Abstract
The mammalian brain and spinal cord contain heterogeneous populations of cycling, immature cells. These include cells with stem cell-like properties as well as progenitors in various stages of early glial differentiation. This latter population is distributed widely throughout gray and white matter and numerically represents an extremely large cell pool. In this review, we discuss the possibility that the glial progenitors that populate the adult CNS are one source of gliomas. Indeed, the marker phenotypes, morphologies, and migratory properties of cells in gliomas strongly resemble glial progenitors in many ways. We review briefly some salient features of normal glial development and then examine the similarities and differences between normal progenitors and cells in gliomas, focusing on the phenotypic plasticity of glial progenitors and the responses to growth factors in promoting proliferation and migration of normal and glioma cells, and discussing known mutational changes in gliomas in the context of how these might affect the proliferative and migratory behaviors of progenitors. Finally, we will discuss the “cancer stem cell” hypothesis in light of the possibility that glial progenitors can generate gliomas.
Keywords: Glial development, Gliomas, Glial progenitors, Oligodendrocytes, Astrocytes, Neural stem cells, PDGF, EGF, Cell migration
Introduction
The cellular sources of gliomas have been a favored subject of speculation by neuropathologists and neuro-oncologists for decades. As neuropathologists, we are all aware of the variety of gliomas, as defined by their architectures, cellular histologies and molecular marker expression profiles. But to complicate matters, gliomas often contain a heterogeneous mix of cell types and architectures. It is not clear how much of this heterogeneity is due to underlying genetic events and how much is due to epigenetic influences or the intermingling of neoplastic and reactive glia.
The recent glioma literature has featured the premise that gliomas arise from neural “stem cells.” Furthermore, the glioma stem cells are able to differentiate to a certain extent along glial lineages, thereby acquiring the phenotypes of immature glia and thus contributing to the cellular heterogeneity of a glioma. Indeed, glioma phenotypes appear to be consistent with those of immature glia, rather than fully differentiated glial cells. The idea of neural stem cells giving rise to gliomas is made less clear by the lack of a uniform definition of a “neural stem cell.” While we do not argue against the possibility that some gliomas indeed arise from “stem-like” cells, in this review we consider another possible cellular origin of gliomas, glial progenitor cells. The developing and even the adult mammalian CNS contain many cycling cells that belong to early glial lineages. These cells are distributed widely, many residing in white matter, and although in percentile terms constitute a small fraction of total CNS cells, a large brain would contain a large number in aggregate (see below).
In this review, we will give an overview of CNS glial development, focusing on issues relevant to gliomas, including lineages, cell migration, gene expression, phenotypic plasticity and growth factor responsiveness. We will then consider diffusely infiltrating glial tumors, including astrocytomas, oligodendrogliomas and glioblastomas, in the context of glial progenitor biology. We believe that many of the biological behaviors of gliomas and many of the histological findings with which neuropathologists are so familiar reflect normal characteristics of glial progenitors in conditions in which these cells lose their normal controls on proliferation and differentiation.
In discussing CNS glial lineages we will focus on the following important points:
The developing CNS appears to contain a mix of progenitors, which can give rise to oligodendrocytes and astrocytes, astrocytes and neurons, oligodendrocytes and neurons, or all three major cell types, as well as cells with restricted fates that generate only one type of cell. How and when these immature cells become restricted to one or more lineages is not exactly known.
Oligodendrocyte progenitors appear to have a greater capacity to proliferate in response to growth factors than do astrocyte progenitors; in fact, oligodendrocyte progenitors can be kept immature and proliferative indelinitely when stimulated with the proper growth factors (including platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF));
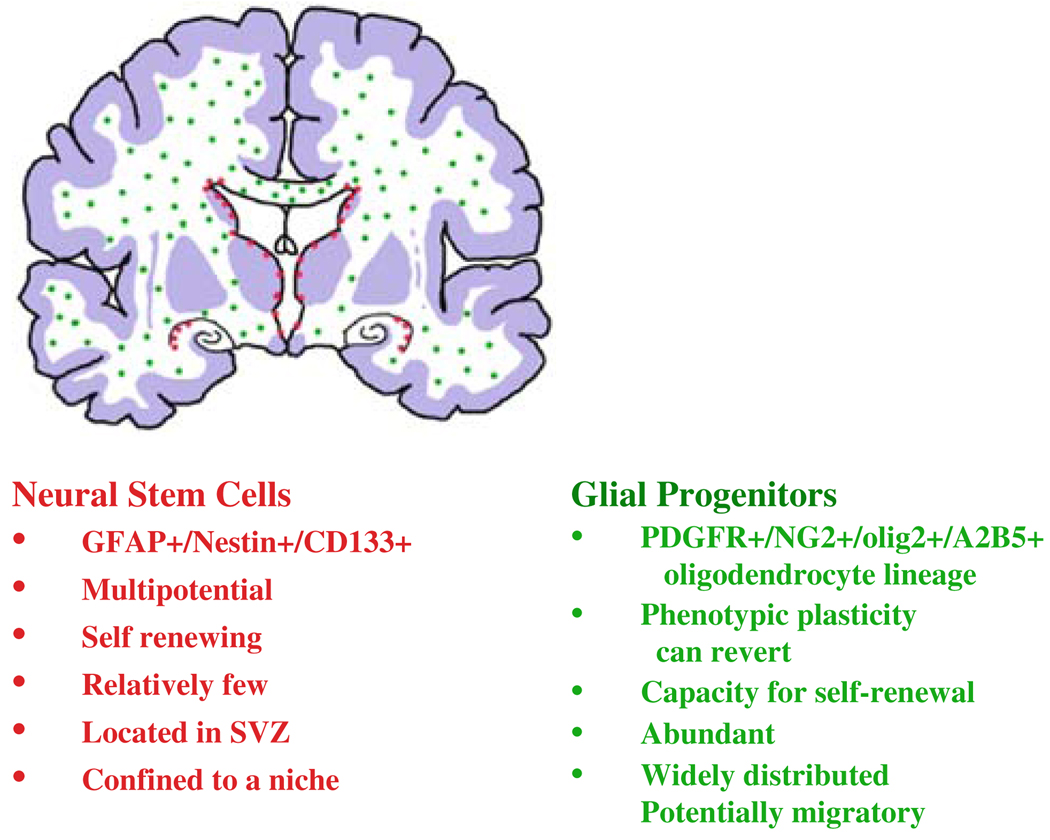
A subset of glial progenitors remains immature and cycling throughout life—these cells are abundant (1– 4% in white matter) making them arguably the largest population of cycling cells in the adult brain; they are widely distributed throughout the brain, but appear to be most abundant in subcortical white matter, where most gliomas occur;
Glial progenitors can revert to a less mature state when stimulated with growth factors.
Many recent studies have focused on the idea that gliomas contain “cancer stem cells” and that these cells are unique in their capacity to initiate to growth and recurrence. In this paper, we focus on the different but related question of cell of origin—what are the cells in the normal brain that have the capacity to give rise to brain tumors? Specifically, we will put forth the argument that glial progenitors have the capacity to form brain tumors and the gliomas are largely composed of cells that closely resemble glial progenitors. At the end of the paper, we will review the current concept of cancer stem cells as it applies to gliomas, comparing and contrasting them to the concept of progenitor-like glioma cells and discussing the implications of these ideas to the cancer stem cell hypothesis.
An overview of mammalian gliogenesis
In this review, we will focus on gliogenesis in the forebrain, since the large majority of diffusely infiltrating gliomas arise in the forebrain. The precursors of astrocytes and oli-godendrocytes originate, as do all neural cells, from the embryonic ventricular zone (VZ). During embryonic and early postnatal life, VZ cells delaminate from their epithelium and some of them develop into small, proliferative, and highly migratory cells that populate the adjacent sub-ventricular zone (SVZ). To colonize the brain, these migratory precursors exit from the SVZ and then travel in some cases long distances into gray and white matter, where they either differentiate into mature glia or remain as immature, resident progenitors [106, 107]. Oligodendrocyte precursors are generated both ventrally and dorsally. Ventral precursors not only populate the ventral forebrain, but they also migrate extensively into the dorsal forebrain, primarily through the intermediate zone in a tangential (parallel to the pial surface) path [41]. Early astrocyte origins are less clear. Some astrocytes arise directly from radial glia, and thus do not go through a migratory phase, while others come from migratory SVZ cells and travel into the overlying white and gray matter. It is important to note that glial precursors, both astrocytes and oligodendrocytes, continue to divide during the migratory phase, and even continue dividing after they have ceased migrating. This phenomenon, which generates clonal clusters of glia, appears to be much more characteristic of the oligodendrocyte than the astrocyte lineage, suggesting that oligodendrocyte precursors possess a greater capacity to proliferate than do astrocyte precursors [107]. Details of astrocyte and oligodendrocyte development are given in recent reviews [30, 31, 50, 66].
While most glial precursors either differentiate into mature glia or die during early postnatal development, a subset remains immature and cycling through adult life (Fig. 1, Fig. 2). The proportion of such cells with respect to the total cell number is higher in white matter than in gray matter. It is estimated that these cells account for up to 4% of the total cells in the adult white matter, making them arguably the largest population of cycling cells in the adult brain [28, 38, 70, 75]). This population is not a homogeneous one, but rather appears to be a mixture of immature cells that represent progenitors in different stages of glial lineages: (1) long-term thymidine labeling in vivo suggests that both oligodendrocytes and astrocytes are derived from these cells in the normal adult brain; (2) if removed from the brain, this population contains subsets that can be differentiated into astrocytes, oligodendrocytes, and neurons; (3) a small percentage can generate neurospheres, indicating a potential stem-like behavior.
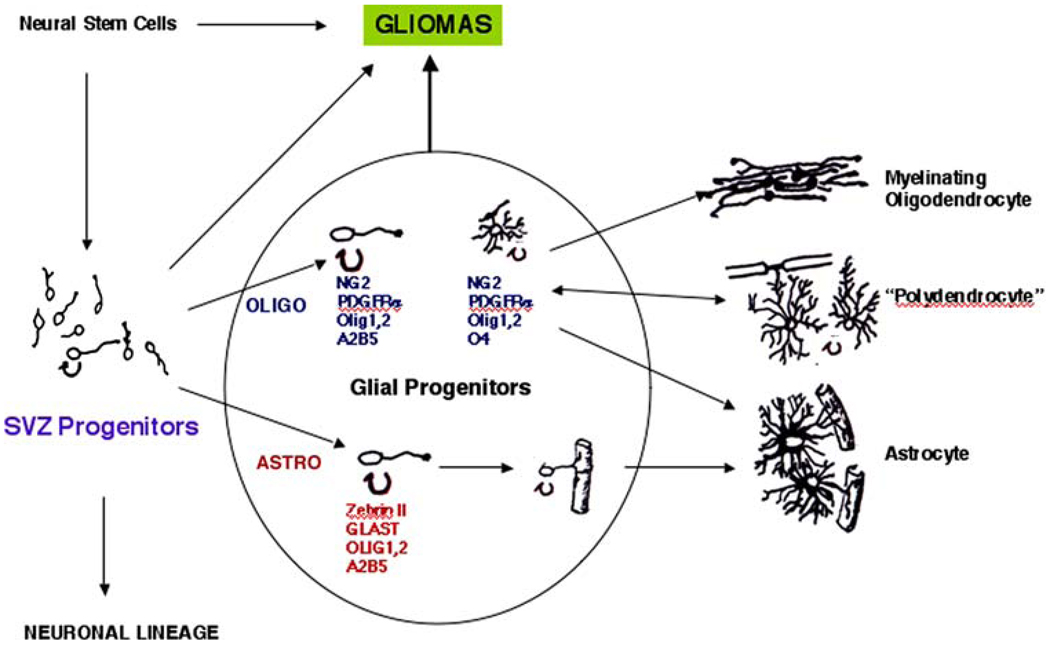
Fig. 1.
Lineage diagram for the development of oligodendrocytes and astrocytes from forebrain SVZ cells, representing some of the immature, intermediate forms of glia as well as the mature forms. We are considering “Glial Progenitors” as a heterogeneous population of immature, cycling cells, which could serve as a source of gliomas. Also noted are “Neural Stem Cells”, which generate SVZ cells, and represent other source of gliomas. SVZ cells also generate neurons, as listed, but we have not considered neuronal or neuronal/glial tumors here. ZebrinII, also known as aldolase C, is expressed by VZ cells, radial glia, SVZ astrocytes, and developing astrocytes [57, 91]. GLAST is a glutamate transporter expressed by some radial glia and by astrocytes [24]. Besides Olig1 and Olig2, a number of other transcription factors are critical for oligodendrocyte development, and include Nkx 2.2, Nkx6.2, Hes 5, Sox5, 6, 10, Mash1 (AskI1), Myt1; Zpf488 [reviewed in 66, 100]. Space limitations preclude a lengthy discussion of transcription factor requirements in gliogenesis
Fig. 2.
Diagram of a coronal section of a human brain showing the localization of neural stem cells in subependymal areas and hippocampus and the widespread localization of glial progenitors in white matter. Not shown here are the smaller number of cycling progenitors in gray matter areas. The description of Glial Progenitors refers to those of the oligodendrocyte lineage, since those appear to be the largest population and can be delineated by markers
Lineages
The progressive restriction of cell fates during CNS development generates populations of gliogenic progenitors. Furthermore, there is good evidence that at least some astrocytes and oligodendrocytes arise from common glial progenitor cells. For example, progenitor cells that are restricted to a glial fate have been isolated from the developing CNS [50, 69]. In vivo lineage analysis studies using retroviral labeling of progenitors in the SVZ indicate that some progenitors give rise to mixed clones composed of astrocytes and oligodendrocytes [107]. However, in some CNS regions and at certain times in embryonic development, astrocytes and oligodendrocytes do not appear to arise from common glial progenitors. In the spinal cord, the majority of oligodendrocytes are generated from a small, ventral domain in the embryonic VZ that Wrst generates motor neurons and then oligodendrocytes (known as the olig2/PMN domain, since the cells therein express the bHLH transcription factor, Olig2, and develop Wrst into motor neurons) [52, 108]. However, the sequential generation of neurons and then oligodendrocytes from a common VZ region does not necessarily imply that the two kinds of mature cells have a common, lineage-restricted progenitor [104]. Lineage tracing with inducible Cre systems shows that a subset of astrocytes and ependymal cells are also generated from the olig2/PMN domain [58].
What induces a glial progenitor to acquire an astrocytic or oligodendrocytic fate is not clearly understood, although a number of factors, such as BMPs and IL-6 family members will promote astrocytic differentiation, while other factors, such as sonic hedgehog, PDGF, and insulin-like growth factor-1 (IGF1) will promote oligodendrocyte differentiation [66, 68]. The bHLH transcription factor, olig2, which is expressed in early oligodendrocytes and astrocytes, represses the transcription of pro-neuronal genes [95, 109], and therefore presumably allows the progenitors to respond to gliogenic signals. The full cascade of signaling pathways and transcription factors involved in this process has yet to be worked out. Other transcription factors play important roles in gliogenesis, including Olig1, Mash1, Nkx2.2, Sox10, Hes5, and others [see for review, 66, 100].
Figure 1 depicts a lineage chart for oligodendrocyte and astrocyte development from forebrain SVZ cells in rodents, showing some of the markers associated with different developmental stages. We have grouped “Glial Progenitors” as a heterogeneous population of cells, some or all of which could generate gliomas. Note that early progenitors express the gangliosides recognized by the A2B5 antibody as well as PSA-NCAM. However, these early markers are not specific to glial lineages, being also expressed by neuronal precursors [see for e.g., 81, 84].
Oligodendrocyte precursors can be identified by the expression of markers, including the NG2 chondroitin sulfate proteoglycan and the PDGF receptor alpha (PDGFRα) (Fig. 1). Cells that express these markers have two major fates. The first is to develop into myelinating oligodendrocytes. The second is to develop into cells characterized by a lacy, highly process-bearing morphology, termed “synanto-cytes” [12] or “polydendrocytes” [67]. This latter cell type, which also expresses the NG2 proteoglycan, is found in all regions of the CNS. Its functions are not clear, although some extend processes to Nodes of Ranvier, and some receive synaptic-like inputs from neurons, at least in the hippocampus [76]. This cell type, or a subset thereof, continue to proliferate throughout life and, in fact, represent one of the largest populations of cycling cells in the adult brain [2, 35]. The NG2+ cells have been thought to be oligodendrocyte precursor cells, able to differentiate into myelinating oligodendrocytes either during normal glial turnover, or during remyelination [77]. However, recent evidence suggests that NG2+ cells can also generate protoplasmic astrocytes [110].
Oligodendrocytes proceed through a relatively well-defined sequence of differentiation (Fig. 1). Early stage progenitors are A2B5+, which does not distinguish them from neuronal progenitors, and then acquire a series of markers, beginning with olig1 and 2, PDGFRα, NG2 proteoglycan, sulfatides (recognized by the O4 monoclonal antibody), and later, galactocerebroside, proteolipid protein, MAG, myelin basic protein, and other components of the myelin sheath. As oligodendrocytes mature, they eventually lose PDGFRα and NG2 expression. This differentiation occurs over the course of about a week in vitro, which is thought to resemble the time it takes for oligodendrocytes to differentiate in the brain. There is some degree of overlap among these populations.
The stages of astrocyte development are not as well characterized as those for oligodendrocytes. Figure 1 shows some of the markers used to define astrocyte lineage cells, although many of these markers are also found in radial glial cells. Several laboratories have isolated astrocyte precursors, defined as immature cells from the developing CNS that can differentiate into astrocytes in culture [48, 49, 63, 64, 79]. These precursors vary in their fate restrictions and in their growth factor requirements for promoting differentiation, but many require added factors, such as IL-6 family members (LIF, CNTF), or BMPs to promote astrocyte differentiation.
The regulation of astrocyte precursor and astrocyte proliferation is complex. Nearly all known astrocyte precursors respond to the basic fibroblast growth factor (bFGF), or FGF-2, which promotes precursor survival and to an extent, proliferation. However, in contrast to oligodendrocyte precursors, which can be kept cycling indefinitely with growth factors (see below), astrocyte precursors will halt proliferation despite the continued presence of bFGF [48]. Epidermal growth factor (EGF) will stimulate astrocyte proliferation in culture, but its effect is inhibited by bFGF [36]. Several growth factors will stimulate thymidine incorporation into astrocytes in culture, but this incorporation is inhibited by transforming growth factor-beta-1 (TGF-beta-1) [99]. We find that astrocyte precursors, as they differentiate, begin to secrete TGF-beta-1, (G. Lin and J.E. Goldman unpublished observations). Thus, astrocytes appear to have cell autonomous feedback systems that limit their proliferation. One of the important brakes on astrocyte precursor cycling appears to be p53. As we discuss below, the loss of p53 function may be an early event in astrocytoma genesis. However, the loss of p53 is not sufficient for transformation, a process that in normal astrocytes requires further genetic changes. For example, human astrocytes in culture show a progressive loss of proliferation blocks when p53 and p16INK4A are lost and then the catalytic subunit of telomerase (hTERT) is constitutively expressed [21]. All three changes transform the cells fully. Finally, the cell cycle kinase inhibitor p27Kip1 also inhibits astrocyte proliferation in culture settings [42, 65]. Thus, the ability to continue cycling with growth factors in the absence of genetic changes appears to be a fundamental difference between oligodendrocyte progenitors and astrocyte precursors.
Isolating glial progenitors from the adult CNS is an important technical strategy if we are to consider such progenitors as possible sources of gliomas. The monoclonal antibodies against surface glycosphingolipids, A2B5 and O4, have proven useful in isolating adult glial progenitors from dissociated tissue preparation (human and rodent) since the lipid antigens are not cleaved by protease treatment [59, 70, 102, for examples]. Normally, adult glial progenitors are non-migratory and cycle slowly (at least the A2B5+ progenitors isolated and examined in vitro) [102], but they can be induced to migrate and proliferate rapidly when stimulated by growth factors. The normal behavior and fate of adult glial progenitors in vivo has not been as well characterized. Progenitors in adult rat and mouse sub-cortical white matter can be labeled with replication-deficient retroviruses that express reporter genes, and the fates of these cycling cells followed [26, 27]. In the normal brain, most of the retrovirus-labeled cells differentiate along the oligodendrocyte lineage [3], while others remain immature and cycling. During a demyelinating-remyelinating lesion, these progenitors differentiate into myelinating oli-godendrocytes, thus participating in lesion repair. They do not migrate far from the site of viral labeling, although if they are forced to overexpress PDGF, they become highly migratory and infiltrative (see below).
Phenotypic plasticity in glial development
Lineage “switching”
The normal lineages of glial development should not be confused with the phenotypic plasticity of glial precursors. There are several experimental examples in which immature glial cells of one lineage can be induced to express genes of another lineage. Indeed, immature glia can express markers we associate with astrocyte and oli-godendrocyte lineages simultaneously under the right conditions. This plasticity of immature glia may underlie the phenotypic heterogeneity one sees in many gliomas (see below). This type of plasticity was first described for the oligodendrocyte precursors of the optic nerve, the “O-2A” cells, which will halt their development into oligo-dendrocytes and begin to express astrocyte markers, such as GFAP if they are removed from the nerve and cultured in the presence of serum or IL-6 family members (CNTF, LIF) [47]. Thus, early glial precursor cells can show developmental plasticity under defined circumstances. These observations also find an analogy in oligodendro-gliomas, which often express GFAP in a subset of cells (see below).
GFAP itself, a characteristic intermediate filament of mature astrocytes, is expressed by radial glia in the embryonic primate brain [11, 15, 45]. Furthermore, the recent identification of GFAP+ “stem” cells in the adult mammalian SVZ [19] (which are likely derived from embryonic radial glia) has broadened the specificity of GFAP as a purely astrocyte marker (although some might claim that these “stem” cells are in fact astrocytes). Nevertheless, they lose GFAP expression as they differentiate into neurons and oligodendrocytes.
Precursor “reversion”
A second type of plasticity concerns the “reversion” to a less mature state. For example, A2B5–/O4+ cells isolated from adult rat white matter will become A2B5+/O4– when treated in vitro with PDGF [59]. PDGF can also induce reversion of O4+ cells isolated from neonatal brain. A2B5+ glial precursor cells isolated from the adult optic nerve or cerebral hemispheres cycle in culture more rapidly when exposed to PDGF [102]. PDGF will also induce cycling and induce non-migratory precursors in the adult rodent white matter to begin migrating and proliferating in vivo [3]. Furthermore, oligodendrocyte progenitors isolated from the P6 rat optic nerve can be reprogrammed to acquire a multipotential stem cell-like phenotype in culture if treated with the appropriate growth factors [43].
Phenotypic heterogeneity of gliomas
Diffusely infiltrating gliomas, including astrocytomas, oli-godendrogliomas, and glioblastomas, are the most common type of primary brain tumors. These tumors can show a variety of histological and cytological features. Neuropathologists have created diagnostic categories with which to divide these tumors into different types and grades. Astro-cytomas are predominantly composed of cells with morphologic and immunophenotypic similarities to astrocytes (cells have oval nuclei, coarse processes and express GFAP), whereas oligodendrogliomas more closely resemble immature cells of the oligodendroglial lineage (round nuclei, fewer processes, less GFAP expression). In practice, however, it is common to encounter gliomas that contain a mixture of cells with astrocytic or oligodendroglial features. In contrast, it is rare for gliomas to contain neoplastic cells that display neuronal features (see recent articles on glio-neuronal tumors [20, 51]. The phenotypic heterogeneity seen in many infiltrating gliomas likely reflects the inherent lineage plasticity of glial progenitors.
Growth factor signaling in glial progenitors and gliomas
Several growth factors have been implicated in regulating gliogenesis during normal brain development. Among these, PDGF, and EGF are two of the best characterized and most firmly established to play important roles in the regulation of normal glial development and gliomagenesis. Both PDGFRs and EGFR belong to the larger family of receptor tyrosine kinases. The signaling cascades that are activated by these receptors have been extensively studied in a number of systems. The details vary depending on the cell type studied, but some general principles have emerged. (1) The binding of ligands to the extracellular domains induces receptors to dimerize and trans-phosphorylate tyrosine residues on their cytoplasmic domains. This leads to the activation of several second-messenger signaling pathways, including (among others) the well-characterized RAS-MAP and PI3K-AKT pathways. These signaling cascades involve the reversible phosphorylation events that regulate the associations and enzymatic activity of the various signaling molecules. The development of phospho-specific antibodies has made it possible to characterize the activation of these pathways in situ and at the cellular level. Recent studies have used phospho-specific antibodies to characterize the activation of signaling pathways in human glioma specimens. Human gliomas often show elevated levels of activation of these signaling pathways [23, 73]. Furthermore, animal studies have shown that constitutive activation of these pathways can drive glial progenitors to form tumors (see below).
PDGF-PDGFR
In the oligodendrocyte lineage, the regulation of proliferation and differentiation is clearly under the control of specific growth factors, particularly PDGF. PDGF supplementation in cultures will keep glial precursors cycling and inhibit their differentiation for a limited number of passages, after which they differentiate along the oligodendrocyte lineage. However, if PDGF is given in combination with FGF the cells will remain proliferative and immature indefinitely [10, 60]. PDGF is also a mitogen for early glial precursors, so it keeps them immature and promotes migration as well [61, 88]. Transgenic mice that express PDGF under the control of the GFAP promoter or NSE promoter overproduce PDGF and overproduce oligodendrocyte progenitors [14, 98, 103]. Progenitor numbers are directly correlated to the levels of PDGF overexpression, suggesting that the availability of PDGF is a major determinant in the generation and survival of oligodendrocyte progenitors [98].
PDGF can drive glial progenitors to behave like glioma cells
If one carries PDGF expression to an extreme in vivo, by forcing glial precursors themselves to express PDGF, the precursors continue to divide and migrate, and form what look very much like malignant gliomas [3, 17, 86, 96]. In a recent study [3], we used stereotactic injections to infect glial progenitors in the adult white matter with retroviruses that express PDGF. Tumors that closely resembled human glioblastoma formed in 100% of the animals by 2 weeks post injection. The tumors were composed of a massive expansion of cells with the immunophenotype of oligodendrocyte progenitors (olig2+/NG2+/PDGFRα+). The cells did not express GFAP, EGFR, or neuronal markers. The tumors also contained numerous GFAP+ astrocytes, but less than 1% of the retrovirus-infected cells were GFAP+. This study provided the first in vivo evidence that adult glial progenitors have the proliferative and self-renewing capacity needed to form malignant tumors. Remarkably, the tumors contained a mixture of retrovirus-infected and uninfected cells, suggesting that PDGF was driving tumor formation via autocrine and paracrine signaling. Furthermore, these findings suggest that even genetically normal progenitors can be driven to behave in a malignant manner if exposed to high enough levels of growth factor. This raises the fascinating possibility that human gliomas may similarly contain normal progenitor cells that have been recruited to proliferate within the mitogen-rich environment of the tumor and in this way significantly contribute to the growth of the tumor. This is not to say that human gliomas do not contain a clonal expansion of genetically transformed cells. There is an enormous body of evidence to show that they do. However, gliomas are remarkably heterogeneous and often contain multiple genotypes within a single tumor. Also, due to their infiltrative growth pattern, glioma cells intermingle with non-neoplastic cells in the surrounding brain tissue, including both astrocytes and glial progenitors, both of which have an inherent capacity to proliferate in response to brain injury and mitogenic stimulation. Thus, infiltrating gliomas may contain large numbers of non-neoplastic glia that are recruited to proliferate within the mitogenic environment of the tumor. At present, however, there is no definitive way to distinguish a reactive/recruited progenitor or astrocyte from a glioma cell and the degree to which recruited progenitors contribute to growth of human gliomas remains an open question. However, transplanting human glioma cells that express PDGF into nude rats induces the massive expansion of rat glial progenitors that contribute significantly to tumor growth providing compelling evidence that such recruitment may occur in the human tumors (Lopez et al. in preparation).
EGF-EGFR
EGF is mitogenic for early neural progenitor cells, is expressed in early glia, but its receptor, EGFR, eventually is down-regulated as the cells mature. However, the constitutive expression of EGFR will keep glial precursors in a proliferative and migratory state in the neonatal or adult rodent CNS in vivo [1, 39]. Forcing the constitutive overex-pression of EGFR in neonatal or adult rat white matter glial progenitors via infection with retrovirus that expresses an EGFR-GFP fusion protein results in a diffuse hypercellu-larity in the white matter that continues to expand over the course of several months. The EGFR-GFP+ cells continue to express markers of immature glial progenitors (PDGFRα+/NG2+/olig2+) and seem to be “stuck” in a highly migratory and proliferative state [39]. Approximately 30% of the rats spontaneously formed large, solid tumors composed of EGFR-GFP+ cells in addition to the diffuse hypercellularity (Ivkovic in preparation).
EGF and PDGF in gliomagenesis
These same growth factors have also been implicated in the pathogenesis of human gliomas. The EGFR gene is amplified in 30–40% of primary glioblastomas and EGFR is overexpressed in over 60% of glioblastomas [8, 72]. Some glioblastomas express mutated growth factor receptors, such as the constitutively active EGFR VIII mutant. Much attention has been focused on the role of ligand-independent signaling via these receptors. However, it is more common for gliomas to overexpress the full length, ligand-responsive form of EGFR [8], suggesting that glioma cell proliferation may be in part dependent on environmentally derived growth factors. Similarly, PDGF ligands and receptors are overexpressed in many human gliomas, suggesting the possibility of both autocrine and paracrine PDGF signaling loops [32, 97]. Furthermore, higher-grade more aggressive gliomas tend to express higher levels of PDGF, suggesting that PDGF’s effects on glioma growth are dose dependent. Several experimental systems have confirmed that both PDGF and EGF have powerful effects on glioma cell migration, proliferation and survival. Furthermore, in vitro studies have shown that glioma cells can be stimulated to proliferate and migrate by growth factors such as EGF and PDGF [53, 54, 78, 97].
The interaction between growth factor signaling and tumor suppressors
Evidence suggests that gliomagenesis involves the combined effects of two types of signaling events: (1) elevated levels of growth factor stimulation and (2) loss of tumor suppressors that normally serve to modulate growth factor signaling and block uncontrolled growth. For example, Pten, which is mutated in approximately 30% of all glioblastomas is known to regulate growth factor signaling via its interactions with the pI3 kinase pathway [55, 90]. Thus, the loss of Pten potentiates signaling via both EGFR and PDGFRα. Indeed, the response to chemotherapies that target EGFR (Gefitinib and Erlotinib) depends on the combined genetic status of EGFR and Pten [62, 83]. Although it is well established that PTEN is also an important modulator of PDGF signaling, relatively little is known about the interactions between PTEN and PDGF signaling in gliomas. There is evidence that the expression of PDGFRα and loss of heterozygosity of chromosome 10q23 are seen together in a significant proportion of glioblastomas [33, 94], raising the possibility of a functional cooperation in gliomagenesis. This idea is supported by in vitro evidence that PTEN forms a ternary complex with PDGFRα and Na/H exchange regulatory factor 1 (NHERF1) to regulate PDGF signaling via the PI3K pathway [94]. Furthermore, genetic deletion of PTEN, while not sufficient to initiate tumor formation on its own, will facilitate tumor formation in glioma-prone mice that contain additional genetic lesions [44, 101, 105]. Similarly, we have found that genetic deletion of pten from adult glial progenitors will significantly facilitate PDGF-driven tumor formation. Furthermore, cell culture studies have shown that deleting pten will render glial progenitors more responsive to the proliferative effects of PDGF stimulation (Ellis, Assanah and Canoll, in preparation).
Mutations in p53 are frequently seen in low-grade astrocytomas and secondary GBMs, often in association with expression of PDGF and PDGFRα [33, 72]. These studies suggest that loss of p53 function may be an early event in the process of gliomagenesis. Loss of p53 is not sufficient to transform astrocytes fully, but may cooperate with other genetic alterations to facilitate tumor formation. Astrocytes isolated from p53 null mice are immortalized and grow more rapidly than do astrocytes from mice with intact p53. After extended passages in vitro in the presence of appropriate growth factors these p53−/− astrocytes will become fully transformed, and acquire the capacity to form tumors when injected into nude mice [9]. Although the loss of p53 was not sufficient to induce the formation of brain tumors in mice, 60% of the p53−/− mice (but not the p53 ± or p53+/+ littermates) formed glioblastoma-like tumors after prenatal exposure to the mutagen ethylnitrosourea (ENU) [29]. Another study showed that PDGF expressing retrovirus induced tumor formation faster and more consistently when injected into the brains of neonatal p53−/− mice than when the same virus was injected to wild-type mice [34]. Similarly, our preliminary studies using Cre expressing retrovirus to selectively delete p53 from adult white matter progenitors in p53lox/lox mice greatly facilitate PDGF-driven tumor formation (Ellis, Ludwig and Canoll unpublished results). Together, these studies suggest that loss of tumor suppressor genes such as pten or p53 is not sufficient to initiate tumor formation, but that these genetic lesions can cooperate with growth factor stimulation to facilitate tumor formation and progression.
Glioma infiltration recapitulates progenitor migration
Glial precursors migrate widely through the CNS. They continue to divide after they have migrated out of the SVZ. They do not appear to migrate randomly through the developing CNS, but rather take preferential pathways, including radial glia, axonal tracts, and blood vessels [40, 93, and P. Canoll, unpublished observations]. Migratory precursors display an overall unipolar or bipolar morphology (Fig. 3). However, time-lapse imaging of migrating precursors in living brain slices clearly shows that they transiently elaborate and withdraw multiple branches or sub-branches [40]. In general, those precursors migrating through white matter tend to be less branched than those in gray matter, possibly because of the simpler and more linear extracellular environment of white matter.
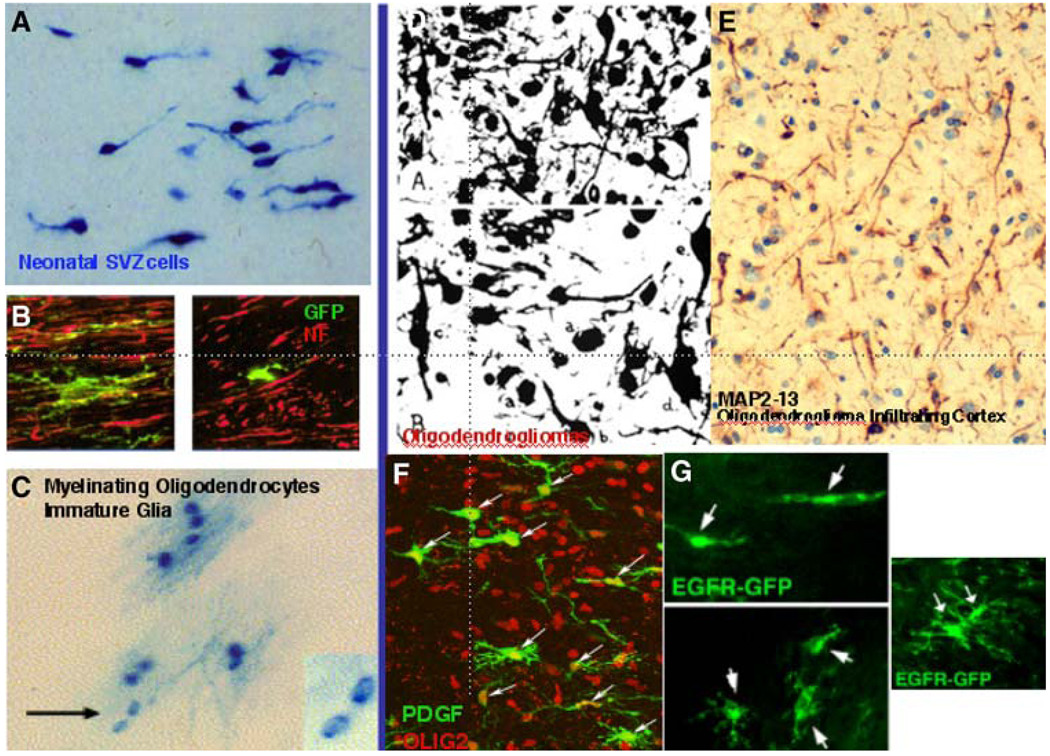
Fig. 3.
Morphology of glial progenitors and gliomas, illustrating similarities. a SVZ cells in a neonatal rat forebrain, here labeled with ret-roviruses that express beta-galactosidease. The unipolar and bipolar forms are typical of these pregenitors. b Cycling cells in the subcortical white matter of an adult rat forebrain, here labeled with retroviruses that express eGFP and double labeled for neurofilaments (red). The forms of these cells vary from complex (left) to simpler (right). c Clonal clusters of oligodendrocytes in an adult rat brain, labeled with a beta-galactosidase-expressing retrovirus. Most of the cells are mye-linating oligodendrocytes, except for two cells with larger nuclei and no attached myelin (arrow and inset) [107]. d Silver staining of oligodendrogliomas, showing a pleiomorphic population, many of which appear unipolar or bipolar [5]. e Oligodendroglioma cells infiltrating human neocortex, imaged by ABC-peroxidase method with an antibody against MAP2-13, also appear simple in morphology [92]. f Progenitor cells in an adult rat forebrain expressing PDGF by retroviral injection generate tumors that look like malignant gliomas. The infected cells (green, arrows) appear uni- or bipolar or more complex, with a few processes; all express olig2 (red). g Progenitor cells in the adult rat forebrain white matter expressing EGFR-GFRP by retroviral injection. These cells appear uni-or bipolar or more complex with a few processes [39]
The morphologies of precursors (and the morphologies of glioma cells) are not readily discernible in standard H&E-stained sections, which emphasize nuclear characteristics. Experimental labeling of precursors with fluorescent markers shows the complexity and moment-to-moment variation in precursor shapes. In the case of human gliomas, cytoplasmic labeling with an antibody raised against Exon 13 of beta tubulin will show cellular processes [92], recalling the classic silver carbonate labeling of gliomas started in the 1920s and 1930s [5] (Fig. 3). Of course, what we observe in histological sections shows the appearance of individual cells at a particular time, but we should not conclude that these morphologies represent stable states.
We propose that glioma infiltration recapitulates the migration of glial progenitor cells that occurs during brain development. This idea is strongly supported by a recent study using the time-lapse microscopy to monitor the migration of GFP expressing glioma cells in living CNS slices [6, 22]. Similar to progenitor cells, the migrating gli-oma cells typically displayed a unipolar morphology, with a prominent leading process that extends and retracts with rapid dynamics. The cell body and nucleus moves in a saltatory fashion with spurts of nuclear translocations separated by periods of immobility. Both glioma cells and progenitors frequently turn or reverse direction, suggesting that they are responding to local attractive and/or repulsive guidance cues.
Some important differences were noted. Most notably, the migrating glioma cells frequently proliferated en route, pausing for about an hour to undergo mitosis before resuming migration. In contrast, migrating glial progenitor cells were rarely seen to undergo cell division in slice culture. However, adding PDGF to the slice culture media stimulates migrating glial progenitors to proliferate en route in a way that closely resembles glioma cells (Assanah et al. in preparation).
Cells of origin of gliomas and the implications to the cancer stem cell hypothesis
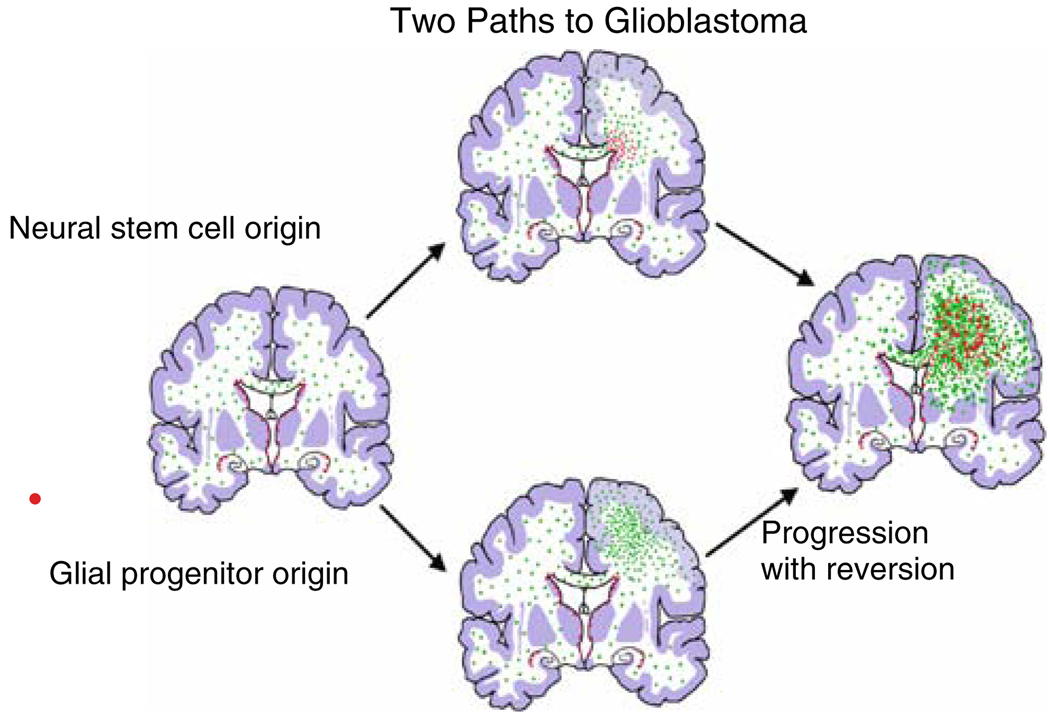
Gliomas are heterogeneous and it is very unlikely that all gliomas arise from the same cell of origin. Well-established clinical features of gliomas provide important insight into the obligate criteria for a cell of origin: (1) the majority of gliomas occur in adults and therefore the cells of origin must be found in the adult brain, (2) gliomas can arise anywhere in the CNS, therefore, the cells of origin must be widely distributed throughout the brain, (3) gliomas are predominantly composed of cells with a distinctly glial phenotype and only rarely contain neoplastic cells with neuronal features. Thus, gliomas either arise from, or quickly evolve into, cells with a glial restricted potential. For many years astrocytes had been considered a prime candidate for a cell of origin of astrocytomas and glioblastomas. Indeed, cell culture studies and animal models have shown that GFAP+ cells have the capacity to form tumors when subjected to the right combinations of genetic lesions and environmental conditions [4, 9, 105]. The recent discovery that some human gliomas contain a subset of cells with stem cell-like properties has focused attention on neural stem cells of the adult SVZ as a likely “cell of origin” for human glioblastomas [18, 25, 29, 37, 82] (Fig. 4). This analogy to the neurogenic adult SVZ is often used as a perspective from which to view CNS “cancer stem cells”, but it is not clear that it is an appropriate perspective, since it reflects only neurogenesis in the adult brain and does not take into account the plasticity and self-renewing capacity of glial progenitors.
Fig. 4.
Diagram representing the generation of glioblastomas from neural stem cells or glial progenitors. The progenitors in this depiction form lower grade gliomas first and then high-grade tumors
One feature of adult neural stem cells is that they divide asymmetrically, generating another stem cell and a neural progenitor cell, which is destined to differentiate. It has been proposed that cancer stem cells go through an asymmetrical division, generating another cell that retains the capacity to self-renew and a daughter cell that has a limited capacity to self-renew [7].
However, the definition and existence of glioma “cancer stem cells” is at present unclear. Investigators have postulated the following characteristics: (1) glioma cancer stem cells are rare in the total tumor population; (2) they have a unique capacity to self-renew endlessly and therefore the only cells capable of giving rise to gliomas and glioma recurrences; (3) they proliferate slowly and are relatively resistant, compared with other cells in the tumor, to chemotherapy and radiation; (4) they have the ability to generate progeny with a limited capacity to self-renew and therefore non-tumorigenic; (5) they are multipotential, giving rise to all of the different cell types in a tumor.
One of the major limitations to our current understanding of cancer stem cells is the lack of definitive markers that can be used distinguish cancer stem cells from other cells in the tumor. Several studies have proposed that CD133 (also known as prominin) can be used to identify and isolate cancer stem cells and that these cells have a unique capacity to initiate tumor growth [89]. However, low-grade gliomas, and a significant proportion of glioblastomas do not contain CD133+ cells [71, 80], raising the issue of what cells give rise to these tumors. Possible candidates are glial progenitors and progenitor-like glioma cells, which are found in abundance in both low- and high-grade gliomas. Furthermore, normal glial progenitors have the capacity to revert to a stem cell-like phenotype in vitro [43]. This phenotypic reversion raises the possibility that the “de-differentiation” of progenitors generates “stem-like” cells in high-grade gliomas. Thus, the presence of stem-like cells in human gliomas should not be taken as proof that gliomas arise from neural stem cells.
There is accumulating evidence that CD133+ cells are not the only elements in gliomas with significant tumorigenic potential. Human gliomas also contain an abundance of cells that express markers normally expressed by adult glial progenitors, including PDGFRα, NG2, olig2, and A2B5 [16, 46, 56, 68, 71, 87]. Furthermore, A2B5+/CD133—cells freshly isolated from human high-grade gliomas form tumors when injected into nude rodents [71], providing another possible link between glial progenitorlike cells and tumorigenesis. The idea that glial progenitors can give rise to gliomas is strongly supported by the results of our animal model studies showing the adult glial progenitors have the capacity to form GBM-like tumors when infected with a PDGF expressing retrovirus [3]. These findings greatly expand the pool of cells in the adult brain that must be considered as potential “cells of origin” (Fig. 2).
We have considered how epigenetic mechanisms promote plasticity in the glial progenitor population and how this plasticity correlates with and perhaps helps to explain the phenotypic heterogeneity in many gliomas. As a final point, consider whether a progenitor to stem cell transition could take place in gliomas, a point already made in principle [43]. Might the plasticity of glial progenitors in the context of gliomas allow for their conversion to cells with stem-like properties? Given that the local environment of blood vessels appears to provide a niche for stem cells in the normal CNS [74, 85], might one anticipate that the interactions of glioma cells with blood vessels would confer on the tumor cells some stem-like characteristics that might elude glioma cells in other microenvironments?
Alternatively, cells with progenitor properties that are distinct from “cancer stem cells”, as defined above, can themselves cause tumor growth and tumor recurrence. Thus, targeting “cancer stem cells” may not be adequate to eliminate tumor growth.
A recent study has shown that CD133+ “stem-like” cells in brain tumors are located next to blood vessels. Furthermore, inhibiting angiogenesis in brain tumor xenografts with anti-VEGF inhibited tumor growth and reduced the numbers of self-renewing stem-like cells that could be isolated from the tumors [13]. These findings suggest that cancer stem cells are dependent on a perivascular niche that is generated by robust vascular proliferation that occurs during tumor progression. This may also explain why stemlike cells are not seen in low-grade gliomas [71, 80]. It also suggests that the infiltrative margin of gliomas where the migratory glioma cells are intermingled with relatively normal brain parenchyma may be highly enriched in progenitor-like glioma cells and relatively devoid of stem-like cells.
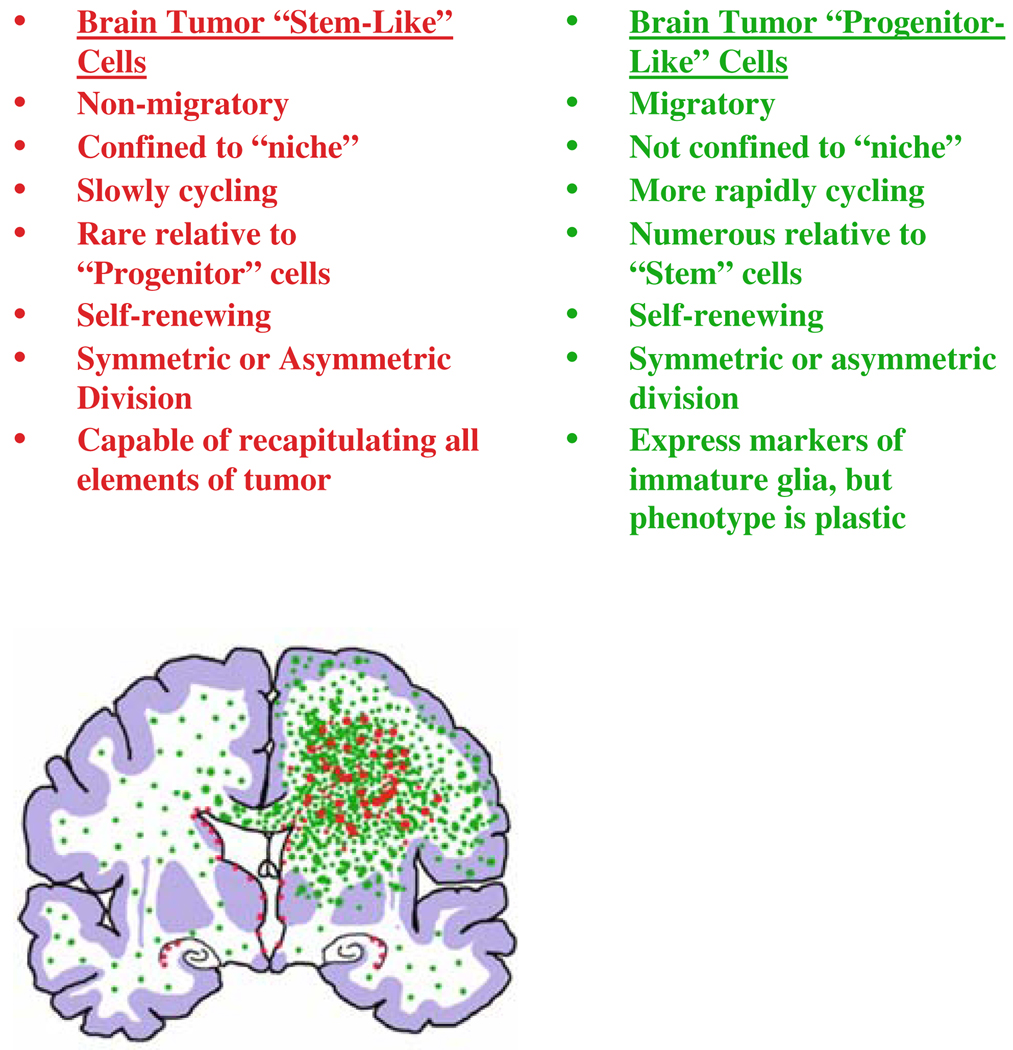
At present, there is no consensus on how to define brain tumor “cancer stem cells.” The cancer stem cell” hypothesis assumes a hierarchical nature of relationships among cells in a tumor, with a unidirectional progression from stem-like cells that have an inexhaustible capacity to self-renew to progenitor-like cells that have a limited capacity to self-renew. However, we would argue that “progenitorlike” glioma cells retain the capacity to proliferate and self-renew, and can contribute to tumor growth, although in a different way than stem-like cells (Fig. 5). Both populations should be taken into consideration when addressing therapeutic approaches.
Fig. 5.
Possible characteristics of brain tumor “stem-like” cells and brain tumor “progenitor-like” cells
Acknowledgments
Supported in part by NIH Grants KO8-NS045070 (P.C.) and NS17125 (J.E.G.)
References
- 1.Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25:11092–11106. doi: 10.1523/JNEUROSCI.2981-05.2005. doi: 10.1523/JNEUROSCI.2981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. doi:10.1002/1098-1136(200009)31:3<219::AID-GLIA30>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. doi: 10.1016/S1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 5.Bailey P, Bucy PC. Oligodendrogliomas of the brain. J Pathol Bacteriol. 1929;32:735–751. doi: 10.1002/path.1700320403. [Google Scholar]
- 6.Beadle C, Assanah MC, Monzo P, Vallee R, Rosenfeld SS, Canoll P. The role of myosin II in glioma invasion of the brain. Mol Biol Cell. 2008;19:3357–3368. doi: 10.1091/mbc.E08-03-0319. doi: 10.1091/mbc.E08-03-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger F, Gay E, Pelletier L, Tropel P, Wion D. Development of gliomas: potential role of asymmetrical cell division of neural stem cells. Lancet Oncol. 2004;5:511–514. doi: 10.1016/S1470-2045(04)01531-1. doi: 10.1016/S1470-2045(04)01531-1. [DOI] [PubMed] [Google Scholar]
- 8.Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H. Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol. 2004;14:131–136. doi: 10.1111/j.1750-3639.2004.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogler O, Nagane M, Gillis J, Huang HJ, Cavenee WK. Malignant transformation of p53-deficient astrocytes is modulated by environmental cues in vitro. Cell Growth differ. 1999;10:73–86. [PubMed] [Google Scholar]
- 10.Bogler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci USA. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borit A, McIntosh GC. Myelin basic protein and glial fibrillary acidic protein in human fetal brain. Neuropathol Appl Neurobiol. 1981;7:279–287. doi: 10.1111/j.1365-2990.1981.tb00099.x. doi: 10.1111/j.1365-2990.1981.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 12.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, et al. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. doi:10.1016/S0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 15.Choi BH. Glial fibrillary acidic protein in radial glia of early human fetal cerebrum: a light and electron microscopic immunoperoxidase study. J Neuropathol Exp Neurol. 1986;45:408–418. doi: 10.1097/00005072-198607000-00003. doi: 10.1097/00005072-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Colin C, Baeza N, Tong S, Bouvier C, Quilichini B, Durbec P, et al. In vitro identification and functional characterization of glial precursor cells in human gliomas. Neuropathol Appl Neurobiol. 2006;32:189–202. doi: 10.1111/j.1365-2990.2006.00740.x. doi: 10.1111/j.1365-2990.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 17.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dirks PB. Cancer: stem cells and brain tumours. Nature. 2006;444:687–688. doi: 10.1038/444687a. doi: 10.1038/444687a. [DOI] [PubMed] [Google Scholar]
- 19.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 20.Edgar MA, Rosenblum MK. Mixed glioneuronal tumors: recently described entities. Arch Pathol Lab Med. 2007;131:228–233. doi: 10.5858/2007-131-228-MGTRDE. [DOI] [PubMed] [Google Scholar]
- 21.Evans RJ, Wyllie FS, Wynford-Thomas D, Kipling D, Jones CJ. A p53-dependent, telomere-independent proliferative life span barrier in astrocytes consistent with the molecular genetics of glioma development. Cancer Res. 2003;63:4854–4861. [PubMed] [Google Scholar]
- 22.Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 23.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 24.Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stemlike neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 26.Gensert JM, Goldman JE. In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia. 1996;17:39–51. doi: 10.1002/(SICI)1098-1136(199605)17:1<39::AID-GLIA4>3.0.CO;2-2. doi:10.1002/(SICI)1098-1136(199605)17:1<39::AID-GLIA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. doi: 10.1016/S0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 28.Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. doi: 10.1002/neu.1043. [PubMed] [Google Scholar]
- 29.Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman JE. Lineages of astrocytes and oligodendrocytes. In: Kettenmann H, Ransom BR, editors. Neuroglia. 2nd edn. New York: Oxford University Press; 2005. pp. 72–84. [Google Scholar]
- 31.Guillemot F. Cell fate specification in the mammalian tel-encephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–3219. [PubMed] [Google Scholar]
- 33.Hermanson M, Funa K, Koopmann J, Maintz D, Waha A, Wes-termark B, et al. Association of loss of heterozygosity on chromosome 17p with high platelet-derived growth factor alpha receptor expression in human malignant gliomas. Cancer Res. 1996;56:164–171. [PubMed] [Google Scholar]
- 34.Hesselager G, Uhrbom L, Westermark B, Nister M. Complementary effects of platelet-derived growth factor autocrine stimulation and p53 or Ink4a-Arf deletion in a mouse glioma model. Cancer Res. 2003;63:4305–4309. [PubMed] [Google Scholar]
- 35.Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498:525–538. doi: 10.1002/cne.21065. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huff KR, Schreier W. Fibroblast growth factor inhibits epidermal growth factor-induced responses in rat astrocytes. Glia. 1990;3:193–204. doi: 10.1002/glia.440030306. doi: 10.1002/glia.440030306. [DOI] [PubMed] [Google Scholar]
- 37.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 38.Imamoto K, Paterson JA, Leblond CP. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. I. Sequential changes in oligodendrocytes. J Comp Neurol. 1978;180:115–128. doi: 10.1002/cne.901800108. doi: 10.1002/cne.901800108. [DOI] [PubMed] [Google Scholar]
- 39.Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J Neurosci. 2008;28:914–922. doi: 10.1523/JNEUROSCI.4327-07.2008. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron. 1999;23:461–472. doi: 10.1016/s0896-6273(00)80800-4. doi: 10.1016/S0896-6273(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 41.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koguchi K, Nakatsuji Y, Nakayama K, Sakoda S. Modulation of astrocyte proliferation by cyclin-dependent kinase inhibitor p27(Kip1) Glia. 2002;37:93–104. doi: 10.1002/glia.10017. doi: 10.1002/glia.10017. [DOI] [PubMed] [Google Scholar]
- 43.Kondo T, Raff M. Oligodendrocyte precursor cells repro-grammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 44.Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68:3286–3294. doi: 10.1158/0008-5472.CAN-07-6867. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- 46.Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 47.Lillien LE, Raff MC. differentiation signals in the CNS: type-2 astrocyte development in vitro as a model system. Neuron. 1990;5:111–119. doi: 10.1016/0896-6273(90)90301-u. doi: 10.1016/0896-6273(90)90301-U. [DOI] [PubMed] [Google Scholar]
- 48.Lin G, Goldman JE. An FGF-responsive astrocyte precursor isolated from the neonatal forebrain. Glia. 2008 doi: 10.1002/glia.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, et al. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276(1):31–46. doi: 10.1016/j.ydbio.2004.08.018. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Rao MS. Glial progenitors in the CNS and possible lineage relationships among them. Biol Cell. 2004;96:279–290. doi: 10.1016/j.biolcel.2004.02.001. doi: 10.1016/j.biolcel.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, et al. Common developmental requirement for olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. doi: 10.1016/S0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 53.Lund-Johansen M, Bjerkvig R, Humphrey PA, Bigner SH, Bigner DD, Laerum OD. effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 1990;50:6039–6044. [PubMed] [Google Scholar]
- 54.Lund-Johansen M, Forsberg K, Bjerkvig R, Laerum OD. effects of growth factors on a human glioma cell line during invasion into rat brain aggregates in culture. Acta Neuropathol. 1992;84:190–197. doi: 10.1007/BF00311394. doi: 10.1007/BF00311394. [DOI] [PubMed] [Google Scholar]
- 55.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phos-phatidylinositol 3, 4, 5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 56.Mao X, Barfoot R, Hamoudi RA, Noble M. Alleletyping of an oligodendrocyte-type-2 astrocyte lineage derive from a human glioblastoma multiforme. J Neurooncol. 1998;40:243–250. doi: 10.1023/a:1006158010388. doi: 10.1023/A:1006158010388. [DOI] [PubMed] [Google Scholar]
- 57.Marshall CA, Goldman JE. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, et al. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendro-cytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293:358–369. doi: 10.1016/j.ydbio.2006.02.029. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 59.Mason JL, Goldman JE. A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- 60.McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- 61.McKinnon RD, Waldron S, Kiel ME. PDGF alpha-receptor signal strength controls an RTK rheostat that integrates phos-phoinositol 3’-kinase and phospholipase Cgamma pathways during oligodendrocyte maturation. J Neurosci. 2005;25:3499–3508. doi: 10.1523/JNEUROSCI.5049-04.2005. doi: 10.1523/JNEUROSCI.5049-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 63.Mi H, Barres BA. Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neu-rosci. 1999;19:1049–1061. doi: 10.1523/JNEUROSCI.19-03-01049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakatsuji Y, Miller RH. Density dependent modulation of cell cycle protein expression in astrocytes. J Neurosci Res. 2001;66:487–496. doi: 10.1002/jnr.1240. doi: 10.1002/jnr.1240. [DOI] [PubMed] [Google Scholar]
- 66.Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55:1287–1299. doi: 10.1002/glia.20540. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- 67.Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- 68.Noble M, Mayer-Proschel M. Growth factors, glia and gliomas. J Neurooncol. 1997;35:193–209. doi: 10.1023/a:1005898228116. doi: 10.1023/A:1005898228116. [DOI] [PubMed] [Google Scholar]
- 69.Noble M, Pröschel C, Mayer-Pröschel M. Getting a GR(i)P on oligodendrocyte development. Dev Biol. 2004;265:33–52. doi: 10.1016/j.ydbio.2003.06.002. doi: 10.1016/j.ydbio.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, et al. Identification and isolation of multipo-tential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. (2nd) 2003;9:439–447. doi: 10.1038/nm837. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 71.Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, et al. Identification of A2B5 + CD133- tumor-initiating cells in adult human gliomas. Neurosurg. 2008;62:505–514. doi: 10.1227/01.neu.0000316019.28421.95. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- 72.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 73.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. doi:10.1002/1096-9861(20001002)425:4<479::AID-CNE2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 75.Paterson JA, Privat A, Ling EA, Leblond CP. Investigation of glial cells in semithin sections. 3. Transformation of sube-pendymal cells into glial cells, as shown by radioautography after 3 H-thymidine injection into the lateral ventricle of the brain of young rats. J Comp Neurol. 1973;149:83–102. doi: 10.1002/cne.901490106. doi: 10.1002/cne.901490106. [DOI] [PubMed] [Google Scholar]
- 76.Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–521. doi: 10.1016/j.conb.2006.08.009. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Polito A, Reynolds R. NG2-expressing cells as oligoden-drocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pollack IF, Randall MS, Kristofik MP, Kelly RH, Selker RG, Vertosick FT., Jr Response of low-passage human malignant gliomas in vitro to stimulation and selective inhibition of growth factor-mediated pathways. J Neurosurg. 1991;75:284–293. doi: 10.3171/jns.1991.75.2.0284. [DOI] [PubMed] [Google Scholar]
- 79.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 80.Rebetz J, Tian D, Persson A, Widegren B, Salford LG, Englund E, Gisselsson D, Fan X. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS ONE. 2008;3:e1936. doi: 10.1371/journal.pone.0001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rousselot P, Lois C, Alvarez-Buylla A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J Comp Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- 82.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 83.Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 84.Schnitzer J, Schachner M. Cell type specificity of a neural cell surface antigen recognized by the monoclonal antibody A2B5. Cell Tissue Res. 1982;224:625–636. doi: 10.1007/BF00213757. doi: 10.1007/BF00213757. [DOI] [PubMed] [Google Scholar]
- 85.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 86.Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 87.Shoshan Y, Nishiyama A, Chang A, Mork S, Barnett GH, Cowell JK, et al. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci USA. 1999;96:10361–10366. doi: 10.1073/pnas.96.18.10361. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simpson PB, Armstrong RC. Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia. 1999;26:22–35. doi:10.1002/(SICI)1098-1136 (199903)26:1<22::AID-GLIA3>3.0.CO;2-M. [PubMed] [Google Scholar]
- 89.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 90.Stambolic V, Suzuki A, De la, Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. doi: 10.1016/S0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 91.Staugaitis SM, Zerlin M, Hawkes R, Levine JM, Goldman JE. Aldolase C/zebrin II expression in the neonatal rat forebrain reveals cellular heterogeneity within the subventricular zone and early astrocyte differentiation. J Neurosci. 2001;21:6195–6205. doi: 10.1523/JNEUROSCI.21-16-06195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzuki SO, Kitai R, Llena J, Lee SC, Goldman JE, Shafit-Zagardo B. MAP-2e, a novel MAP-2 isoform, is expressed in gliomas and delineates tumor architecture and patterns of infiltration. J Neuropathol Exp Neurol. 2002;61:403–412. doi: 10.1093/jnen/61.5.403. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci. 2003;23:4240–4250. doi: 10.1523/JNEUROSCI.23-10-04240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi Y, Morales FC, Kreimann EL, Georgescu MM. PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J. 2006;25:910–920. doi: 10.1038/sj.emboj.7600979. doi: 10.1038/sj.emboj.7600979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, et al. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev. 2000;99:143–148. doi: 10.1016/s0925-4773(00)00466-4. doi: 10.1016/S0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- 96.Uhrbom L, Hesselager G, Nister M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58:5275–5279. [PubMed] [Google Scholar]
- 97.van der Valk P, Lindeman J, Kamphorst W. Growth factor profiles of human gliomas. Do non-tumour cells contribute to tumour growth in glioma? Ann Oncol. 1997;8:1023–1029. doi: 10.1023/a:1008265905505. doi: 10.1023/A:1008265905505. [DOI] [PubMed] [Google Scholar]
- 98.van Heyningen P, Calver AR, Richardson WD. Control of progenitor cell number by mitogen supply and demand. Curr Biol. 2001;11:232–241. doi: 10.1016/s0960-9822(01)00075-6. doi: 10.1016/S0960-9822(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 99.Vergeli M, Mazzanti B, Ballerini C, Gran B, Amaducci L, Massacesi L. Transforming growth factor-beta 1 inhibits the proliferation of rat astrocytes induced by serum and growth factors. J Neurosci Res. 1995;40:127–133. doi: 10.1002/jnr.490400114. doi: 10.1002/jnr.490400114. [DOI] [PubMed] [Google Scholar]
- 100.Wegner M. A matter of identity: transcriptional control in oligodendrocytes. J Mol Neurosci. 2008;35:3–12. doi: 10.1007/s12031-007-9008-8. doi: 10.1007/s12031-007-9008-8. [DOI] [PubMed] [Google Scholar]
- 101.Wei Q, Clarke L, Scheidenhelm DK, Qian B, Tong A, Sabha N, et al. High-grade glioma formation results from postnatal pten loss or mutant epidermal growth factor receptor expression in a transgenic mouse glioma model. Cancer Res. 2006;66:7429–7437. doi: 10.1158/0008-5472.CAN-06-0712. doi: 10.1158/0008-5472.CAN-06-0712. [DOI] [PubMed] [Google Scholar]
- 102.Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2A perinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 104.Wu S, Wu Y, Capecchi MR. Motoneurons and oligoden-drocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–590. doi: 10.1242/dev.02236. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]
- 105.Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van D, yke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172–5180. doi: 10.1158/0008-5472.CAN-04-3902. doi: 10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- 106.Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate Wlament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15:7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zerlin M, Milosevic A, Goldman JE. Glial progenitors of the neonatal subventricular zone differentiate asynchronously, leading to spatial dispersion of glial clones and to the persistence of immature glia in the adult mammalian CNS. Dev Biol. 2004;270:200–213. doi: 10.1016/j.ydbio.2004.02.024. doi: 10.1016/j.ydbio.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 108.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype speciWcation. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. doi: 10.1016/S0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. doi: 10.1016/S0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- 110.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]