Abstract
A catalyst system for the Suzuki-Miyaura cross-coupling reactions of aryl and vinyl tosylates and mesylates has been developed. This catalyst displays excellent functional group tolerance and allows the coupling of heteroarylboronic acids with aryl tosylates and mesylates to be performed in high yields. Moreover, reactions employing alkylboronic acids, as well as heteroaryl, vinyl, and allylic pinacol boronate esters were conducted with high efficiencies.
There has been a substantial increase in the utility of Pd-catalyzed Suzuki-Miyaura cross-coupling reactions in the last decade.1 Catalyst systems based on new ligands have allowed reactions of aryl halides and arylboronic acids to be performed at low catalyst loadings and with excellent functional group tolerance.2 More recently, considerable attention has been devoted to effectively coupling the less reactive aryl or vinyl tosylates3 and mesylates.4 These substrates are more attractive than the corresponding triflates and nonaflates because they are cheaper and more stable.5
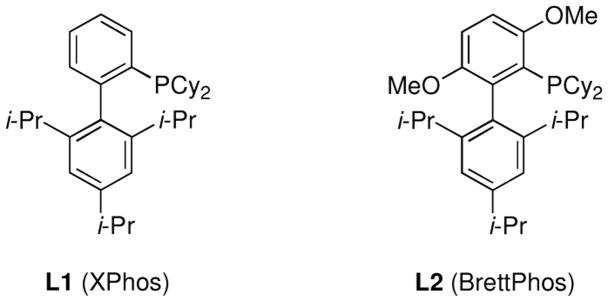
In 2003 we reported a catalyst system, comprised of ligand L1 (XPhos, Figure 1), for the cross-coupling of arylboronic acids with aryl or vinyl tosylates.3d Although this system provided good results in many instances, reactions involving heteroarylboronic acids or electron-rich aryl tosylates were not disclosed. More recently, Kwong has disclosed an active catalyst, based on an indoyl phosphine ligand, for the Suzuki-Miyaura cross-coupling reactions of aryl mesylates4b and tosylates.3a This system showed good functional group tolerance and generally required ≤2 mol % Pd; however, no examples using electron-deficient, heteroaryl, alkyl, or alkenylboronic acids, as well as cross-couplings to form tri-ortho substituted biaryls, were performed.
Figure 1.
Biarylphosphine ligands.
Herein, we report a catalyst for the Suzuki-Miyaura cross-coupling of aryl tosylates and mesylates with boronic acids and esters. This new system is highly active and general for these reactions and, more importantly, extends the reaction scope to substrates that had previously been ineffective coupling partners.
We began our study by examining the reaction of a heteroarylboronic acid with an aryl mesylate. We hypothesized that a catalyst based on L2, which we have previously shown to be efficient for C–N cross-coupling reactions of aryl mesylates,6 would be effective for these difficult Suzuki-Miyaura reactions. Using Pd(OAc)2, L2, and K3PO4 in t-AmOH furan-3-boronic acid was successfully combined with 4-tert-butylphenylmethane-sulfonate in a 98% GC yield (Table 1). With L1, previously our best ligand for the coupling of aryl tosylates with arylboronic acids,3d,7 only 71% of the desired product was obtained. Most other solvents that are commonly employed for cross-coupling reactions gave reduced yields in this reaction; however, use of t-BuOH gave similar results as with t-AmOH. We chose to use t-AmOH for the remainder of our studies because it is a liquid at room temperature and has a higher boiling point than t-BuOH.8 Furthermore, carbonate and fluoride bases gave diminished yields compared to K3PO4.
Table 1.
Condition Screen for the Suzuki-Miyaura Reaction of Furan-3-boronic Acid and 4-tert-Butylphenylmethanesulfonate.a
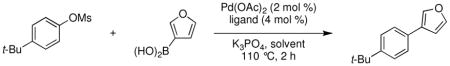 | ||||
|---|---|---|---|---|
| entrya | ligand | solvent | base | yield (GC) |
| 1 | L2 | t-AmOH | K3PO4 | 98% |
| 2 | L1 | t-AmOH | K3PO4 | 71% |
| 3 | L2 | DME | K3PO4 | 73% |
| 4 | L2 | 1,4-dioxane | K3PO4 | 79% |
| 5 | L2 | toluene | K3PO4 | 36% |
| 6 | L2 | t-BuOH | K3PO4 | 99% |
| 7 | L2 | t-AmOH | K2CO3 | 51% |
| 8 | L2 | t-AmOH | Cs2CO3 | 71% |
| 9 | L2 | t-AmOH | CsF | 83% |
Reaction conditions: ArOMs (1.0 mmol), ArB(OH)2 (2.0 mmol), base (3.0 mmol), Pd(OAc)2(2 mol %), ligand (4 mol %), solvent (2 mL), 110 °C, 2 h.
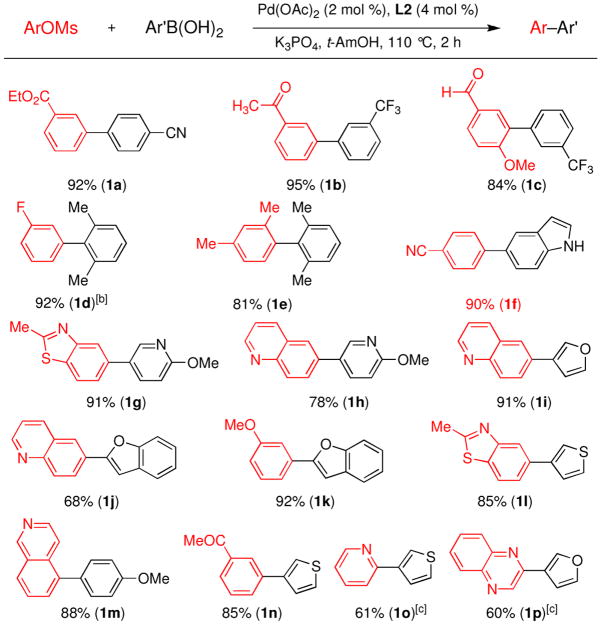
With our optimized reaction conditions we set out to explore the scope of this catalyst system for the coupling of arylboronic acids with aryl mesylates. Common functional groups such as esters, nitriles, aldehydes, and ketones were all well tolerated under these conditions (Figure 2, entries 1a–1c). A hindered substrate, 2,6-dimethylphenyl-boronic acid, was successfully coupled to form a di-ortho-substituted biaryl in an excellent yield with only 0.5 mol % Pd. This same substrate was also successfully combined with 2,4-dimethylphenylmethanesulfonate in an 81% yield (Figure 2, entry 1e). This is the first example of a Suzuki-Miyaura reaction of an aryl mesylate to form a tri-ortho-substituted biaryl.4b
Figure 2.
Suzuki-Miyaura reactions of aryl mesylates using a catalyst system based on L2.a
[a] Reaction conditions: ArOMs (1.0 mmol), ArB(OH)2 (2.0 mmol), K3PO4 (3.0 mmol), Pd(OAc)2 (2 mol %), L2 (4 mol %), t-AmOH (2 mL/mmol), 110 °C, 2 h. Isolated yields based upon an average of 2 runs. [b] 0.5 mol % Pd(OAc)2 and 1 mol % L2 were used. [c] Used K3PO4·H2O as the base and toluene as the solvent.
Due to the ubiquity of heterocycles in biologically active molecules we focused our attention on using heteroarylboronic acids as substrates, which have not previously been coupled with aryl mesylates. These substrates have often been difficult partners for Suzuki-Miyaura reactions due to their instability;9 however, we postulated that with our new catalyst system the desired reaction would be faster than the decomposition pathway. Using a catalyst based on L2, 3-pyridyl, 3-furyl, 2-benzofuryl, 5-indolyl, and 3-thiophenylboronic acids were all reacted with aryl mesylates in good to excellent yields (Figure 2, entries 1f–1p). It is worth pointing out that the 5-indolylboronic acid was successfully coupled in a high yield without the need for a protecting group on the nitrogen. In addition to heteroarylboronic acids, heteroarylmesylates also proved to be viable coupling partners. Mesylates derived from quinolines, isoquinolines, and benzothiazoles were successfully utilized and yielded the biaryl product in adequate yields (Figure 2, entries 1g–1j, 1l, 1m). Unfortunately, employing mesylates containing a nitrogen ortho to the sulfonate gave modest yields due to competing hydrolysis to form the undesired phenol (Figure 2, entries 1o and 1p).
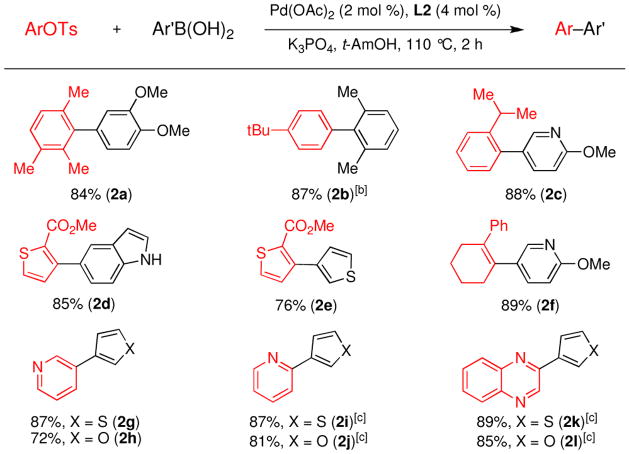
Using a catalyst comprised of L2 and Pd(OAc)2, aryl tosylates were coupled with an array of arylboronic acids with higher efficiencies than in the reactions with the corresponding aryl mesylates. Ortho substitution on both the aryl tosylate and the arylboronic acid were well tolerated (Figure 3, entries 2a–2f). For example, the di-ortho-substituted 2,6-dimethylphenylboronic acid was successfully reacted with an aryl tosylate in an excellent yield with only 0.1% Pd; 5 times less Pd than what was required for the reaction of 2,6-dimethylphenylboronic acid with an aryl mesylate. Heteroarylboronic acids were also successfully employed in these reactions for the first time. The 3-thiophenyl, 3-furyl, 3-pyridyl, and 5-indolylboronic acids were coupled with aryl and heteroaryl tosylates in satisfactory yields (Figure 3, entries 2c–2l). We also found that heteroaryl tosylates that contained a nitrogen ortho to the sulfonate gave considerably higher yields in these reactions than the corresponding heteroaryl mesylates. Hydrolysis to give the phenol was not observed in these reactions (Figure 3, entries 2i–2l).
Figure 3.
Suzuki-Miyaura Coupling of Aryl and Vinyl Tosylates Using L2.a
[a] Reaction conditions: ArOTs (1.0 mmol), ArB(OH)2 (2.0 mmol), K3PO4 (3.0 mmol), Pd(OAc)2 (2 mol %), L2 (4 mol %), t-AmOH (2 mL/mmol), 110 °C, 2 h. Isolated yields based upon an average of 2 runs. [b] 0.1 mol % Pd(OAc)2 and 0.2 mol % L2 were used. [c] Used K3PO4•H2O as the base and toluene as the solvent.
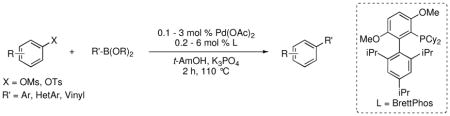
Alkylboronic acids were also viable coupling partners using this new catalyst system; however, the scope was much more limited than what we had seen with aryl-boronic acids (Table 2, entries 1–5). For example, methyl-boronic acid was reacted with an aryl mesylate and an aryl tosylate in high yields. By switching to a small secondary alkyl substrate, cyclopropylboronic acid, the efficiency of the coupling diminished and only a 58% yield was obtained. When the larger cyclopentylboronic acid was employed the reaction completely shut down and none of the desired product was formed.10
Table 2.
Suzuki–Miyaura Coupling of Mesylates and Tosylates With Alkyl, Vinyl and Heterocyclic Nucleophiles Using L2.a
| entry | mesylate/tosylate | nucleophile | Product |
|---|---|---|---|
| 1 | CH3B(OH)2 |
86% |
|
| 2 | 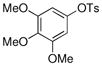 |
CH3B(OH)2 |
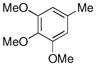 85% |
| 3 | CH3B(OH)2 |
78% |
|
| 4 |
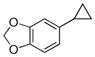 58%b |
||
| 5 | — 0% |
||
| 6 | 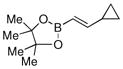 |
 88% |
|
| 7 |  |
 |
 92% |
| 8 | 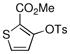 |
 |
 72% |
| 9 | 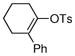 |
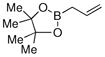 |
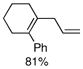 81% |
| 10 |
70% |
||
| 11 | 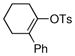 |
 |
 88% |
| 12 |  |
 |
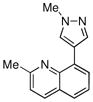 90% |
| 13 | 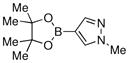 |
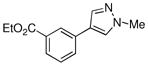 84% |
|
| 14 | 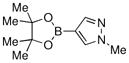 |
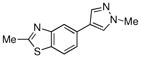 85% |
Reaction conditions: ArX (1.0 mmol), RB(OH)2 (2.0 mmol), K3PO4 (3.0 mmol), Pd(OAc)2 (2 mol %), L2 (4 mol %), t-AmOH (2 mL/mmol), 110 °C, 2 h. Isolated yields based upon an average of 2 runs.
ArOTs (1.0 mmol), RB(OH)2 (3.0 mmol), Pd(OAc)2 (4 mol %), L2(8 mol %), K3PO4•H2O (3.0 mmol), t-AmOH (1 mL/mmol), 120 °C, 4 h.
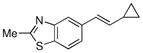
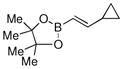
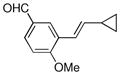
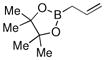
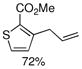
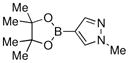
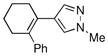
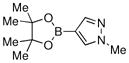
Finally, we were also able to couple pinacol boronate esters with aryl tosylates and mesylates using a catalyst comprised of L2 (Table 2, entries 6–14). These substrates are attractive because they are more stable and more amenable to storage than boronic acids.9b,11 Vinyl and allylic boronate esters could be reacted with aryl mesylates and aryl tosylates in appreciable yields.12 Further, 1-methylpyrazole-4-boronic acid pinacol ester could be coupled with aryl or vinyl tosylates and mesylates in high yields.
In summary, we have developed a versatile catalyst system, based on L2, for the Suzuki-Miyaura cross-coupling reactions of aryl or vinyl tosylates and mesylates. Using this catalyst an array of aryl mesylates and boronic acids, which contained sensitive functional groups, were successfully coupled in high yields. This system also allowed the cross-coupling of heteroarylboronic acids with both aryl tosylates and aryl mesylates for the first time. Finally, alkylboronic acids, as well as vinyl, allylic, and heteroaryl pinacol boronate esters proved to be proficient coupling partners in these reactions.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM-46059) for financial support of this project. We thank Merck, Boehringer Ingelheim, Amgen, BASF (Pd(OAc)2), Nippon Chemical, and Chemetall (Cs2CO3) for additional support. BPF thanks Merck for a fellowship.
Footnotes
Supporting Information Available. Procedural and spectral data is available free of charge via http://pubs.acs.org.
References
- 1.(a) Martin R, Buchwald SL. Acc Chem Res. 2008;41:1461. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Doucet H. Eur J Org Chem . 2008:2013. [Google Scholar]; (c) Corbet J, Mignani G. Chem Rev. 2006;106:2651. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]
- 2.(a) Billingsley K, Buchwald SL. J Am Chem Soc. 2007;129:3358. doi: 10.1021/ja068577p. [DOI] [PubMed] [Google Scholar]; (b) Marion N, Navarro O, Mei J, Stevens ED, Scott NM, Nolan SP. J Am Chem Soc. 2006;128:4101. doi: 10.1021/ja057704z. [DOI] [PubMed] [Google Scholar]; (c) Zapf A, Beller M. Chem Commun. 2005:431. doi: 10.1039/b410937f. [DOI] [PubMed] [Google Scholar]; (d) Barder TE, Walker SD, Martinelli JR, Buchwald SL. J Am Chem Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 3.(a) So CM, Lau CP, Chan ASC, Kwong FY. J Org Chem. 2008;73:7731. doi: 10.1021/jo8014819. [DOI] [PubMed] [Google Scholar]; (b) Wilson DA, Wilson CJ, Rosen BM, Percec V. Org Lett. 2008;10:4879. doi: 10.1021/ol801972f. [DOI] [PubMed] [Google Scholar]; (c) Zhang L, Meng T, Wu J. J Org Chem. 2007;72:9346. doi: 10.1021/jo7019064. [DOI] [PubMed] [Google Scholar]; (d) Nguyen HN, Huang X, Buchwald SL. J Am Chem Soc. 2003;125:11818. doi: 10.1021/ja036947t. [DOI] [PubMed] [Google Scholar]; (e) Brenstrum T, Gerristma DA, Adjabeng GM, Frampton CS, Britten J, Robertson AJ, McNulty J, Capretta A. J Org Chem. 2004;69:7635. doi: 10.1021/jo048875+. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kuroda J, Inamota K, Hiroya K, Doi T. Eur J Org Chem. 2009:2251. [Google Scholar]; (b) So CM, Lau CP, Kwong FY. Angew Chem, Int Ed. 2008;47:8059. doi: 10.1002/anie.200803193. [DOI] [PubMed] [Google Scholar]
- 5.(a) Munday RH, Martinelli JR, Buchwald SL. J Am Chem Soc. 2008;130:2754. doi: 10.1021/ja711449e. [DOI] [PubMed] [Google Scholar]; (b) Klapers A, Campos KR, Chen C, Volante RP. Org Lett. 2005;7:1185. doi: 10.1021/ol050117y. [DOI] [PubMed] [Google Scholar]
- 6.Fors BP, Watson DA, Biscoe MR, Buchwald SL. J Am Chem Soc. 2008;130:13552. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A catalyst comprised of L1 has also been used for other Pd-catalyzed C–C cross-coupling reactions of aryl mesylates: Zhang L, Qing J, Yang P, Wu J. Org Lett. 2008;10:4971. doi: 10.1021/ol802049t.Ackermann L, Althammer A, Fenner S. Angew Chem, Int Ed. 2008;121:207.
- 8.t-BuOH: mp = 25 °C, bp = 85 °C; t-AmOH: mp = −12 °C, bp = 102 °C.
- 9.(a) Knapp DM, Gillis EP, Burke MD. J Am Chem Soc. 2009;131:6961. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Deng JZ, Paone DV, Ginnetti AT, Kurihara H, Dreher SD, Weissman SA, Stauffer SR, Burgey CS. Org Lett. 2009;11:345. doi: 10.1021/ol802556f. [DOI] [PubMed] [Google Scholar]; (c) Billingsley KL, Buchwald SL. Angew Chem, Int Ed. 2008;47:4695. doi: 10.1002/anie.200801465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For Suzuki-Miyaura cross-coupling of secondary alkyl trifluoroborates with aryl chlorides, see: Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257. doi: 10.1021/ja8031423.
- 11.(a) Yang DX, Colletti SL, Wu K, Song M, Li GY, Shen HC. Org Lett. 2009;11:381. doi: 10.1021/ol802642g. [DOI] [PubMed] [Google Scholar]; (b) Billingsley KL, Buchwald SL. J Org Chem. 2008;73:5589. doi: 10.1021/jo800727s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tivola PB, Deagostino A, Prandi C, Venturello P. Org Lett. 2002;4:1275. doi: 10.1021/ol0255817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.