of approximately 1 cm was made, and the cecum exposed, ligated, and punctured using a 23-gauge needle. Care was taken not to obstruct the bowel. A small amount of feces was extruded from the cecum to ensure patency of the puncture sites. Sham control mice were subjected to the same protocol except the cecum was not ligated or punctured. Thereafter, the cecum was replaced in the abdominal cavity, and the abdominal muscles and skin closed. A small skin incision was made on the ventral portion of right hindlimb and a time-release pellet containing recombinant human IGF-I formulated to release approximately 2 µg/day for a period of 6 days (3 mm diameter) was placed proximal to the belly of the gastrocnemius. The native IGF-I peptide is 95% conserved between mice and humans [19] and there is a high degree of conservation of IGF-I action over a wide range of species (e.g., humans to fish) [19,20]. Similarly, a placebo pellet was implanted next to the left gastrocnemius. In addition, muscles from naive control mice - which underwent only the surgical preparation but no pellet implantation - were used as controls (“sham”). The skin incision was closed using a single wound clip. After surgery, all mice were housed individually and each received 1 ml of warmed (37 °C) 0.9% sterile saline containing 0.05 mg/kg of buprenorphine administered subcutaneously every 12 h for the remainder of the experimental protocol. Control mice were pair-fed to match the food consumption of septic mice. Mice were killed 5 days after CLP and gastrocnemius and heart excised, and blood collected from the inferior vena cava into a heparinized syringe. The 5-day time point was selected because preliminary studies demonstrated that at this time it was possible to detect a net increase in muscle weight in response to IGF-I stimulation (data not shown); data from studies using shorter periods of time post-CLP had greater variability.
All experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine and adhered to the National Institutes of Health guidelines for the use of experimental animals.
Immunoprecipitation and Western blot analysis
The tissue preparation was the same as previously described [4,17,21]. A portion of fresh muscle was homogenized using a 1:5 ratio of ice-cold buffer consisting of (in mmol/L) 20 HEPES, pH 7.4, 2 EGTA, 50 NaF, 100 KCl, 0.2 EDTA, 50 β-glycerophosphate, 1 DTT, 0.1 PMSF, 1 benzamidine, 0.5 sodium vanadate, plus a protease inhibitor cocktail tablet from Roche (Indianapolis, IN), and clarified by centrifugation. The samples were subjected to SDS-PAGE and the proteins electrophoretically transferred to PVDF membranes. The blots were incubated with either primary antibodies (unless otherwise noted from Cell Signaling, Beverly, MA) total S6K1 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-specific S6K1 (Thr389), and total (Bethyl Laboratories, Montgomery, TX) and phospho-specific 4E–BP1 (Thr37/46). Blots were washed with TBS-T (1X TBS including 0.1% Tween-20) and incubated with secondary antibody (horseradish peroxidase conjugated goat anti-mouse or goat anti-rabbit) IgG at room temperature. The blots were developed with enhanced chemiluminescence Western blotting reagents (Amersham), and exposed to X-ray film in a cassette equipped with a DuPont Lightning Plus intensifying screen. After development, the film was scanned (Microtek ScanMaker IV) and analyzed using National Institutes of Health Image 1.6 software.
The eIF4E·4EBP1 and eIF4E·eIF4G complexes were quantified as described [4,17,21]. From an aliquot of supernatant, eIF4E was immunoprecipitated using an anti-eIF4E monoclonal antibody (kindly provided by Drs. Jefferson and Kimball; Hershey, PA). Antibody-antigen complexes were collected using magnetic beads, subjected to SDS-PAGE, and proteins transferred to a PVDF membrane. Blots were incubated with a mouse anti-human eIF4E antibody, rabbit anti-rat 4E–BP1 antibody, or rabbit anti-eIF4G antibody.
RNAse protection assay (RPA)
Primer selection for mouse genes of interest (i.e., atrogin-1, MuRF1, TNFα, IL-1 and IL-6) has been previously published by our laboratory [22,23]. A 2 µl aliquot of template was labeled using T7 Polymerase, RNaisin and DNase (Promega, Madison, WI), NTPs and tRNA (Sigma) and 32P-UTP (Amersham, Piscataway, NJ). Unless otherwise noted, the entire RPA procedure including labeling conditions, component concentrations, sample preparation, and gel electrophoresis was performed according to previously published protocols (BD Biosciences Pharmingen, San Diego, CA). Hybridization buffer was 80% formamide and 20% stock buffer (200 mM Pipes, pH 6.4, 2 M NaCl, 5 mM EDTA). Ten micrograms of total RNA were hybridized overnight at 56o C in a dry bath incubator (Fisher Scientific, Pittsburgh, PA) without the use of mineral oil. Samples were treated with RNAse A+T1 (Sigma) in 1x RNAse buffer (10 mM Tris-HCl, pH 7.5, 5 mM EDTA, 300 mM NaCl) followed by Proteinase K (Fisher Scientific) in 1x Proteinase K buffer (50 mM Tris pH 8.0, 1 mM EDTA, 1% Tween 20). Following ethanol precipitation, samples were resuspended in loading buffer [98% formamide (v/v), 0.05% xylene cyanol (w/v), 0.05% bromphenol blue (w/v), 10 mM EDTA]. Polyacrylamide gels were run, transferred to chromatography paper, and dried (FB GD 45 Gel Dryer, Fisher Scientific). Gels were exposed to a PhosphorImager screen (Molecular Dynamics Inc., Sunnyvale, CA) and the resultant data were quantified using Molecular Dynamics’s ImageQuant software (ImageQuant version 5.2). Although the data were normalized to L32 the same results were obtained if data were normalized to GAPDH (data not shown).
IGF-I and insulin determinations
Plasma samples were acid-ethanol extracted and cryoprocipitated to remove IGFBPs [14]. IGF-I of human origin (e.g., from the implanted pellet) was differentiated from endogenous rat IGF-I by use of species-specific radioimmunassays (RIA; Diagnostic Systems Laboratory, Webster, TX). The plasma insulin concentration was also determined by RIA (Linco Research, Inc., St. Louis, MO).
Statistical analysis
Data for each condition are summarized as means ± SE where the number of mice per treatment group is indicated in the legends to the figure or table. Unless otherwise indicated, statistical evaluation of the data was performed using 2-way ANOVA with post-hoc Student-Neuman-Keuls test when the interaction was significant. Differences were considered significant when P < 0.05.
RESULTS
Acute regulation of local IGF-I
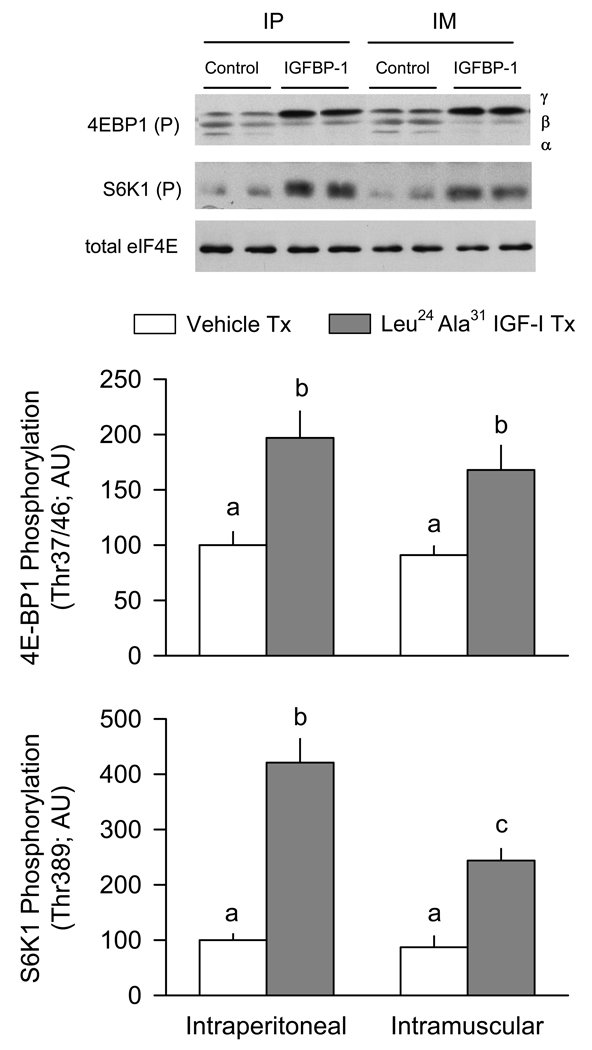
The first series of studies provided proof-of-principle that regulation of the local IGF-I concentration was capable of acutely modulating endpoints related to muscle protein synthesis. First, mice were administered Leu24Ala31 IGF-I which increases free IGF-I [16]. The intraperitoneal injection of Leu24Ala31 IGF-I into 5-h fasted mice increased the phosphorylation of 4E–BP1 and S6K1, compared to values from vehicle-treated control mice (Figure 1). Although we did not directly determine rates of muscle protein synthesis in this or subsequent studies, the phosphorylation of these proteins is accepted as a functional readout of mTOR (mammalian target of rapamycin) activity and correlates with proportional changes in muscle protein synthesis [1]. Similarly, injection of Leu24Ala31 IGF-I directly into muscle also increased phosphorylation of these two proteins. The total amount of 4E–BP1 and S6K1 protein in muscle was not different between groups (data not shown). The intramuscular injection of Leu24Ala31 IGF-I did not appear to “spill-over” into the general circulation because the extent of 4E–BP1 and S6K1 phosphorylation in the vehicle-injected contralateral muscle was not different from levels in muscle of control mice injected intraperitoneally with vehicle. To provide confirmation that intramuscular injected Leu24Ala31 IGF-I did not appreciably spill-over into the blood, plasma insulin levels were determined. The intraperitoneal injection of Leu24Ala31 IGF-I decreased the insulin concentration (74 ± 18 pmol/L; n = 6), compared to values in vehicle-treated control mice (144 ± 24 pmol/L; n = 7; P < 0.05). Such a hypoinsulinenic response was not detected when Leu24Ala31 IGF-I was injected intramuscularly (138 ± 25 pmol/L; n = 5; P = NS compared to vehicle-treated control value).
Figure 1.
Effect of intraperitoneal or intramuscular administration of Leu24Ala31 IGF-I on the phosphorylation of 4E–BP1 and S6K1. For mice injected intraperitoneally (IP), Leu24Ala31 IGF-I or vehicle (saline) was administered to two separate groups of mice. For mice administered Leu24Ala31 IGF-I intramuscularly (IM), the IGF-I analogue was locally injected into the right gastrocnemius and saline injected into the contralateral muscle of the same mouse. Muscles were sampled 20 min after injection of Leu24Ala31 IGF-I and used to assess phosphorylation of 4E–BP1 (Thr37/46) and S6K1 (Thr389). Inset, representative Western blots for these proteins is shown in the top panel. For 4E–BP1, three isoforms of the protein were detected (e.g., γ-, β-, and α). Total eIF4E is included as a loading control. The total amount of 4E–BP1 and S6K1 in muscle did not differ among the various treatment groups (data not shown). For bar graphs. values are means ± SEM; N = 7, 6, 5 and 5, respectively. Values with different letters are statistically different, P < 0.05.
Conversely, the second study was performed to determine whether bioavailable IGF-I in muscle could be decreased by injecting IGFBP-1, one of the six naturally occurring IGF binding proteins. The intraperitoneal injection of IGFBP-1 decreased 4E–BP1 phosphorylation by 60% in muscle from freely fed mice (Figure 2). Systemically administered IGFBP-1 decreases the circulating concentration of free IGF-I [14] and, because of its low molecular weight, would also be expected to cross the capillary endothelium where it would neutralize interstitial IGF-I [24]. In this regard, injection of IGFBP-1 directly into the gastrocnemius reduced 4E–BP1 phosphorylation. Such a change was not detected in the contralateral muscle injected with vehicle suggesting the local intramuscular injection of IGFBP-1 did not spill-over into the general circulation. The absence of a physiologically relevant amount of IGFBP-1 spill-over into the general circulation was confirmed by the lack of hyperinsulinemia. That is, mice injected intraperitoneal with IGFBP-1 were hyperinsulinemic (208 ± 29 pmol/L; n = 7; P < 0.05), compared to either control mice injected with vehicle (148 ± 26 pmol/L; n = 7) or mice where IGFBP-1 was injected intramuscularly (152 ± 21 pmol/L; n = 5). No effect of exogneous IGFBP-1 on S6K1 phosphorylation was observed because of the low constitutive phosphorylation of this protein under basal conditions (data not shown). The total 4E–BP1 and S6K1 protein in muscle was not different between groups (data not shown).
Figure 2.
Effect of intraperitoneal or intramuscular administration of IGFBP-1 on the phosphorylation of 4E–BP1. For mice injected intraperitoneally (IP), IGFBP-1 or vehicle (saline) was administered to two separate groups of mice. For mice injected intramuscularly (IM), IGFBP-1 was locally injected into the right gastrocnemius and saline injected into the contralateral muscle of the same mouse. Muscles were sampled 20 min after injection of IGFBP-1 and used to assess the phosphorylation state of 4E–BP1 (Thr37/46). Inset, a representative Western blot for phosphorylated 4E–BP1 is shown in the top panel and the γ-and β-isoforms identified. Total eIF4E was included as a loading control. Thr389-phosphorylation of S6K1 was not quantified in these mice because the low basal phosphorylation of this protein in muscle prevents seeing a treatment-induced decrease. The total amount of 4E–BP1 and S6K1 in muscle did not differ among the various treatment groups (data not shown). For bar graph, values are means ± SEM; N = 7, 7, 5 and 5, respectively. Values with different letters are statistically different, P < 0.05.
IGF-I effect in control and septic muscle
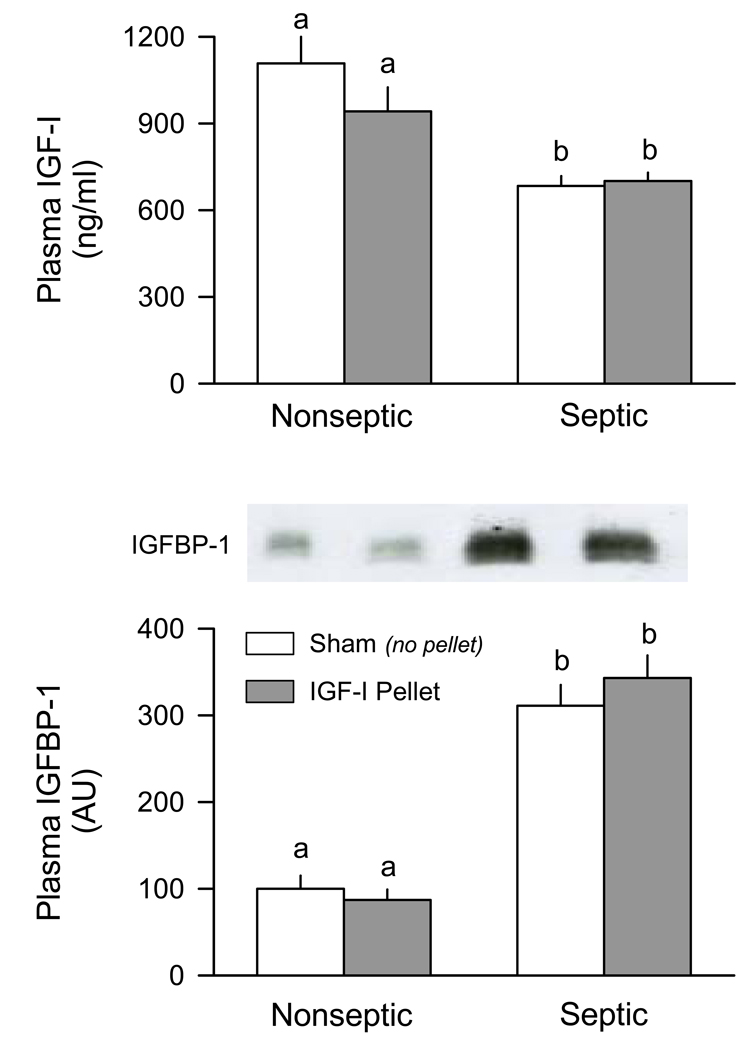
Figure 3 (top panel) illustrates the plasma IGF-I concentration was decreased approximately 30% in mice 5-days after CLP, compared to nonseptic pairfed control values. Importantly, the IGF-I in the circulation was not different between septic mice that had no pellets implanted (i.e., sham) and those in which one hindlimb was implanted with an IGF-containing pellet. Conversely, sepsis increased the plasma IGFBP-1 concentration nearly 3-fold (Figure 3, bottom panel) and, again, there was no difference in the sepsis-induced change between septic mice with an IGF-I containing pellet and sham-treated septic animals. The plasma insulin concentrations were not different between control and septic mice, and did not differ between sham and pellet-containing mice (data not shown). Finally, the heart weight was not significantly different between control and septic mice, and did not differ between sham and pellet-containing mice (data not shown). Collectively, these data indicate sepsis produces characteristic changes in the IGF system which have previously been reported in rats [25] and implantation of a pellet containing IGF-I juxtaposed to the gastrocnemius did not “leak” IGF-I into the general circulation.
Figure 3.
Plasma concentrations of IGF-I and IGFBP-1 in nonseptic (control) and septic mice implanted with IGF-I containing pellet. Mice (both nonseptic and septic) in the “pellet” group had an IGF-I containing pellet implanted near the right gastrocnemius and a placebo pellet implanted near the left gastrocnemius. Sham mice (both nonseptic and septic) had no pellets implanted. Plasma IGF-I was determined by radioimmunoassay and IGFBP-1 by Western blot analysis. Inset, a representative autoradiograph for IGFBP-1; each lane was loaded with the same amount of total protein and corresponds to one of the four experimental groups. Values are means ± SEM; N = 7, 7, 8 and 8, respectively. Values with different letters are statistically different, P < 0.05.
The gastrocnemius was removed from animals in the various groups and the IGF-I protein and mRNA content quantified. The total IGF-I protein was decreased almost 50% in muscle from septic mice, compared to muscle from pair-fed nonseptic control mice (Figure 4, top panel) A comparable decrease in IGF-I protein was detected in the left gastrocnemius of septic mice implanted with the placebo pellet. In the muscle of nonseptic mice which received the IGF-I containing pellet, the total IGF-I content tended to be increased, compared to the contralateral muscle of the same mouse, but this change did not achieve statistical significance. Using species-specific RIAs, it was determined that in nonseptic mice the IGF-I pellet decreased endogenous IGF-I and this was largely “balanced” by the presence of exogenous human IGF-I from the pellet. In contrast, in septic mice the IGF-I pellet did not decrease endogenous IGF-I and thereby increased the total IGF-I protein in muscle back to values not different from the nonseptic mice under basal conditions.
Figure 4.
IGF-I protein and mRNA content of gastrocnemius in nonseptic (control) and septic mice implanted with either a placebo or IGF-I containing pellet. Mice (both nonseptic and septic) in the “pellet” group had an IGF-I containing pellet implanted near the right gastrocnemius and a placebo pellet implanted near the left gastrocnemius. Sham mice (both nonseptic and septic) had no pellets implanted. For muscle IGF-I protein content (top panel), the third bar in each group is stacked with the open portion indicating the exogenous IGF-I derived from the implanted pellet and the cross-hatched portion indicating the endogenously produced rat IGF-I. Bottom panel, IGF-I mRNA expression of gastrocnemius determined by ribonuclease protection assay. Inset, representative autoradiographs for IGF-I and L32 mRNA are shown with each lane corresponding to one of the six experimental groups. Values were normalized to L32 and the sham nonseptic value set at 1.0 arbitrary units (AUs). Values are means ± SEM; N = 7, 7, 8, 8, 8 and 8, respectively. Values with different letters are statistically different, P < 0.05.
IGF-I mRNA expression was reduced 40% in muscle from septic mice, compared to nonseptic control values (Figure 4, bottom panel). The IGF-I mRNA content was also reduced in gastrocnemius implanted with the IGF-I containing pellet. Such an IGF-I induced decrease in IGF-I mRNA was not detected in muscle from septic mice which was similarly treated.
There was a 20% reduction in the wet weight of the gastrocnemius, compared to muscles from pair-fed control mice, over the 5-day duration of the septic insult (Figure 5, top panel). This sepsis-induced decrease was observed in sham mice as well as in the gastrocnemius from mice receiving the placebo pellet. In contrast, the weight of the gastrocnemius next to the IGF-I containing pellet was increased to a comparable extent in both nonseptic and septic mice. Comparable changes were noted for the concentration of protein in muscles from nonseptic and septic mice in the presence and absence of IGF-I, with the exception that the “increase” in protein detected in the muscle from nonseptic control mice with the placebo pellet did not achieve statistical significance (Figure 5, bottom panel).
Figure 5.
Wet weight and protein of gastrocnemius obtained from nonseptic (control) and septic mice implanted with either a placebo or IGF-I containing pellet. Mice (both nonseptic and septic) in the “pellet” group had an IGF-I containing pellet implanted near the right gastrocnemius and a placebo pellet implanted near the left gastrocnemius. Sham mice (both nonseptic and septic) had no pellets implanted. Values are means ± SEM; N = 7, 7, 8, 8, 8 and 8, respectively. Values with different letters are statistically different, P < 0.05.
Tissue cytokine mRNA content
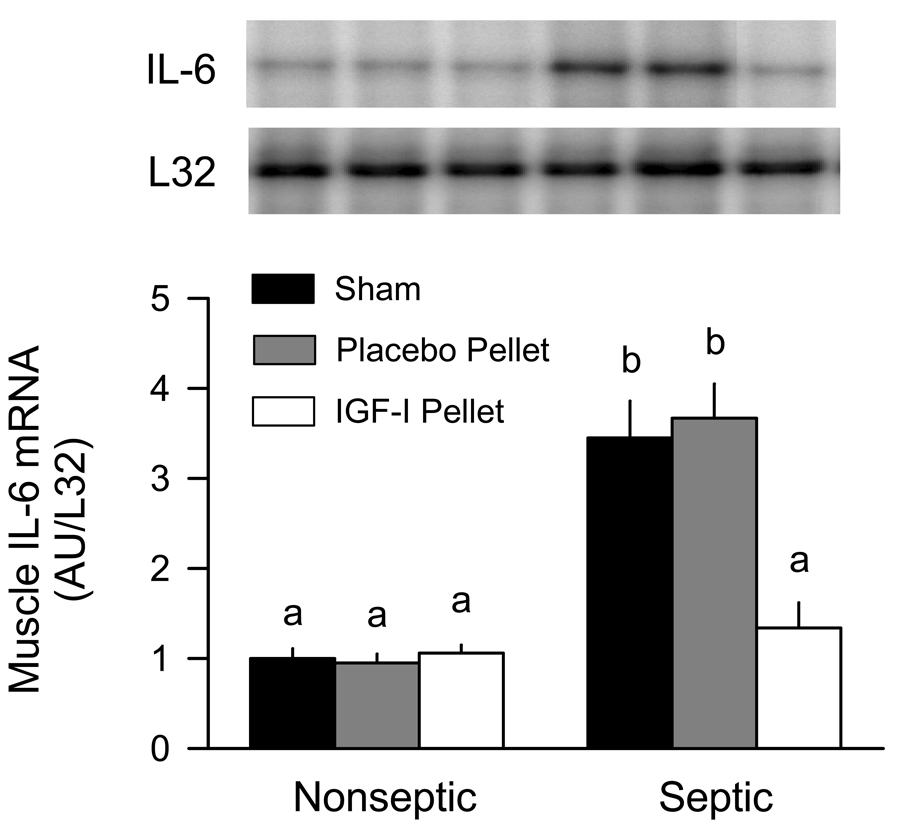
The IL-6 mRNA content in muscle from 5-day septic mice was increased approximately 3.5-fold above time-matched nonseptic control values (Figure 6). This sepsis-induced increase in IL-6 was also seen in muscle with the implanted placebo pellet. In contrast, IL-6 mRNA expression in muscle from septic mice implanted with the IGF-I containing pellet was comparable to values in nonseptic mice. The IGF-I pellet did not alter IL-6 mRNA expression in nonseptic animals, although constitutive expression was nominal under basal conditions. At the 5-day time period, no sepsis- or IGF-I change was detectable for either TNFα or IL-1 mRNA expression in skeletal muscle (data not shown). RPA analysis for TNFα, IL-6 and IL-1 was also performed on liver from control and septic mice. There was no detectable change in the mRNA content for these three cytokines in liver from control and septic mice (data not shown).
Figure 6.
IL-6 mRNA content in skeletal muscle obtained from nonseptic (control) and septic mice implanted with either a placebo or IGF-I containing pellet. Mice (both nonseptic and septic) in the “pellet” group had an IGF-I containing pellet implanted near the right gastrocnemius and a placebo pellet implanted near the left gastrocnemius. Sham mice (both nonseptic and septic) had no pellets implanted. Inset, representative autoradiographs for IL-6 and L32 mRNA are shown with each lane corresponding to one of the six experimental groups. For bar graph, values are means ± SEM; N = 7, 7, 8, 8, 8 and 8, respectively. Values with different letters are statistically different, P < 0.05.
Surrogate markers of muscle protein synthesis and degradation
In general, sepsis decreased the phosphorylation of 4E–BP1, compared to nonseptic values (Figure 7A). Moreover, this change was consistent with the reduced amount of the active eIF4E·eIF4G complex (Figure 7B) and increased amount of the inactive eIF4E·4EBP1 complex (Figure 7C). In muscle of nonseptic control mice, the IGF-I pellet increased 4E–BP1 phosphorylation and caused redistribution of eIF4E from 4E–BP1 to eIF4G. Muscle-directed IGF-I also tended to increase S6K1 phosphorylation in muscle from both nonseptic and septic mice, but neither change achieved statistical significance (Figure 7D).
Figure 7.
Phosphorylation of 4E–BP1 and S6K1, and formation of the active eIF4F complex in nonseptic (control) and septic mice implanted with either a placebo or IGF-I containing pellet. Mice (both nonseptic and septic) in the “pellet” group had an IGF-I containing pellet implanted near the right gastrocnemius and a placebo pellet implanted near the left gastrocnemius. Sham mice (both nonseptic and septic) had no pellets implanted. Panel A, Thr37/46 phosphorylation of 4E–BP1 in gastrocnemius; total 4E–BP1 was not different among the various groups (data not shown). Panels B and C, quantitation of the amount of eIF4E associated with either eIF4G or 4E–BP1 in muscle, respectively. Western blot analysis for either eIF4G or 4E–BP1 was performed on the eIF4E which was immunoprecipitated from muscle. Total eIF4E in tissue homogenates and eIF4E in the immunoprecipitate was not different between groups (data not shown). Panel D, Thr-389 phosphorylation of S6K1; total S6K1 protein was not different among the various groups (data not shown). Values are means ± SEM; N = 7, 7, 8, 8, 8 and 8, respectively. Values with different letters are statistically different, P < 0.05.
In catabolic conditions, atrogin-1 and MuRF1 mRNA content is often correlated with the rate of muscle proteolysis [26]. Sepsis increased the mRNA content for both atrogin-1 and MuRF1 in gastrocnemius, compared to values from nonseptic control mice (Figure 8). IGF-I decreased atrogin-1 expression in both nonseptic and septic mice, with the decrement in the latter group being greater in magnitude. In contrast, we failed to detect an IGF-I induced decrease in the expression of MuRF1 in either group of mice at the time point assessed.
Figure 8.
Atrogin-1 and MuRF1 mRNA content in skeletal muscle obtained from nonseptic (control) and septic mice implanted with either a placebo or IGF-I containing pellet. Mice (both nonseptic and septic) in the “pellet” group had an IGF-I containing pellet implanted in the right gastrocnemius and a placebo pellet implanted in the left gastrocnemius. Sham mice (both nonseptic and septic) had no pellets implanted. Inset, representative autoradiographs for atrogin-1, MuRF1 and L32 are shown with each lane corresponding to one of the six experimental groups. For bar graphs, values were normalized to L32 and the sham nonseptic value set at 1.0 arbitrary units (AUs). Values are means ± SEM; N = 7, 7, 8, 8, 8 and 8, respectively. Values with different letters are statistically different, P < 0.05.
DISCUSSION
The results of the present study suggest direct modulation of IGF-I availability within muscle of control mice is capable of acutely regulating the phosphorylation of proteins central to maintaining protein synthesis. In this regard, increasing IGF-I bioavailability by the intramuscular injection of Leu24Ala31 IGF-I appeared to increase mTOR activity as evidenced by enhanced phosphorylation of both 4E–BP1 and S6K1 [27]. These data extend previous work showing the addition of Leu24Ala31 IGF-I to cultured myocytes increases protein synthesis and that this stimulation resulted from a displacement of native IGF-I from various IGFBPs and not from its direct interaction with the IGF-I receptor [28]. Conversely, sequestration of bioactive IGF-I within muscle after the intramuscular injection of IGFBP-1 reduced phosphorylation of 4E–BP1. These data are also consistent with the reported reduction in muscle protein synthesis observed when IGFBP-1 was infused intravenously at a dose sufficient to reduce the circulating concentration of free IGF-I [14] or when myocytes were cultured with excess IGFBP-1 [29]. In the present study, the intramuscular and intraperitoneal injection of either Leu24Ala31 IGF-I or IGFBP-1 produced qualitatively similar changes in 4E–BP1 phosphorylation. However, we cannot draw conclusions regarding the sensitivity of muscle protein synthesis to these two different routes of administration because complete dose response curves were not generated. Regardless, our data clearly demonstrate local regulation of IGF-I has the potential to acutely impact muscle protein balance.
In preliminary studies, repeated intramuscular injections of Leu24Ala31 IGF-I were performed in an attempt to produce a sustained effect over several days. However, multiple injections into the muscle produced muscle inflammation as manifested by an increase in inflammatory cytokine mRNA content and by altered phosphorylation of 4E–BP1 (Lang unpublished observations) Hence, we replaced this invasive method in subsequent studies by the implantation of a time-release pellet in close proximity to the gastrocnemius. Muscle from the contralateral leg implanted with the placebo pellet had cytokine mRNA levels as well as surrogate markers of protein synthesis and degradation which were not different from muscle of naive control mice, suggesting the implantation procedure per se did not alter muscle protein balance. Furthermore, based on the circulating concentrations of IGF-I, IGFBP-1 and insulin as well as the heart weight, all of which were not different between sham and pellet-implanted mice, the effect of the IGF-I containing pellet on the target muscle appears to result solely from an elevation in the local muscle concentration of IGF-I.
Muscle-directed IGF-I increased the gastrocnemius mass in both control and septic mice, and the increment in weight compared to the contralateral muscle was not different between groups. The IGF-I pellet successfully restored IGF-I protein content in the target muscle of septic mice to basal control levels. In contrast, the IGF-I pellet did not significantly increase IGF-I protein in muscle of control mice, although a trend was noted. This differential effect between control and septic mice in response to IGF-I appears to result from a reduction in IGF-I synthesis (e.g., as evidenced by the decrease in IGF-I mRNA content) in muscle of control but not septic mice. The ability of locally directed IGF-I to prevent the sepsis-indued atrophy in gastrocnemius led to a corresponding increase in muscle protein. These results differ from those of Criswell et al [30] where muscle-specific over-expression of IGF-I failed to prevent atrophy produced by disuse. Although the reason for this difference is unknown, it is noteworthy that the atrophic response produced by disuse was not associated with a reduction in muscle IGF-I, whereas the IGF-I content was markedly reduced by sepsis. The ability of muscle-directed IGF-I to increase muscle protein and mass in septic animals is consistent with similar responses produced in various catabolic conditions when IGF-I is administered intravenously [31–37]. One limitation of the current study is that we did not determine whether locally administered IGF-I increased the number or size of myofibers. However, IGF-I is known to stimulate both cellular proliferation and differentiation s22 [8]. Moreover, the local infusion of IGF-I in adult rats produces a proportional increase in the muscle DNA and protein suggesting a hypertrophic response, possibly via stimulation of satellite cells [11].
Under basal conditions, sepsis and endotoxin deplete muscle protein in part via a decrease in mTOR activity and inhibition of mRNA translation [4,17]. In the current study this is evidenced by the reduction in 4E–BP1 phosphorylation and the redistribution of eIF4E from the active eIF4E·eIF4G complex to the inactive eIF4E·4EBP1 complex. Although protein synthesis was not directly determined, the changes in 4E–BP1 phosphorylation and eIF4 distribution are consistent with other studies which report a significant correlation between these parameters and protein synthesis per se in the same muscle [1]. Muscle-directed IGF-I increased the phosphorylation of 4E–BP1 (and tended to increase S6K1 phosphorylation), suggesting an increased mTOR activity. Furthermore, locally administered IGF-I also shifted a larger amount of eIF4E into the active eIF4E·eIF4G complex. While these IGF-I produced changes were relatively smaller than those seen in muscle from nonseptic mice exposed to IGF-I, they nonetheless maintained all of these endpoints at values comparable to those seen in control muscle under basal conditions. The exact mechanism for this blunted response in septic muscle was not determined but may be related to the increased concentration of IGFBP-1 in the blood and, presumably, in the muscle.
The mRNA content for atrogin-1 and MuRF1, which are collectively referred to as “atrogenes,” is upregulated in various atrophic conditions [23,26,38]. Although some reports question the causal relationship between atrogin-1 and MuRF1 mRNA expression and protein degradation per se [39], an increased mRNA content for these muscle-specific atrogenes appears to be directly proportional to increased proteolysis in many conditions. Our present data indicate both atrogin-1 and MuRF1 mRNAs were increased 5 days after induction of sepsis and these findings are consistent with previous reports indicating an upregulation at earlier times points in response to sepsis and endotoxin [40,41]. Muscle-directed IGF-I selectively decreased the sepsis-induced increase in atrogin-1 mRNA. In contrast, IGF-I failed to decrease MuRF1 mRNA in septic mice. As a result of this divergent response between the two atrogenes, it is not possible to unequivocally conclude whether the ability of locally directed IGF-I to prevent sepsis-induced wasting is mediated in part by a reduction in muscle proteolysis. In this regard, while a sustained intravenous administration of IGF-I has been reported to decrease muscle proteolysis in some catabolic conditions, such as burns and aging [42,43], it appears relatively ineffective at preventing the increased degradation produced by sepsis [44]. Therefore, additional studies which directly quantitate protein breakdown are required to definitively determine whether the ability of locally directed IGF-I to ameliorate the sepsis-induced decrease in muscle mass is mediated solely by its stimulatory effect on protein synthesis or in part by slowing the accelerated rate of protein degradation.
Elevations in one or more of the inflammatory cytokines, TNFα, IL-1 or IL-6, either directly or indirectly have been implicated in the muscle atrophy produced in a variety of conditions [25,45,46]. In general, the increase in the circulating and tissue content of these cytokines occurs relatively early (4–24 h) after infection and wanes with time [19,47]. Of the three inflammatory cytokines studied (e.g., TNFα, IL-1 and IL-6), only the latter was significantly elevated in skeletal muscle at the 5-day time point used in the current study, However, based on information in the literature, it is likely that all of these cytokines were elevated in both liver and muscle at earlier points (e.g., < 24 h) during the septic insult [48]. In contrast, in the current study, the hepatic expression for all three cytokines was not different from basal nonseptic levels at this relatively late time point. Due to technical problems, plasma cytokine levels were not assessed in our study and therefore we cannot determine whether the elevation in muscle IL-6 mRNA resulted in an elevation in its circulating concentration. The ability of the IGF-I pellet to prevent the sepsis-induced atrophy in adjacent muscle was associated with a reduction in IL-6 mRNA content. Such a reduction in cytokine production is consistent with previous reports that the systemic administration of IGF-I can attenuate the elevation in circulating TNFα, IL-1 and IL-6 levels produced by sepsis and burns [49–51]. The mechanism for the suppressive effect of IGF-I on muscle IL-6 mRNA content has not been elucidated but our preliminary studies would suggest an indirect effect of the growth factor because pre-incubation of C2C12 myoblasts with different concentrations of IGF-I did not prevent or ameliorate the endotoxin-induced increase in IL-6 mRNA content (preliminary data, Frost and Lang). Furthermore, these data are consistent with the growth defect and reduced IGF-I levels observed in transgenic mice over expressing IL-6 [52], the ability of IL-6 to decrease blood borne IGF-I [53], and the ability of locally infused IL-6 to decrease muscle protein synthesis [54]. Collectively, our data suggest it is possible to modulate IGF-I bioavailability within muscle per se and that agents increasing the availability of IGF-I within muscle might be effective in ameliorating the sepsis-induce loss of muscle mass without having undesirable effects on metabolic processes in distant organs.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Institutes of Health GM-38032.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 2.Long CL, Jeevanandam M, Kim BM, et al. Whole body protein synthesis and catabolism in septic man. Am J Clin Nutr. 1977;30:1340–1344. doi: 10.1093/ajcn/30.8.1340. [DOI] [PubMed] [Google Scholar]
- 3.Hall-Angeras M, Angeras U, Zamir O, et al. Effect of the glucocorticoid receptor antagonist RU 38486 on muscle protein breakdown in sepsis. Surgery. 1991;109:468–473. [PubMed] [Google Scholar]
- 4.Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab. 2004;287:E721–E730. doi: 10.1152/ajpendo.00132.2004. [DOI] [PubMed] [Google Scholar]
- 5.Andreyev HJ, Norman AR, Oates J, et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–509. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 6.Kotler DP, Tierney AR, Wang J, et al. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 7.Smith IJ, Lecker SH, Hasselgren PO. CALPAIN ACTIVITY AND MUSCLE WASTING IN SEPSIS. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 9.Bark TH, McNurlan MA, Lang CH, et al. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol. 1998;275:E118–E123. doi: 10.1152/ajpendo.1998.275.1.E118. [DOI] [PubMed] [Google Scholar]
- 10.Coleman ME, DeMayo F, Yin KC, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 11.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg AL. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol. 1967;213:1193–1198. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- 13.Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93:1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- 14.Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology. 2003;144:3922–3933. doi: 10.1210/en.2002-0192. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen DL, Won WB, Siu J, et al. Insulin-like growth factor binding protein-1 induces insulin release in the rat. Endocrinology. 1997;138:2073–2080. doi: 10.1210/endo.138.5.5143. [DOI] [PubMed] [Google Scholar]
- 16.Loddick SA, Liu XJ, Lu ZX, et al. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc Natl Acad Sci U S A. 1998;95:1894–1898. doi: 10.1073/pnas.95.4.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E–BP1, and S6K1 in skeletal muscle. J Cell Physiol. 2005;203:144–155. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 18.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 19.Rotwein P. Structure, evolution, expression and regulation of insulin-like growth factors I and II. Growth Factors. 1991;5:3–18. doi: 10.3109/08977199109000267. [DOI] [PubMed] [Google Scholar]
- 20.Upton Z, Yandell CA, Degger BG, et al. Evolution of insulin-like growth factor-I (IGF-I) action:in vitro characterization of vertebrate IGF-I proteins. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:35–41. doi: 10.1016/s0305-0491(98)10111-6. [DOI] [PubMed] [Google Scholar]
- 21.Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12:291–299. doi: 10.2119/2006-00071.Lang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R698–R709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 23.Krawiec BJ, Nystrom GJ, Frost RA, et al. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab. 2007;292:E1555–E1567. doi: 10.1152/ajpendo.00622.2006. [DOI] [PubMed] [Google Scholar]
- 24.Lee PD, Conover CA, Powell DR. Regulation and function of insulin-like growth factor-binding protein-1. Proc Soc Exp Biol Med. 1993;204:4–29. doi: 10.3181/00379727-204-43630. [DOI] [PubMed] [Google Scholar]
- 25.Lang CH, Frost RA. Role of growth hormone, insulin-like growth factor-I, and insulin-like growth factor binding proteins in the catabolic response to injury and infection. Curr Opin Clin Nutr Metab Care. 2002;5:271–279. doi: 10.1097/00075197-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 27.Avruch J, Hara K, Lin Y, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 28.Lang CH, Frost RA. Effect of insulin-like growth factor proteins on skeletal muscle protein metabolism during normal and catabolic conditions. In: Houston MS, Holly JJP, Feldman EL, editors. IGF Nutrition in Health and Disease. Totowa, NJ: Humana Press Inc; 2004. pp. 193–209. [Google Scholar]
- 29.Frost RA, Lang CH. Differential effects of insulin-like growth factor I (IGF-I) and IGF-binding protein-1 on protein metabolism in human skeletal muscle cells. Endocrinology. 1999;140:3962–3970. doi: 10.1210/endo.140.9.6998. [DOI] [PubMed] [Google Scholar]
- 30.Criswell DS, Booth FW, DeMayo F, et al. Overexpression of IGF-I in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol. 1998;275:E373–E379. doi: 10.1152/ajpendo.1998.275.3.e373. [DOI] [PubMed] [Google Scholar]
- 31.Debroy MA, Wolf SE, Zhang XJ, et al. Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J Trauma. 1999;47:904–910. doi: 10.1097/00005373-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Lang CH, Frost RA, Svanberg E, et al. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab. 2004;286:E916–E926. doi: 10.1152/ajpendo.00554.2003. [DOI] [PubMed] [Google Scholar]
- 33.Svanberg E, Frost RA, Lang CH, et al. IGF-I/IGFBP-3 binary complex modulates sepsis-induced inhibition of protein synthesis in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1145–E1158. doi: 10.1152/ajpendo.2000.279.5.E1145. [DOI] [PubMed] [Google Scholar]
- 34.Svanberg E, Ohlsson C, Kimball SR, et al. rhIGF-I/IGFBP-3 complex, but not free rhIGF-I, supports muscle protein biosynthesis in rats during semistarvation. Eur J Clin Invest. 2000;30:438–446. doi: 10.1046/j.1365-2362.2000.00652.x. [DOI] [PubMed] [Google Scholar]
- 35.Tomas FM, Knowles SE, Owens PC, et al. Increased weight gain, nitrogen retention and muscle protein synthesis following treatment of diabetic rats with insulin-like growth factor (IGF)-I and des(1–3)IGF-I. Biochem J. 1991;276(Pt 2):547–554. doi: 10.1042/bj2760547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomas FM. The anti-catabolic efficacy of insulin-like growth factor-I is enhanced by its early administration to rats receiving dexamethasone. J Endocrinol. 1998;157:89–97. doi: 10.1677/joe.0.1570089. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Iresjo BM, Karlsson L, et al. Provision of rhIGF-I/IGFBP-3 complex attenuated development of cancer cachexia in an experimental tumor model. Clin Nutr. 2000;19:127–132. doi: 10.1054/clnu.1999.0090. [DOI] [PubMed] [Google Scholar]
- 38.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol. 2007;292:R328–R336. doi: 10.1152/ajpregu.00561.2006. [DOI] [PubMed] [Google Scholar]
- 39.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1777–R1789. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frost RA, Nystrom GJ, Jefferson LS, et al. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E501–E512. doi: 10.1152/ajpendo.00359.2006. [DOI] [PubMed] [Google Scholar]
- 41.Yu Z, Li P, Zhang M, et al. Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS ONE. 2008;3:e2086. doi: 10.1371/journal.pone.0002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dardevet D, Sornet C, Savary I, et al. Glucocorticoid effects on insulin- and IGF-I-regulated muscle protein metabolism during aging. J Endocrinol. 1998;156:83–89. doi: 10.1677/joe.0.1560083. [DOI] [PubMed] [Google Scholar]
- 43.Fang CH, Li BG, James JH, et al. Protein breakdown in muscle from burned rats is blocked by insulin-like growth factor i and glycogen synthase kinase-3beta inhibitors. Endocrinology. 2005;146:3141–3149. doi: 10.1210/en.2004-0869. [DOI] [PubMed] [Google Scholar]
- 44.Fang CH, Li BG, Sun X, et al. Insulin-like growth factor I reduces ubiquitin and ubiquitin-conjugating enzyme gene expression but does not inhibit muscle proteolysis in septic rats. Endocrinology. 2000;141:2743–2751. doi: 10.1210/endo.141.8.7593. [DOI] [PubMed] [Google Scholar]
- 45.Kotler DP. Cachexia. Ann Intern Med. 2000;133:622–634. doi: 10.7326/0003-4819-133-8-200010170-00015. [DOI] [PubMed] [Google Scholar]
- 46.Vary TC. Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr Metab Care. 1998;1:217–224. doi: 10.1097/00075197-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang CH, Nystrom G, Frost RA. Beta-adrenergic blockade exacerbates sepsis-induced changes in tumor necrosis factor alpha and interleukin-6 in skeletal muscle and is associated with impaired translation initiation. J Trauma. 2008;64:477–486. doi: 10.1097/01.TA.0000249375.43015.01. [DOI] [PubMed] [Google Scholar]
- 49.Inoue T, Saito H, Fukushima R, et al. Growth hormone and insulinlike growth factor I enhance host defense in a murine sepsis model. Arch Surg. 1995;130:1115–1122. doi: 10.1001/archsurg.1995.01430100093018. [DOI] [PubMed] [Google Scholar]
- 50.Jeschke MG, Barrow RE, Herndon DN. Insulinlike growth factor I plus insulinlike growth factor binding protein 3 attenuates the proinflammatory acute phase response in severely burned children. Ann Surg. 2000;231:246–252. doi: 10.1097/00000658-200002000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeschke MG, Barrow RE, Suzuki F, et al. IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med. 2002;8:238–246. [PMC free article] [PubMed] [Google Scholar]
- 52.De BF, Alonzi T, Moretta A, et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–650. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemet D, Eliakim A, Zaldivar F, et al. Effect of rhIL-6 infusion on GH-->IGF-I axis mediators in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1663–R1668. doi: 10.1152/ajpregu.00053.2006. [DOI] [PubMed] [Google Scholar]
- 54.Haddad F, Zaldivar F, Cooper DM, et al. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]