Aortitis is the all-encompassing term ascribed to inflammation of the aorta. The most common causes of aortitis are the large vessel vasculitides, giant cell arteritis (GCA) and Takayasu arteritis, although it is also associated with several other rheumatologic diseases. Infectious aortitis is a rare but potentially life-threatening disorder. In some cases, aortitis is an incidental finding at the time of histopathologic examination following surgery for aortic aneurysm. As the clinical presentation of aortitis is highly variable, the cardiovascular clinician must have a high index of suspicion to establish an accurate diagnosis of this disorder in a timely fashion. In this manuscript, we review the pathophysiology, epidemiology, diagnostic approach, and management of aortitis.
Pathophysiology and Classification
Aortitis is the pathologic term for inflammation of the aortic wall. The classification of aortitis broadly includes underlying rheumatologic and infectious diseases, along with isolated aortitis (Table 1). The most common rheumatologic causes of aortitis are the large vessel vasculitides, giant cell arteritis (GCA) and Takayasu arteritis, although aortitis is also associated with systemic lupus erythematosus, rheumatoid arthritis, the HLA-B27 associated spondyloarthropathies, ANCA-associated vasculitides, Behç;et's disease, Cogan's syndrome, and sarcoidosis. Infectious etiologies include tuberculosis, syphilis, salmonella and other bacteria. Aortitis also may occur in isolation (i.e. there is no associated rheumatologic or infectious disease) or accompany idiopathic retroperitoneal fibrosis or an inflamed abdominal aortic aneurysm.
Table 1.
Causes of Aortitis
| Inflammatory |
| Large-Vessel Vasculitis |
| Giant cell arteritis (temporal arteritis) |
| Takayasu arteritis |
| Rheumatoid arthritis |
| Systemic lupus erythematosis |
HLA-B27 associated spondyloarthropathies
|
Other vasculitides
|
| Sarcoidosis |
| Isolated Aortitis |
| Isolated idiopathic (thoracic) aortitis |
Chronic periaortitis
|
| Infectious |
Bacterial
|
| Luetic (Syphilis) |
| Mycobacterial (i.e., M. tuberculosis) |
| Other |
ANCA – Anti-neutrophil cytoplasmic antibody
Infectious Aortitis
While the majority of cases of aortitis are non-infectious in nature, the possibility of an infectious cause must always be considered, as treatment strategies for infectious and non-infectious aortitis diverge widely. A number of organisms have been associated with infectious aortitis, most commonly Salmonella and Staphylococcal species, along with Streptococcus pneumonia.1, 2 In most cases of bacterial aortitis, a segment of the aortic wall with pre-existing pathology, such as an atherosclerotic plaque or aneurysm sac, is seeded by bacteria via the vasa vasorum.1, 2 Tuberculous aortitis, an uncommon problem in the developed world, may occur as a result of direct seeding of the thoracic aorta from adjacent infected tissues, such as infected lymph nodes or lung lesions, or by military spread.2 Syphilitic or luetic aortitis, now exceedingly rare, typically involves the ascending aorta and is associated with thoracic aortic aneurysm. The classic histopathologic finding is “tree-barking” of the aortic intima.2 There is a chronic inflammatory infiltrate of the medial and adventitial vasa vasorum, which ultimately leads to medial necrosis and a wrinkled appearance of the intima.2 Small microgummas may be visualized within the media, and Treponema pallidum organisms may rarely be identified with specialized staining (Warthin-Starry).2
Non-infectious Aortitis
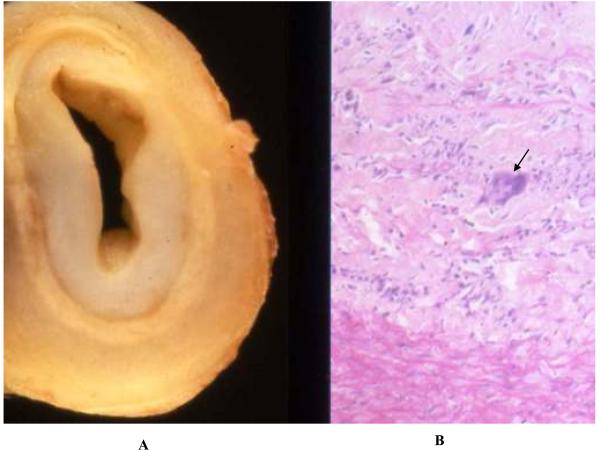
The most common causes of aortitis are the large-vessel vasculitides GCA and Takayasu arteritis. Although the epidemiological and clinical features of these two disorders are distinct (see Clinical Presentation), there may be significant overlap in histopathologic findings (Figure 1). Both GCA and Takayasu arteritis are associated with an inflammatory cellular infiltrate of the aortic media, adventitia, and vasa vasorum that contains a predominance of lymphocytes, macrophages, and multinucleated giant cells.2, 3 Over time, there is scarring of the aortic media and destruction of the elastic lamina.2, 3 Tree-barking may be seen in both of these disorders, not only in syphilis-associated aortitis.4 Granuloma formation and multinucleated giant cells may be seen in both GCA and Takayasu arteritis.2, 3 There are a few pathologic features that may be used to distinguish Takayasu arteritis and GCA. Takayasu arteritis is more commonly associated with extensive intimal and adventitial fibrosis or scarring with resultant luminal narrowing.2, 4, 5 Aortic wall thickness is generally greater among patients with Takayasu aortitis than giant cell aortitis.2, 5 Giant cell arteritis is more commonly associated with extensive medial inflammation and necrosis and the formation of aortic aenurysms.2, 5 Giant cell arteritis is also characterized by focal arterial inflammation and “skip-lesions” are common, a finding which can lead to false negative findings on temporal artery biopsy.3
Figure 1.
Pathologic findings in Takayasu arteritis. Shown are gross (Panel A) and histologic (Panel B) pathology of the innominate artery of a 27 year-old woman with Takayasu arteritis with arch vessel involvement. There is extensive intimal thickening with attenuation of the media and adventitial fibrosis (Panel A). Histology revealed degeneration of the media with a dense inflammatory infiltrate, including giant cells (arrow). (Image courtesy Dr. Richard N. Mitchell, Department of Pathology, Brigham and Women's Hospital).
The pathogenesis of both GCA and Takayasu arteritis is unknown. Both are felt to be antigen-driven cell mediated autoimmune processes, although the specific antigenic stimuli have not been identified.3 Both GCA and Takayasu arteritis have been associated with specific HLA-linked antigens and a resultant genetic predisposition. Giant cell arteritis has been associated with HLA-DR4, while Takayasu arteritis has been associated with HLA-BW52, -DR2, and -MB1 in Japanese patients and possibly with HLA-DR4 in non-Asian patients.3, 6-8 HLA-DR1 has a negative association with the development of Takayasu arteritis in Caucasians and may be protective.8
In addition to GCA and Takayasu arteritis, other rheumatologic disorders including rheumatoid arthritis, systemic lupus erythematous, Wegener's granulomatosis, Behçet's disease, polyarteritis nodosum, and microscopic polyangiitis may lead to aortitis.2, 5, 9-13 In the case of rheumatoid-associated aortitis, rheumatoid nodules are in the aortic wall in up to 50% of pathologic specimens.2 In addition to these disorders, aortitis has been reported in the HLA B-27 associated seronegative spondyloarthropathies Reiter's syndrome and ankylosing spondylitis.14, 15 There are case reports of aortitis associated with sarcoidosis.16 Cogan syndrome is an usual disorder characterized by episodes of interstitial keratitis and vestibuloauditory dysfunction (i.e., eye and ear symptoms), in which aortitis occurs in up to 10% of cases.17
Aortitis also occurs in association with idiopathic retroperitoneal fibrosis (Ormond's disease), inflammatory abdominal aortic aneurysm, and perianeurysmal retroperitoneal fibrosis, a group of clinical disorders now categorized as chronic periaortitis.18-22 These disorders are unique in that inflammation, while symptomatic, is limited to the aorta and peri-aortic tissues, rather than a manifestation of a widespread vasculitis. Similarly in a significant percentage of cases, the diagnosis of aortitis is an incidental histopathologic finding, since there is no rheumatologic disorder, infection, or symptoms attributable directly to the aortitis.5, 23 Such cases of idiopathic isolated aortitis are typically localized to the ascending thoracic aorta and occur in association with ascending aortic aneurysm. Patchy necrosis of the aortic media is the primary histologic finding, along with an inflammatory cellular infiltrate, which may include multinucleated giant cells.5
Epidemiology
The epidemiology of aortitis as a distinct clinical entity is poorly characterized. One survey-based study of 1,290 hospitals in Japan determined a crude incidence of 0.01 cases of aortitis per 100,000 children per year.24 While comparable data on the incidence of aortitis in Western populations or adults are not available, the epidemiology of the large-vessel vasculitides GCA and Takayasu arteritis, the most common causes of aortitis, have been studied in some depth.
Giant cell arteritis is a disorder in persons of advanced age and is more common among Caucasian women.25 In the Olmsted County, Minnesota population, the average age and sex-adjusted incidence of GCA among individuals aged 50 years or older during a fifty year period was 18.8/100,000 per year.25 The overall incidence of GCA increased substantially during the fifty year follow-up period of the study, perhaps due to advances in diagnostic modalities. In this cohort, the incidence of GCA was more than two-fold higher among women than men (24.4 vs. 10.3 per 100,000 per year, respectively) and the mean age at the time of diagnosis was 75 years.25 The long-term survival of patients with GCA did not significantly differ from age-matched control patients without GCA. Not surprisingly, a similar incidence of GCA as reported in Olmsted County has been reported in Scandinavian countries.26, 27 The reported incidence of GCA is lower in other racial groups, including Southern Europeans, Israelis, and Hispanic Americans.28-31 There is little published data regarding the epidemiology of GCA in non-Western countries, although one Japanese study reported a very low prevalence of 1.5/100,000 individuals aged 50 years or older.32
The epidemiology of Takayasu arteritis is not as well characterized as that of GCA. In general, Takayasu arteritis is a disorder of young women, and is rare relative to GCA.27 Data from Olmstead County, Minnesota, based upon a very small number of cases, estimated an incidence of 2.6 per 1 million residents per year, which is higher than that reported in any other epidemiologic series.27, 33 An incidence of 0.4-1 case per 1 million residents per year has been reported in Germany.27, 34 Although originally described by the Japanese ophthalmologist Dr. Mikoto Takayasu, there is no data to support an increased incidence of this disorder in Japan.27, 35
There are circumstances in which the prevalence of aortitis is substantially higher than in that of the general population. In a Cleveland Clinic case series of 1,204 consecutive pathologic specimens taken from patients who underwent aortic surgery over twenty years, the prevalence of aortitis on surgical pathology specimens was 4.3%.23 Most of the surgeries were performed for aortic aneurysmal disease. In nearly 70% of patients with evidence of aortitis, there was no underlying systemic disease, and the vascular inflammation was a truly incidental finding in the majority of these cases of isolated idiopathic aortitis.23
Clinical Presentation
The clinical presentation of aortitis varies across a spectrum of symptoms and clinical signs, ranging from back or abdominal pain with fever to acute severe aortic insufficiency to an incidentally-identified large thoracic aortic aneurysm (Table 2). Acute aortic syndromes, including aortic dissection and rupture can occur in persons with aortitis.9, 36, 37 Inflammation-associated thrombus formation in the aortic lumen with peripheral embolization has also been reported.38, 39 The underlying cause of the aortitis and its concomitant manifestations determine the presenting symptoms. The location of aortic inflammation (e.g., ascending thoracic versus abdominal aorta) and the presence of coexisting arteritis in other blood vessels also determine clinical presentation. Due to the varied presentation of aortitis, and the often non-specific nature of its symptoms and signs, the index of suspicion of the evaluating clinician must be high to establish an accurate diagnosis in a timely fashion.
Table 2.
Varied Spectrum of Clinical Presentation of Aortitis
| Back or abdominal pain with fever |
| Systemic inflammatory syndrome due to vasculitis |
Aneurysmal disease
|
Cardiac abnormalities
|
| Aortic thrombosis with distal embolization |
| Aortic dissection or rupture (rare) |
| Upper and/or lower extremity claudication with pulse deficits |
| Hypertension in a young patient (Takayasu arteritis) |
Giant Cell Arteritis
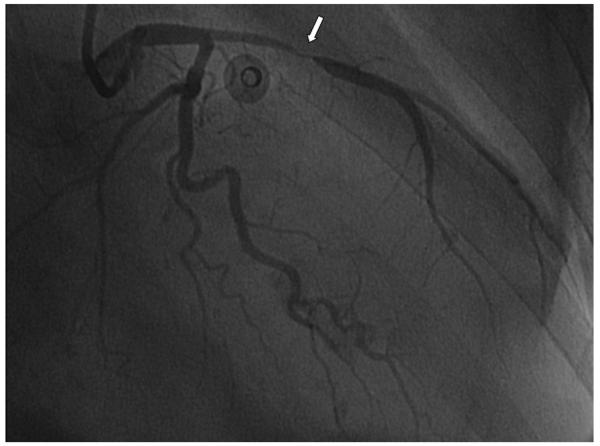
The classic clinical presentation of GCA is headache, temporal artery abnormalities on physical examination, and elevated markers of inflammation in an older adult. Specific criteria have been established for the diagnosis of GCA by the American College of Rheumatology (Table 3), with the presence of three or more of five diagnostic criteria conferring a sensitivity of 94% and a specificity of 91% for the diagnosis.40 In addition to the classic headache and finding of temporal artery thickening, common manifestations of GCA include polymyalgia rheumatica, scalp tenderness, jaw claudication (due to involvement of the branches of the external carotid artery), visual field changes (due to involvement of the ophthalmic, posterior ciliary, or retinal arteries), and mono or polyneuropathy.41 Giant cell arteritis less commonly presents with arm or leg claudication because of arterial occlusive disease that involves the aortic branch vessels and large peripheral arteries, particularly the subclavian and axillary arteries.41 In these cases, the classic radiologic finding is long-segment, tapering lesions in the axillary and subclavian arteries and significant wall thickening. Femoropopliteal or tibioperoneal involvement with similar radiographic findings also occurs.42 This may cause intermittent claudication. Coronary GCA, manifest as tapering lesions in the coronary arteries and myocardial infarction, also has been reported (Figure 2).43 The frequency of aortic involvement in GCA is not known. It is suggested that all patients with temporal GCA who present with symptoms suggestive of extracranial vascular involvement undergo an imaging study to evaluate the aorta and large vessels.41
Table 3.
| 1. Age at disease onset >=50 years |
| Development of symptoms or findings beginning at age 50 or older |
| 2. New headache |
| New onset of or new type of localized pain in the head |
| 3. Temporal artery abnormality |
| Temporal artery tenderness to palpation or decreased pulsation, unrelated to arteriosclerosis of cervical arteries |
| 4. Elevated erythrocyte sedimentation rate |
| Erythrocyte sedimentation rate >=50 mm/hour by the Westergren method |
| 5. Abnormal artery biopsy |
| Biopsy specimen with artery showing vasculitis characterized by a predominance of mononuclear cell infiltration or granulomatous inflammation, usually with multinucleated giant cells |
The presence of three or more criteria is consistent with the diagnosis of giant cell arteritis with a sensitivity of 94% and a specificity of 91%
(Adapted from Hunder, et al. 40 with permission of the publisher.)
Figure 2.
Coronary giant cell arteritis. This 75 year-old woman presented with non-ST segment elevation myocardial infarction and concomitant bilateral arm claudication.43 There is a tapering lesion in the left anterior descending artery (arrow), typical of GCA. (Reproduced from Jang, et al.43, with permission of the publisher).
There is an association between a history of GCA and the development of aortic aneurysm, particularly thoracic aortic aneurysm, as a manifestation of extracranial involvement.36, 37 In the Olmsted County cohort, patients treated for GCA had a 17-fold increase in the incidence of thoracic aortic aneurysm compared to age and gender-matched controls.37 While 18% of patients with GCA and aortic aneurysm were diagnosed with thoracic aortic aneurysm at the time of diagnosis in this population, the majority developed aneurysm during follow-up, a median of 5.8 years after the initial diagnosis. More than half of the patients with GCA-associated thoracic aneurysm died of acute aortic dissection. In this cohort, GCA was also associated with a more than two-fold (relative risk 2.4) increased risk of developing abdominal aortic aneurysm, a median of 2.5 years after initial presentation with GCA.37 Risk factors for the development of aortic and large-vessel complications in GCA have been identified, including the presence of a murmur of aortic insufficiency at diagnosis, concomitant hyperlipidemia, and coronary artery disease.36 Presentation with classic cranial symptoms and signs of temporal arteritis (i.e., headache, scalp tenderness, abnormal temporal artery pulsations, higher erythrocyte sedimentation rate) was a negative predictor of an aortic complication36. Evidence of GCA has also been identified on histopathologic specimens of patients undergoing thoracic aortic aneurysm repair, including those not known to have aortic involvement5, 23. In some of these cases there was a known history of temporal arteritis, in others suggestive symptoms, and in some, this is a truly isolated finding (idiopathic isolated aortitis).
Takayasu arteritis
In contrast to GCA, Takayasu arteritis is a much rarer disorder with a predilection for young women. The average age at diagnosis is 25-30 years, and anywhere from 75-97% of patients are female.44-46 As is the case for GCA, specific diagnostic criteria for Takayasu arteritis have been established47 (Table 4). The presence of three or more of five criteria has a sensitivity of 91% and a specificity of 98% for this diagnosis.47
Table 4.
| 1. Age at disease onset < 40 years |
| Development of symptoms or findings related to Takayasu arteritis at age less than 40 years |
| 2. Claudication of extremities |
| Development and worsening of fatigue and discomfort in muscles of one or more extremity while in use, especially the upper extremities |
| 3. Decreased brachial artery pulse |
| Decreased pulsation of one or both brachial arteries |
| 4. Blood pressure difference >10 mm Hg |
| Difference of >10 mm Hg in systolic blood pressure between arms |
| 5. Bruit over subclavian arteries or aorta |
| Bruit audible on auscultation over one or both subclavian arteries or abdominal aorta |
| 6. Arteriogram abnormality |
| Arteriographic narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper or lower extremities, not due to arteriosclerosis, fibromuscular dysplasia, or similar causes; changes usually focal or segmental |
The presence of any three or more criteria is consistent with a diagnosis of Takayasu arteritis with a sensitivity of 91% and a specificity of 98%.
(Adapted from Arend, et al.47 with permission of the publisher.)
The most common presentation of Takayasu arteritis includes symptoms due to arterial occlusive disease of the aorta, aortic arch, and large vessels. Other common names for Takayasu arteritis, including pulseless disease and aortic-arch syndrome, reflect its clinical presentation. Nearly all patients with Takayasu arteritis either present initially or ultimately develop large vessel manifestations of the disease, including hypertension due to supra-renal aortic or renal artery occlusive disease, pulse deficits and/or vascular bruits, and upper and/or lower extremity claudication.44, 45 A comprehensive vascular examination, including measurement of blood pressure in both arms and palpitation and auscultation of pulses in all major vascular regions is a critical component of the clinical evaluation of all patients with suspected Takayasu arteritis. In addition, we recommend measurement of blood pressure in all four extremities for such patients.
Aortic involvement in Takayasu arteritis is very common, with angiographic abnormalities demonstrated on aortography in nearly all patients.44-46 The abdominal aorta is the most common site of involvement (Figure 3, followed by the descending thoracic aorta and aortic arch)44-46. At the time of aortography, stenotic lesions in the aorta are most frequently detected, although aortic aneurysm is also common and has been reported in up to 45% of patients in published case series.44-46, 48, 49 Case series have also reported rapid aortic aneurysm expansion, aortic rupture, and the development of aortic aneurysm at the site of anastomoses of prior reconstructive surgery among patients with Takayasu arteritis.48-50 Branch vessel disease is highly prevalent in Takayasu arteritis, with the subclavian, innominate, renal, common carotid, vertebral, and mesenteric arteries most often involved.44, 45
Figure 3.
Aortic occlusive disease in a patient with Takayasu arteritis and bilateral leg claudication. Shown are MRA images demonstrating severe, diffuse stenosis of the infrarenal abdominal aorta and common iliac arteries (Panels A and B). A prominent collateral vessel is noted (arrow). The patient underwent endovascular revascularization (Panel C, volume rendered CTA). (Image courtesy Drs. Gary Hoffman and Paul Schoenhagen, Cleveland Clinic Foundation).
While presentation with symptoms or signs related to aortic or arterial occlusive disease is most common, other symptoms may be present at the time of diagnosis. Some patients with Takayasu arteritis may present with neck pain due to carotid arterial inflammation.44, 46 Constitutional symptoms, neurologic symptoms, and musculoskeletal symptoms are less common.44 Nearly 40% of patients with Takayasu arteritis develop cardiac abnormalities, including acute myocardial infarction, angina pectoris, and acute aortic insufficiency.44 In these cases, the cardiac pathology is directly related to the aortic inflammation, including aortic insufficiency as a result of aortic root dilatation, and coronary ostial stenoses due to aortitis (Figure 4).44, 51 Takayasu arteritis may also involve the pulmonary arteries and present with symptoms mimicking pulmonary embolism or with isolated pulmonary hypertension.52 The prevalence of angiographically proven pulmonary artery involvement among patients with Takayasu arteritis is poorly characterized, with values ranging from 14.3% to 70% percent in published series.53-56 Stenotic or occlusive lesions are the most common abnormalities noted in the pulmonary arteries, although aneurysmal dilatation has also been described.45
Figure 4.
Takayasu arteritis involving the coronary ostia. Shown are high-grade ostial stenoses of the left main coronary artery (Panel A) and right coronary artery (Panel B). Coronary involvement in association with Takayasu arteritis is generally ostial in location and due to aortitis at the aortic root. (Reproduced from Kang, et al.51, with permission of the publisher).
Aortitis in Association with Other Rheumatologic Disorders
Aortitis should be suspected among patients presenting with evidence of systemic inflammatory disorders or vasculitis with any clinical features suggestive of aortic involvement (Table 2). Acute aortic insufficiency, evidence of arterial occlusive disease involving the extremities or renal or visceral vessels, unexplained aortic dissection, or a new finding of an aortic aneurysm in a patient without risk factors should raise the possibility of aortitis. In some cases, the combination of aortic findings with other pathognomonic clinical features may point to a particular entity. For example, Behçet's disease may present with aortic aneurysm or acute insufficiency in association with uveitis and oral and/or genital ulceration.11 A patient with longstanding ankylosing spondylitis who develops aortic insufficiency should be suspected of having aortic valvulitis or ascending thoracic aneurysm.15 A young adult patient presenting with vertigo and acute ocular symptom (e.g., interstitial keratitis, iritis, subconjunctival or conjunctive hemorrhage), possibly with fever (Cogan syndrome) should undergo careful cardiac examination and echocardiography to rule out the presence of acute aortic insufficiency.17
Idiopathic Isolated Aortitis
Patients with the isolated aortitis variants, such as chronic periaortitis of the abdominal aorta or idiopathic isolated aortitis of the thoracic aorta, may present with symptoms related to aortic inflammation or may be diagnosed incidentally on the basis of surgical pathology from an aortic procedure. Patients with inflammatory abdominal aortic aneurysms, perianeurysmal aortitis, or Ormond's disease may present with constitutional symptoms, back or abdominal pain, and elevated inflammatory markers.18-20 Inflammatory abdominal aortic aneurysm accounts for 3-10% of all abdominal aortic aneurysms. This entity is more strongly associated with tobacco smoking, younger age at presentation, and family history of aortic aneurysm.18, 22 Idiopathic isolated abdominal periaortitis has also been reported in the absence of aortic aneurysm or retroperitoneal fibrosis.57 Acute renal failure due to ureteral obstruction has been reported in patients with retroperitoneal fibrosis.20 In rare cases, claudication due to arterial occlusive disease also occurs.58 In the case of idiopathic isolated aortitis of the thoracic aorta, the inflammation and aortitis is sub-clinical in nature and usually diagnosed incidentally at the time of histopathology review following thoracic aortic aneurysm surgery.23
Infectious Aortitis
The diagnosis of infectious aortitis is difficult to establish. This disorder generally presents in older individuals with preexisting aortic pathology, such as abdominal aortic aneurysm or atherosclerosis of the abdominal aorta.1 Common presenting symptoms are back or abdominal pain. Bacteremia may or may not be present.1 Aortitis should also be suspected among older patients with atherosclerosis and sepsis with bacteremia from a typical organism, such as Salmonella species.1, 59, 60 In such cases, immediate cross- sectional aortic imaging (CTA or MRA) should be performed to exclude the possibility of infectious aortitis. Establishing the diagnosis of infectious aortitis or mycotic aortic aneurysm early on is critical, as this condition is associated with a high rate of rupture and subsequent mortality if left untreated.1, 59-62 The diagnosis of tuberculous aortitis is very difficult to establish, as this disorder is exceedingly rare and can mimic Takayasu arteritis.63 Delay in diagnosis may occur, and patients may be treated with immunosuppressive therapy before the diagnosis of tuberculosis is made. As such, the possibility of tuberculous aortitis should be considered among patients with aortitis or atypical aortic aneurysms who have a history of pulmonary or extrapulmonary tuberculosis or chronic immunosupression or who present with a suspicious finding such as a cavitary lung lesion, pleural effusion, or lymphadenitis.63 In such cases, a definitive diagnosis should be pursued before glucocorticoids are given.
Diagnostic Testing
When the diagnosis of aortitis is suspected on the basis of clinical presentation, expedient imaging of the entire aorta with an appropriate modality is critical to establish the diagnosis. Modern imaging tools for the aorta include computed tomography angiography (CT), magnetic resonance angiography (MRA), and ultrasonography. Positron emission tomography (PET) scanning has emerged for targeted imaging of vascular inflammation, and may be particularly useful when combined with traditional cross sectional imaging modalities.
Angiography
Though once the gold-standard for diagnosing aortitis, particularly aortitis due to Takayasu arteritis, technological advances in non-invasive cross sectional imaging techniques have generally led to the replacement of conventional aortography with CTA or MRA. In addition to their non-invasive nature, CTA and MRA have the additional advantage of imaging the components of the aortic wall and periaortic structures, rather than the lumen only, as is the case of conventional angiography. Invasive aortography is generally reserved for cases in which diagnosis of an acute aortic syndrome is uncertain, despite non-invasive imaging, or for performance of catheter-based revascularization procedures in select patients.
Non-Invasive Angiographic Imaging (CTA and MRA)
Computed tomography, generally with the administration of iodinated contrast (CTA), is widely available in most medical centers and also allows for the rapid exclusion of aortic pathologies which may mimic acute aortitis, including aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcer. In addition, CTA allows for accurate assessment of stenotic lesions of the aorta or large arteries, most common in Takayasu arteritis, as well as the presence and extent of aortic aneurysm or thrombus. Computed tomography in the setting of acute aortitis may demonstrate thickening of the aortic wall and peri-aortic inflammation, although milder degrees of inflammation or wall edema may not be apparent.64 It is considered less sensitive than other modalities for evaluation of the degree of inflammation in the aortic wall.64 Computed tomography is of use in the long-term follow-up of patients with treated aortitis, particularly for monitoring progression of thoracic aortic aneurysm. Unlike MRA, computed tomography can also be used to assess the extent of aortic and arterial calcification, a complication of long-standing, “burnt out” aortitis (Figure 5).65 Aside from concerns regarding decreased sensitivity for assessment of disease activity in aortitis, the primary limitation of CTA is the requisite radiation exposure and need for iodinated contrast for optimal vascular imaging.
Figure 5.
Computed tomogram of severe aortic and coronary calcification in a patient with a remote history of aortitis (“porcelain aorta”). Shown are volume-rendered CT images demonstrating severe calcification of the thoracic and abdominal aorta (arrowheads), as well as the coronary arteries (arrows). There is diffuse aneurysmal dilatation of the ascending thoracic aorta. (Image courtesy Dr. Paul Schoenhagen, Departments of Radiology and Cardiovascular Medicine, Cleveland Clinic Foundation).
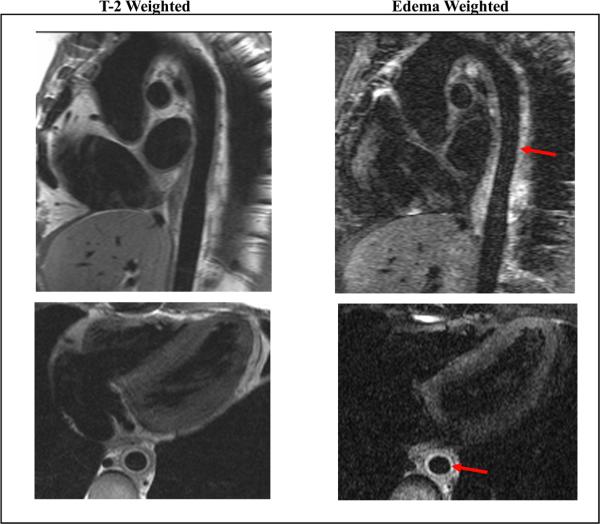
Magnetic resonance angiography, generally with gadolinium contrast enhancement, continues to is emerging as a non-invasive imaging modality of choice for aortitis, particularly aortitis associated with GCA and Takayasu arteritis.64 Magnetic resonance imaging may be used to image the entire aorta without radiation exposure or iodinated contrast, and it provides excellent resolution of the aortic wall. Areas of active aortitis may appear as vessel wall edema, enhancement, or wall thickening on MRA66 (Figure 6). Specific MRI imaging protocols have been developed to enhance characterization of the blood vessel wall in the setting of arteritis, including the so-called “edema weighted” technique.67 Similar to CTA, MRA can detect aortic aneurysm and areas of aortic or arterial stenosis, the large-vessel complications of aortic wall inflammation. Improvement of vessel wall edema with immunosuppressive therapy has been demonstrated in patients with Takayasu arteritis and patients with GCA undergoing immunosuppressive therapy and followed with serial imaging studies.68, 69 One study in a larger cohort of patients with Takayasu arteritis found that MRI assessment of vessel wall edema did not fully correlate with clinical disease activity.66 These investigators have hypothesized that the finding of vessel wall edema may not be due to active inflammation, rather tissue remodeling after inflammation has resolved.66 While the role of MRA for prospective assessment of disease activity in aortitis remains controversial, it is clearly an invaluable non-invasive tool for the diagnosis of aortitis, as well as associated aneurysm formation or arterial stenosis.
Figure 6.
Magnetic resonance angiography in a patient with Takayasu arteritis and active aortitis. Shown are oblique sagittal (top row) and oblique axial (bottom row) images of the thoracic aorta with T-2 weighted black blood (left column) and edema-weighted imaging (right column). There is wall thickening of the descending thoracic aorta (best seen on axial images) with increased signal consistent with edema and inflammation (arrows). Periaortic inflammation and lymphadenopathy were also present. (Image courtesy Dr. Paul Schoenhagen, Departments of Radiology and Cardiovascular Medicine, Cleveland Clinic Foundation).
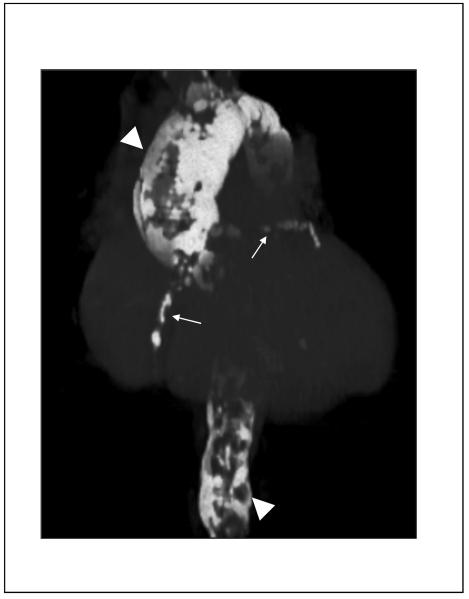
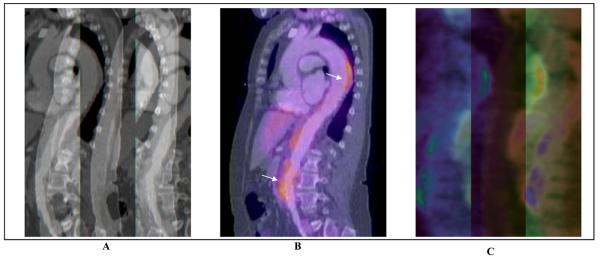
Recently, the use of 18-fluorodeoxyglucose positron emission tomography (18F-FDG PET), either alone or in combination with contrast-enhanced CTA or MRA, has emerged as a potential tool for initial diagnosis and for assessment of disease activity of aortitis due to either GCA or Takayasu arteritis.69-72 Recent imaging series have reported a sensitivity of 60-92% and a specificity of 88-100% of 18F-FDG PET for diagnosis of active inflammation in arteritis, but these studies have been limited by small sample size, heterogeneous patient population, and inconsistent choice of a reference standard.70, 72, 73 Hybrid imaging with 18F-FDG PET and either CTA or MRA allows for more precise anatomic localization of disease activity, with increased uptake of 18F-FDG felt to be a surrogate marker of increased activity of inflammatory cells. The presence of wall thickening, arterial stenosis, luminal thrombus, and aneurysm cannot be assessed by PET alone; CTA and MRA are complementary to PET for complete evaluation of the patient with aortitis (Figure 7). The role of 18F-FDG PET in the diagnosis and follow-up of patients with aortitis will ultimately need to be established with larger clinical studies.
Figure 7.
Combination of 18F-FDG PET and computed tomography for assessment of Takayasu arteritis. Shown are sagittal plane contrast-enhanced computed tomographic (CTA) images of the thoracic and abdominal aorta (Panel A), 18F-FDG PET – CTA overlay (Panel B), and 18F-FDG PET alone (Panel C). There are areas of FDG-uptake, consistent with inflammation, visualized in the descending thoracic and abdominal aorta. (Image courtesy Dr. Paul Schoenhagen, Departments of Radiology and Cardiovascular Medicine, Cleveland Clinic Foundation).
Ultrasound
Though generally not utilized as a primary diagnostic modality for aortitis, abdominal ultrasound or transthoracic or transesophageal echocardiography may demonstrate circumferential thickening of the aortic wall. Abdominal ultrasonography is useful for the diagnosis of abdominal aortic aneurysm occurring as a complication of aortitis, on in association with chronic periaortitis or inflammatory aneurysm. In addition, echocardiography plays a key role in assessment of the aortic root and aortic valve in the setting of aortitis of the ascending thoracic aorta associated with aortic insufficiency and aneurysm formation.74-76
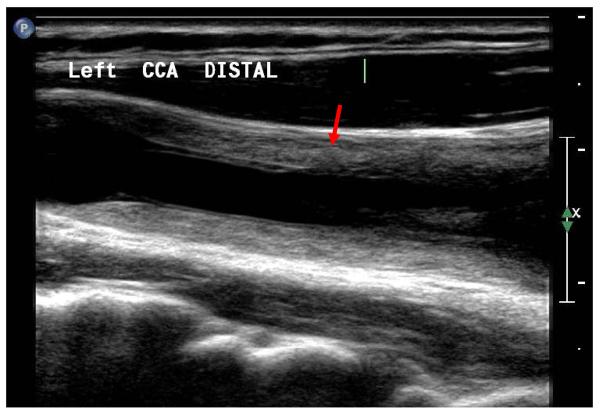
Recently, peripheral vascular ultrasound has also been used in the diagnosis and surveillance of both GCA and Takayasu arteritis. The presence of a hypoechoic (i.e., dark) halo around the vessel lumen on color duplex ultrasound of the temporal arteries (“halo sign”) is associated with active inflammation on temporal artery biopsy.77-79 In addition, the absence of a temporal artery “halo sign” has a negative predictive value of 92-96% for temporal arteritis on biopsy.77, 78 Reduction and complete resolution of the periluminal “halo” may occur in response to glucocorticoid therapy.77 A recent study identified abnormalities of the abdominal aorta on duplex ultrasound, including aortic wall thickening, small aneurysms, and the “halo sign” among a majority of patients with biopsy-proven GCA.80 Takayasu arteritis may present with extensive, concentric arterial wall thickening on color duplex ultrasound of involved arterial segments, reflective of vessel wall inflammation (Figure 8).79, 81, 82 The thickening is generally brighter than the “halo sign” of GCA and has been named the “macaroni sign”.79, 81 Marked thickening of the intimal-medial complex may be seen in the subclavian and common carotid arteries, in particular, and is often bilateral.81, 83-85 Duplex ultrasound may also identify areas of arterial stenosis or occlusion in the setting of both GCA and Takayasu arteritis. Thickness of the carotid intimal-medial complex has shown early promise as a quantifiable marker of disease activity among patients with Takayasu arteritis, and may have potential as a marker of therapeutic response among patients receiving immunosuppressive therapy.86, 87
Figure 8.
High resolution B-mode ultrasound of the common carotid artery a patient with active Takayasu arteritis. There is marked, diffuse, concentric thickening of the vessel wall with a heterogeneous appearance (arrow). The intimal-medial complex is markedly thickened. This finding has been termed the “macaroni sign”.81 No atherosclerotic plaque is visualized. Similar findings were present in the contralateral common carotid artery.
Laboratory Testing
While the diagnosis of aortitis is generally made on the basis of clinical presentation and aortic imaging, key laboratory tests are helpful. The initial evaluation of a patient with suspected aortitis should include markers of inflammation, namely erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP), a complete blood count, assessment of kidney and liver function, and blood cultures to exclude the unlikely but critical diagnosis of infectious aortitis. Additional laboratory testing should be based upon the clinical assessment of the patient and the differential diagnosis of the underlying cause. A rheumatologic panel, including anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, and rheumatoid factor may be helpful in the appropriate clinical setting. Skin testing for tuberculosis and serologic testing for syphilis should be reserved for those rare cases in which the clinical suspicion of these very unlikely disorders is high. In cases of aortitis associated with retroperitoneal fibrosis, secondary causes such as lymphoma should be considered, and a biopsy should be obtained if there is clinical suspicion of malignancy on the basis of clinical presentation and imaging studies.
While ESR and CRP are typically markedly elevated in cases of aortitis due to GCA and other systemic vasculitides, these inflammatory markers may be unreliable for prediction of disease activity among patients with Takayasu arteritis.44, 88, 89 Accordingly, the American College of Rheumatology diagnostic criteria for GCA includes elevated ESR, while the criteria for Takayasu arteritis do not include elevated markers of inflammation.40, 47 Recent clinical investigation has focused on the identification of novel and more sensitive laboratory markers for disease activity among patients with Takayasu arteritis, with IL-6, IL-18, and certain matrix metalloproteinases showing promise in small studies.88, 90, 91
Management
Once the diagnosis of aortitis has been established, the approach to management depends upon the underlying cause. The goals of therapy include both the immediate treatment of aortic inflammation, or infection in rare cases, and surveillance and management of aortic and arterial complications.
Infectious Aortitis
The uncommon case of infectious aortitis requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon. The initial treatment of suspected infectious aortitis is intravenous antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly Staphylococcal species and gram negative rods.1 Antibiotics should be initiated as soon as the diagnosis of infectious aortitis is suspected, and while awaiting microbiologic data. The antibiotic regimen can then be tailored, based upon the results of culture and sensitivity data. Due to case series reporting a very high mortality rate among patients with gram-negative infectious aortitis treated with medical therapy alone, a combination strategy of intensive antibiotic therapy and surgical debridement, with aneurysm repair if necessary, is generally recommended, although no clinical trials have explored the optimal management of such patients.1, 60-62, 92-94 There are small case series, each with no more than a few cases, of combined medical and endovascular management of mycotic aneurysm.95-98 Despite aggressive therapy, mortality associated with infectious aortitis remains high in reported series, largely due to a high rate of aortic rupture.1, 59, 61, 62, 93, 94 While no clinical studies have established the optimal duration of antibiotic therapy for aortitis, a treatment course of at least 6 to 12 weeks following surgical debridement and clearance of blood cultures is generally recommended, with a longer course reserved for patients with immunosuppressive disorders.1
Aortitis Associated with Large Vessel Vasculitis
Immunosuppressive therapy is the primary treatment of non-infectious aortitis due to large-vessel vasculitis, and patients are ideally managed by a multi-disciplinary team that includes a rheumatologist and medical and surgical cardiovascular specialists. Once the diagnosis of non-infectious aortitis due to GCA or Takayasu arteritis has been established, oral glucocorticoid therapy should be initiated. In general, an initial starting dose of 40-60 milligrams prednisone daily (for GCA) or 1 milligram/kilogram prednisone daily (for Takayasu arteritis) is prescribed, although the optimal starting dose of steroid therapy for patients with aortitis has not been established.41, 44, 99 The prednisone dose is gradually tapered over time with close monitoring of symptoms, vascular signs, inflammatory markers, and imaging studies. Most patients with arteritis are treated with steroids over a period of months, or even years, in attempt to achieve complete remission.44, 100
Despite glucocorticoid therapy, the relapse rate for both GCA and Takayasu arteritis is high, up to 50% or greater.44, 100-102 In addition to recurrent symptoms, re-elevation of inflammatory markers may be a helpful sign of relapsed disease, particularly among patients with GCA.41, 88 Due to decreased sensitivity of inflammatory markers for monitoring disease activity in Takayasu arteritis, the diagnosis of relapse is more difficult.88 The NIH criteria for disease activity in Takayasu arteritis define active disease as new or worsening of two or more of the following: systemic symptoms attributable to arteritis, elevated ESR, vascular symptoms or examination findings, or typical angiographic abnormalities.44 The role of edema-weighted MRI, 18F-FDG PET, vascular ultrasound, and novel inflammatory biomarkers (e.g., IL-6) in the diagnosis of relapse in Takayasu arteritis are areas of ongoing investigation.
Due to the need for long-term therapy and the potential for steroid-related adverse effects, medications to prevent osteoporosis, gastric ulceration, and Pneumocystis (carinii) jiroveci pneumonia should be prescribed, as indicated, for patients with aortitis requiring long-term glucocorticoid therapy.103 Patients should also be carefully monitored for the development of steroid-induced diabetes mellitus or hypertension, secondary infections, and cataracts, common complication of long-term glucocorticoid therapy.100, 104 Patients with visual symptoms related to GCA are generally admitted to the hospital for intravenous glucocorticoid therapy, followed by oral therapy, although the likelihood of permanent visual deficits remains high, even among those aggressively treated.105, 106 Patients with severe symptoms related to aortitis, such as acute aortic insufficiency or coronary artery involvement, should similarly be treated in hospital with initial intravenous therapy.
Several studies have investigated potential steroid-sparing regimens for both GCA and Takayasu arteritis, because of the potential need for long-term immunosuppressive therapy. Daily administration of prednisone (45 mg daily) was found to be superior to every other day treatment (90mg) in the management of GCA.107 Recently, a small randomized trial compared standard oral prednisone therapy to initial induction therapy with intravenous methylprednisolone (15mg/kg) followed by oral therapy among patients with GCA.108 At 78 weeks of follow-up, patients randomized to initial intravenous induction therapy had a lower oral steroid requirement and were less likely to relapse, although there was no significant difference in adverse clinical events between the two groups.108 Three randomized clinical trials have investigated the potential benefit of the addition of methotrexate to standard oral steroid therapy for the initial treatment of GCA with conflicting findings, and such combination therapy is not generally recommended.109-111 A recent small trial of the anti-tumor necrosis factor agent, infliximab, for the maintenance of remission in patients with GCA found no benefit.112 Adjunctive, steroid-sparing therapy has also been investigated in the management of Takayasu arteritis, particularly among patients with difficult to treat or relapsing disease.99, 113 In small studies, methotrexate, azathioprine, mycophenolate mofetil, and infliximab have shown promise.114-117 These agents are an option for use in steroid-refractory patients, although data from large randomized clinical trials are needed to determine their optimal role.
Isolated Idiopathic Aortitis
In rare cases, active aortitis on histopathology may be found incidentally in patients with thoracic or abdominal aneurysm who have undergone surgical repair.4, 5, 23 Such patients may have no known history of vasculitis and no systemic signs or symptoms to suggest active or prior disease. The optimal management of such patients is uncertain, and the decision to treat with a course of glucocorticoid therapy should be considered on a case-by-case basis depending on the clinical presentation of the patient and the location and extent of inflammation. Inflammatory markers, such as the ESR or CRP, will be unreliable during recovery from major aortic surgery, but may be helpful in long-term follow-up. In such patients, imaging of the entire aorta for evidence of active disease or aneurysm in other vascular beds should be considered. Patients with isolated idiopathic aortitis require careful follow-up, as small case series have identified a propensity toward aneurysm formation in other vascular beds over time.5, 23
Surgical and Endovascular Therapy
In addition to immunosuppressive therapy, patients with aortitis due to large-vessel vasculitis must be closely monitored for the development of vascular complications, particularly evidence of aortic aneurysm or new aortic or arterial occlusive disease. A comprehensive vascular examination should be performed at each visit. Measurement of bilateral arm and ankle pressures is useful to assess for arterial occlusive disease, particularly among patients with Takayasu arteritis. Patients with known thoracic or abdominal aneurysm due to treated aortitis should be monitored periodically for aneurysm expansion, with indications for surgical correction the same as for other etiologies.118 All patients with Takayasu arteritis or GCA with known aortic involvement should undergo periodic imaging for the development of thoracic or abdominal aortic aneurysm, given the known risk of these complications.36, 37, 48, 49 It is preferable if inflammation of the aorta can be treated with a course of immunosuppressive therapy prior to surgery. Ideally, patients should be in clinical remission before elective repair of an aortitis-related aneurysm. Open aortic reconstructive surgery is generally the standard of treatment for aortic aneurysms associated with aortitis, although endovascular techniques have recently been employed with early reported successes.95, 119-121 While endovascular treatment has the theoretical advantage of avoiding extensive manipulation of inflamed aortic tissue, there have been no head-to-head trials of the optimal strategy for management of aortic aneurysm in patients with aortitis. As endovascular techniques for the treatment of aortic aneurysm in all anatomic locations evolve, particularly the thoracic aorta, this will undoubtedly be an area of future investigation.
For patients with severe symptoms from aortic or arterial occlusive disease due to GCA or Takayasu arteritis, such as secondary hypertension or arm or leg claudication, surgical or catheter-based revascularization of the aorta or large arteries is a therapeutic option (Figure 3). As is the case for treatment of aneurysmal disease, elective surgery is ideally deferred until the patient has achieved a clinical remission with immunosuppressive therapy and inflammation has been controlled. Secondary hypertension due to severe aortic or renal arterial occlusive disease from Takayasu arteritis has been treated with balloon angioplasty, although the rate of restenosis is high.44, 122 The addition of endovascular stenting has improved outcome in these patients, to some extent, though the restenosis rate remains substantial in recent case series.123-125 In certain cases, surgical bypass grafting may be associated with more durable long-term outcome for arterial occlusive disease than endovascular procedures among patients with Takayasu arteritis.125, 126 Among patients with GCA with severe arm claudication or critical limb ischemia due to long-segment axillary or subclavian artery stenosis or occlusion, treatment generally involves surgical revascularization with upper extremity arterial bypass grafting from the common carotid artery to the axillary or brachial artery. Large cases series exploring outcomes in such cases are limited. Endovascular therapy has recently been applied to select arterial occlusive lesions in patients with GCA with successful results in case series, however the typical long-segment nature of the occlusive lesions of GCA is associated with a significant risk of restenosis.127, 128
Acknowledgment
The authors thank Mr. Abizer Sakarwala for research assistance in preparation of this manuscript.
Funding Sources: Dr. Creager receives support as Program Director for a Research Career Development Program in Vascular Medicine (K12 HL083786) from the National Heart, Lung, and Blood Institute (NHLBI). He is the Simon C. Fireman Scholar in Cardiovascular Medicine at Brigham and Women's Hospital.
Footnotes
Conflicts of Interest None
Contributor Information
Heather L. Gornik, Assistant Professor of Medicine, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University Medical Director, Non-Invasive Vascular Laboratory Department of Cardiovascular Medicine The Cleveland Clinic Foundation 9500 Euclid Avenue/Desk S60 Cleveland, Ohio 44120 (216) 445-3689 gornikh@ccf.org
Mark A. Creager, Professor of Medicine, Harvard Medical School Simon C. Fireman Scholar in Cardiovascular Medicine Director, Vascular Center Brigham and Women's Hospital 75 Francis Street Boston, Massachusetts 02115 (617) 732-5267 mcreager@partners.org.
References
- 1.Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious Aortitis. Curr Treat Options Cardiovasc Med. 2005;7:89–97. doi: 10.1007/s11936-005-0010-6. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Burke A. Nonatherosclerotic Diseases of the Aorta and Miscellaneous Disease of the Main Pulmonary Arteries and Large Veins. In: Silver M, Gotlieb A, Schoen F, editors. Cardiovascular Pathology. 3rd ed. Churchill Livingstone; Philadelphia: 2001. pp. 107–137. [Google Scholar]
- 3.Gravanis MB. Giant cell arteritis and Takayasu aortitis: morphologic, pathogenetic and etiologic factors. Int J Cardiol. 2000;75(Suppl 1):S21–33. doi: 10.1016/s0167-5273(00)00184-4. discussion S35-26. [DOI] [PubMed] [Google Scholar]
- 4.Tavora F, Burke A. Review of isolated ascending aortitis: differential diagnosis, including syphilitic, Takayasu's and giant cell aortitis. Pathology. 2006;38:302–308. doi: 10.1080/00313020600820898. [DOI] [PubMed] [Google Scholar]
- 5.Miller DV, Isotalo PA, Weyand CM, Edwards WD, Aubry MC, Tazelaar HD. Surgical pathology of noninfectious ascending aortitis: a study of 45 cases with emphasis on an isolated variant. Am J Surg Pathol. 2006;30:1150–1158. doi: 10.1097/01.pas.0000213293.04026.ec. [DOI] [PubMed] [Google Scholar]
- 6.Moriuchi J, Wakisaka A, Aizawa M, Yasuda K, Yokota A, Tanabe T, Itakura K. HLA-linked susceptibility gene of Takayasu Disease. Hum Immunol. 1982;4:87–91. doi: 10.1016/0198-8859(82)90054-4. [DOI] [PubMed] [Google Scholar]
- 7.Volkman DJ, Mann DL, Fauci AS. Association between Takayasu's arteritis and a B-cell alloantigen in North Americans. N Engl J Med. 1982;306:464–465. doi: 10.1056/NEJM198202253060806. [DOI] [PubMed] [Google Scholar]
- 8.Khraishi MM, Gladman DD, Dagenais P, Fam AG, Keystone EC. HLA antigens in North American patients with Takayasu arteritis. Arthritis Rheum. 1992;35:573–575. doi: 10.1002/art.1780350514. [DOI] [PubMed] [Google Scholar]
- 9.Guard RW, Gotis-Graham I, Edmonds JP, Thomas AC. Aortitis with dissection complicating systemic lupus erythematosus. Pathology. 1995;27:224–228. doi: 10.1080/00313029500169023. [DOI] [PubMed] [Google Scholar]
- 10.Chirinos JA, Tamariz LJ, Lopes G, Del Carpio F, Zhang X, Milikowski C, Lichtstein DM. Large vessel involvement in ANCA-associated vasculitides: report of a case and review of the literature. Clin Rheumatol. 2004;23:152–159. doi: 10.1007/s10067-003-0816-0. [DOI] [PubMed] [Google Scholar]
- 11.Sakane T, Takeno M, Suzuki N, Inaba G. Behcet's disease. N Engl J Med. 1999;341:1284–1291. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Ogino H, Matsuda H, Minatoya K, Sasaki H, Kitamura S. Multiple and repetitive anastomotic pseudoaneurysms with polyarteritis nodosa. Ann Thorac Surg. 2008;85:317–319. doi: 10.1016/j.athoracsur.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 13.Hosoda Y, Iri H, Hata J, Wakasugi A. Granulomatous aortitis associated with necrotizing angiitis and glomerulonephritis. Acta Pathol Jpn. 1973;23:129–138. doi: 10.1111/j.1440-1827.1973.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 14.Morgan SH, Asherson RA, Hughes GR. Distal aortitis complicating Reiter's syndrome. Br Heart J. 1984;52:115–116. doi: 10.1136/hrt.52.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lautermann D, Braun J. Ankylosing spondylitis--cardiac manifestations. Clin Exp Rheumatol. 2002;20:S11–15. [PubMed] [Google Scholar]
- 16.Weiler V, Redtenbacher S, Bancher C, Fischer MB, Smolen JS. Concurrence of sarcoidosis and aortitis: case report and review of the literature. Ann Rheum Dis. 2000;59:850–853. doi: 10.1136/ard.59.11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS. Cogan syndrome: studies in thirteen patients, long-term follow-up, and a review of the literature. Medicine (Baltimore) 1980;59:426–441. [PubMed] [Google Scholar]
- 18.Hellmann DB, Grand DJ, Freischlag JA. Inflammatory abdominal aortic aneurysm. Jama. 2007;297:395–400. doi: 10.1001/jama.297.4.395. [DOI] [PubMed] [Google Scholar]
- 19.Vaglio A, Buzio C. Chronic periaortitis: a spectrum of diseases. Curr Opin Rheumatol. 2005;17:34–40. doi: 10.1097/01.bor.0000145517.83972.40. [DOI] [PubMed] [Google Scholar]
- 20.Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004;43:1441–1446. doi: 10.1093/rheumatology/keh326. [DOI] [PubMed] [Google Scholar]
- 21.Kuwana M, Wakino S, Yoshida T, Homma M. Retroperitoneal fibrosis associated with aortitis. Arthritis Rheum. 1992;35:1245–1247. doi: 10.1002/art.1780351020. [DOI] [PubMed] [Google Scholar]
- 22.Tang T, Boyle JR, Dixon AK, Varty K. Inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2005;29:353–362. doi: 10.1016/j.ejvs.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Rojo-Leyva F, Ratliff NB, Cosgrove DM, 3rd, Hoffman GS. Study of 52 patients with idiopathic aortitis from a cohort of 1,204 surgical cases. Arthritis Rheum. 2000;43:901–907. doi: 10.1002/1529-0131(200004)43:4<901::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Fujikawa S, Okuni M. A nationwide surveillance study of rheumatic diseases among Japanese children. Acta Paediatr Jpn. 1997;39:242–244. doi: 10.1111/j.1442-200x.1997.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 25.Salvarani C, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51:264–268. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 26.Nordborg C, Johansson H, Petursdottir V, Nordborg E. The epidemiology of biopsy-positive giant cell arteritis: special reference to changes in the age of the population. Rheumatology (Oxford) 2003;42:549–552. doi: 10.1093/rheumatology/keg172. [DOI] [PubMed] [Google Scholar]
- 27.Lane SE, Watts R, Scott DG. Epidemiology of systemic vasculitis. Curr Rheumatol Rep. 2005;7:270–275. doi: 10.1007/s11926-005-0036-5. [DOI] [PubMed] [Google Scholar]
- 28.Salvarani C, Macchioni P, Zizzi F, Mantovani W, Rossi F, Castri C, Capozzoli N, Baricchi R, Boiardi L, Chiaravalloti F, Portioli I. Epidemiologic and immunogenetic aspects of polymyalgia rheumatica and giant cell arteritis in northern Italy. Arthritis Rheum. 1991;34:351–356. doi: 10.1002/art.1780340313. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Gay MA, Garcia-Porrua C, Rivas MJ, Rodriguez-Ledo P, Llorca J. Epidemiology of biopsy proven giant cell arteritis in northwestern Spain: trend over an 18 year period. Ann Rheum Dis. 2001;60:367–371. doi: 10.1136/ard.60.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnenblick M, Nesher G, Friedlander Y, Rubinow A. Giant cell arteritis in Jerusalem: a 12-year epidemiological study. Br J Rheumatol. 1994;33:938–941. doi: 10.1093/rheumatology/33.10.938. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez EB, Varner WT, Lisse JR, Daniels JC, Hokanson JA. Giant-cell arteritis in the southern United States. An 11-year retrospective study from the Texas Gulf Coast. Arch Intern Med. 1989;149:1561–1565. [PubMed] [Google Scholar]
- 32.Kobayashi S, Yano T, Matsumoto Y, Numano F, Nakajima N, Yasuda K, Yutani C, Nakayama T, Tamakoshi A, Kawamura T, Ohno Y, Inaba Y, Hashimoto H. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum. 2003;49:594–598. doi: 10.1002/art.11195. [DOI] [PubMed] [Google Scholar]
- 33.Hall S, Barr W, Lie JT, Stanson AW, Kazmier FJ, Hunder GG. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore) 1985;64:89–99. [PubMed] [Google Scholar]
- 34.Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, Gross WL. Stable incidence of primary systemic vasculitides over five years: results from the German vasculitis register. Arthritis Rheum. 2005;53:93–99. doi: 10.1002/art.20928. [DOI] [PubMed] [Google Scholar]
- 35.Koide K. Takayasu arteritis in Japan. Heart Vessels Suppl. 1992;7:48–54. doi: 10.1007/BF01744544. [DOI] [PubMed] [Google Scholar]
- 36.Nuenninghoff DM, Hunder GG, Christianson TJ, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3522–3531. doi: 10.1002/art.11353. [DOI] [PubMed] [Google Scholar]
- 37.Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995;122:502–507. doi: 10.7326/0003-4819-122-7-199504010-00004. [DOI] [PubMed] [Google Scholar]
- 38.Silver AS, Shao CY, Ginzler EM. Aortitis and aortic thrombus in systemic lupus erythematosus. Lupus. 2006;15:541–543. doi: 10.1191/0961203306lu2342cr. [DOI] [PubMed] [Google Scholar]
- 39.Vaideeswar P, Deshpande JR. Non-atherosclerotic aorto-arterial thrombosis: A study of 30 cases at autopsy. J Postgrad Med. 2001;47:8–14. [PubMed] [Google Scholar]
- 40.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW, Jr, Masi AT, McShane DJ, Mills JA, Wallace SL, Zvaifler NJ. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 41.Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002;347:261–271. doi: 10.1056/NEJMra011913. [DOI] [PubMed] [Google Scholar]
- 42.Tato F, Hoffmann U. Clinical presentation and vascular imaging in giant cell arteritis of the femoropopliteal and tibioperoneal arteries. Analysis of four cases. J Vasc Surg. 2006;44:176–182. doi: 10.1016/j.jvs.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 43.Jang JJ, Gorevic PD, Olin JW. Images in vascular medicine: Giant cell arteritis presenting with acute myocardial infarction. Vasc Med. 2007;12:379. doi: 10.1177/1358863X07083176. [DOI] [PubMed] [Google Scholar]
- 44.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS. Takayasu arteritis. Ann Intern Med. 1994;120:919–929. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 45.Mwipatayi BP, Jeffery PC, Beningfield SJ, Matley PJ, Naidoo NG, Kalla AA, Kahn D. Takayasu arteritis: clinical features and management: report of 272 cases. ANZ J Surg. 2005;75:110–117. doi: 10.1111/j.1445-2197.2005.03312.x. [DOI] [PubMed] [Google Scholar]
- 46.Park MC, Lee SW, Park YB, Chung NS, Lee SK. Clinical characteristics and outcomes of Takayasu's arteritis: analysis of 108 patients using standardized criteria for diagnosis, activity assessment, and angiographic classification. Scand J Rheumatol. 2005;34:284–292. doi: 10.1080/03009740510026526. [DOI] [PubMed] [Google Scholar]
- 47.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW, Jr., Masi AT, McShane DJ, Mills JA, Stevens MB, Wallace SL, Zvaifler NJ. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 48.Sueyoshi E, Sakamoto I, Hayashi K. Aortic aneurysms in patients with Takayasu's arteritis: CT evaluation. AJR Am J Roentgenol. 2000;175:1727–1733. doi: 10.2214/ajr.175.6.1751727. [DOI] [PubMed] [Google Scholar]
- 49.Matsumura K, Hirano T, Takeda K, Matsuda A, Nakagawa T, Yamaguchi N, Yuasa H, Kusakawa M, Nakano T. Incidence of aneurysms in Takayasu's arteritis. Angiology. 1991;42:308–315. doi: 10.1177/000331979104200408. [DOI] [PubMed] [Google Scholar]
- 50.Miyata T, Sato O, Koyama H, Shigematsu H, Tada Y. Long-term survival after surgical treatment of patients with Takayasu's arteritis. Circulation. 2003;108:1474–1480. doi: 10.1161/01.CIR.0000089089.42153.5E. [DOI] [PubMed] [Google Scholar]
- 51.Kang WC, Han SH, Oh KJ, Ahn TH, Shin EK. Images in cardiovascular medicine. Implantation of a drug-eluting stent for the coronary artery stenosis of Takayasu arteritis: de novo and in-stent restenosis. Circulation. 2006;113:e735–737. doi: 10.1161/CIRCULATIONAHA.105.540336. [DOI] [PubMed] [Google Scholar]
- 52.Lie JT. Isolated pulmonary Takayasu arteritis: clinicopathologic characteristics. Mod Pathol. 1996;9:469–474. [PubMed] [Google Scholar]
- 53.Liu YQ, Jin BL, Ling J. Pulmonary artery involvement in aortoarteritis: an angiographic study. Cardiovasc Intervent Radiol. 1994;17:2–6. [PubMed] [Google Scholar]
- 54.Yamada I, Shibuya H, Matsubara O, Umehara I, Makino T, Numano F, Suzuki S. Pulmonary artery disease in Takayasu's arteritis: angiographic findings. AJR Am J Roentgenol. 1992;159:263–269. doi: 10.2214/ajr.159.2.1352939. [DOI] [PubMed] [Google Scholar]
- 55.Sharma S, Kamalakar T, Rajani M, Talwar KK, Shrivastava S. The incidence and patterns of pulmonary artery involvement in Takayasu's arteritis. Clin Radiol. 1990;42:177–181. doi: 10.1016/s0009-9260(05)81929-4. [DOI] [PubMed] [Google Scholar]
- 56.He NS, Liu F, Wu EH, Zhang CL, Yang JG, Tan J, Gao S, Yang LC, Zhou YB. Pulmonary artery involvement in aorto-arteritis. An analysis of DSA. Chin Med J (Engl) 1990;103:666–672. [PubMed] [Google Scholar]
- 57.Zeina AR, Gleb S, Naschitz JE, Loberman Z, Barmeir E. Isolated periaortitis: clinical and imaging characteristics. Vasc Health Risk Manag. 2007;3:1083–1086. [PMC free article] [PubMed] [Google Scholar]
- 58.Lance NJ, Levinson DJ. Aortitis and periaortic fibrosis. J Rheumatol. 1991;18:1095–1099. [PubMed] [Google Scholar]
- 59.Bardin JA, Collins GM, Devin JB, Halasz NA. Nonaneurysmal suppurative aortitis. Arch Surg. 1981;116:954–956. doi: 10.1001/archsurg.1981.01380190082019. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez Guerrero ML, Aguado JM, Arribas A, Lumbreras C, de Gorgolas M. The spectrum of cardiovascular infections due to Salmonella enterica: a review of clinical features and factors determining outcome. Medicine (Baltimore) 2004;83:123–138. doi: 10.1097/01.md.0000125652.75260.cf. [DOI] [PubMed] [Google Scholar]
- 61.Muller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33:106–113. doi: 10.1067/mva.2001.110356. [DOI] [PubMed] [Google Scholar]
- 62.Reddy DJ, Shepard AD, Evans JR, Wright DJ, Smith RF, Ernst CB. Management of infected aortoiliac aneurysms. Arch Surg. 1991;126:873–878. doi: 10.1001/archsurg.1991.01410310083012. discussion 878-879. [DOI] [PubMed] [Google Scholar]
- 63.Gajaraj A, Victor S. Tuberculous aortoarteritis. Clinical radiology. 1981;32:461–466. doi: 10.1016/s0009-9260(81)80307-8. [DOI] [PubMed] [Google Scholar]
- 64.Kissin EY, Merkel PA. Diagnostic imaging in Takayasu arteritis. Curr Opin Rheumatol. 2004;16:31–37. doi: 10.1097/00002281-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi T, Ando M, Okita Y, Tagusari O, Hanabusa Y, Kitamura S. Redo aortic valve replacement with “porcelain” aorta in an aortitis patient. A case report. J Cardiovasc Surg (Torino) 2005;46:77–79. [PubMed] [Google Scholar]
- 66.Tso E, Flamm SD, White RD, Schvartzman PR, Mascha E, Hoffman GS. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum. 2002;46:1634–1642. doi: 10.1002/art.10251. [DOI] [PubMed] [Google Scholar]
- 67.Flamm SD, White RD, Hoffman GS. The clinical application of ‘edema-weighted’ magnetic resonance imaging in the assessment of Takayasu's arteritis. Int J Cardiol. 1998;66(Suppl 1):S151–159. doi: 10.1016/s0167-5273(98)00163-6. discussion S161. [DOI] [PubMed] [Google Scholar]
- 68.Bley TA, Ness T, Warnatz K, Frydrychowicz A, Uhl M, Hennig J, Langer M, Markl M. Influence of corticosteroid treatment on MRI findings in giant cell arteritis. Clin Rheumatol. 2007;26:1541–1543. doi: 10.1007/s10067-006-0427-7. [DOI] [PubMed] [Google Scholar]
- 69.Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K, Conrad M, Vosshenrich R. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30:730–736. doi: 10.1007/s00259-003-1144-y. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi Y, Ishii K, Oda K, Nariai T, Tanaka Y, Ishiwata K, Numano F. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med. 2005;46:917–922. [PubMed] [Google Scholar]
- 71.Meller J, Grabbe E, Becker W, Vosshenrich R. Value of F-18 FDG hybrid camera PET and MRI in early takayasu aortitis. Eur Radiol. 2003;13:400–405. doi: 10.1007/s00330-002-1518-8. [DOI] [PubMed] [Google Scholar]
- 72.Webb M, Chambers A, A AL-N, Mason JC, Maudlin L, Rahman L, Frank J. The role of 18F-FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging. 2004;31:627–634. doi: 10.1007/s00259-003-1429-1. [DOI] [PubMed] [Google Scholar]
- 73.Walter MA, Melzer RA, Schindler C, Muller-Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32:674–681. doi: 10.1007/s00259-004-1757-9. [DOI] [PubMed] [Google Scholar]
- 74.Soto ME, Espinola-Zavaleta N, Ramirez-Quito O, Reyes PA. Echocardiographic follow-up of patients with Takayasu's arteritis: five-year survival. Echocardiography. 2006;23:353–360. doi: 10.1111/j.1540-8175.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 75.Harris KM, Malenka DJ, Plehn JF. Transesophageal echocardiographic evaluation of aortitis. Clin Cardiol. 1997;20:813–815. doi: 10.1002/clc.4960200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsui KL, Lee KW, Chan WK, Chan HK, Hon SF, Leung TC, Lee KL, Tsoi TH, Li SK. Behcet's aortitis and aortic regurgitation: a report of two cases. J Am Soc Echocardiogr. 2004;17:83–86. doi: 10.1016/j.echo.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt WA, Kraft HE, Vorpahl K, Volker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337:1336–1342. doi: 10.1056/NEJM199711063371902. [DOI] [PubMed] [Google Scholar]
- 78.LeSar CJ, Meier GH, DeMasi RJ, Sood J, Nelms CR, Carter KA, Gayle RG, Parent FN, Marcinczyk MJ. The utility of color duplex ultrasonography in the diagnosis of temporal arteritis. J Vasc Surg. 2002;36:1154–1160. doi: 10.1067/mva.2002.129648. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt WA. Technology Insight: the role of color and power Doppler ultrasonography in rheumatology. Nat Clin Pract Rheumatol. 2007;3:35–42. doi: 10.1038/ncprheum0377. quiz 59. [DOI] [PubMed] [Google Scholar]
- 80.Agard C, Hamidou MA, Said L, Ponge T, Connault J, Chevalet P, Masseau A, Pistorius MA, Brisseau JM, Planchon B, Barrier JH. [Screening of abdominal aortic involvement using Doppler sonography in active giant cell (temporal) arteritis at the time of diagnosis. A prospective study of 30 patients] Rev Med Interne. 2007;28:363–370. doi: 10.1016/j.revmed.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 81.Maeda H, Handa N, Matsumoto M, Hougaku H, Ogawa S, Oku N, Itoh T, Moriwaki H, Yoneda S, Kimura K, Kamada T. Carotid lesions detected by B-mode ultrasonography in Takayasu's arteritis: “macaroni sign” as an indicator of the disease. Ultrasound Med Biol. 1991;17:695–701. doi: 10.1016/0301-5629(91)90101-2. [DOI] [PubMed] [Google Scholar]
- 82.Buckley A, Southwood T, Culham G, Nadel H, Malleson P, Petty R. The role of ultrasound in evaluation of Takayasu's arteritis. J Rheumatol. 1991;18:1073–1080. [PubMed] [Google Scholar]
- 83.Lefebvre C, Rance A, Paul JF, Beguin C, Bletry O, Amoura Z, Piette JC, Fiessinger JN. The role of B-mode ultrasonography and electron beam computed tomography in evaluation of Takayasu's arteritis: a study of 43 patients. Semin Arthritis Rheum. 2000;30:25–32. doi: 10.1053/sarh.2000.8375. [DOI] [PubMed] [Google Scholar]
- 84.Sun Y, Yip PK, Jeng JS, Hwang BS, Lin WH. Ultrasonographic study and long-term follow-up of Takayasu's arteritis. Stroke. 1996;27:2178–2182. doi: 10.1161/01.str.27.12.2178. [DOI] [PubMed] [Google Scholar]
- 85.Raninen RO, Kupari MM, Pamilo MS, Pajari RI, Poutanen VP, Hekali PE. Arterial wall thickness measurements by B mode ultrasonography in patients with Takayasu's arteritis. Ann Rheum Dis. 1996;55:461–465. doi: 10.1136/ard.55.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukudome Y, Abe I, Onaka U, Fujii K, Ohya Y, Fukuhara M, Kaseda S, Esaki M, Fujishima M. Regression of carotid wall thickening after corticosteroid therapy in Takayasu's arteritis evaluated by B-mode ultrasonography: report of 2 cases. J Rheumatol. 1998;25:2029–2032. [PubMed] [Google Scholar]
- 87.Seth S, Goyal NK, Jagia P, Gulati G, Karthikeyan G, Sharma S, Talwar KK. Carotid intima-medial thickness as a marker of disease activity in Takayasu's arteritis. Int J Cardiol. 2006;108:385–390. doi: 10.1016/j.ijcard.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 88.Salvarani C, Cantini F, Boiardi L, Hunder GG. Laboratory investigations useful in giant cell arteritis and Takayasu's arteritis. Clin Exp Rheumatol. 2003;21:S23–28. [PubMed] [Google Scholar]
- 89.Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with Takayasu arteritis. A preliminary report from The International Network for the Study of the Systemic Vasculitides (INSSYS) Int J Cardiol. 1998;66(Suppl 1):S191–194. doi: 10.1016/s0167-5273(98)00181-8. discussion S195. [DOI] [PubMed] [Google Scholar]
- 90.Park MC, Lee SW, Park YB, Lee SK. Serum cytokine profiles and their correlations with disease activity in Takayasu's arteritis. Rheumatology (Oxford) 2006;45:545–548. doi: 10.1093/rheumatology/kei266. [DOI] [PubMed] [Google Scholar]
- 91.Matsuyama A, Sakai N, Ishigami M, Hiraoka H, Kashine S, Hirata A, Nakamura T, Yamashita S, Matsuzawa Y. Matrix metalloproteinases as novel disease markers in Takayasu arteritis. Circulation. 2003;108:1469–1473. doi: 10.1161/01.CIR.0000090689.69973.B1. [DOI] [PubMed] [Google Scholar]
- 92.Jarrett F, Darling RC, Mundth ED, Austen WG. The management of infected arterial aneurysms. J Cardiovasc Surg (Torino) 1977;18:361–366. [PubMed] [Google Scholar]
- 93.Oskoui R, Davis WA, Gomes MN. Salmonella aortitis. A report of a successfully treated case with a comprehensive review of the literature. Arch Intern Med. 1993;153:517–525. doi: 10.1001/archinte.153.4.517. [DOI] [PubMed] [Google Scholar]
- 94.Soravia-Dunand VA, Loo VG, Salit IE. Aortitis due to Salmonella: report of 10 cases and comprehensive review of the literature. Clin Infect Dis. 1999;29:862–868. doi: 10.1086/520450. [DOI] [PubMed] [Google Scholar]
- 95.Krohg-Sorensen K, Hafsahl G, Fosse E, Geiran OR. Acceptable short-term results after endovascular repair of diseases of the thoracic aorta in high risk patients. Eur J Cardiothorac Surg. 2003;24:379–387. doi: 10.1016/s1010-7940(03)00330-0. [DOI] [PubMed] [Google Scholar]
- 96.Chan YC, Morales JP, Taylor PR. The management of mycotic aortic aneurysms: is there a role for endoluminal treatment? Acta Chir Belg. 2005;105:580–587. doi: 10.1080/00015458.2005.11679783. [DOI] [PubMed] [Google Scholar]
- 97.Stanley BM, Semmens JB, Lawrence-Brown MM, Denton M, Grosser D. Endoluminal repair of mycotic thoracic aneurysms. J Endovasc Ther. 2003;10:511–515. doi: 10.1177/152660280301000316. [DOI] [PubMed] [Google Scholar]
- 98.Semba CP, Sakai T, Slonim SM, Razavi MK, Kee ST, Jorgensen MJ, Hagberg RC, Lee GK, Mitchell RS, Miller DC, Dake MD. Mycotic aneurysms of the thoracic aorta: repair with use of endovascular stent-grafts. J Vasc Interv Radiol. 1998;9:33–40. doi: 10.1016/s1051-0443(98)70479-8. [DOI] [PubMed] [Google Scholar]
- 99.Koening CL, Langford CA. Takayasu's Arteritis. Curr Treat Options Cardiovasc Med. 2008;10:164–172. doi: 10.1007/s11936-008-0018-9. [DOI] [PubMed] [Google Scholar]
- 100.Proven A, Gabriel SE, Orces C, O'Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–708. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 101.Hachulla E, Boivin V, Pasturel-Michon U, Fauchais AL, Bouroz-Joly J, Perez-Cousin M, Hatron PY, Devulder B. Prognostic factors and long-term evolution in a cohort of 133 patients with giant cell arteritis. Clin Exp Rheumatol. 2001;19:171–176. [PubMed] [Google Scholar]
- 102.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56:1000–1009. doi: 10.1002/art.22404. [DOI] [PubMed] [Google Scholar]
- 103.Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Arthritis Rheum. 2001;44:1496–1503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 104.Nesher G, Sonnenblick M, Friedlander Y. Analysis of steroid related complications and mortality in temporal arteritis: a 15-year survey of 43 patients. J Rheumatol. 1994;21:1283–1286. [PubMed] [Google Scholar]
- 105.Danesh-Meyer H, Savino PJ, Gamble GG. Poor prognosis of visual outcome after visual loss from giant cell arteritis. Ophthalmology. 2005;112:1098–1103. doi: 10.1016/j.ophtha.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 106.Chan CC, Paine M, O'Day J. Steroid management in giant cell arteritis. Br J Ophthalmol. 2001;85:1061–1064. doi: 10.1136/bjo.85.9.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med. 1975;82:613–618. doi: 10.7326/0003-4819-82-5-613. [DOI] [PubMed] [Google Scholar]
- 108.Mazlumzadeh M, Hunder GG, Easley KA, Calamia KT, Matteson EL, Griffing WL, Younge BR, Weyand CM, Goronzy JJ. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310–3318. doi: 10.1002/art.22163. [DOI] [PubMed] [Google Scholar]
- 109.Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, Cohen P, Calabrese LH, Dickler H, Merkel PA, Fortin P, Flynn JA, Locker GA, Easley KA, Schned E, Hunder GG, Sneller MC, Tuggle C, Swanson H, Hernandez-Rodriguez J, Lopez-Soto A, Bork D, Hoffman DB, Kalunian K, Klashman D, Wilke WS, Scheetz RJ, Mandell BF, Fessler BJ, Kosmorsky G, Prayson R, Luqmani RA, Nuki G, McRorie E, Sherrer Y, Baca S, Walsh B, Ferland D, Soubrier M, Choi HK, Gross W, Segal AM, Ludivico C, Puechal X. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309–1318. doi: 10.1002/art.10262. [DOI] [PubMed] [Google Scholar]
- 110.Jover JA, Hernandez-Garcia C, Morado IC, Vargas E, Banares A, Fernandez-Gutierrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:106–114. doi: 10.7326/0003-4819-134-2-200101160-00010. [DOI] [PubMed] [Google Scholar]
- 111.Spiera RF, Mitnick HJ, Kupersmith M, Richmond M, Spiera H, Peterson MG, Paget SA. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA) Clin Exp Rheumatol. 2001;19:495–501. [PubMed] [Google Scholar]
- 112.Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, Salvarani C, Xu W, Visvanathan S, Rahman MU. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–630. doi: 10.7326/0003-4819-146-9-200705010-00004. [DOI] [PubMed] [Google Scholar]
- 113.Koening CL, Langford CA. Novel therapeutic strategies for large vessel vasculitis. Rheum Dis Clin North Am. 2006;32:173–186. doi: 10.1016/j.rdc.2005.09.006. xi. [DOI] [PubMed] [Google Scholar]
- 114.Hoffman GS, Leavitt RY, Kerr GS, Rottem M, Sneller MC, Fauci AS. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994;37:578–582. doi: 10.1002/art.1780370420. [DOI] [PubMed] [Google Scholar]
- 115.Valsakumar AK, Valappil UC, Jorapur V, Garg N, Nityanand S, Sinha N. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu's arteritis. J Rheumatol. 2003;30:1793–1798. [PubMed] [Google Scholar]
- 116.Hoffman GS, Merkel PA, Brasington RD, Lenschow DJ, Liang P. Anti-tumor necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum. 2004;50:2296–2304. doi: 10.1002/art.20300. [DOI] [PubMed] [Google Scholar]
- 117.Shinjo SK, Pereira RM, Tizziani VA, Radu AS, Levy-Neto M. Mycophenolate mofetil reduces disease activity and steroid dosage in Takayasu arteritis. Clin Rheumatol. 2007;26:1871–1875. doi: 10.1007/s10067-007-0596-z. [DOI] [PubMed] [Google Scholar]
- 118.Gornik HL, Creager MA. Diseases of the Aorta. In: Topol EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. Lipincott, Williams, & Wilkins; Philadelphia: 2007. [Google Scholar]
- 119.Zehr KJ, Mathur A, Orszulak TA, Mullany CJ, Schaff HV. Surgical treatment of ascending aortic aneurysms in patients with giant cell aortitis. Ann Thorac Surg. 2005;79:1512–1517. doi: 10.1016/j.athoracsur.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 120.Engelke C, Sandhu C, Morgan RA, Belli AM. Endovascular repair of thoracic aortic aneurysm and intramural hematoma in giant cell arteritis. J Vasc Interv Radiol. 2002;13:625–629. doi: 10.1016/s1051-0443(07)61658-3. [DOI] [PubMed] [Google Scholar]
- 121.Baril DT, Carroccio A, Palchik E, Ellozy SH, Jacobs TS, Teodorescu V, Marin ML. Endovascular treatment of complicated aortic aneurysms in patients with underlying arteriopathies. Ann Vasc Surg. 2006;20:464–471. doi: 10.1007/s10016-006-9091-2. [DOI] [PubMed] [Google Scholar]
- 122.D'Souza SJ, Tsai WS, Silver MM, Chait P, Benson LN, Silverman E, Hebert D, Balfe JW. Diagnosis and management of stenotic aorto-arteriopathy in childhood. J Pediatr. 1998;132:1016–1022. doi: 10.1016/s0022-3476(98)70401-9. [DOI] [PubMed] [Google Scholar]
- 123.Tyagi S, Kaul UA, Arora R. Endovascular stenting for unsuccessful angioplasty of the aorta in aortoarteritis. Cardiovasc Intervent Radiol. 1999;22:452–456. doi: 10.1007/s002709900431. [DOI] [PubMed] [Google Scholar]
- 124.Bali HK, Jain S, Jain A, Sharma BK. Stent supported angioplasty in Takayasu arteritis. Int J Cardiol. 1998;66(Suppl 1):S213–217. doi: 10.1016/s0167-5273(98)00171-5. discussion S219-220. [DOI] [PubMed] [Google Scholar]
- 125.Liang P, Tan-Ong M, Hoffman GS. Takayasu's arteritis: vascular interventions and outcomes. The Journal of rheumatology. 2004;31:102–106. [PubMed] [Google Scholar]
- 126.Delis KT, Gloviczki P. Middle aortic syndrome: from presentation to contemporary open surgical and endovascular treatment. Perspect Vasc Surg Endovasc Ther. 2005;17:187–203. doi: 10.1177/153100350501700302. [DOI] [PubMed] [Google Scholar]
- 127.Monte R, Gonzalez-Gay MA, Garcia-Porrua C, Lopez-Alvarez MJ, Pulpeiro JR. Successful response to angioplasty in a patient with upper limb ischaemia secondary to giant cell arteritis. Br J Rheumatol. 1998;37:344. doi: 10.1093/rheumatology/37.3.344a. [DOI] [PubMed] [Google Scholar]
- 128.Both M, Aries PM, Muller-Hulsbeck S, Jahnke T, Schafer PJ, Gross WL, Heller M, Reuter M. Balloon angioplasty of arteries of the upper extremities in patients with extracranial giant-cell arteritis. Ann Rheum Dis. 2006;65:1124–1130. doi: 10.1136/ard.2005.048470. [DOI] [PMC free article] [PubMed] [Google Scholar]