Most pharmaceuticals are either chemically synthesized small molecules or are derived directly from natural sources such as plants and human blood. An increasing number of drugs comprising recombinant proteins, antibodies and nucleic acids are also produced in living organisms that have been genetically engineered for the purpose (Walsh, 2006). In parallel to this growing market of so-called biopharmaceuticals (Lawrence, 2007; Ledford, 2006), research has focused on the development of improved production platforms, notably genetically modified plants and animals. Recombinant biopharmaceuticals have, for example, been expressed in maize kernels, tobacco leaves, goat's milk and chicken eggs (Giddings et al, 2000; Dove, 2000). The production of recombinant pharmaceutical proteins in higher organisms is termed ‘pharming', a portmanteau of pharmaceuticals and farming, which reflects the combination of ‘red' (biomedical and/or pharmaceutical) and ‘green' (agricultural and/or food) biotechnology. The idea behind pharming is that it will enable the faster, more flexible and cost-effective production of pharmaceuticals compared with current synthetic production processes.
The use of recombinant DNA technology to alter organisms for a specific purpose has raised its fair share of controversy. While initial public resistance to producing biopharmaceuticals in lower organisms has largely disappeared—for instance, synthesizing human insulin in bacteria—there is still considerable opposition to genetically modified higher organisms and their use in agriculture or medicine. In light of the often hostile public reaction to other applications of biotechnology, a better understanding of the public's views and attitudes to pharming might help to guide the interaction of the research community and industry with the public at large.
…a better understanding of the public's views and attitudes to pharming might help to guide the interaction of the research community and industry with the public at large
Here, we present public perceptions of plant and animal pharming in 15 advanced industrial societies—12 European countries plus Israel, Japan and the USA—that represent a spectrum of social values and socioeconomic conditions (for full details, see supplementary Methods online). Previous social research into pharming has been limited to qualitative analyses or surveys of regional samples in the USA, which have focused largely on public attitudes to “plant molecular farming” (Kirk & McIntosh, 2005; Einsiedel & Medlock, 2005; Nevitt et al, 2006; Knight, 2007). By contrast, our study was designed with a broader scope in terms of the societies studied, sample size (1,500 respondents in each country) and coverage of both plant and animal pharming. We also used a more complex and robust set of metrics through which to assess views and attitudes, taking multiple measures of both the dependent (‘support of pharming') and explanatory variables. Finally, we included a number of specific scenarios for our interviewees to consider: various plants and their mode of cultivation—open fields, containment, confinement—and the types of animal and medium—such as milk, egg, urine and blood—in which the protein or drug is produced.
Nearly two decades ago, Dancker D. L. Daamen and co-authors (1990) postulated that general attitudes towards science and technology are unstable and weak theoretical constructs. Although these are assumed to have a universal scope, they nevertheless fail to aggregate and account for the public's views on a range of disparate scientific areas. Analyses, according to this line of reasoning, should focus on an intermediate level between views on science at large and specific scientific developments, namely clusters or subsets of science that share several attributes, such as reproductive technologies, computer and communication technologies or nuclear energy. In turn, the literature on attitudes towards biotechnology has shown that a distinction must be drawn between the two subsets of ‘green' and ‘red' biotechnologies, as these tend to meet with opposing public perceptions—negative and positive respectively (Gaskell et al, 1997; Frewer et al, 1997; Fischhoff & Fischhoff, 2001; Sturgis et al, 2005; Pardo & Calvo, 2006).
Pharming is situated somewhere between the red and green groups of technologies, and might elicit both positive and negative responses. Our study therefore addresses the crucial question of whether we are facing a single, undifferentiated and holistic evaluation of pharming, or potentially varied, nuanced and plural perceptions. Indeed, the results show that even a biotechnology subset such as pharming elicits markedly different responses from the public, depending on both the goals and the specifics of the means—that is, the types of animal being used, the medium in which the drug is produced, the plants used and where these plants are grown. This highly differentiated landscape of perceptions of pharming has important implications for how those working in the field engage with the public (Knight, 2006).
The public tends to approach pharming like any new and complex subject: by using different evaluative perspectives simultaneously (Pardo et al, 2002). Our study sought to explore these perspectives, from the abstract—pharming for unspecified biomedical goals—to a more specific set of scenarios that ranged from fighting critical diseases to treating minor ailments and cosmetic applications. In addition to these two perspectives, we explored how two types of variable affect public attitudes towards pharming. The first concerned specific attributes relating to the biotechnology being considered: is it useful, moral, reckless, natural, risky; does it involve ‘playing God', ‘scientific arrogance', the interests of pharmaceutical multinationals; in the case of animal pharming, is it incompatible with the dignity of animals or does it cause animals to suffer? The second assessed the influence of more abstract factors such as individual worldviews, including perceptions of nature and values, scientific knowledge and trust in the scientific community and regulatory agencies.
A majority of individuals have a negative perception of the genetic modification of organisms, although this critical view has softened during the past five years. A large body of social research has been dedicated to understanding the reasons for opposition to this promising technology (Bauer & Gaskell, 2002; Priest, 2001; Sturgis et al, 2005; Gaskell & Bauer, 2006). These studies have singled out a lack of perceived utility as one of the most powerful factors. Our own data confirm that the perception of usefulness has the strongest correlation with support for plant pharming in all countries (from 0.6 to 0.8 on a scale from 0 (totally disagree) to 10 (totally agree); supplementary Table S1 online), with a similar result seen in the case of animal pharming (supplementary Table S2 online). Although this suggests that in the trade-off between means and ends the perceived utility has a significant role, the correlation matrix indicates that many other evaluative angles reinforce or diminish the level of support. The perception that the technique is of interest to a few multinational companies is, however, weakly correlated with pharming support and in contrast with the genetic modification of food crops, does not seem to influence the public's perception of pharming.
Pharming is situated somewhere between the red and green groups of technologies, and might elicit both positive and negative responses
The means and ends trade-off falls towards the side of acceptance when plants are being genetically modified to obtain pharmaceutical drugs. The dominant view is that this is a “useful” technique that should be “supported” and although it is perceived as going “against nature”, it is not considered to be either “immoral” or “reckless”. Perceptions of “risk” are more divided. The majority do not believe that plant pharming for pharmaceuticals is a product of the “arrogance of scientists” (supplementary Fig S1 and Table S3 online), and they trust the laws and controls established by each country's government to regulate it (supplementary Fig S2 and Table S4 online).
As with plants, public perception of the usefulness of animal pharming shows the greatest correlation with support in most countries: between 0.6 and 0.7. In this case, however, another perception of a similar magnitude arises, but in the opposite direction: the view that it “is an unacceptable exploitation of animals” produced values between 0.6 and 0.7 in nine out of the 15 countries. Obviously the usefulness of the goals being pursued does not fully outweigh concerns about the treatment of animals as production facilities. In principle, this dual perception could generate ambivalent attitudes. The negative correlation values of support for this type of pharming with the perception of “immorality”, “risk” and “recklessness”; and with the ideas that it goes “against nature”, is “incompatible with the inherent dignity of animals” and will cause them a great deal of “suffering”, are equal to or higher than 0.5 in most countries. By contrast, the views that animal pharming is “playing God”, will “transmit diseases from animals to humans”, is a “product of the arrogance of scientists” and that this area is a “product of the interests of a small number of pharmaceutical multinationals” display relatively low levels of correlation with support for animal pharming (supplementary Table S2 online). Trust in the regulator, although it reaches the mid-point or above in six out of 15 countries, is significantly lower than in the case of plant pharming (supplementary Table S5 and Fig S3 online). As the mean values of virtually all evaluative criteria in most countries indicate a convergent negative view of animal pharming, this translates into a low level of support in spite of the perceived usefulness (supplementary Table S6 and Fig S4 online).
Reservations about biotechnology are considerably attenuated or even dispelled in contexts where two conditions are met. First, the development in question is geared towards achieving biomedical goals: health is among the most prized desires of the population. Second, it does not involve the utilization of animals, save for a few exceptional cases. This condition highlights the different status accorded to plants and animals in most societies, which generates starkly contrasting attitudes towards the modification of their genetic blueprints.
Often the arguments for and against biotechnology remain relatively abstract and tend to be defined by the trade-off between the type of goals pursued, biomedical in our case, and the methods applied—genetic modification of plants or animals. Yet, from an analytical standpoint, and for the purpose of communication with the public, it is interesting to gauge the sensitivity or elasticity of people's attitudes in response to the presence of more specific goals and means. A low response to these specifics would suggest we are up against evaluative structures of a holistic or ideological kind, resistant to change and favouring confrontation rather than dialogue with the public.
Specific goals are either medical—the treatment of a particular disease—or socioeconomic, for example the increased production of biopharmaceuticals or cost savings relative to other production platforms. The analysis of the means must factor in the type of plants to be modified and their cultivation site, or the animal species and the medium in which the drug is obtained.
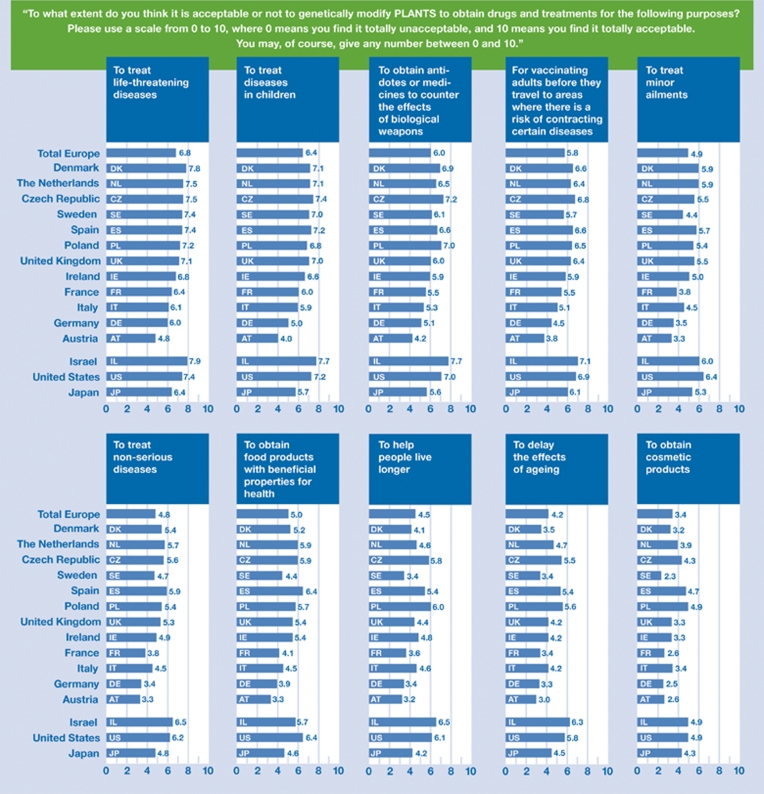
The purpose of the drug that is produced has a significant effect on the acceptance and/or rejection of pharming with regard to plants, and a moderate effect with regard to animals. Treatments for life-threatening and childhood diseases, antidotes to counteract the effects of biological weapons, and vaccines noticeably increase acceptance. Perceptions become more varied for purposes such as cures for minor ailments. The use of plant pharming to produce drugs for anti-ageing purposes and, even more so, for obtaining cosmetic products is rejected by a majority in all the societies studied (Fig 1). In most countries, respondents also tended to accept plant pharming for the production of cheap drugs for less developed societies and to overcome shortages, whereas views are more divided about obtaining cheaper drugs for the population of the advanced world (supplementary Fig S5 online).
…the results show that even a biotechnology subset such as pharming elicits markedly different responses from the public, depending on boththe goals and the specifics of the means
Figure 1.
Acceptance of plant pharming for biomedical purposes
The goal is also a significant factor in the acceptance of animal pharming, albeit against a background of overall rejection. The only purpose that generates a moderate level of support in almost all countries is the treatment of serious diseases and, in some countries, childhood diseases (Fig 2). Furthermore, the majority of respondents in nearly all countries disapprove of the genetic modification of animals to attain socioeconomic goals, including producing drugs for the populations of less developed countries (supplementary Fig S6 online).
Figure 2.
Acceptance of animal pharming for biomedical purposes
The specifics of the means used also determine the level of support for pharming. In the case of plant pharming, one particular aspect—the place where the plants are grown—further differentiates citizens' views: all societies find the use of containment in greenhouses acceptable and reject cultivation in open fields, even when distant from fields with non-genetically modified plants. Less intensely, the type of crop also produces a differentiated response, with a clear preference for non-food plants (supplementary Table S7 and Figs S7,S8 online). Against a background of negative views on animal pharming in almost all the societies studied, the use of fruit flies and, to a lesser extent, mice, meets with moderate acceptance in some countries. The use of fish inspires widespread rejection in most countries (supplementary Fig S9 online), a reaction that is even stronger in the case of sheep, cows, pigs and chimpanzees. Opposition also varies according to the medium in which the drug is produced; it is less intense when the drug is to be obtained from the milk or egg of genetically modified animals than when it is to be obtained from the urine or blood (supplementary Fig S10 online).
Preferences for one or other method of production to obtain a given medicine with an identical composition show a precise hierarchy: a clear majority of citizens prefer drugs to be isolated from wild plant species, followed at a distance by chemical synthesis and the genetic modification of plants. Genetic modification of animal cells in culture and, even more so, of whole animals is seldom chosen as a desirable source in most countries (supplementary Table S8 and Fig S11 online). The predisposition to personally use biopharmaceuticals derived from pharming is consistent with the dual views on plant and animal pharming: positive for plant pharming and negative for drugs obtained through animal pharming (supplementary Table S9 and Fig S12 online).
Our results—that public opinion differentiates according to the specific means and aims of pharming—indicate that the public do not adhere to ideological or undifferentiated arguments, but are rather open to different scenarios and, accordingly, to argumentation and dialogue. Yet, in addition to evaluative angles at a short distance from pharming, a characterization of public perceptions in this area needs to include more general or distant factors that are known to shape attitudes towards biotechnology, and which tend to be more resistant to short-term change. The following results refer to the consolidated European sample.
The worldviews that people hold about the effects of scientific and technological developments on their lives have an important role. Individuals with an optimistic view of technological progress also take a more favourable view of a particular, although controversial, technology such as pharming (supplementary information S1 online). Another important worldview is the perception of nature and naturalness and the role of science and technology in its transformation (Sjöberg, 2000; Siipi, 2008). A more romantic vision of nature translates into less support for both types of pharming (supplementary information S2 online).
Views and attitudes towards animals also affect support for their genetic modification to obtain biopharmaceutical drugs (Crettaz von Roten, 2008). A view of greater “animal–human closeness” tends to be associated with less support for animal pharming (supplementary Fig S13 and information S3 online). In particular, attitudes towards animals' rights to life generate considerable differences in the mean values of support for animal pharming (supplementary Fig S14 and information S4 online).
A general predisposition to feeling threatened by potential risks—or “general fear”—was not mirrored in predispositions towards pharming (supplementary information S5 online). By contrast, a higher level of elementary “scientific knowledge” increases support for pharming, although the size of its independent contribution is small (supplementary information S6 online).
…the public do not adhere to ideological or undifferentiated arguments, but are rather open to different scenarios and, accordingly, to argumentation and dialogue
Finally, regulatory “trust” is a key variable to account for predispositions to pharming (Siegrist, 2000; Priest et al, 2003). A high level of trust in regulatory agencies might substitute or compensate for a modest level of understanding of all the complexities when evaluating research areas such as pharming. Differences in the level of support of both plant and animal pharming, by level of trust in national regulatory agencies, are statistically significant and of considerable magnitude (supplementary information S7 online). Trust in other organizations also makes a marked difference in support for pharming; specifically, trust in the National Ministry of Health and in national scientific institutions translates as a higher level of acceptance (supplementary information S8 online).
There are large differences in the perceptions of animal and plant pharming in the 15 countries that we studied. The USA, Israel, Denmark, Spain, Poland and the Czech Republic express the most positive views of plant pharming: it is considered to be very useful with no high risks, and against nature but not immoral. A second group— formed of the Netherlands, the UK, Ireland, Sweden, Italy and France—approves of plant pharming, albeit with less intensity: it is perceived as very useful, not immoral, but anti-natural, and there are divided opinions about the risks entailed. At the other end, Germany, Japan and, in particular, Austria, mainly reject plant pharming: they perceive it as useful, but it is nonetheless viewed as risky, reckless and immoral.
In a generally unfavourable climate for animal pharming, the countries that are least critical are Spain and Israel, followed by the Czech Republic and Denmark. Although citizens in these societies see animal pharming as risky, immoral and going against nature, they also seem convinced about its usefulness and accept it for specific biomedical goals: the treatment of life-threatening diseases, diseases in children, or to obtain antidotes or medicines to counter the effects of biological weapons. These are countries with high levels of public trust in regulatory agencies and scientific institutions, and that express positive expectations about science and a generally high acceptance of the use of animals in biomedical research. They also consider that an animal's right to life should be recognized, although in a different way to that of a human.
An intermediate group, comprising the USA, Poland, the Netherlands, the UK, Ireland, Italy and Japan, regard animal pharming as useful but immoral, reckless, risky and very anti-natural. They place only modest trust in the laws and controls to regulate animal pharming; finally, they hold positive expectations of science. The countries most critical of animal pharming are Germany, Austria, France and Sweden. In these societies, strong rejection is associated with a low perception of its usefulness—except in Sweden—and, particularly, a heightened perception of its risks, immorality, recklessness and the idea that it is an unacceptable exploitation of animals, incompatible with their inherent dignity. With the exception of Sweden, respondents in these countries also express low levels of trust about the regulation of animal pharming. Austria further stands out for its lower expectations about science in general and stronger rejection of the use of animals in biomedical research.
Trust is a crucial variable and there is a significant stock of trust in regulatory agencies and scientific institutions
Germany and, more so, Austria, show a divergent profile from the other countries in rejecting both plant and animal pharming to varied extents. Furthermore, the modulation of means and ends does little to alter opposition. Conversely, France and Sweden show changes in acceptance between plant and animal pharming. The views expressed, especially in Sweden, are more sensitive to the specifics of means and goals (supplementary Tables S10,S11 online).
The public climate for biotechnology has improved significantly during the past few years (Gaskell et al, 2002). Reservations about genetic engineering in many advanced societies can neither be simply equated with stigma nor attributed to mere ignorance. Pharming is a biotechnology area embedded in a complex space of perceptions, characterized by high expectations for reaching desirable goals, but also by significant reservations about the means. In addition, in the public's view, not all biomedical goals warrant the genetic modification of plants and, particularly, of animals (Lassen et al, 2006).
Our data show a potentially differentiated landscape of perceptions of pharming, rather than a holistic view, whether positive or negative. In the present context, it is of paramount importance that the scientific community working in this area does not confine itself to the by-now-outdated and, in isolation, not very effective recipe of “more knowledge” for the public—even if this is certainly needed. Instead, researchers should be willing to adopt a more complex view of the various cognitive and evaluative dimensions embedded in people's perceptions. Special care should be taken in the case of animal pharming, as it activates prevalent images of closeness to humans, animal rights and the suffering of animals, which in turn can trigger strong resistance.
Hype about pharming as the tool for solving the world's medical needs does not resonate with most individuals. A more nuanced and piecemeal framework, taking into account the relevance of the goals and the different profile of the means, would offer an opportunity for productive dialogue with the public (Anon, 2008). Trust is a crucial variable and there is a significant stock of trust in regulatory agencies and scientific institutions. A transparent, unbiased regulatory framework alongside an objective communication of the significant scientific advances taking place, and their plausible practical application, could have a major role in sustaining a fruitful interaction with the public and in gaining its acceptance and support.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Rafael Pardo
Margret Engelhard
Kristin Hagen
Rikke Bagger Jørgensen
Eckard Rehbinder
Angelika Schnieke
Mariana Szmulewicz
Felix Thiele
Footnotes
The authors declare that they have no conflict of interest.
References
- Anon (2008) Join the dots. Nat Biotechnol 26: 837. [DOI] [PubMed] [Google Scholar]
- Bauer M, Gaskell G (eds) (2002) Biotechnology: The Making of a Global Controversy. Cambridge, UK: Cambridge University Press [Google Scholar]
- Crettaz von Roten F (2008) Mapping perceptions of animal experimentation: trend and explanatory factors. Soc Sci Q 89: 537–549 [Google Scholar]
- Daamen DDL, van der Lans IA, Midden CJH (1990) Cognitive structures in the perception of modern technologies. Sci Technol Human Values 15: 202–225 [Google Scholar]
- Dove A (2000) Milking the genome for profit. Nat Biotechnol 18: 1045–1048 [DOI] [PubMed] [Google Scholar]
- Einsiedel EF, Medlock J (2005) A public consultation on plant molecular farming. AgBioForum 8: 26–32 [Google Scholar]
- Fischhoff B, Fischhoff I (2001) Publics' opinions about biotechnologies. AgBioForum 4: 155–162 [Google Scholar]
- Frewer LJ, Howard C, Shepherd R (1997) Public concerns about general and specific applications of genetic engineering: risk, benefit and ethics. Sci Technol Human Values 22: 98–124 [DOI] [PubMed] [Google Scholar]
- Gaskell G et al. (2002) Europeans and Biotechnology in 2002. A Report to the EC Directorate General for Research from the Project ‘Life Sciences in European Society' QLG7-CT-1999–00286 Eurobarometer 58.0. Brussels, Belgium: The European Opinion Research Group
- Gaskell G et al. (1997) Europe ambivalent on biotechnology. Nature 387: 845–847 [DOI] [PubMed] [Google Scholar]
- Gaskell G, Bauer M (eds) (2006) Genomics and Society: Legal, Ethical, Social Dimensions. London, UK: Earthscan [Google Scholar]
- Giddings G et al. (2000) Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol 18: 1151–1155 [DOI] [PubMed] [Google Scholar]
- Kirk D, McIntosh K (2005) Social acceptance of plant-made vaccines: indications from a public survey. AgBioForum 8: 228–234 [Google Scholar]
- Knight AJ (2007) Intervening effects of knowledge, morality, trust, and benefits on support for animal and plant biotechnology applications. Risk Anal 27: 1553–1563 [DOI] [PubMed] [Google Scholar]
- Knight AJ (2006) Does application matter? An examination of public perception of agricultural biotechnology applications. AgBioForum 9: 121–128 [Google Scholar]
- Lassen J, Gjerris M, Sandøe P (2006) After Dolly—Ethical limits to the use of biotechnology on farm animals. Theriogenology 65: 992–1004 [DOI] [PubMed] [Google Scholar]
- Lawrence S (2007) Billion dollar babies—biotech drugs as blockbusters. Nat Biotechnol 25: 380–382 [DOI] [PubMed] [Google Scholar]
- Ledford H (2006) The farmyard drug store. Nature 443: 16–17 [DOI] [PubMed] [Google Scholar]
- Nevitt J, Mills BF, Reaves DW, Norton GW (2006) Public perceptions of tobacco biopharming. AgBioForum 9: 104–110 [Google Scholar]
- Pardo R, Calvo F (2006) Are Europeans really antagonistic to biotech? Nat Biotechnol 24: 393–395 [DOI] [PubMed] [Google Scholar]
- Pardo R, Midden C, Miller JD (2002) Attitudes toward biotechnology in the European Union. J Biotechnol 98: 9–24 [DOI] [PubMed] [Google Scholar]
- Priest SH (2001) A Grain of Truth: The Media, the Public and Biotechnology. New York, NY, USA: Rowman & Littlefield [Google Scholar]
- Priest SH, Bonfadelli H, Rusanen M (2003) The “trust gap” hypothesis: predicting support for biotechnology across national cultures as a function of trust in actors. Risk Anal 23: 751–766 [DOI] [PubMed] [Google Scholar]
- Siegrist M (2000) The influence of trust and perceptions of risks and benefits on the acceptance of gene technology. Risk Anal 20: 195–204 [DOI] [PubMed] [Google Scholar]
- Siipi H (2008) Dimensions of naturalness. Ethics & the Environment 13: 71–103 [Google Scholar]
- Sjöberg L (2000) Perceived risk and tampering with nature. J Risk Res 3: 353–367 [Google Scholar]
- Sturgis P, Cooper H, Fife-Schaw C (2005) Attitudes to biotechnology: estimating the opinions of a better-informed public. New Genet Soc 24: 33–58 [DOI] [PubMed] [Google Scholar]
- Walsh G (2006) Biopharmaceutical benchmarks. Nat Biotechnol 24: 769–776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information