Abstract
During a couple of decades, a number of antihypertensive drugs have been developed, and the choice of hypertension treatment has been expanded. Among antihypertensive drugs, calcium channel blockers, which inhibit L-type voltage-gated calcium channels, are potent vasodilators, and have been used as a first- or second-line drug. Dihydropyridine-class calcium channel blockers are categorized into three generations according to the length of activity, and long-acting calcium channel blockers cause less activation of sympathetic nervous system, and are reported to offer beneficial action compared with short-action agents. Furthermore, novel types of calcium channel blockers have been developed that possess the blocking action on other calcium channel subtypes (T- and N-type), and exert agent-specific action apart from their class effects, such as the effects on heart rate and renin/aldosterone release. These additional benefits conferred by T/N-type calcium channel blockade are anticipated to provide organ protective actions in the treatment of hypertension, in addition to the blood pressure-lowering effect of L-type calcium channel blockade. In conclusion, novel calcium channel blockers with sustained activity and T/N-type calcium channel blocking action could provide more beneficial effects than classical blockers, and may expand the clinical utility of these agents.
Keywords: N-type calcium channels, T-type calcium channels, tachycardia, heart failure, renal protection
INTRODUCTION
Hypertension is one of the most important risk factors for cardiovascular diseases, including cerebral infarction, ischemic heart diseases and heart failure. Indeed, a 5–6 mmHg decrement in diastolic blood pressure is demonstrated to reduce the risk of stroke and coronary heart disease [1,2]. Several classes of antihypertensive agents have been in clinical use, including diuretics, α-blockers, β-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II type 1 receptor blockers (ARB), and organic calcium channel blockers (CCBs). Among them, CCBs exert potent antihypertensive action and are widely used as a first line antihypertensive drug with few contraindication [3–7]. Traditionally, CCBs exert dilator action on vascular smooth muscle cells by inhibiting calcium entry through L-type calcium channels. Recently, novel types of CCBs have been developed that express unique characteristics. Thus, certain CCBs manifest blocking activity on N- (cilnidipine) and/or T- (mibefradil and efonidipine) type calcium channels as well as L-type channels, and it is surmised that these properties produce additional benefits associated with reductions in cardiovascular events and renal injury. For example, the blockade of N-type or T-type calcium channels in cardiac pacemaker cells may suppress heart rates, which could therefore reduce cardiac events and improve survival [8,9]. Although this premise appears intriguing, it remains to be established whether these subclasses of calcium channels contribute to the development of cardiovascular events in a clinical setting.
As there are many interesting review articles of calcium antagonists [10], we attempt to characterize the action of various CCBs based on the target channel subclasses, especially on T-type and N-type channels in this review. Furthermore, the roles of these properties in the development of organ injury, including heart and kidney, are also assessed.
1. CLASSIFICATION OF CALCIUM CHANNELS AND CCBS
The voltage-gated calcium channel consists of 4 subunits, α1, α2-δ, β and γ. An α1 subunit is the dominant component of the calcium channels and constitutes pore structure for ion conduction. Ten different α1 subunits have been reported and each of them has specific distribution and ion conductance of its channels (Table 1). These distinct subunits characterize the channel properties of L-, N-, T-, P-, Q- and R-type calcium channels [11,12]. Of these channels, L-type calcium channels are the main targets of the CCB. Either α1S (Cav1.1), α1C (Cav1.2), α1D (Cav1.3) or α1F (Cav1.4) subunit is a component of L-type calcium channels. The α1 subunits of T-type channels include α1G (Cav3.1), α1H (Cav3.2) and α1I (Cav3.3). The α1I distributes in the brain, and α1G in the brain, sinoatrial node, atrioventricular node, Purkinje fiber and along the nerve. The α1H subunits prevail in sinoatrial node, atrioventricular node, Purkinje fiber, brain, kidney and liver. The subunits of P/Q, N and R-type channels are α1A (Cav2.1), α1B (Cav2.2), and α1E (Cav2.3), and these channels are distributed in the brain, neuron, and pituitary gland [13].
Table 1.
Pharmacology and Distribution of Calcium Channels
| Current | α1 subunit | Channel | Distribution | Inhibitors |
|---|---|---|---|---|
| P | α1A | CaV2.1 | neurons | ω-agatoxin IVA |
| Q | α1A | CaV2.1 | neurons | ω-agatoxin IVA |
| N | α1B | CaV2.2 | neurons | ω-conotoxin GIVA |
| R | α1E | CaV2.3 | neurons | SNX-482 |
| L | α1S | CaV1.1 | skeletal muscle | DHP/PAA/BZP |
| α1C | CaV1.2 | heart, endocrine, neurons | DHP/PAA/BZP | |
| α1D | CaV1.3 | endocrine, neurons | DHP/PAA/BZP | |
| α1F | CaV1.4 | retina | N/A | |
| T | α1G | CaV3.1 | neurons, heart | N/A |
| α1H | CaV3.2 | neurons, heart | N/A | |
| α1I | CaV3.3 | neurons | N/A |
DHP: dihydropyridines, PAA: phenylalkylamines, BZP: benzothiazepines
From Triggle [13] with modifications
Based on the chemical structure, CCBs are categorized into 3 subgroups; benzothiazepines (e.g., diltiazem and clenazem), phenylalkylamines (e.g., verapamil and gallopamil) and dihydropyridines (e.g., nifedipine, nicardipine, felodipine, amlodipine, aranidipine, azelnidipine, cilnidipine, efonidipine, manidipine and nilvadipine). The differences in chemical structures would provide heterogeneity in the action of these agents. All CCBs block the calcium influx by binding to the α1 subunit [14], and inhibit cell excitability. Benzothiazepines [15] and dihydropyridines binds to the specific amino acid residue of the α1 subunit exposed to the cell surface. In contrast, phenylalkylamines bind to the specific region of the α1 subunit located at the inner surface of the cell membrane [16,17]. Benzothiazepines and phenylalkylamines have negative inotropic or chronotropic effect, whereas dihydropyridines do not show negative chronotropic effect because of its reflex tachycardia due to peripheral vasodilation.
Although the CCB inhibits calcium currents through L-type calcium channels, some CCBs possess the ability to block other calcium channels. Mibefradil is a well-known T-type calcium channel blocker, and has a similar structure with phenylalkylamines, but possesses weak L-type calcium channel blocking activity [18]. Efonidipine is developed as a long acting dihydropyridine-class CCB, and found to possess both L-type and T-type calcium channel blocking action [19]. Recently, some of dihydropyridines, including benidipine [20], nilvadipine [21], and aranidipine [22] are also reported to possess T-type calcium channel blocking activity. Cilnidipine is a recently developed CCB, and possesses both L- and N-type calcium channel blocking activity [23]. Since N-type calcium is distributed along the nerve and in the brain, cilnidipine is anticipated to exert specific action on nerve activity, such as inhibition of the sympathetic nervous system. In this regard, recent studies have demonstrated that amlodipine possesses N-type calcium channel blocking action [24].
Based on the difference in the formula and the length of their action, dihydropyridine-class CCBs are clinically classified to 1st, 2nd, and 3rd generations [25]. The first generation drugs are the original formula of nifedipine, nicardipine, verapamil, and diltiazem. Second generation drugs have better pharmacokinetic profile that encompasses longer action than 1st generation drugs and enhanced vascular selectivity. They are subdivided to 2 groups; slow release formula (e.g., nifedipine SR (slow release), felodipine ER (extended release) and diltiazem SR) and newer chemical structures (e.g., benidipine, manidipine, nilvadipine and nitrendipine). The third generation drugs have defined as long acting drugs, and two distinct types of CCBs belong to this generation; one is characterized by a sustained blood concentration with a long half-life. Amlodipine is in the ionized state at a physiological pH and combines slowly with the receptor and binds firmly to various tissue compartments [26]. The other category is characterized by lipophilic and highly histotropic properties,(e.g., lercanidipine and lacidipine) which subsequently provides long-acting pharmacokinetics of this agent [26,27]. Azelnidipine is also lipophilic [28,29] and has the high affinity to vascular tissue [30], and these properties have yielded long activity. In general, longer acting and lipophilic CCBs tend to have slow onsets.
2. CCBS AND THE HEART
Framingham study reported that heart rate is one of the most important factors for the mortality of cardiovascular disease. CASTEL study also reported that heart rate is the strong predictor of the cardiovascular death in men [31]. It was conventionally believed that CCBs were not an appropriate treatment for heart diseases because they elicit the reflex tachycardia associated with excessive decrease in blood pressure, activation of sympathetic nervous system and renin-angiotensin system, cardiac overload and labile hypertension [32–35]. Actually, several lines of clinical studies have reported unfavorable effects of CCBs in the treatment of heart diseases. Boden et al. [36] reported that nifedipine increased the heart rate and the incidence of angina pectoris, when compared with β-blockers or nitrate. In The MDPIT Trial Research Group, diltiazem exerted no overall effect on mortality and incidence of reinfarction of the postinfarction patients [37]. Elkayam et al. reported that the deterioration of heart failure was higher in the nifedipine-treated group than that of the isosorbide dinitrate-treated group [38]. Furberg et al. [39] analyzed 16 randomized trials using nifedipine and reported that the mortality was increased in a dose-dependent manner in these trials. Most of these problems are associated with the use of short acting dihydropyridines and the subsequent occurrence of reflex tachycardia and rapid blood pressure decrease. To counter this confounding issue, novel types of CCBs have been developed.
The first category of the drugs is the CCB with sustained action to avoid reflex tachycardia, and amlodipine, lercanidipine and azelnidipine are included in this class. Controversy attends the impact of CCBs on heart failure. Although lowering blood pressure improves or prevents the development of heart failure, some CCBs are known to deteriorate cardiac function in patients with heart failure. Several lines of clinical trials also have witnessed that newly-developed CCBs prevent the cardiovascular/cerebrovascular events [40–43]. For example, amlodipine is demonstrated to exert salutary action in several clinical trials. In PRAISE study [44], amlodipine improved the morbidity and mortality in the patients with non-ischemic cardiomyopathy, but not the ones with ischemic heart diseases. In PREVENT study, amlodipine retarded the progress of coronary atherosclerosis, and it did not increase the incidence of cardiovascular events [45]. The VALUE study has demonstrated that there is no difference in the incidence of heart failure or stroke between amlodipine- and valsartan-treated patients, and the incidence of myocardial infarction is rather lower in amlodipine-treated group [40]. Amlodipine is also demonstrated to reduce cardiovascular events compared with enalapril and placebo did in the CAMELOT study [46]. Amlodipine is reported to improve the cardiac function in ischemia-reperfusion injury [47] and does not increase the mortality and morbidity of heart failure patients [41,44]. Recently, ASCOT-BPLA study reported that amlodipine-based regimen reduced the incidences of cardiovascular diseases and inhibited the progress of diabetes compared with that of atenolol-based regimen, though combination of perindopril with amlodipine and that of bendroflumethiazide with atenolol could affect on the results [48]. With regard to other recent CCBs, PATE-Hypertension demonstrated that the incidence of cardiovascular events was not different between manidipine-treated group (27.8/1000) and in delapril-treated group (22.5/1000) [43]. Long-term treatment with azelnidipine is demonstrated to decrease heart rate in hypertensive animal model [49] and in a randomized double-blind study of 46 patients with essential hypertension [29]. Arita et al. [50] demonstrated that azelnidipine decreased blood pressure both at rest and at exercise without an increase in cardiac output, heart rate or plasma norepinephrine/epinephrine compared to other antihypertensive agents, including metoprolol, doxazosin, trichlormethiazide and nifedipine.
In accordance with these new antagonists, evidence has been accumulated that sustained nifedipine release tablets whereby nifedipine is gradually released in the gastrointestinal tract offer favorable benefit in patients with cardiovascular disease. For example, in the JMIC-B trial, nifedipine-retard has been as effective as ACE inhibitors in preventing the cardiac events and mortality in patients with hypertension and coronary artery disease [51,52]. Furthermore, although in the ACTION trial, addition of nifedipine GITS to the conventional treatment of angina pectoris has no effect on major cardiovascular event-free survival in patients with stable angina pectoris [51,52], nifedipine GITS significantly reduces the incidence of any stroke or transient ischemic attack and the need for coronary angiography by 21% in normotensives and 16% in hypertensives. Thus, different drug absorption systems (nifedipine vs. nifedipine-retard/nifedipine-GITS) may influence the vulnerability to cardiovascular events.
The second group is characterized by the possession of the blocking action on multiple calcium channels in addition to L-type calcium channels, into which group cilnidipine and efonidipine are categorized. It is well established that verapamil and diltiazem can be used for rate control, because both agents block calcium-dependent conduction in the AV node and effective for supraventricular tachycardia. In contrast, dihydropyridines have been reported to cause tachycardia since they potentially activate sympathetic nervous system [53].
Furthermore, T-type calcium channels are distributed in pacemaker cells, atrial cells and Purkinje fibers in the heart [54], although L-type calcium channel are mostly found in the myocardium. Under the condition of atrial fibrillation, L-type calcium channels and sodium channels are down-regulated and inactivated [55], whereas calcium influx through T-type calcium channels remains active [56]. This small persistent calcium influx from T-type calcium channels would induce calcium overload and is responsible for the mechanism for continuous atrial fibrillation. Fareh et al. [57] reported that mibefradil prevented atrial fibrillation-promoting electrophysiological remodeling by atrial tachycardia in dogs subjected to rapid atrial pacing for 7 days. Masumiya et al. [21] reported that efonidipine suppress selectively the later phase of pacemaker depolarization through inhibition of both L- and T-type calcium currents of rabbit sinoatrial node tissue and decreased the heart rate. Indeed, efonidipine has potent negative chronotropic but weak inotropic activity [58]. Ohashi et al. [59] have recently demonstrated that efonidipine exerts a more sustained action to prevent atrial electrical remodeling compared with verapamil in dogs underwent rapid atrial pacing. Since atrial fibrillation downregulates L-type calcium channels but maintains T-type calcium channel activity, it is expected that calcium influx is refractory to L-type calcium channel blockade, but more susceptible to T-type channel blockade.
In the clinical studies, several chronotropic and inotropic effects have been reported. Harada et al. [9] demonstrated that administration of efonidipine decreased heart rate, reduced sympathetic nervous activity and enhanced parasympathetic nerve activity in patients with a high heart rate. Using a more T-type-selective calcium channel blocker, mibefradil, Lee et al. [60] reported an improvement in treadmill exercise parameters, compared with diltiazem CD in patients with chronic stable angina in the PRIDE trial. Pellizzer et al. [61] demonstrated that mibefradil increased parasympathetic nervous system activity, although no differences existed between effect of L- and T-type CCB on sympathetic nervous system activity and baroreflex sensitivity.
It has recently been reported that the increased current of T-type calcium channels is responsible for the progression of heart failure in the myocardium of rat ventricle [62]. Generally, α1H (an α1 subunit of T-type calcium channels) is highly expressed in the embryonal myocardium, and is decreased with the growth of the animal [63]. In contrast, α1G (an α1 subunit of T-type calcium channels) is rarely expressed in the embryonal myocardium. In the myocardium of the UM-X7.1 cardiomyopathic hamster, the expression of α1G subunits is increased, compared with that of the control hamster, and a T-type calcium channel blocker, mibefradil, retards the progression of heart failure [64]. Izumi et al. [65] demonstrated that endothelin1 activation increased the T-type calcium current and the expression of the α1G mRNA level in ventricular myocytes in Dahl salt-sensitive rats with heart failure, and these increases were eliminated by the chronic bosentan treatment. Mibefradil is demonstrated to reduce interstitial and perivascular fibrosis and improve cardiac function in myocardial infarction-induced heart failure in rats [66]. Unfortunately, mibefradil caused fatal arrhythmia in MACH-1 study [67], and this arrhythmia is presumably due to the interaction with other drugs that are also metabolized by the cytochrome P-450 3A4 enzyme. Although this interaction is not a class effect of the T-type CCB, new calcium antagonists of this class have not been developed till lately. Recently, a clinical study examining the effect of efonidipine, which possesses potent activity on T-type as well as L-type calcium channels, on the development of heart failure is in progress, and is expected to demonstrate additional benefits of the T-type CCB [68]. This JATOS study group has released its tentative report that morbidity of 12 months treatment of efonidipine was 20.9/1,000, and its mortality was 1.7/1,000 [69], similar with morbidity of amlodipine treatment (VALUE: 33.4/1,000) or Nitrendipine treatment (Syst-Eur: 23.3/1,000, Syst-China:21.4/1,000) in clinical studies [5,6,40]. This study is in progress, and the positive outcome of the final report is expected.
As N-type channels are distributed along nerves, cilnidipine would affect the autonomic nervous system and subsequently decrease the blood pressure without inducing reflex tachycardia [70]. Cilnidipine improved the peripheral vascular resistance, mean blood pressure, atrial rate and increased the cardiac output in the chronic atrioventricular block dogs [8], and attenuated the incidences of ventricular premature beats during ischemia and reperfusion and decreased myocardial interstitial noradrenaline levels during ischemia and reperfusion in a rabbit model of myocardial infarction [71]. Recently, cilnidipine is reported to possess the blocking effect for T-type calcium current [72], and that effect may affect on the cilnidipine treatment.
In the clinical study, cilnidipine is demonstrated to suppress cardiac sympathetic overactivity in patients with essential hypertension [73]. Furthermore, Konda et al. [74] demonstrated that cilnidipine decreased mean blood pressure, and inhibited the changes in heart rate and plasma norepinephrine concentration induced by bilateral carotid artery occlusion in anesthetized dog. It also reduced myocardial interstitial noradrenaline levels and decreased the incidence of ventricular premature beats in rabbit myocardial infarction model [71]. Cilnidipine also reduces white coat effect in patients with essential hypertension [75]. Sakata et al. demonstrated that cilnidipine treatment improved the cardiac sympathetic overactivity of hypertension patients without changing plasma renin activity and plasma norepinephrine concentration, when amlodipine treatment did not change both of them by using 123I-metaiodo-benzylguanidine (MIBG) cardiac imaging [73]. However, the effects of cilnidipine on cardiovascular events await large clinical trials.
3. CCBS AND ATHEROSCLEROSIS
A growing body of evidence has been accumulated that some of CCBs have anti-atherosclerotic action and/or possess antioxidant activity, both of which favor cardiovascular protection. Amlodipine and benidipine are demonstrated to prevent myocardial remodeling induced by chronic nitric oxide inhibition in rats [76]. Pretreatment of benidipine suppressed vascular cell adhesion molecule (VCAM) -1, intracellular cell adhesion molecule (ICAM) -1, and the induction of monocyte chemoattractant protein and interleukin-8 in vitro [77]. Roth et al. [78] demonstrated that amlodipine, felodipine, manidipine, verapamil, or diltiazem significantly decreased both the constitutive and platelet-derived growth factor β-dependent collagen deposition in the extracellular matrix formed by human vascular smooth muscle cells and fibroblasts. Finally, direct evaluation of the coronary artery with IVUS showed less progression of atherosclerosis in amlodipine-treated patients [46,79].
It has recently been demonstrated that T-type calcium channels are expressed in rat pulmonary microvascular endothelial cells, and mibefradil inhibits the calcium influx through these calcium channels [80]. Since T-type calcium current is induced by ischemia, it increases the intracellular calcium concentration and activates the nitric oxide synthesis [81]. Chronic treatment with mibefradil enhanced endothelium-dependent relaxations in arteries from Dahl salt sensitive rats with high salt diet [82]. Mibefradil, but not amlodipine or verapamil, is reported to inhibit the leukocyte adhesion [83]. Azelnidipine inhibited 8-iso-PGF2 alpha production, compared with nifedipine or amlodipine in cultured human arterial endothelial cells under hydrogen peroxide stimulation [84], and reduced tumor necrosis factor-α-induced interleukin-8 expression in endothelial cells [85]. Furthermore, Nomura et al. [86] reported that efonidipine improved platelet-derived microparticles, CD62P-, CD63-, PAC-1-, and annexin V-positive platelets, sICAM-1, sVCAM-1, sP-selectin, and sE-selectin levels in the hypertensive patients with diabetes. Thus, these studies suggest that novel CCBs can improve endothelial dysfunction and could be effective in the treatment of hypertension with diabetes.
4. CCBS AND CHRONIC KIDNEY DISEASES
Hypertension is one of the most important risk factors for the progression of renal disease [87,88]. In the follow-up study of the multiple risk factor intervention trial (MRFIT), a strong, graded relation between blood pressure and end-stage renal disease was identified [89]. Moreover, chronic renal dysfunction [90], proteinuria [42], and albuminuria [91,92] are independent risk factors of cerebrovascular and cardiovascular diseases. Thus, the treatment of hypertension constitutes a crucial strategy to minimize the development of renal disease and to reduce the risk of cardiovascular events. Among antihypertensive drugs, ACE inhibitors and ARB are the treatment of choice in patients with renal disease since proteinuria per se accelerates the renal dysfunction [93,94] and these classes of agents can reduce proteinuria [95,96]. In AASK study, the ability of amlodipine to retard the progression of renal disease is less than that of ramipril [97]. In the IDNT study, the risk of a doubling of the serum creatinine concentration and a relative risk of end-stage renal disease were lower in the irbesartan group than in the amlodipine group [98]. However, these studies do not suggest that the adverse effects of calcium antagonists on renal function. Furthermore, there is the possible renal protective action of the CCB. Several recent studies have demonstrated that long-acting nifedipine exerts beneficial action on the progression of renal injury [99]. Kumagai et al. [100] compared the effects of amlodipine and ACE inhibitors on the progression of renal disease in hypertensive patients with renal impairment, and demonstrated that increase in serum creatinine and creatinine clearance in amlodipine-treated group was comparable to that of ACE inhibitor-treated group during the one-year follow-up period. In the ALLHAT study, there were no different relative risk of cardiovascular diseases and mortality among chlorthalidone, amlodipine, and lisinopril [41].
As CCBs are demonstrated to elicit predominant dilation of the afferent arteriole but less dilation of the efferent arteriole [101–106], the pressure overload to the glomerulus may result in glomerular hypertension and the subsequent glomerulosclerosis. It is well established that L-type calcium channels prevail on afferent arterioles whereas efferent arterioles lack functional activity of these channels. The segmental difference in the distribution of L-type calcium channels therefore would explain why CCBs cause predominant dilation of the afferent arteriole.
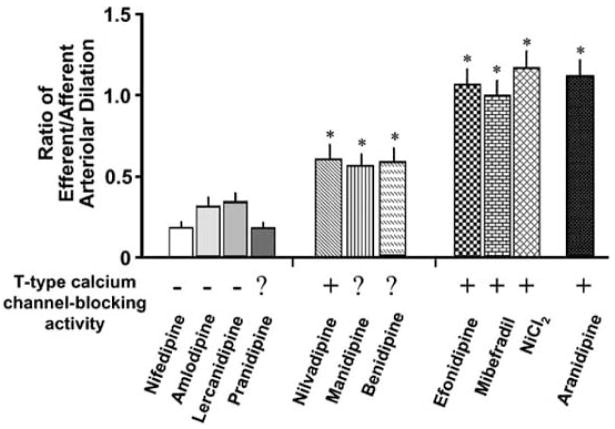
In contrast to predominant action of conventional types of CCBs on the afferent arteriole, several lines of studies have demonstrated that novel CCBs exert dilator action on both afferent and efferent arterioles (Fig. 1) [101–104,106]. Recently, some of CCBs has demonstrated to dilate both afferent and efferent arterioles. Because of lack of L-type calcium channels in the efferent arteriole, the dilatation of the efferent arteriole cannot be attributed to the class effect of the CCB. One of the possible mechanisms for the efferent arteriolar dilation by these CCBs is the blocking action on both L-type and other calcium channels, including T- and N-type calcium channels. It has recently been demonstrated that T-type calcium channels are present in both afferent and efferent arterioles of the rat kidney [107]. Ozawa et al. have demonstrated that mibefradil and nickel chloride dilate the angiotensin II-induced vasoconstriction of these arterioles [103].
Fig. 1.
Divergent vasodilator action of calcium antagonists on efferent arterioles. Based on the relative activity on efferent vs. afferent arterioles, calcium antagonists are classified into 3 groups. The first group of calcium antagonists (e.g., nifedipine) elicits predominant vasodilation of afferent arterioles, with modest action on efferent and efferent arterioles. The second group (e.g., nilvadipine) produces both afferent and efferent arteriolar vasodilation, although the efferent arteriolar vasodilation is less than that on the afferent arteriole. The third group of calcium antagonists (e.g., efonidipine) potently relaxes both afferent and efferent arterioles, with nearly the same activity on these vessels. *p < 0.05 vs. baseline. ? = Not examined. From Hayashi et al. [106] with modifications.
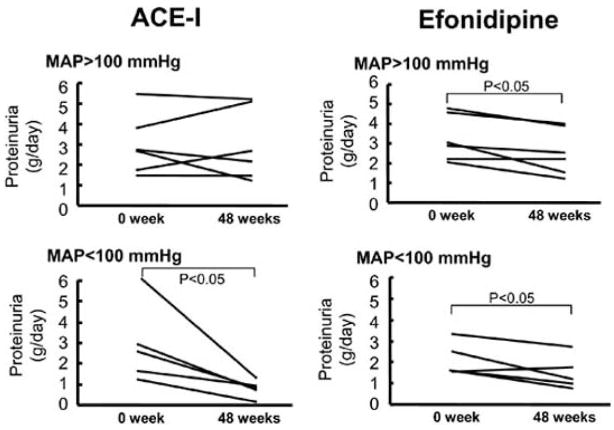
Renal microvascular action of these CCBs could reduce glomerular capillary pressure and therefore ameliorate glomerular hypertension. Fujiwara et al. [108] demonstrated that efonidipine reduced proteinuria as little as ACEI and less than nifedipine in subtotal nephrectomised SHR, though blood pressure decrease was similar level among all of treatments. Hayashi et al. reported that efonidipine reduced proteinuria as much as ACE inhibitors, but caused less side-effects (cough and hyperkalemia) in hypertensive patients (Fig. 2) [109]. Furthermore, Zhou et al. [110] demonstrated that cilnidipine caused decreases in both afferent and efferent arteriolar resistance and ameliorated nephrosclerosis in l-NAME-treated SHR. Cilnidipine is also reported to reduce proteinuria in the hypertensive patients with renal sclerosis [111,112]. Collectively, these CCBs can possess renal protective action from the aspect of renal hemodynamics.
Fig. 2.
Role of systemic blood pressure in the development of proteinuria in patients with proteinuria > 1 g/day treated with angiotensin converting enzyme inhibitors and efonidipine. ACE-I = angiotensin converting enzyme inhibitors; MAP = mean arterial pressure. From Hayashi et al. [109] with modifications.
Although renal renin-angiotensin-aldosterone system plays an important role in mediating the physiological function, this system also participates in the pathological process of the development of renal injury. Both ACE inhibitors and ARB effectively reduce proteinuria independently of blood pressure, and the inhibition of renin-angiotensin system is known to retard the progression of renal dysfunction. Recent investigations suggest that local renin-angiotensin system is enhanced apart from systemic renin-angiotensin system. Kobori et al. [113–115] have demonstrated that enhanced local renal renin-angiotensin system constitutes an important factor for the acceleration of hypertensive nephropathy. Furthermore, evidence has accrued that aldosterone is associated with the increased event of heart failure [116] and induce renal injury independently [117] from renin-angiotensin system. Nifedipine is known to reduce aldosterone selection [118], and mibefradil also reduced renin secretion [119]. Rossier et al. [120] demonstrated that aldosterone secretion appears to be related to T-type channel activity compared with L-type channel activity during activation of bovine adrenal glomerulosa cells. Arima et al. [121] also demonstrated that aldosterone elicited constriction of both afferent and efferent arterioles, and efonidipine reversed this response in the isolated renal microvessels in vitro. In this regards, dual L- and T-type CCB may retard the progression of renal dysfunction by blocking renin-angiotensin-aldosterone system.
CONCLUSION
More than 40 years have passed since the development of the CCB, and several classes of CCBs are in clinical use for the treatment of hypertension because of potent vasodepressor action. Although CCBs, particularly with short acting nature, were deemed as drugs causing cardiovascular events, subsequent studies using long-acting CCBs clearly demonstrate benefits from these agents in the field of cardiovascular and kidney disease. A growing body of studies now reveals that a number of recently-developed CCBs exert inhibitory action on multiple calcium channel subtypes, including L-, T- and N-type channels. Since these channels have been demonstrated to be distributed widely and have substantial effect on cardiovascular and neurohumoral systems, dual or triple blockade of calcium channels may offer additional benefits as a therapeutic strategy of hypertension (Table 2).
Table 2.
Benefits and Defects of CCBs
| CCBs | Benefits | Defects |
|---|---|---|
| Short acting | Prompt, Reliable | Too Sharp |
| Long acting | Mild, Continuous | Taking Long Time to Get Stable Actions |
| L-type selective | Prompt Antihypertensive Action | (not yet reported) |
| L- & T-type | Chronotropic, Inotropic, and Renoprotective Effects | (not yet reported) |
| L- & N-type | Direct Action on Autonomic Nerves | (not yet reported) |
Acknowledgments
We are grateful for the following investigators who have contributed significantly to this work: Drs. Keiji Fujiwara, Takahiko Nagahama, and Takao Saruta.
References
- 1.Moser M, Hebert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. doi: 10.1016/0735-1097(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Gong L, Zhang W, Zhu Y, et al. Shanghai trial of nifedipine in the elderly (STONE) J Hypertens. 1996;14:1237–1245. doi: 10.1097/00004872-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The systolic hypertension in Europe (Syst-Eur) trial investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older chinese patients with isolated systolic hypertension. Systolic hypertension in China (Syst-China) collaborative group. J Hypertens. 1998;16:1823–1829. doi: 10.1097/00004872-199816120-00016. [DOI] [PubMed] [Google Scholar]
- 7.Randomized double-blind comparison of a calcium antagonist and a diuretic in elderly hypertensives. National intervention cooperative study in elderly hypertensives study group. Hypertension. 1999;34:1129–1133. [PubMed] [Google Scholar]
- 8.Takahara A, Sugiyama A, Satoh Y, Nakamura Y, Hashimoto K. Cardiovascular effects of an L/N-type Ca2+ channel blocker cilnidipine assessed in the chronic atrioventricular conduction block dogs. J Pharmacol Sci. 2004;96:219–223. doi: 10.1254/jphs.scj04007x. [DOI] [PubMed] [Google Scholar]
- 9.Harada K, Nomura M, Nishikado A, Uehara K, Nakaya Y, Ito S. Clinical efficacy of efonidipine hydrochloride, a T-type calcium channel inhibitor, on sympathetic activities. Circ J. 2003;67:139–145. doi: 10.1253/circj.67.139. [DOI] [PubMed] [Google Scholar]
- 10.Nathan S, Pepine CJ, Bakris GL. Calcium antagonists: Effects on cardio-renal risk in hypertensive patients. Hypertension. 2005;46:637–642. doi: 10.1161/01.HYP.0000184541.24700.c7. [DOI] [PubMed] [Google Scholar]
- 11.Ertel EA, Campbell KP, Harpold MM, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 12.Miljanich GP, Ramachandran J. Antagonists of neuronal calcium channels: Structure, function, and therapeutic implications. Annu Rev Pharmacol Toxicol. 1995;35:707–734. doi: 10.1146/annurev.pa.35.040195.003423. [DOI] [PubMed] [Google Scholar]
- 13.Triggle DJ. Mechanisms of action of calcium antagonists. In: Epstein M, editor. Calcium antagonists in clinical medicine. 3. Philadelphia: Hanley & Belfus, Inc; 2002. pp. 1–32. [Google Scholar]
- 14.Hofmann F, Lacinova L, Klugbauer N. Voltage-dependent calcium channels: From structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 15.Dilmac N, Hilliard N, Hockerman GH. Molecular determinants of Ca2+ potentiation of diltiazem block and Ca2+-dependent inactivation in the pore region of cav1.2. Mol Pharmacol. 2003;64:491–501. doi: 10.1124/mol.64.2.491. [DOI] [PubMed] [Google Scholar]
- 16.Bangalore R, Baindur N, Rutledge A, Triggle DJ, Kass RS. L-type calcium channels: Asymmetrical intramembrane binding domain revealed by variable length, permanently charged 1,4-dihydropyridines. Mol Pharmacol. 1994;46:660–666. [PubMed] [Google Scholar]
- 17.Kurokawa J, Adachi-Akahane S, Nagao T. 1,5-benzothiazepine binding domain is located on the extracellular side of the cardiac L-type Ca2+ channel. Mol Pharmacol. 1997;51:262–268. doi: 10.1124/mol.51.2.262. [DOI] [PubMed] [Google Scholar]
- 18.Mehrke G, Zong XG, Flockerzi V, Hofmann F. The Ca(++)-channel blocker ro 40–5967 blocks differently T-type and L-type Ca++ channels. J Pharmacol Exp Ther. 1994;271:1483–1488. [PubMed] [Google Scholar]
- 19.Masumiya H, Shijuku T, Tanaka H, Shigenobu K. Inhibition of myocardial L- and T-type Ca2+ currents by efonidipine: Possible mechanism for its chronotropic effect. Eur J Pharmacol. 1998;349:351–357. doi: 10.1016/s0014-2999(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi H, Murai Y, Akaike N. Effect of nilvadipine on the voltage-dependent Ca2+ channels in rat hippocampal ca1 pyramidal neurons. Brain Res. 1998;813:121–127. doi: 10.1016/s0006-8993(98)01018-x. [DOI] [PubMed] [Google Scholar]
- 21.Masumiya H, Tanaka H, Shigenobu K. Effects of Ca2+ channel antagonists on sinus node: Prolongation of late phase 4 depolarization by efonidipine. Eur J Pharmacol. 1997;335:15–21. doi: 10.1016/s0014-2999(97)01150-3. [DOI] [PubMed] [Google Scholar]
- 22.Masumiya H, Tanaka Y, Tanaka H, Shigenobu K. Inhibition of T-type and L-type Ca(2+) currents by aranidipine, a novel dihydropyridine Ca(2+) antagonist. Pharmacology. 2000;61:57–61. doi: 10.1159/000028381. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S, Kameyama K, Hosono M, Hayashi Y, Kitamura K. Effect of cilnidipine, a novel dihydropyridine Ca++-channel antagonist, on N-type Ca++ channel in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1997;280:1184–1191. [PubMed] [Google Scholar]
- 24.Furukawa T, Nukada T, Suzuki K, Fujita Y, Mori Y, Nishimura M, Yamanaka M. Voltage and pH dependent block of cloned N-type Ca2+ channels by amlodipine. Br J Pharmacol. 1997;121:1136–1140. doi: 10.1038/sj.bjp.0701226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luscher TF, Cosentino F. The classification of calcium antagonists and their selection in the treatment of hypertension. A reappraisal. Drugs. 1998;55:509–517. doi: 10.2165/00003495-199855040-00003. [DOI] [PubMed] [Google Scholar]
- 26.van Zwieten PA, Pfaffendorf M. Pharmacology of the dihydropyridine calcium antagonists: Relationship between lipophilicity and pharmacodynamic responses. J Hypertens. 1993;11 (Suppl 6):S3–8. [PubMed] [Google Scholar]
- 27.Epstein M. Lercanidipine: A novel dihydropyridine calcium-channel blocker. Heart Dis. 2001;3:398–407. doi: 10.1097/00132580-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Oizumi K, Nishino H, Koike H, Sada T, Miyamoto M, Kimura T. Antihypertensive effects of CS-905, a novel dihydropyridine Ca++ channel blocker. Jpn J Pharmacol. 1989;51:57–64. doi: 10.1254/jjp.51.57. [DOI] [PubMed] [Google Scholar]
- 29.Kuramoto K, Ichikawa S, Hirai A, Kanada S, Nakachi T, Ogihara T. Azelnidipine and amlodipine: A comparison of their pharmacokinetics and effects on ambulatory blood pressure. Hypertens Res. 2003;26:201–208. doi: 10.1291/hypres.26.201. [DOI] [PubMed] [Google Scholar]
- 30.Sada T, Saito H. Pharmacological profiles and clinical effects of azelnidipine, a long-acting calcium channel blocker. Nippon Yakurigaku Zasshi. 2003;122:539–547. doi: 10.1254/fpj.122.539. [DOI] [PubMed] [Google Scholar]
- 31.Palatini P, Casiglia E, Julius S, Pessina AC. High heart rate: A risk factor for cardiovascular death in elderly men. Arch Intern Med. 1999;159:585–592. doi: 10.1001/archinte.159.6.585. [DOI] [PubMed] [Google Scholar]
- 32.Schanzenbacher P, Liebau G, Deeg P, Kochsiek K. Effect of intravenous and intracoronary nifedipine on coronary blood flow and myocardial oxygen consumption. Am J Cardiol. 1983;51:712–717. doi: 10.1016/s0002-9149(83)80120-9. [DOI] [PubMed] [Google Scholar]
- 33.Opie LH, Messerli FH. Nifedipine and mortality. Grave defects in the dossier. Circulation. 1995;92:1068–1073. doi: 10.1161/01.cir.92.5.1068. [DOI] [PubMed] [Google Scholar]
- 34.Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–625. [PubMed] [Google Scholar]
- 35.Ishikawa K, Nakai S, Takenaka T, et al. Short-acting nifedipine and diltiazem do not reduce the incidence of cardiac events in patients with healed myocardial infarction. Secondary prevention group. Circulation. 1997;95:2368–2373. doi: 10.1161/01.cir.95.10.2368. [DOI] [PubMed] [Google Scholar]
- 36.Boden WE, Korr KS, Bough EW. Nifedipine-induced hypotension and myocardial ischemia in refractory angina pectoris. JAMA. 1985;253:1131–1135. [PubMed] [Google Scholar]
- 37.The effect of diltiazem on mortality and reinfarction after myocardial infarction. The multicenter diltiazem postinfarction trial research group. N Engl J Med. 1988;319:385–392. doi: 10.1056/NEJM198808183190701. [DOI] [PubMed] [Google Scholar]
- 38.Elkayam U, Amin J, Mehra A, Vasquez J, Weber L, Rahimtoola SH. A prospective, randomized, double-blind, crossover study to compare the efficacy and safety of chronic nifedipine therapy with that of isosorbide dinitrate and their combination in the treatment of chronic congestive heart failure. Circulation. 1990;82:1954–1961. doi: 10.1161/01.cir.82.6.1954. [DOI] [PubMed] [Google Scholar]
- 39.Mizumura T, Nithipatikom K, Gross GJ. Bimakalim, an ATP-sensitive potassium channel opener, mimics the effects of ischemic preconditioning to reduce infarct size, adenosine release, and neutrophil function in dogs. Circulation. 1995;92:1236–1245. doi: 10.1161/01.cir.92.5.1236. [DOI] [PubMed] [Google Scholar]
- 40.Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the value trial. Lancet. 2004;363:2049–2051. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 41.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 42.Mancia G, Brown M, Castaigne A, et al. Outcomes with nifedipine GITS or Co-amilozide in hypertensive diabetics and nondiabetics in intervention as a goal in hypertension (INSIGHT) Hypertension. 2003;41:431–436. doi: 10.1161/01.HYP.0000057420.27692.AD. [DOI] [PubMed] [Google Scholar]
- 43.Ogihara T. Practitioner’s trial on the efficacy of antihypertensive treatment in the elderly hypertension (the pate-hypertension study) in Japan. Am J Hypertens. 2000;13:461–467. doi: 10.1016/s0895-7061(99)00215-0. [DOI] [PubMed] [Google Scholar]
- 44.Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective randomized amlodipine survival evaluation study group. N Engl J Med. 1996;335:1107–1114. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 45.Pitt B, Byington RP, Furberg CD, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. Prevent investigators. Circulation. 2000;102:1503–1510. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- 46.Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: The CAMELOT study: A randomized controlled trial. JAMA. 2004;292:2217–2225. doi: 10.1001/jama.292.18.2217. [DOI] [PubMed] [Google Scholar]
- 47.Gross GJ, Farber NE, Pieper GM. Effects of amlodipine on myocardial ischemia-reperfusion injury in dogs. Am J Cardiol. 1989;64:94I–100I. doi: 10.1016/0002-9149(89)90966-1. [DOI] [PubMed] [Google Scholar]
- 48.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the anglo-scandinavian cardiac outcomes trial-blood pressure lowering arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 49.Oizumi K, Nishino H, Miyake S, et al. Hemodynamic changes following long-term administration of CS-905, a novel dihydropyridine calcium blocker, in conscious SHR. Jpn J Pharmacol. 1990;54:1–6. doi: 10.1254/jjp.54.1. [DOI] [PubMed] [Google Scholar]
- 50.Arita M, Hashizume T, Tanigawa K, Yamamoto H, Nishio I. A new Ca-antagonist, azelnidipine, reduced blood pressure during exercise without augmentation of sympathetic nervous system in essential hypertension: A randomized, double-blind, placebo-controlled trial. J Cardiovasc Pharmacol. 1999;33:186–192. doi: 10.1097/00005344-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Poole-Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): Randomised controlled trial. Lancet. 2004;364:849–857. doi: 10.1016/S0140-6736(04)16980-8. [DOI] [PubMed] [Google Scholar]
- 52.Yui Y, Sumiyoshi T, Kodama K, et al. Comparison of nifedipine retard with angiotensin converting enzyme inhibitors in Japanese hypertensive patients with coronary artery disease: The Japan multicenter investigation for cardiovascular diseases-b (JMIC-b) randomized trial. Hypertens Res. 2004;27:181–191. doi: 10.1291/hypres.27.181. [DOI] [PubMed] [Google Scholar]
- 53.Singh BN, Nademanee K. Use of calcium antagonists for cardiac arrhythmias. Am J Cardiol. 1987;59:153B–162B. doi: 10.1016/0002-9149(87)90096-8. [DOI] [PubMed] [Google Scholar]
- 54.Bean BP. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985;86:1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grant AO. Mechanisms of atrial fibrillation and action of drugs used in its management. Am J Cardiol. 1998;82:43N–49N. doi: 10.1016/s0002-9149(98)00585-2. [DOI] [PubMed] [Google Scholar]
- 56.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 57.Fareh S, Benardeau A, Thibault B, Nattel S. The T-type Ca(2+) channel blocker mibefradil prevents the development of a substrate for atrial fibrillation by tachycardia-induced atrial remodeling in dogs. Circulation. 1999;100:2191–2197. doi: 10.1161/01.cir.100.21.2191. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka H, Masumiya H, Sekine T, et al. Myocardial and vascular effects of efonidipine in vitro as compared with nifedipine, verapamil and diltiazem. Gen Pharmacol. 1996;27:451–454. doi: 10.1016/0306-3623(95)02065-9. [DOI] [PubMed] [Google Scholar]
- 59.Ohashi N, Mitamura H, Tanimoto K, et al. A comparison between calcium channel blocking drugs with different potencies for T- and L-type channels in preventing atrial electrical remodeling. J Cardiovasc Pharmacol. 2004;44:386–392. doi: 10.1097/01.fjc.0000138163.11612.7b. [DOI] [PubMed] [Google Scholar]
- 60.Lee DS, Goodman S, Dean DM, et al. Randomized comparison of T-typeversus L-typecalcium-channel blockade on exercise duration in stable angina: Results of the posicor reduction of ischemia during exercise (PRIDE) trial. Am Heart J. 2002;144:60–67. doi: 10.1067/mhj.2002.122869. [DOI] [PubMed] [Google Scholar]
- 61.Pellizzer AM, Kamen PW, Esler MD, Lim S, Krum H. Comparative effects of mibefradil and nifedipine gastrointestinal transport system on autonomic function in patients with mild to moderate essential hypertension. J Hypertens. 2001;19:279–285. doi: 10.1097/00004872-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Huang B, Qin D, Deng L, Boutjdir M, N E-S. Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Cardiovasc Res. 2000;46:442–449. doi: 10.1016/s0008-6363(00)00017-1. [DOI] [PubMed] [Google Scholar]
- 63.Kitchens SA, Burch J, Creazzo TL. T-type Ca2+ current contribution to Ca2+-induced Ca2+ release in developing myocardium. J Mol Cell Cardiol. 2003;35:515–523. doi: 10.1016/s0022-2828(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 64.Villame J, Massicotte J, Jasmin G, Dumont L. Effects of mibefradil, a T- and L-type calcium channel blocker, on cardiac remodeling in the um-x7.1 cardiomyopathic hamster. Cardiovasc Drugs Ther. 2001;15:41–48. doi: 10.1023/a:1011158717901. [DOI] [PubMed] [Google Scholar]
- 65.Izumi T, Kihara Y, Sarai N, et al. Reinduction of T-type calcium channels by endothelin-1 in failing hearts in vivo and in adult rat ventricular myocytes in vitro. Circulation. 2003;108:2530–2535. doi: 10.1161/01.CIR.0000096484.03318.AB. [DOI] [PubMed] [Google Scholar]
- 66.Sandmann S, Min JY, Meissner A, Unger T. Effects of the calcium channel antagonist mibefradil on haemodynamic parameters and myocardial Ca(2+)-handling in infarct-induced heart failure in rats. Cardiovasc Res. 1999;44:67–80. doi: 10.1016/s0008-6363(99)00180-7. [DOI] [PubMed] [Google Scholar]
- 67.Levine TB, Bernink PJ, Caspi A, et al. Effect of mibefradil, a T-type calcium channel blocker, on morbidity and mortality in moderate to severe congestive heart failure: The MACH-1 study. Mortality assessment in congestive heart failure trial. Circulation. 2000;101:758–764. doi: 10.1161/01.cir.101.7.758. [DOI] [PubMed] [Google Scholar]
- 68.Saito F, Takahashi A, Kushiro T. Intervention studies on elderly hypertensive patients in Japan. Nippon Rinsho. 2005;63:1078–1085. [PubMed] [Google Scholar]
- 69.The Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS): Protocol, patient characteristics, and blood pressure during the first 12 months. Hypertens Res. 2005;28:513–520. doi: 10.1291/hypres.28.513. [DOI] [PubMed] [Google Scholar]
- 70.Tominaga M, Ohya Y, Tsukashima A, et al. Ambulatory blood pressure monitoring in patients with essential hypertension treated with a new calcium antagonist, cilnidipine. Cardiovasc Drugs Ther. 1997;11:43–48. doi: 10.1023/a:1007795722576. [DOI] [PubMed] [Google Scholar]
- 71.Nagai H, Minatoguchi S, Chen XH, et al. Cilnidipine, an N+L-type dihydropyridine Ca channel blocker, suppresses the occurrence of ischemia/reperfusion arrhythmia in a rabbit model of myocardial infarction. Hypertens Res. 2005;28:361–368. doi: 10.1291/hypres.28.361. [DOI] [PubMed] [Google Scholar]
- 72.Takeda K, Yamagishi R, Masumiya H, Tanaka H, Shigenobu K. Effect of cilnidipine on l- and T-type calcium currents in guinea pig ventricle and action potential in rabbit sinoatrial node. J Pharmacol Sci. 2004;95:398–401. doi: 10.1254/jphs.scj04001x. [DOI] [PubMed] [Google Scholar]
- 73.Sakata K, Shirotani M, Yoshida H, et al. Effects of amlodipine and cilnidipine on cardiac sympathetic nervous system and neurohormonal status in essential hypertension. Hypertension. 1999;33:1447–1452. doi: 10.1161/01.hyp.33.6.1447. [DOI] [PubMed] [Google Scholar]
- 74.Konda T, Takahara A, Maeda K, Dohmoto H, Yoshimoto R. Effects of a dual L/N-type Ca(2+) channel blocker cilnidipine on neurally mediated chronotropic response in anesthetized dogs. Eur J Pharmacol. 2001;413:117–120. doi: 10.1016/s0014-2999(01)00738-5. [DOI] [PubMed] [Google Scholar]
- 75.Morimoto S, Takeda K, Oguni A, et al. Reduction of white coat effect by cilnidipine in essential hypertension. Am J Hypertens. 2001;14:1053–1057. doi: 10.1016/s0895-7061(01)02159-8. [DOI] [PubMed] [Google Scholar]
- 76.Sanada S, Node K, Minamino T, et al. Long-acting Ca2+ blockers prevent myocardial remodeling induced by chronic no inhibition in rats. Hypertension. 2003;41:963–967. doi: 10.1161/01.HYP.0000062881.36813.7A. [DOI] [PubMed] [Google Scholar]
- 77.Matsubara M, Hasegawa K. Effects of benidipine, a dihydropyridine-Ca2+ channel blocker, on expression of cytokine-induced adhesion molecules and chemoattractants in human aortic endothelial cells. Eur J Pharmacol. 2004;498:303–314. doi: 10.1016/j.ejphar.2004.07.086. [DOI] [PubMed] [Google Scholar]
- 78.Roth M, Eickelberg O, Kohler E, Erne P, Block LH. Ca2+ channel blockers modulate metabolism of collagens within the extracellular matrix. Proc Natl Acad Sci USA. 1996;93:5478–5482. doi: 10.1073/pnas.93.11.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamazaki T, Taniguchi I, Kurusu T, et al. Effect of amlodipine on vascular responses after coronary stenting compared with an angiotensin-converting enzyme inhibitor. Circ J. 2004;68:328–333. doi: 10.1253/circj.68.328. [DOI] [PubMed] [Google Scholar]
- 80.Wei Z, Manevich Y, Al-Mehdi AB, Chatterjee S, Fisher AB. Ca2+ flux through voltage-gated channels with flow cessation in pulmonary microvascular endothelial cells. Microcirculation. 2004;11:517–526. doi: 10.1080/10739680490476367. [DOI] [PubMed] [Google Scholar]
- 81.Fisher AB, Al-Mehdi AB, Manevich Y. Shear stress and endothelial cell activation. Crit Care Med. 2002;30:S192–197. doi: 10.1097/00003246-200205001-00004. [DOI] [PubMed] [Google Scholar]
- 82.Boulanger CM, Desta B, Clozel JP, Vanhoutte PM. Chronic treatment with the Ca2+ channel inhibitor RO 40–5967 potentiates endothelium-dependent relaxations in the aorta of the hypertensive salt sensitive Dahl rat. Blood Press. 1994;3:193–196. doi: 10.3109/08037059409102252. [DOI] [PubMed] [Google Scholar]
- 83.Nebe B, Holzhausen C, Rychly J, Urbaszek W. Impaired mechanisms of leukocyte adhesion in vitro by the calcium channel antagonist mibefradil. Cardiovasc Drugs Ther. 2002;16:183–193. doi: 10.1023/a:1020688019792. [DOI] [PubMed] [Google Scholar]
- 84.Shinomiya K, Mizushige K, Fukunaga M, Masugata H, Ohmori K, Kohno M, Senda S. Antioxidant effect of a new calcium antagonist, azelnidipine, in cultured human arterial endothelial cells. J Int Med Res. 2004;32:170–175. doi: 10.1177/147323000403200210. [DOI] [PubMed] [Google Scholar]
- 85.Yamagishi S, Inagaki Y, Nakamura K, Imaizumi T. Azelnidipine, a newly developed long-acting calcium antagonist, inhibits tumor necrosis factor-alpha-induced interleukin-8 expression in endothelial cells through its anti-oxidative properties. J Cardiovasc Pharmacol. 2004;43:724–730. doi: 10.1097/00005344-200405000-00016. [DOI] [PubMed] [Google Scholar]
- 86.Nomura S, Kanazawa S, Fukuhara S. Effects of efonidipine on platelet and monocyte activation markers in hypertensive patients with and without type 2 diabetes mellitus. J Hum Hypertens. 2002;16:539–547. doi: 10.1038/sj.jhh.1001447. [DOI] [PubMed] [Google Scholar]
- 87.Domanski M, Mitchell G, Pfeffer M, et al. Pulse pressure and cardiovascular disease-related mortality: Follow-up study of the multiple risk factor intervention trial (MRFIT) JAMA. 2002;287:2677–2683. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 88.Vupputuri S, Batuman V, Muntner P, et al. Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension. 2003;42:1144–1149. doi: 10.1161/01.HYP.0000101695.56635.31. [DOI] [PubMed] [Google Scholar]
- 89.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 90.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 91.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 92.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 93.Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the international nifedipine gits study: Intervention as a goal in hypertension treatment (INSIGHT) Lancet. 2000;356:366–372. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 94.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 95.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN group (gruppo italiano di studi epidemiologici in nefrologia) Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 96.Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: A meta-analysis of randomized trials. Angiotensin-converting-enzyme inhibition and progressive renal disease study group. Ann Intern Med. 1997;127:337–345. doi: 10.7326/0003-4819-127-5-199709010-00001. [DOI] [PubMed] [Google Scholar]
- 97.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 98.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 99.de Leeuw PW, Ruilope LM, Palmer CR, et al. Clinical significance of renal function in hypertensive patients at high risk: Results from the INSIGHT trial. Arch Intern Med. 2004;164:2459–2464. doi: 10.1001/archinte.164.22.2459. [DOI] [PubMed] [Google Scholar]
- 100.Kumagai H, Hayashi K, Kumamaru H, Saruta T. Amlodipine is comparable to angiotensin-converting enzyme inhibitor for long-term renoprotection in hypertensive patients with renal dysfunction: A one-year, prospective, randomized study. Am J Hypertens. 2000;13:980–985. doi: 10.1016/s0895-7061(00)00287-9. [DOI] [PubMed] [Google Scholar]
- 101.Hayashi K, Nagahama T, Oka K, Epstein M, Saruta T. Disparate effects of calcium antagonists on renal microcirculation. Hypertens Res. 1996;19:31–36. doi: 10.1291/hypres.19.31. [DOI] [PubMed] [Google Scholar]
- 102.Ozawa Y, Hayashi K, Nagahama T, Fujiwara K, Wakino S, Saruta T. Renal afferent and efferent arteriolar dilation by nilvadipine: Studies in the isolated perfused hydronephrotic kidney. J Cardiovasc Pharmacol. 1999;33:243–247. doi: 10.1097/00005344-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Ozawa Y, Hayashi K, Nagahama T, Fujiwara K, Saruta T. Effect of T-type selective calcium antagonist on renal microcirculation: Studies in the isolated perfused hydronephrotic kidney. Hypertension. 2001;38:343–347. doi: 10.1161/01.hyp.38.3.343. [DOI] [PubMed] [Google Scholar]
- 104.Hayashi K, Ozawa Y, Fujiwara K, Wakino S, Kumagai H, Saruta T. Role of actions of calcium antagonists on efferent arterioles-with special references to glomerular hypertension. Am J Nephrol. 2003;23:229–244. doi: 10.1159/000072054. [DOI] [PubMed] [Google Scholar]
- 105.Loutzenhiser RD, Epstein M, Hayashi K. Renal hemodynamic effects of calcium antagonists. Am J Cardiol. 1989;64:41F–45F. doi: 10.1016/0002-9149(89)90745-5. [DOI] [PubMed] [Google Scholar]
- 106.Hayashi K, Ozawa Y, Saruta T, Epstein M. Renal hemodynamic effects of calcium antagonists. In: Epstein M, editor. Calcium antagonists in clinical medicine. 3. Philadelphia: Hanley & Belfus, Inc; 2002. pp. 559–578. [Google Scholar]
- 107.Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res. 2001;89:630–638. doi: 10.1161/hh1901.097126. [DOI] [PubMed] [Google Scholar]
- 108.Fujiwara K, Kanno Y, Hayashi K, Takenaka T, Saruta T. Renal protective effects of efonidipine in partially nephrectomized spontaneously hypertensive rats. Clin Exp Hypertens. 1998;20:295–312. doi: 10.3109/10641969809052123. [DOI] [PubMed] [Google Scholar]
- 109.Hayashi K, Kumagai H, Saruta T. Effect of efonidipine and ACE inhibitors on proteinuria in human hypertension with renal impairment. Am J Hypertens. 2003;16:116–122. doi: 10.1016/s0895-7061(02)03147-3. [DOI] [PubMed] [Google Scholar]
- 110.Zhou X, Ono H, Ono Y, Frohlich ED. N- and L-type calcium channel antagonist improves glomerular dynamics, reverses severe nephrosclerosis, and inhibits apoptosis and proliferation in an l-NAME/SHR model. J Hypertens. 2002;20:993–1000. doi: 10.1097/00004872-200205000-00035. [DOI] [PubMed] [Google Scholar]
- 111.Rose GW, Kanno Y, Ikebukuro H, et al. Cilnidipine is as effective as benazepril for control of blood pressure and proteinuria in hypertensive patients with benign nephrosclerosis. Hypertens Res. 2001;24:377–383. doi: 10.1291/hypres.24.377. [DOI] [PubMed] [Google Scholar]
- 112.Kojima S, Shida M, Yokoyama H. Comparison between cilnidipine and amlodipine besilate with respect to proteinuria in hypertensive patients with renal diseases. Hypertens Res. 2004;27:379–385. doi: 10.1291/hypres.27.379. [DOI] [PubMed] [Google Scholar]
- 113.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 117.Nishiyama A, Abe Y. Aldosterone and renal injury. Nippon Yakurigaku Zasshi. 2004;124:101–109. doi: 10.1254/fpj.124.101. [DOI] [PubMed] [Google Scholar]
- 118.Fiad TM, Cunningham SK, Hayes FJ, McKenna TJ. Effects of nifedipine treatment on the renin-angiotensin-aldosterone axis. J Clin Endocrinol Metab. 1997;82:457–460. doi: 10.1210/jcem.82.2.3748. [DOI] [PubMed] [Google Scholar]
- 119.Wagner C, Kramer BK, Hinder M, Kieninger M, Kurtz A. T-type and L-type calcium channel blockers exert opposite effects on renin secretion and renin gene expression in conscious rats. Br J Pharmacol. 1998;124:579–585. doi: 10.1038/sj.bjp.0701861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rossier MF, Burnay MM, Vallotton MB, Capponi AM. Distinct functions of T- and L-type calcium channels during activation of bovine adrenal glomerulosa cells. Endocrinology. 1996;137:4817–4826. doi: 10.1210/endo.137.11.8895352. [DOI] [PubMed] [Google Scholar]
- 121.Arima S, Kohagura K, Xu HL, et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol. 2003;14:2255–2263. doi: 10.1097/01.asn.0000083982.74108.54. [DOI] [PubMed] [Google Scholar]