Abstract
Natural killer (NK) cells are innate immune lymphocytes capable of killing target cells and producing immunoregulatory cytokines. Herein, we discuss recent studies that indicate that NK cells span the conventional boundaries between innate and adaptive immunity. For example, it was recently discovered that NK cells have the capacity for memory-like responses, a property that was previously thought to be limited to adaptive immunity. NK cells have also been identified in multiple tissues, and a subset of cells that specialize in the production of the TH17 cytokine IL-22, NK-22s, was recently described in mucosal-associated lymphoid tissue. Finally, we review work that shows that NK cells develop at sites that were traditionally thought to be occupied only by adaptive immune cells, including the thymus and lymph nodes.
Keywords: natural killer cell, innate immunity, memory, IL-22, cytokine
Glossary
CCL20 chemokine (C-C motif) ligand 20
CCR6 chemokine (C-C motif) receptor 6
IFN-γ interferon gamma
IL interleukin
IRF interferon regulatory factor
LIF leukaemia inhibitory factor
LTi lymphoid tissue inducer cells
Ly49C/I killer cell lectin-like receptors Ly49C and I
Ly49H killer cell lectin-like receptor Ly49H
MCMV murine cytomegalovirus
MHC major histocompatibility complex
NKp44 natural cytotoxicity triggering receptor 2
Rag2 recombination activating gene 2
ROR retinoid-related orphan receptor
SCID severe combined immunodeficiency
TH T helper
TRAIL tumour necrosis factor-related apoptosis-inducing ligand
Introduction
The immune response is mediated by two broad systems which provide innate and adaptive immunity and work together to efficiently combat the wide range of pathogens that challenge vertebrates (Janeway & Medzhitov, 2002). While adaptive T and B lymphocytes provide long-lasting specific immunity, the first line of defence against pathogens is the innate immune system. This is best demonstrated in patients with defects in innate immunity, who suffer from uncontrolled, fatal infections (Biron et al, 1989; Bustamante et al, 2008). The innate immune system precedes adaptive immunity from a phylogenetic standpoint and is present in both plants and animals (Janeway & Medzhitov, 2002). Although at first glance innate immunity might appear primitive, innate immune cells can orchestrate discrete immune responses to different infections through the recognition of diverse pathogens by germline-encoded pattern recognition receptors (Kumar et al, 2009). In addition, several studies have revealed specific memory-like responses elicited by the innate immune system of invertebrates (Kurtz & Franz, 2003). Therefore, the innate immune system and its cellular components have recently been recognized to be more intricate and sophisticated than previously thought.
Since the first characterization of natural killer (NK) cells was made more than 30 years ago, these innate immune lymphocytes have been found to serve as a first line of defence against a variety of infections (Biron & Brossay, 2001; Lodoen & Lanier, 2006). NK cells mediate their effects through the recognition and killing of target cells and the production of immunoregulatory cytokines, particularly IFN-γ, which enhance the innate immune response and help to shape the subsequent adaptive immune response (Strowig et al, 2008b; Yokoyama, 2008). Unlike adaptive T and B lymphocytes, NK cells do not rearrange their receptor genes somatically, but rather rely on a fixed number of inhibitory and activating NK cell receptors (NKRs) that are capable of recognizing MHC class I and class I-like molecules, as well as other ligands (Bryceson & Long, 2008). The tolerance of NK cells to self is achieved through mechanisms that require the engagement of inhibitory NKRs with self-MHC before attaining functional competence, a process termed ‘licensing' (Kim et al, 2005; Raulet & Vance, 2006; Jonsson & Yokoyama, 2009). NK cell effector functions can be triggered by the engagement of activating NKRs with cell-surface ligands—which can be encoded by the host or by pathogens—some of which are upregulated in infected cells or tumours (Arase et al, 2002; Smith et al, 2002; Guerra et al, 2008). NK cells also respond to other signals, especially cytokines derived from antigen-presenting cells, which allow them to mediate early host responses against pathogens (Andrews et al, 2003; Moretta et al, 2006).
Recent studies have shed new light on the role of NK cells in the immune response and suggest that these innate lymphocytes have characteristics of both innate and adaptive immunity. Here, we highlight some of the latest advances in NK cell biology: a newly recognized capacity for immunological memory, a subset of NK cells that specializes in producing the TH17 cytokine IL-22, and differentiation of NK cell subsets in the thymus and lymph nodes, traditionally thought of as home to adaptive immune cells.
Memory-like functions of NK cells
Immunological memory has two primary features: antigen specificity and an amplified response following subsequent antigen exposure. Through the somatic recombination of their antigen receptor genes, adaptive immune T and B cells can express an almost unlimited number of antigen receptors that will recognize nearly any foreign antigen. After antigen stimulation and clonal expansion of specific T and B cells, a population of experienced memory lymphocytes persists to help protect the host from subsequent encounters with that same antigen (Murphy et al, 2007). By contrast, cellular components of the innate immune system—such as macrophages, dendritic cells and NK cells—have a limited repertoire of germline-encoded pathogen-recognition receptors and are thought to react in a similar manner after repeated stimulation, therefore lacking immunological memory. However, several recent studies have suggested that NK cells can have memory-like properties (O'Leary et al, 2006; Cooper et al, 2009; Raulet, 2009; Sun et al, 2009).
NK cells participate in contact hypersensitivity (CHS) reactions in a murine model of hapten-induced dermatitis (O'Leary et al, 2006; Yokoyama, 2006). Conventionally thought to be a T-cell-dependent phenomenon, CHS responses were nevertheless found in SCID and Rag2-deficient mice, which lack T cells. However, there were no CHS responses in T-cell-deficient mice that also lacked NK cells. In addition, the adoptive transfer of NK cells from hapten-sensitized mice into naive mice resulted in a delayed-type hypersensitivity reaction when recipients were challenged with the original hapten, but not with different haptens (O'Leary et al, 2006), which is consistent with a memory-like property of NK cells. Interestingly, the CHS phenotype was specifically seen after the transfer of the Ly49C/I+ NK cell subset but not the Ly49C/I− subset. This latter result suggested a potential role for MHC-dependent NK cell licensing in the development of NK cells with memory-like functions.
Licensing is a process whereby interactions between self-MHC class I (H2Kb in this case) and its cognate receptor on an NK cell (Ly49C) render the NK cell functionally competent (Kim et al, 2005; Jonsson & Yokoyama, 2009). The basis for the specificity of the memory-like property of licensed NK cells in CHS is uncertain, as no NKRs are known to recognize haptenated cells. Hapten-induced inflammatory effects—rather than direct NK cell recognition—could influence NK cell function. Alternatively, the haptenation of MHC might alter inhibitory NKR interactions with self-MHC on haptenated cells, thereby removing inhibitory signals and allowing activating signals to prevail.
More recently, NK cells that had been activated exclusively by cytokines were shown to have an NK-intrinsic, enhanced capacity to produce IFN-γ on re-stimulation, which is also consistent with a memory-like phenotype (Cooper et al, 2009). As the overall NKR repertoire is limited to germline-encoded receptors, NK cells often rely on cytokine signalling for their activation during an immune response, rather than on antigen-specific receptors. Indeed, NK cells respond to a variety of inflammatory cytokines that are produced by antigen-presenting cells, such as IL-1, IL-12, IL-15 and IL-18, which provide a common mechanism to elicit efficient NK responses to a wide range of infectious stimuli (Cooper et al, 2001).
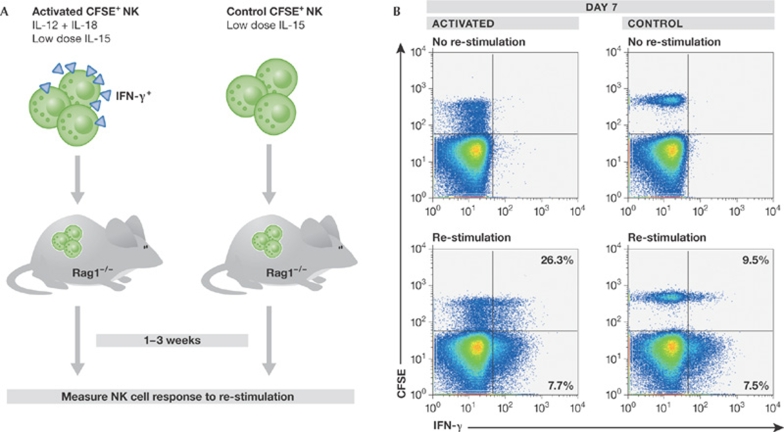
To determine whether NK cells have properties of immunological memory, an in vivo adoptive transfer system was used (Cooper et al, 2009). NK cells that had been activated to produce IFN-γ with IL-12 and IL-18 returned to a resting state after adoptive transfer but—contrary to control cells—were intrinsically able to respond more robustly to re-stimulation with cytokines ex vivo or through the engagement of activating NKRs (Fig 1; Cooper et al, 2009). These findings suggest that, based on a prior experience, NK cells fundamentally change how they respond to subsequent activation. This NK-intrinsic memory-like effect persisted for at least three weeks (Cooper et al, 2009), a relatively long time considering that the half-life of an NK cell has been estimated to be between one week and 17 days (Koka et al, 2003; Jamieson et al, 2004). Whether memory-like NK cells have a survival advantage is unknown. Cytokine-activated NK cells proliferated in vivo after adoptive transfer and, interestingly, daughter cells also had a similar memory-like phenotype, despite never having been activated (Fig 1; Cooper et al, 2009). Thus, memory-like differentiation in NK cells is both stable and heritable, suggesting that a single activation event might result in a population of experienced NK cells with enhanced activity, independent of continued stimulation or even—in the case of daughter cells—a history of prior stimulation. Memory is therefore different from priming, which would be expected to affect only the stimulated cell and not its progeny. Consequently, innate immune responses that are mounted on a regular basis to protect hosts from pathogens could induce the differentiation and continuous renewal of a pool of memory-like NK cells that have enhanced activity when called to duty (Fig 2).
Figure 1.
Cytokine activation of NK cells induces the differentiation of memory-like cells with enhanced IFN-γ production. (A) Adoptive transfer model used to assess NK cell re-stimulation. Splenic NK cells are either activated using cytokines or control-treated, labelled with CFSE and transferred into Rag1-deficient hosts. This system allows the assessment of NK cell responses to re-stimulation. (B) Characterization of CFSE+ NK cells one week after transfer. Activated and control donor CFSE+ NK cells were easily identified by flow cytometry and did not constitutively produce IFN-γ in the absence of re-stimulation (cytometry gates are set on total NK cells). Activated NK cells proliferate after adoptive transfer, as evidenced by the dilution of CFSE, as compared to control NK cells. After re-stimulation with cytokines, significantly more of the previously activated donor NK cells produced IFN-γ compared to controls. The percentages indicate the proportion of IFN-γ+ NK cells in the CFSE+ or CFSE− populations. CFSE, carboxyfluorescein succinimidyl ester; IFN-γ, interferon gamma; NK, natural killer.
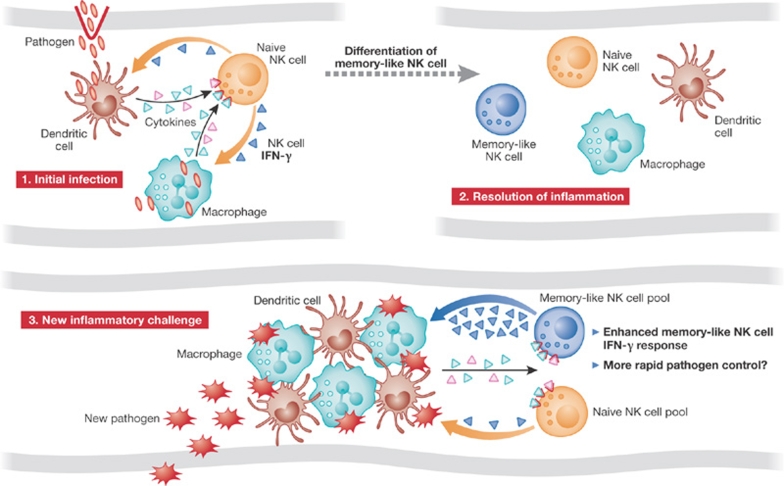
Figure 2.
Proposed mechanism of NK cell memory responses in immunity. Step 1. During an initial infection, NK cells provide a source of early IFN-γ in response to cytokines produced by macrophages and dendritic cells. Step 2. A fraction of activated NK cells might then differentiate into memory-like cells. Step 3. In the context of a new infection, memory-like NK cells would be activated again and trigger an enhanced IFN-γ response, possibly contributing to improved pathogen control. IFN-γ, interferon gamma; NK, natural killer.
In addition to cytokine stimulation, NK cells can be activated through the engagement of a limited number of activating receptors. Some of these receptors recognize pathogen-specific antigens, most notably the murine Ly49H receptor, which is responsible for the genetic resistance of certain mouse strains to infection with MCMV (Brown et al, 2001; Daniels et al, 2001; Lee et al, 2001). Ly49H recognizes an MCMV-encoded antigen, m157 (Arase et al, 2002; Smith et al, 2002). Interestingly, Ly49H+ NK cells specifically expand after MCMV infection, similarly to how antigen-specific T cells proliferate after antigen stimulation (Dokun et al, 2001). Using an adoptive transfer model, Sun and colleagues recently found that Ly49H+ NK cells can persist at least two months after MCMV infection (Sun et al, 2009). The previously activated Ly49H+ NK cells were more responsive to activation in vitro 70 days after the initial MCMV infection, a longer time period than had been evaluated for cytokine-induced NK cell memory-like functions. These Ly49H+ NK cells expressed a more ‘mature' phenotype, including low levels of CD27 and higher levels of Ly6C, KLRG1, and CD43 (Sun et al, 2009). However, as with cytokine-induced memory-like NK cells, no definitive phenotypic markers of NK cell memory were found. Ly49H+ memory-like NK cells also provided better protection than naïve NK cells against MCMV when transferred into newborn mice (Sun et al, 2009). The specificity of Ly49H+ NK cell memory in protecting against MCMV compared with other pathogens was not assessed in this study, and it will be interesting to know if the same NK cell populations can confer resistance to other infections.
Collectively, these studies provide new evidence that NK cells can have memory, an attribute once thought to be limited to adaptive immune lymphocytes. Memory-like NK cells can be generated through activation with cytokines or the engagement of activating receptors. Although the relationship between these two modes of activation in the establishment of memory needs to be clarified, it is conceivable that NK cell memory could be boosted (Sidebar A). These findings could lead to new strategies to enable us to control and manipulate innate immune memory, which would be particularly beneficial for people with impaired adaptive immune memory, such as newborns and immunodeficient patients. In addition, it is worth reflecting on the possibility that other innate immune cells have a form of memory.
Sidebar A | In need of answers.
What is the role of NK cell memory in the host response to infection? Is NK memory specific?
Which factors are important for the differentiation of NK cell memory?
What are the functional differences between cytokine-induced and antigen-induced memory-like NK cells?
Where do NK-22 cells differentiate? From which precursors do these cells arise? What is the developmental relationship between NK-22s and LTi cells?
Do NK-22s have pro-inflammatory as well as anti-inflammatory effects?
What is the role of thymic-derived (or other organ-derived) NK cells in vivo?
Do thymic-derived (or other organ-derived) NK cell precursors commit to this differentiation pathway and subsequently home in on the thymus (or other organ), or is lineage commitment a consequence of additional signals obtained at the target organ?
An innate immune source of IL-22: NK-22 cells
A subset of NK cells that are programmed to secrete IL-22 was recently discovered in the mucosa-associated lymphoid tissue (MALT) of mice and humans (Satoh-Takayama et al, 2008; Cella et al, 2009; Cupedo et al, 2009; Luci et al, 2009; Sanos et al, 2009). These NK cells, known as NK-22s, are specifically activated by IL-23 to secrete IL-22, which belongs to the family of IL-10-related cytokines and has been proposed to have diverse roles in host defence and disease pathogenesis, and has both pro-inflammatory and anti-inflammatory effects (Zenewicz & Flavell, 2008). Importantly, IL-22 in the gut and other mucosal surfaces seems to protect the epithelial cell barrier between host and pathogen (Ouyang et al, 2008).
In humans, NK-22s were identified in an NKp44+ subset of CD56+CD3− NK cells found predominantly in the mucosal areas of the tonsil and the ileal Peyer's patches (Cella et al, 2009). Intriguingly, NKp44+ NK cells were not proficient at prototypic NK cell functions, including cytotoxicity and IFN-γ production (Ferlazzo & Munz, 2004; Cella et al, 2009). Instead, NKp44+ NK cells constitutively produced IL-22, IL-26 and LIF transcripts. Although IL-22 and IL-26 are part of the cytokine profile of TH17 CD4+ T cells (Liang et al, 2006), NKp44+ NK cells did not produce IL-17. The activation of NKp44+ NK cells with various inflammatory cytokines revealed that IL-23 selectively induced the production of IL-22 in NKp44+ NK cells that express CCR6 (Cella et al, 2009), which were therefore named NK-22 cells, indicating their unique capacity to produce this cytokine. Antigen-presenting cells provide an endogenous source of IL-23 in response to pathogens in vivo, and NK-22s also produce IL-22 when cultured with activated allogeneic monocytes. In addition, NK-22 cells secrete CCL20—which is the ligand for CCR6—suggesting that these NK cells can promote their own accumulation and influence the immune cell composition of their microenvironment. A similar subset of IL-23-responsive murine NK cells was identified in Peyer's patches (Cella et al, 2009). Murine NK-22s are NKp46+CD127+CD3− with variable NK1.1 expression, and they upregulate the production of IL-22 upon stimulation with IL-23 (Satoh-Takayama et al, 2008; Cella et al, 2009; Sanos et al, 2009), suggesting the conservation of this unique NK cell subset from mice to humans.
NK-22 cells express several TH17 transcription factors, including RORγ, aryl hydrocarbon receptor, RORα and IRF4 (Satoh-Takayama et al, 2008; Cella et al, 2009; Cupedo et al, 2009; Luci et al, 2009; Sanos et al, 2009). However, peripheral NK cells cannot differentiate into NK-22s under TH17 polarizing conditions in vitro, which suggests that the gut and mucosal microenvironment are important for differentiation of NK-22s from local progenitors (Cella et al, 2009). Studies of intestinal NK cells in germ-free mice also show that commensal bacteria are important for the differentiation and function of NK-22s (Satoh-Takayama et al, 2008; Sanos et al, 2009). The transcription factor RORγ also seems to be important for the differentiation of NK-22s, as mice lacking RORγ show significant decreases in NK-22 cell numbers and an absence of the IL-22 transcript in gut NK cells (Satoh-Takayama et al, 2008; Luci et al, 2009). In humans, a subset of immature NK cell precursors in the tonsil was found to express high levels of constitutive IL-22 but not of IL-17 (Hughes et al, 2009). Together, these findings suggest that NK-22 precursors could reside in secondary lymphoid tissues, where they would differentiate in response to microbial-driven inflammation.
An important question is whether NK-22 cells are generated during conventional NK cell development (Sidebar A). Human lymph node NK cells develop through four distinct stages (described in greater detail below; Freud & Caligiuri, 2006), and precursors that express IL-22 and IL-26 were found in stage 3 cells, which are defined as CD56−CD34−cKit+CD94− (Hughes et al, 2009). Thus, it is possible that some of these NK precursors continue to produce IL-22 indefinitely, whereas others go on to stage 4, thereby becoming classical CD56+ NK cells that produce IFN-γ. In mice, IL-22-producing NK cells include a major subset of NK1.1− cells, which—in contrast to conventional NK cells—do not require IL-15 for differentiation, suggesting that they develop through an alternative pathway. However, a minor subset of NK1.1+ cells is IL-15-dependent (Satoh-Takayama et al, 2008) and therefore could be derived from the classical developmental path of NK cells. NK-22 cells could also develop from a local progenitor present in mucosae. Two recent studies showed that human fetal LTi cells and mouse adult LTi-like cells secrete IL-17 and IL-22 and share striking phenotypic and transcriptional similarities to NK-22 cells, including the expression of CD127 (IL-7Ra) and RORγt (Cupedo et al, 2009; Takatori et al, 2009). LTi are rare cells that are involved in the formation of secondary lymphoid organs, including gut-associated Peyer's patches. These findings have instigated an as yet unresolved debate as to the true origin of these cells: LTi-like and NK-22 cells could be two sequential developmental stages of the same cell type or, alternatively, could originate from the same precursor cell through divergent pathways.
NK-22 cells and mucosal immunity
NK-22 cells rapidly produce IL-22 after being activated by IL-23 and are probably important for mucosal homeostasis and the protection of mucosal sites during infection and inflammation. The IL-22 receptor is expressed on several types of epithelial tissue—particularly that of the skin and gastrointestinal tract (Wolk et al, 2004)—rather than on immune cells. IL-22 is thought to protect and maintain epithelial barriers through the upregulation of anti-apoptotic molecules and bactericidal proteins (Zenewicz & Flavell, 2008; Aujla & Kolls, 2009). Indeed, NK-22 cell-conditioned media induces colon epithelial cell proliferation, the activation of anti-apoptotic pathways and the secretion of IL-10, which is an anti-inflammatory cytokine that is important for the protection of mucosal cells (Cella et al, 2009).
Early host defence against infection of the gastrointestinal tract by Citrobacter (C.) rodentium has been shown to depend on IL-22 (Zheng et al, 2008) and NK-22 cells appear in the small intestine lamina propria after mice are infected with C. rodentium (Cella et al, 2009). Furthermore, the depletion of NK cells from C. rodentium-infected Rag2−/− mice—which lack T-cell-produced IL-22—resulted in accelerated mortality, suggesting a protective role for NK-22s in this IL-22-dependent infection (Satoh-Takayama et al, 2008; Cella et al, 2009). IL-22 has also been shown to be protective against inflammatory diseases, including hepatitis, autoimmune myocarditis and inflammatory bowel disease (Radaeva et al, 2004; Chang et al, 2006; Zenewicz et al, 2007; Zenewicz et al, 2008). A recent study in Rag1−/− mice demonstrated that NK cells can serve as a source of protective IL-22 in two murine models of inflammatory bowel disease (Zenewicz et al, 2008). Collectively, these studies suggest that after an inflammatory response is elicited at mucosal barriers and IL-23 is produced by resident antigen-presenting cells, NK-22s might provide an innate immune source of IL-22 that can help to protect the host mucosa and control inflammation (Fig 3).
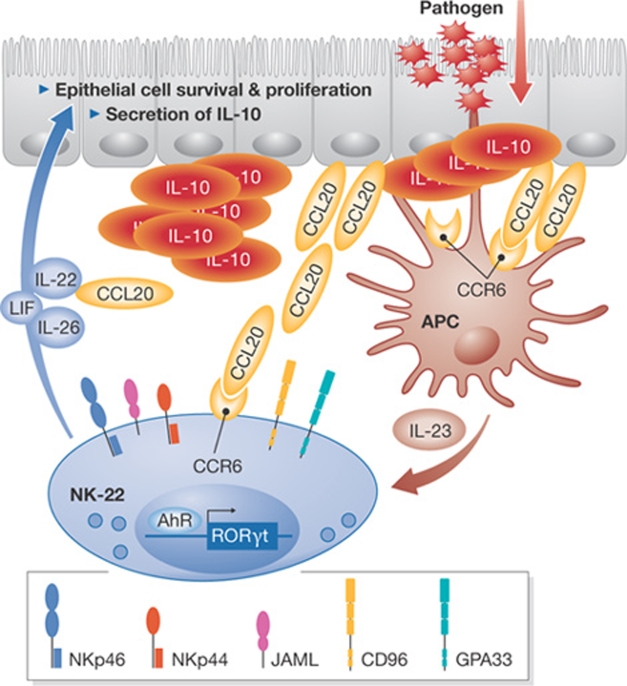
Figure 3.
Role of human NK-22 cells as a potential innate source of IL-22 for mucosal immunity. NK-22 cells express RORγt and home in on the lamina propria of the mucosa and on mucosal-associated lymphoid tissues through the CCR6–CCL20 interaction. Human NK-22 cells express adhesion molecules—such as CD96, JAML, and GPA33—which facilitate NK-22 epithelial cell interactions. Mucosal dendritic cells secrete IL-23 on interaction with microbial components, which stimulates NK-22 to secrete IL-22, IL-26, LIF and CCL20. IL-22, IL-26 and LIF promote epithelial cell survival, proliferation and secretion of the anti-inflammatory cytokine IL-10. CCL20 could facilitate the self-recruitment of NK-22 cells into the mucosa. CCL20, chemokine (C-C motif) ligand 20; CCR6, chemokine (C-C motif) receptor 6; GPA33, glycoprotein A33; JAML, junctional adhesion molecule-like; LIF, leukemia inhibitory factor; NK, natural killer; ROR, retinoid-related orphan receptor.
IL-22 promotes host defences in many cases, but it is also thought to be involved in the pathogenesis of some diseases including psoriasis and multiple sclerosis (Zenewicz & Flavell, 2008). The primary adaptive immune source of IL-22 is TH17 CD4+ T cells, which also produce IL-17—a cytokine associated with autoimmune disease pathogenesis—and are thought to have a pathogenic role in the same diseases (Iwakura et al, 2008). In contrast to TH17 cells, MALT-associated NK-22 cells specialize in IL-22 production and do not produce IL-17 (Cella et al, 2009). Whether NK-22s mediate inflammatory diseases is not yet known, although their lack of IL-17 suggests that this NK cell subset has the potential to exert protective IL-22-mediated effects at mucosal sites without eliciting TH17-associated pathology. If this is the case, the dual role of IL-22 as being both protective and detrimental to the host would be explained by the cellular source of IL-22 and the local cytokine milieu (Sidebar A).
Non-classical sites of NK cell differentiation
NK cells were once thought to arise only from the bone marrow, but it is now clear that NK cells with specific functions and surface markers are present in a variety of other organs, including the liver, thymus, lymph nodes, uterus and MALT (Freud & Caligiuri, 2006; Huntington et al, 2007; Riley & Yokoyama, 2008). For example, immature murine NK cells present in the liver express the tumour necrosis factor family ligand, TRAIL, and can suppress the metastasis of TRAIL-sensitive tumours in vivo (Takeda et al, 2001). Uterine NK cells—which are the most prevalent decidual immune cells during early pregnancy—accumulate at the site of embryo implantation and produce IFN-γ, which is important for appropriate vascular remodelling and endometrial decidualization (Riley & Yokoyama, 2008; Murphy et al, 2009). As we have developed a better understanding of the distribution and range of functions of NK cells, questions have arisen as to the developmental origins of tissue-specific NK cell subsets. Do these cells arise in the bone marrow and circulate to different sites, or does the local microenvironment dictate the differentiation of NK cell subsets? Here, we focus on the thymus and lymph nodes, both of which support the differentiation of adaptive immune cells and NK cell subsets (Freud & Caligiuri, 2006; Di Santo, 2008).
Bipotent progenitors that are able to differentiate into T cells or NK cells are present in the murine and human thymus (Sanchez et al, 1994; Spits et al, 1995; Carlyle et al, 1997), and recent studies in mice by the Di Santo laboratory have characterized a thymic pathway of NK cell development (Vosshenrich et al, 2006). Thymic NK cells express high levels of the IL-7 receptor α-chain, CD127, and are dependent on IL-7, IL-15 and the common cytokine receptor γ-chain—shared by the IL-2, -4, -7, -9, -15 and -21 receptors—for development (Vosshenrich et al, 2006; Cheng et al, 2009). By contrast, CD127− NK cells derived from the bone marrow do not require IL-7 for differentiation or survival. The transcription factor GATA 3 is also essential for the differentiation of thymic NK cells; it is expressed at high levels in thymic NK cells, but not expressed in splenic NK cells (Vosshenrich et al, 2006). Compared with conventional splenic NK cells, CD127+ thymic NK cells efficiently produce cytokines, but have a low cytotoxic capacity (Vosshenrich et al, 2006). Interestingly, NK cells that also express CD127 and are phenotypically similar to thymic NK cells comprise 15–30% of the lymph node NK compartment, but a very low percentage of the splenic or liver NK compartment. A thymic transplant model showed that this CD127+ lymph node NK cell subset is probably thymic-derived (Vosshenrich et al, 2006), suggesting that NK cells from the thymus preferentially circulate to and/or are retained in the lymph nodes. However, CD127+ NK cells might not have a strict thymic requirement, as it was subsequently shown that these NK cells are present in athymic mice (Stewart et al, 2007). The characterization of additional markers is clearly needed to identify thymic-derived NK cells, as a minor population of CD127+NK1.1+ cells which is negative for cell-surface CD3 might actually be T cells (Stewart et al, 2007). Whether the CD127+ thymic NK precursor differentiates in the thymus or bone marrow, and the developmental stages of this cytokine-producing NK cell subset, are unknown.
Peripheral blood human NK cells can be divided into functional subsets on the basis of the cell-surface density of CD56; CD56bright NK cells have an enhanced capacity for cytokine production and CD56dim NK cells are characterized by a higher cytotoxic potential (Cooper et al, 2001). A minority (5–15%) of peripheral blood and splenic NK cells are CD56bright, whereas most NK cells in the lymph nodes are CD56bright (Fehniger et al, 2003; Ferlazzo et al, 2004). Four discrete stages have been identified during the development of human lymph node NK cells from CD34+ precursors (Freud & Caligiuri, 2006; Freud et al, 2006), providing direct evidence for NK cell differentiation in the lymph node. Human lymph nodes contain an enriched CD34+CD45RA+ haematopoietic precursor population in the parafollicular region, which is adjacent to areas of T cells and CD56bright NK cells (Freud et al, 2005). After in vitro culture with the cytokines IL-2 or IL-15, or with activated autologous lymph node T cells, these precursors can give rise to the predominant lymph node CD56bright NK cell population. It is unclear whether lymph node CD56bright NK cells are terminally differentiated or develop into CD56dim NK cells, as suggested by in vitro studies that show the conversion of peripheral blood CD56bright NK cells into CD56dim NK cells after culture on synovial fibroblasts (Chan et al, 2007) or under other conditions (Romagnani et al, 2007). In mice, CD56 is not a useful marker of NK cells, which makes it difficult to relate human NK cell subsets to mouse subsets on the basis of CD56 expression. By contrast, recent evidence suggests that murine CD127+ thymic NK cells are similar to human CD56bright NK cells (Vosshenrich et al, 2006). In addition, another subset of CD27highCD11bhigh murine NK cells are also enriched in lymph nodes and have enhanced cytokine production, therefore constituting another potential counterpart to human CD56bright NK cells (Hayakawa & Smyth, 2006). These findings could help to characterize the developmental relationship of these NK cell subsets to conventional NK cells, as well as their clinical relevance, in more detail.
Role of NK cells in the thymus and lymph node
The role of thymic and lymph node NK cells during an immune response remains unclear. Both NK cell subsets readily produce cytokines and have lower cytotoxic capacity than conventional NK cells, which suggests that they might serve an important immunoregulatory function at these sites. In vivo evidence for this is lacking, although tonsilar NK cells cultured with activated dendritic cells are able to inhibit EBV-induced B cell transformation in an IFN-γ-dependent manner (Strowig et al, 2008a), suggesting that NK cells might limit local infections before the activation of antigen-specific T cells. A complementary hypothesis is that NK cells might help to prime the adaptive immune response, which is supported by several studies suggesting that the production of IFN-γ by NK cells and their interactions with dendritic cells can prime the polarization of TH1 adaptive immune responses (Martin-Fontecha et al, 2004; Mailliard et al, 2005; Morandi et al, 2006; Agaugue et al, 2008). NK cells probably also continue to interact with activated TH1 T cells in the lymph node, as CD56bright cells constitutively express the high-affinity heterotrimeric IL-2 receptor (IL-2Rαβγ), and IL-2 derived from activated T cells can co-stimulate CD56bright NK cell IFN-γ production (Fehniger et al, 2003). Overall, the role of NK cells in the thymus and lymph node seems to be complex, although further studies of the developmental pathways and functional capacities of these NK cell subsets will provide additional insight into their roles during the immune response (Sidebar A).
Conclusion
Since NK cells were first identified on the basis of their capacity to kill targets without prior sensitization, these innate immune lymphocytes have been recognized to have broad functions and distribution. Recent studies have demonstrated that NK cells cross over the traditional boundaries of innate and adaptive immunity with their capacity for memory-like responses. The specificity of NK memory-like responses is unknown, however their induction in response to cytokine stimulation suggests that they are nonspecific. NK cells can also specialize in the production of the TH17 cytokine IL-22, and NK-22 cells seem to be important in maintaining mucosal homeostasis during inflammation. Finally, NK cell development in the thymus and lymph nodes, sites that are home to adaptive immune cells, suggests that these innate immune lymphocytes are important during the coordination of an adaptive immune response.
Megan A. Cooper
Marco Colonna
Wayne M. Yokoyama
Acknowledgments
Work in the Yokoyama laboratory is supported by the Howard Hughes Medical Institute and grants AI34385, AI33903, AI51345, AI57160 and AR48335 from the National Institutes of Health (NIH). The Colonna laboratory is supported by the NIH. M.A.C. is supported by the NIH under Ruth L. Kirschstein National Research Service Award T32 HD043010 from the NICHD.
References
- Agaugue S, Marcenaro E, Ferranti B, Moretta L, Moretta A (2008) Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood 112: 1776–1783 [DOI] [PubMed] [Google Scholar]
- Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA (2003) Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol 4: 175–181 [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL (2002) Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296: 1323–1326 [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Kolls JK (2009) IL-22: a critical mediator in mucosal host defense. J Mol Med 87: 451–454 [DOI] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL (1989) Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 320: 1731–1735 [DOI] [PubMed] [Google Scholar]
- Biron CA, Brossay L (2001) NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol 13: 458–464 [DOI] [PubMed] [Google Scholar]
- Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM (2001) Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292: 934–937 [DOI] [PubMed] [Google Scholar]
- Bryceson YT, Long EO (2008) Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol 20: 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J, Boisson-Dupuis S, Jouanguy E, Picard C, Puel A, Abel L, Casanova JL (2008) Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol 20: 39–48 [DOI] [PubMed] [Google Scholar]
- Carlyle JR, Michie AM, Furlonger C, Nakano T, Lenardo MJ, Paige CJ, Zuniga-Pflucker JC (1997) Identification of a novel developmental stage marking lineage commitment of progenitor thymocytes. J Exp Med 186: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457: 722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P (2007) CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol 179: 89–94 [DOI] [PubMed] [Google Scholar]
- Chang H et al. (2006) Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol 177: 3635–3643 [DOI] [PubMed] [Google Scholar]
- Cheng M, Charoudeh HN, Brodin P, Tang Y, Lakshmikanth T, Hoglund P, Jacobsen SE, Sitnicka E (2009) Distinct and overlapping patterns of cytokine regulation of thymic and bone marrow-derived NK cell development. J Immunol 182: 1460–1468 [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22: 633–640 [DOI] [PubMed] [Google Scholar]
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM (2009) Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA 106: 1915–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H (2009) Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol 10: 66–74 [DOI] [PubMed] [Google Scholar]
- Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM (2001) Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med 194: 29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo JP (2008) Natural killer cells: diversity in search of a niche. Nat Immunol 9: 473–475 [DOI] [PubMed] [Google Scholar]
- Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM (2001) Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2: 951–956 [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA (2003) CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101: 3052–3057 [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Munz C (2004) NK cell compartments and their activation by dendritic cells. J Immunol 172: 1333–1339 [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C (2004) The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol 172: 1455–1462 [DOI] [PubMed] [Google Scholar]
- Freud AG et al. (2005) A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22: 295–304 [DOI] [PubMed] [Google Scholar]
- Freud AG, Caligiuri MA (2006) Human natural killer cell development. Immunol Rev 214: 56–72 [DOI] [PubMed] [Google Scholar]
- Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA (2006) Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med 203: 1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH (2008) NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Smyth MJ (2006) CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 176: 1517–1524 [DOI] [PubMed] [Google Scholar]
- Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA (2009) Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH17 cytokine interleukin-22. Blood 113: 4008–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington ND, Vosshenrich CA, Di Santo JP (2007) Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol 7: 703–714 [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Nakae S, Saijo S, Ishigame H (2008) The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226: 57–79 [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH (2004) Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol 172: 864–870 [DOI] [PubMed] [Google Scholar]
- Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20: 197–216 [DOI] [PubMed] [Google Scholar]
- Jonsson AH, Yokoyama WM (2009) Natural killer cell tolerance licensing and other mechanisms. Adv Immunol 101: 27–79 [DOI] [PubMed] [Google Scholar]
- Kim S et al. (2005) Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436: 709–713 [DOI] [PubMed] [Google Scholar]
- Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A (2003) Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα-deficient mice. J Exp Med 197: 977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S (2009) Pathogen recognition in the innate immune response. Biochem J 420: 1–16 [DOI] [PubMed] [Google Scholar]
- Kurtz J, Franz K (2003) Innate defence: evidence for memory in invertebrate immunity. Nature 425: 37–38 [DOI] [PubMed] [Google Scholar]
- Lee SH, Girard S, Macina D, Busa M, Zafer A, Belouchi A, Gros P, Vidal SM (2001) Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet 28: 42–45 [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203: 2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodoen MB, Lanier LL (2006) Natural killer cells as an initial defense against pathogens. Curr Opin Immunol 18: 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C et al. (2009) Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 10: 75–82 [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P (2005) IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med 202: 941–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F (2004) Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 5: 1260–1265 [DOI] [PubMed] [Google Scholar]
- Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C (2006) NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol 36: 2394–2400 [DOI] [PubMed] [Google Scholar]
- Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, Moretta A (2006) Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev 214: 219–228 [DOI] [PubMed] [Google Scholar]
- Murphy KP, Travers P, Walport M (2007) Janeway's Immunobiology 7th edn. London, UK: Taylor & Francis [Google Scholar]
- Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA (2009) Interferon gamma in successful pregnancies. Biol Reprod 80: 848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH (2006) T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 7: 507–516 [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y (2008) The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28: 454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Pan HN, Hong F, Gao B (2004) Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39: 1332–1342 [DOI] [PubMed] [Google Scholar]
- Raulet DH (2009) Natural killer cells: remembrances of things past. Curr Biol 19: R294–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH, Vance RE (2006) Self-tolerance of natural killer cells. Nat Rev Immunol 6: 520–531 [DOI] [PubMed] [Google Scholar]
- Riley JK, Yokoyama WM (2008) NK cell tolerance and the maternal–fetal interface. Am J Reprod Immunol 59: 371–387 [DOI] [PubMed] [Google Scholar]
- Romagnani C et al. (2007) CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol 178: 4947–4955 [DOI] [PubMed] [Google Scholar]
- Sanchez MJ, Muench MO, Roncarolo MG, Lanier LL, Phillips JH (1994) Identification of a common T/natural killer cell progenitor in human fetal thymus. J Exp Med 180: 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A (2009) RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol 10: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N et al. (2008) Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29: 958–970 [DOI] [PubMed] [Google Scholar]
- Smith HR et al. (2002) Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA 99: 8826–8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Lanier LL, Phillips JH (1995) Development of human T and natural killer cells. Blood 85: 2654–2670 [PubMed] [Google Scholar]
- Stewart CA, Walzer T, Robbins SH, Malissen B, Vivier E, Prinz I (2007) Germ-line and rearranged Tcrd transcription distinguish bona fide NK cells and NK-like gammadelta T cells. Eur J Immunol 37: 1442–1452 [DOI] [PubMed] [Google Scholar]
- Strowig T, Brilot F, Arrey F, Bougras G, Thomas D, Muller WA, Munz C (2008) Tonsilar NK cells restrict B cell transformation by the Epstein–Barr virus via IFN-gamma. PLoS Pathog 4: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Brilot F, Munz C (2008) Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol 180: 7785–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL (2009) Adaptive immune features of natural killer cells. Nature 457: 557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ (2009) Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206: 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Yamaguchi N, Yagita H, Okumura K (2001) Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell Immunol 214: 194–200 [DOI] [PubMed] [Google Scholar]
- Vosshenrich CA et al. (2006) A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol 7: 1217–1224 [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21: 241–254 [DOI] [PubMed] [Google Scholar]
- Yokoyama WM (2006) Contact hypersensitivity: not just T cells! Nat Immunol 7: 437–439 [DOI] [PubMed] [Google Scholar]
- Yokoyama WM (2008) Mistaken notions about natural killer cells. Nat Immunol 9: 481–485 [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27: 647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Flavell RA (2008) IL-22 and inflammation: leukin' through a glass onion. Eur J Immunol 38: 3265–3268 [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA (2008) Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29: 947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y et al. (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14: 282–289 [DOI] [PubMed] [Google Scholar]