Abstract
Background
An adaptable technique for micropatterning biomaterial scaffolds has enormous implications in controlling cell function and in the development of tissue-engineered (TE) microvasculature. In this paper, we report a technique to embed microscale patterns onto a collagen–glycosaminoglycan (CG) membrane as a first step towards the creation of TE constructs with built-in microvasculature.
Method of Approach
The CG membranes were fabricated by homogenizing a solution of Type I bovine collagen and chondroitin 6-sulfate in acetic acid and vacuum filtering the solution subsequently. The micropatterning technique consisted of three steps: surface dissolution of base matrix using acetic acid solution, feature resolution by application of uniform pressure and feature stability by glutaraldehyde crosslinking.
Results
Application of the new technique yielded patterns in CG membranes with a spatial resolution in the order of 2-3 microns. We show that such a patterned matrix is conducive to the attachment of bovine aortic endothelial cells (BAEC's).
Conclusions
The patterned membranes can be used for the development of complex three-dimensional TE products with built-in flow channels, as templates for topographically directed cell growth, or as a model system to study various microvascular disorders where feature scales are important. The new technique is versatile; topographical patterns can be custom-made for any predetermined design with high spatial resolution and the technique itself can be adapted for use with other scaffold materials.
Keywords: Microfabrication, spatially resolved surface patterning, microvasculature, tissue engineering, biomaterial scaffolds
Background
Spatio-temporal patterning of biomaterials such as proteins and biodegradable polymers at the micro- and nanoscales has enormous potential in cell and tissue engineering applications. Micropatterning has been used to control cell growth and function [1, 2]. Spatially-controlled growth and elongation of various mammalian cell types on several different synthetic biomaterials have been demonstrated [3]. Cell growth has been shown to be controlled by feature geometry in systems patterned with microchannels [4]. Directed cell growth and motility have applications in the development of tissue-engineered systems including nerve repair [5, 6] and endothelialization of microvasculature [7].
Vacanti et al. have developed endothelialized flow channels in the synthetic non-degradable polymer, poly(dimethylsiloxane) (PDMS) using MEMS technology along with soft lithography and microfluidics [8]. More recently, others have tried to develop microvasculature in biodegradable polymers like poly(lactide-co-glycolide) and poly(glycerol sebacate) (PGS) [9, 10]. Endothelial cells grown in PGS microchannels, however, did not achieve confluence even after two weeks of culture. A confluent endothelial lining is critical for a microvasculature system because thrombosis can occur where the confluence of the layer is breached. A similar system built with native extracellular matrix proteins such as collagen can lead to the creation of a confluent layer.
A micropatterned biodegradable biomaterial can be used to tissue engineer microvasculature. Without an intrinsic vasculature system, the transport of nutrients and oxygen to implanted cells in tissue-engineered (TE) constructs becomes limited by diffusion. This results in the formation of a necrotic core of dead tissue at the center of the TE construct [10, 11]. Currently, due in part to this problem, most TE products are either targeted towards avascular tissues such as cartilage or quasi two-dimensional skin and bladder tissues [8].
Despite the advances in microfabrication methodologies, very few studies have been carried out to pattern collagen for tissue engineering applications. Vernon et al. showed that cells aligned and elongated in the direction of channels made of microgrooves of air-dried collagen hydrogel membranes [4]. A composite system consisting of microfabricated PDMS and collagen was developed by Norman et al.[12]. In this system, however, the patterning was primarily in the non-biodegradable siloxane polymer which prevents its scale-up for applications in tissue engineering. Tang et al. developed a micromolding technique for collagen hydrogels [13] and showed cells can be embedded in microchannels of collagen gels. Collagen hydrogels are, however, fragile and difficult to manipulate for use in tissue engineering.
This paper describes a technique of forming micropatterns in collagen-glycosaminoglycan (CG) membranes. The pioneering work of Yannas et al. [14, 15] has shown that CG materials can be used for tissue-engineered constructs because CG is biocompatible and promotes cell adhesion and proliferation. Glycosaminoglycans, such as chondroitin 6-sulfate, strengthen collagen by forming salt links and hydrogen bonds that contribute up to 25% of the total collagen stabilizing forces [16]. In addition, these membranes have very high mechanical strength (tensile strength: 4-13 MPa) [17]. For these reasons, we believe that, in many cases, CG systems provide a better scaffold for cell attachment and growth than other biodegradable polymer systems. In this work, we micropattern CG membranes using microfabrication techniques similar to soft lithography. In addition, we report results of endothelial cell adhesion on the patterned CG matrix. Our results suggest the potential of CG membrane soft lithography for creating tissue-engineered systems with built in microvasculature.
Method of Approach
A. Fabricating CG membranes
CG membranes were fabricated using a method adopted from Yannas et al.[15]. In short, 0.55 g of Type I bovine collagen (Sigma, St. Louis, MO) was added to 200 ml of 0.5% (v/v) aqueous solution of acetic acid. The solution was then blended in an ice bath cooled vessel at a speed of 13,500 rpm using an overhead homogenizer (IKA Works, Wilmington, NC). After 20 minutes of blending, 0.055 g of chondroitin 6-sulfate dissolved in 10 ml of 0.5% (v/v) aqueous solution of acetic acid was added drop wise to the collagen solution. The solution was homogenized for an additional 20 minutes at 13,500 rpm. The solution was then transferred to a 350 ml Buchner filter funnel (Chemglass, Vineland, NJ) and vacuum filtered until all filtrate was removed. The residual collagen-glycosaminoglycan (CG) membrane formed can then be removed. By adjusting the volume of the solution used in the above technique, CG membranes of varying thickness (50-500 microns) can be obtained. In this work, we used CG membranes of average thickness about 350 microns.
B. Micropatterning in CG Membranes
CG membranes prepared as described above were stored in de-ionized water at 4°C prior to use. The flow network patterns to be cast on the membranes were obtained on Silicon wafer templates by the standard techniques of photo-lithography using SU-8 2075 (MicroChem, Newton, MA) as the photoresist [18]. Briefly, patterns of interest were drawn in AutoCAD software (AutoDesk, CA) and were sent for plastic mask fabrication to Advance Reproductions (North Andover, MA). In this work, we chose bifurcating flow patterns that maximized mass transport characteristics of the flow networks [19]. The plastic mask containing the patterns was then placed on a photoresist-coated silicon wafer (4″ diameter, University Wafer, MA) and the wafer was exposed to UV light of wavelength 365 nm for selective photopolymerization of the photoresist. The unpolymerized portions of photoresist were dissolved in a solvent to yield a silicon wafer template containing photoresist patterns (Fig. 1). For collagen soft lithography, the wafer was cleaned with 100% ethanol and N2-dried prior to the experiment. The entire procedure was performed at room temperature. CG membrane samples of appropriate sizes, matching those of the patterns, were cut using a scalpel. The sample surfaces were treated with acetic acid solution followed by carefully placing the patterns on top of the samples, with the pattern side facing down. Weights were immediately placed on top of the membrane-pattern sandwich. After 5 minutes of exposure, glutaraldehyde solution was added to submerge the membrane as well as the pattern. During the crosslinking of CG membranes by glutaraldehyde, which took at least 6 hours, the network pattern was cast onto the membrane sample. The factors that affected the formation of patterns on CG membranes, namely, acetic acid and glutaraldehyde solution concentrations, crosslinking time, and the applied pressure during the casting process, were varied (Table 1). The patterned membranes were rinsed 3-4 times in DI water to remove excess glutaraldehyde. Some of the patterned membranes were sent for pre-treatment for SEM image analysis, and some others were sent for histology processing.
Figure 1.
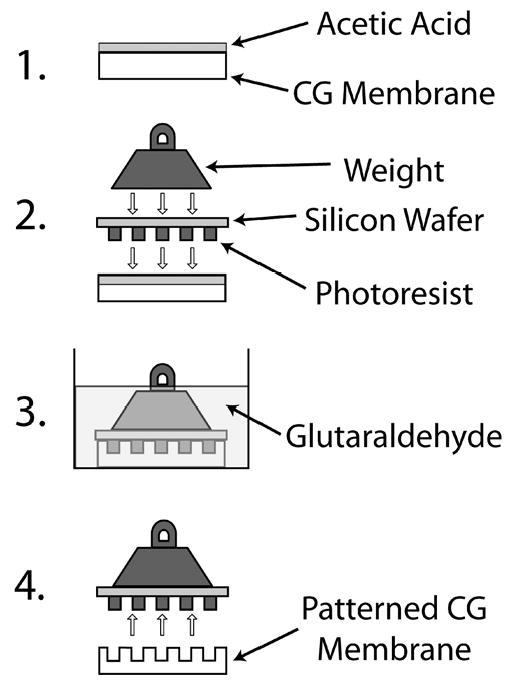
Schematic of the patterning technique. 1. Surface dissolution - Acetic acid is applied to the CG membrane surface. 2. Feature resolution - After the top layer of the CG membrane has been dissolved the silicon wafer with photoresist is positioned on the membrane surface and pressure is applied. 3. Feature stabilization - The system is immersed in glutaraldehyde to crosslink the membrane and to stabilize the patterned features. 4. Separation - The silicon wafer and CG membrane are separated resulting in a patterned CG membrane.
Table 1.
Experimental range and optimal values of process parameters used in micropatterning of CG membranes
| Process Parameter | Experimental Range | Optimal Values |
|---|---|---|
| Acetic acid concentration | 0 – 3 (M) | 1 – 3 (M) |
| Applied pressure | 365 – 880 (Pa) | 880 (Pa) |
| Glutaraldehyde concentration | 0 – 3 (% v/v) | 1 – 3 (% v/v) |
| Crosslinking time | 0 – 24 (hrs) | 6 – 24 (hrs) |
C. SEM imaging
The patterned membrane samples were dehydrated for scanning electron microscopy (SEM) imaging. We used the method of air-drying of the samples by the evaporation of hexamethyldisilazane (HMDS) [20]. Briefly, the samples were initially dehydrated in solutions of 70%, 80% and 90% ethanol and finally twice in 100% ethanol for 15 minutes each in that order. The samples were then stored in 100% ethanol overnight. After all the water within the samples was replaced with ethanol, they were immersed in 100% HMDS twice for 3 minutes each time. Subsequently they were dried in a fume hood and Pd-sputter-coated. SEM imaging was then carried out in a Philips XL-30 environmental scanning electron microscope (FEI, Hillsboro, OR).
D. Histology Processing
In order to quantify the depth of the micropatterned channels and the sharpness of the feature walls, the membrane samples were embedded in poly(ethyl-methacrylate) and sectioned. The sections were imaged using fluorescence microscopy to analyze the morphological characteristics of the patterned features. Image analysis was carried out using Image-Pro Plus (Media Cybernetics, Bethesda, MD).
E. Endothelial Cell Culture on CG Patterned Matrix
Bovine aortic endothelial cells (BAEC's, p5-6) were cultured using Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS). Sterile patterned CG membranes were fabricated using the procedure mentioned above in a sterile environment, and conditioned at physiological pH using 1× Phosphate Buffered Saline (PBS). Fibronectin (20 μg/ml) was added to the surfaces of the patterned matrices and the samples were incubated at 37 °C and 5% CO2 for an hour. Subsequently, BAEC's (at 80% confluence) were seeded directly on top of the micropatterned channels and allowed to attach for 2 hours. The membrane samples were then submerged in culture medium and incubated for 6 hours. Finally samples were fixed with 4% buffered formaldehyde solution and dehydrated for SEM imaging.
Results and Discussion
In this paper, we describe a technique to cast microscale patterns on to a CG biopolymeric matrix. We chose CG as our scaffold material because it is a natural biodegradable material with adequate biocompatibility and mechanical strength for integration with the host tissue. The micropatterned CG membranes can be used to control directed cell growth and also to create microvasculature within tissue-engineered constructs.
The pressure casting technique can be divided into three main steps, a schematic of which is shown in Fig. 1. The first step involved partial surface dissolution of the CG membrane samples using acetic acid solution. We chose acetic acid for its action as a strong solvent of collagen. The next step involved application of a uniform pressure on the sample-pattern sandwich. The final step consisted of crosslinking the sample with glutaraldehyde solution while still under pressure. The wafer was then removed to expose the micropattern that was permanently embedded into the CG surface.
The resolution of micropatterns on the CG surface was dependant on several factors. The first factor was obviously the initial resolution of the patterned wafer used in the pressure casting technique. In this work, we utilized simple photo-lithography techniques that allowed for photoresist patterning resolution of approximately one micron. More advanced techniques for submicron patterning can also be employed but the tissue engineering benefits of submicron patterning are not evident [4].
The other factors consisted of the parameters for each step, which were optimized to yield consistent, high resolution patterned membranes. The dissolution step is important to make samples pliable for the subsequent patterning steps. A higher concentration of acetic acid than used in membrane formation is required to form a surface layer amenable to casting. The applied pressure facilitates good feature resolution of the flow network patterns. We, however, noted an upper limit to the pressure as an excess of applied pressure compressed the porous network of the native CG membrane (data not shown). This can lead to a membrane with poor bulk transport characteristics. Table 1 lists the ranges of parameters that were investigated. It also lists the optimal parameter values that led to CG membranes with stable high resolution micropatterns.
Figure 2 summarizes results of the patterning technique. Figure 2A is an SEM image of the surface of flow channel networks cast on to a CG membrane. The flow channels and the islands (space between the channels) have sharp edges, consistent over the entire patterned area. The original pattern design (Fig. 2C) is reproduced reasonably well. The resolution of the pattern in CG membrane occurs mainly as a result of a balance between the application of adequate pressure uniformly across the casting domain and an optimum concentration of acetic acid solution. Non-optimal concentrations of acetic acid or applied pressure result in either poorly resolved patterns or highly altered/flattened-out structures (data not shown). Figure 2B is an SEM image of the smallest island on the patterned membrane. It shows a pattern with dimensions down to 2-3 microns indicative of the high resolution that can be obtained using this method. In a relevant study [4], Vernon et al. showed microgrooved dry collagen membranes of feature resolution ∼1 micron can be obtained by air drying collagen hydrogels of starting thickness ∼1.7 mm on microfabricated templates for 24-48 hours. The technique described in the current work complements the above study with additional benefits; it is faster (6-8 hours), and involves wet processing that leads to feature definition and stability at the application level.
Figure 2.
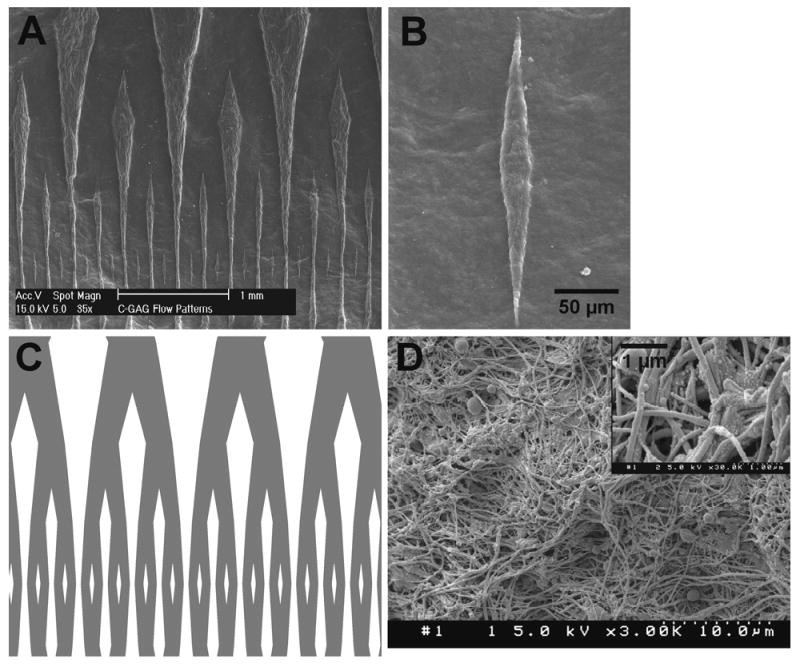
(A) SEM image of flow networks cast on to a CG membrane using micropatterning (B) SEM image of the smallest “island” on the patterned CG membrane (C) Corresponding AutoCAD design of the flow networks used for micropatterning (D) SEM image of the individual collagen fibers on the surface of a patterned CG membrane (with inset at higher magnification)
Figure 2D shows the surface of the membrane at a higher magnification with individual collagen fibers. The native collagen structure is not altered during the micropatterning process. It results in a uniformly porous matrix structure, but the pores are on the submicron level. These pores are large enough to allow diffusion of nutrients and waste products through the membrane and small enough to retain cells such as endothelial cells to adhere to the surface and proliferate. Such an arrangement will allow for efficient endothelialization of the entire flow channel network. Moreover, due to the dense fiber structure (Fig. 2D), the CG membranes used in this technique are substantially stronger than collagen gels [13], and are easy to handle and manipulate. Also, because collagen is a natural protein it is easy for cells to remodel it to form native extra-cellular matrix structure. The degree of degradability, however, depends on the crosslinking procedure employed, as native collagen material can be more easily degraded than crosslinked ones.
The characteristics of the feature walls obtained using the micropatterning technique were determined by fluorescence imaging of sections perpendicular to the direction of the channels. Glutaraldehyde crosslinking leads to a fluorescent construct that can be easily imaged. Figure 3A is a typical cross-sectional fluorescent image of the channels showing the feature walls. It runs across a complex patterned section with channel widths ranging from 50 microns up to 300 microns. The depths of the wider channels (see inset) are of the order of 100 microns, similar to that of the original wafer template that was used to fabricate the patterns. On the other hand, the narrower channels (see inset) are not as deep (∼ 40 microns) and the corresponding feature walls are more rounded. This could be partly due to the steps involved in histology processing, where dehydration of the samples leads to shrinkage affecting smaller features disproportionately. This could also be the limiting depth that can be obtained with the current technique for micron-scale features.
Figure 3.
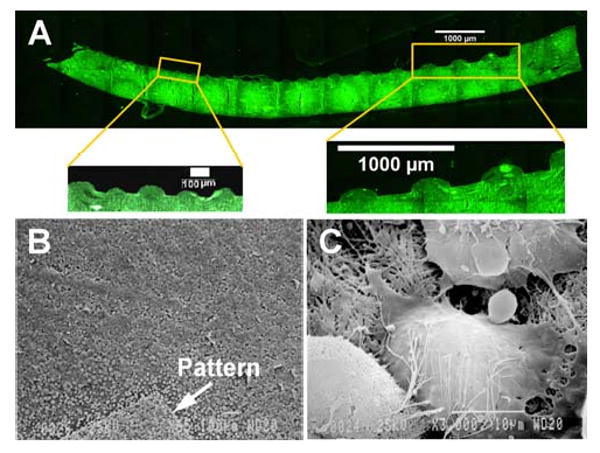
(A) Fluorescent image of the cross-section of a patterned CG membrane perpendicular to the channel direction, showing the morphological characteristics of the patterned features. The insets show the wide and narrow width channels at higher magnification. (B) SEM image of bovine aortic endothelial cells (BAEC's) seeded onto a sample patterned CG flow channel network (the pattern is marked by an arrow) (C) SEM image of an individual cell with extension of processes and establishment of cell-cell junctions
Stability of the flow networks is an important consideration for suitability of their use as tissue-engineered microvasculature. The crosslinking step plays a critical role in ensuring stability of the features cast on to the CG membrane. When no glutaraldehyde was applied, the patterns were ill-formed and unstable. The patterns obtained by using the optimal process parameters listed in Table 1 have been stable for several months (data not shown). Furthermore, the patterns retain their structure even after being subjected to vacuum drying and subsequent wetting. The application of glutaraldehyde crosslinks the CG membrane and stabilizes the flow structures as the patterns are being cast in the presence of acetic acid solution and uniform pressure. It may be possible to use other crosslinking methods such as EDC/NHS treatment [21] if there is concern about calcification that often occurs in glutaraldehyde crosslinked collagen constructs in vivo, though this has not been characterized in this work.
Utilizing the procedure described above, one can form two-dimensional flow patterns on CG membranes. Tissue engineering applications involving complex tissues, however, require three-dimensional networks. Currently, fabricating a three-dimensional flow structure in collagen-based biomaterials is a technically challenging problem. A combination of the above method and the method of stereolithography can be developed [22]. This can lead to three-dimensional structures but achieving micron-scale resolution will be a problem. A technically feasible but simpler method can involve layering of multiple CG membranes with 2-D patterns.
Endothelialization is an important step to achieve a functional microvasculature. To test the adhesion of endothelial cells and biocompatibility, we seeded BAEC's on the glutaraldehyde crosslinked CG membranes with flow patterns. Endothelial cells attached to the base matrix and formed cell-matrix and cell-cell bonding (Fig. 3). Figure 3B is an SEM image of a layer of endothelial cells attached to the crosslinked CG surface, with the pattern marked by an arrow. Figure 3C is a magnified image of a single endothelial cell that shows extension of cell processes and establishment of cell-cell junctions. These results suggest that glutaraldehyde crosslinked CG membranes are compatible substrates for endothelial cell adhesion and can be used to obtain ‘vascularized’ networks.
Conclusions
In summary, we have successfully cast flow network patterns on to a natural collagen-based scaffold material. The cast networks are very stable with a feature resolution in order of 2-3 microns. This is the first step in our effort to develop tissue-engineered (TE) products with built-in microvasculature. Establishment of microfluidics and subsequent endothelialization of the CG flow networks will form a functional microvascular network to be integrated within scaffolds for tissue engineering. In addition, these patterned membranes can be used as templates for topographically directed cell growth or as a model system to study various microvascular disorders where feature scales are important. Furthermore, the pressure casting technique described in this paper is amenable for adaptation in other scaffold materials, and can be used for the development of complex three-dimensional TE products with built-in flow channels and surface topography.
Acknowledgments
The research was funded in part by grants from the Whitaker Foundation and the National Institutes of Health (EB006203). The authors acknowledge Mr. Shubin Yu of the Electronics Design Center (EDC) for his guidance in the microfabrication of the silicon wafer templates.
References
- 1.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering Cell Shape and Function. Science. 1994;264(5159):696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 2.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric Control of Cell Life and Death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 3.Folch A, Toner M. Microengineering of Cellular Interactions. Annu Rev Biomed Eng. 2000;2:227–256. doi: 10.1146/annurev.bioeng.2.1.227. [DOI] [PubMed] [Google Scholar]
- 4.Vernon RB, Gooden MD, Lara SL, Wight TN. Microgrooved Fibrillar Collagen Membranes as Scaffolds for Cell Support and Alignment. Biomaterials. 2005;26(16):3131–3140. doi: 10.1016/j.biomaterials.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Lu Q, Simionescu A, Vyavahare N. Novel Capillary Channel Fiber Scaffolds for Guided Tissue Engineering. Acta Biomater. 2005;1(6):607–614. doi: 10.1016/j.actbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Schmalenberg KE, Uhrich KE. Micropatterned Polymer Substrates Control Alignment of Proliferating Schwann Cells to Direct Neuronal Regeneration. Biomaterials. 2005;26(12):1423–1430. doi: 10.1016/j.biomaterials.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Wang YC, Ho CC. Micropatterning of Proteins and Mammalian Cells on Biomaterials. Faseb J. 2004;18(3):525–527. doi: 10.1096/fj.03-0490fje. [DOI] [PubMed] [Google Scholar]
- 8.Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, Detmar M, Vacanti JP. Endothelialized Networks with a Vascular Geometry in Microfabricated Poly(Dimethyl Siloxane) Biomed Microdevices. 2004;6(4):269–278. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- 9.Bettinger CJ, Orrick B, Misra A, Langer R, Borenstein JT. Microfabrication of Poly (Glycerol-Sebacate) for Contact Guidance Applications. Biomaterials. 2006;27(12):2558–2565. doi: 10.1016/j.biomaterials.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized Microvasculature Based on a Biodegradable Elastomer. Tissue Eng. 2005;11(12):302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 11.Neumann T, Nicholson BS, Sanders JE. Tissue Engineering of Perfused Microvessels. Microvasc Res. 2003;66(1):59–67. doi: 10.1016/s0026-2862(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 12.Norman JJ, Desai TA. Control of Cellular Organization in Three Dimensions Using a Microfabricated Polydimethylsiloxane-Collagen Composite Tissue Scaffold. Tissue Eng. 2005;11(34):378–386. doi: 10.1089/ten.2005.11.378. [DOI] [PubMed] [Google Scholar]
- 13.Tang MD, Golden AP, Tien J. Molding of Three-Dimensional Microstructures of Gels. J Am Chem Soc. 2003;125(43):12988–12989. doi: 10.1021/ja037677h. [DOI] [PubMed] [Google Scholar]
- 14.Yannas IV, Burke JF. Design of an Artificial Skin. I. Basic Design Principles. J Biomed Mater Res. 1980;14(1):65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 15.Yannas IV, Burke JF, Gordon PL, Huang C, Rubenstein RH. Design of an Artificial Skin. Ii. Control of Chemical Composition. J Biomed Mater Res. 1980;14(2):107–132. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- 16.Nimni ME. Collagen. CRC Press; Boca Raton, Fla: 1988. [Google Scholar]
- 17.Charulatha V, Rajaram A. Influence of Different Crosslinking Treatments on the Physical Properties of Collagen Membranes. Biomaterials. 2003;24(5):759–767. doi: 10.1016/s0142-9612(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz H, Despont M, Fahrni N, LaBianca N, Renaud P, Vettiger P. Su-8: A Low-Cost Negative Resist for Mems. Journal of Micromechanics and Microengineering. 1997;7(3):121–124. [Google Scholar]
- 19.Janakiraman V, Mathur K, Baskaran H. Optimal Planar Flow Network Designs for Tissue Engineered Constructs with Built-in Vasculature. Ann Biomed Eng. 2007;35(3):337–347. doi: 10.1007/s10439-006-9235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braet F, De Zanger R, Wisse E. Drying Cells for Sem, Afm and Tem by Hexamethyldisilazane: A Study on Hepatic Endothelial Cells. J Microsc. 1997;186(Pt 1):84–87. doi: 10.1046/j.1365-2818.1997.1940755.x. [DOI] [PubMed] [Google Scholar]
- 21.Pieper JS, Hafmans T, Veerkamp JH, van Kuppevelt TH. Development of Tailor-Made Collagen-Glycosaminoglycan Matrices: Edc/Nhs Crosslinking, and Ultrastructural Aspects. Biomaterials. 2000;21(6):581–593. doi: 10.1016/s0142-9612(99)00222-7. [DOI] [PubMed] [Google Scholar]
- 22.Arcaute K, Mann BK, Wicker RB. Stereolithography of Three-Dimensional Bioactive Poly(Ethylene Glycol) Constructs with Encapsulated Cells. Ann Biomed Eng. 2006;34(9):1429–1441. doi: 10.1007/s10439-006-9156-y. [DOI] [PubMed] [Google Scholar]


