Abstract
The GroEL/GroES chaperonin folding chamber is an encapsulated space of ~65 Å diameter with a hydrophilic wall, inside of which many cellular proteins reach the native state. The question of whether the cavity wall actively directs folding reactions or is playing a passive role has been open. We review past and recent observations and conclude that the chamber functions as a passive “Anfinsen cage” that prevents folding monomers from multimolecular aggregation.
Keywords: GroEL, chaperonin, aggregation, cis cavity
Chaperonins are large oligomeric double ring assemblies that carry out an essential function in the cell assisting many newly translated proteins to fold to their native forms [1–3]. The bacterial chaperonin, GroEL, the most studied of this family, is a tetradecamer of 57 kDa subunits, assembled as two back-to-back seven-membered rings, each with a central cavity containing a hydrophobic lining to which a non-native polypeptide substrate can bind [4]. The co-chaperonin GroES, a seven-membered ring of 10 kDa subunits, associates as a “lid” structure with either end of GroEL in an ATP-dependent manner to form an enclosed cavity with a now hydrophilic wall character where folding of non-native substrate proteins takes place [5–10; see Fig. 1].
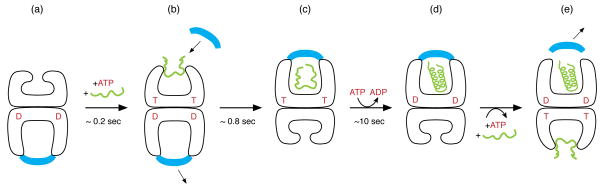
Fig. 1. Chaperonin reaction cycle.
An asymmetric GroEL-GroES-ADP complex (a) is the normal acceptor state for ATP (red; also indicated as T) and non-native polypeptide (green), binding them (b) in the open ring opposite the one bound by GroES (blue) and ADP (red D). ATP binding produces small rigid body apical domain movements in the bound ring (b), enabling GroES binding, attended by large rigid body movements that produce the stable folding-active cis complex end-state (c). This folding-active state is the longest-lived state of the reaction cycle, ~10 sec, followed by ATP hydrolysis (c→d), which then gates the entry of ATP and polypeptide into the opposite trans ring, rapidly discharging the cis ligands (e) and initiating a new folding active cycle on the ATP/polypeptide-bound ring.
While the steps of the ATP-driven GroEL/GroES reaction cycle have been generally understood for nearly ten years, how this system acts on substrate polypeptides to assist their proper folding has remained unclear. It has been established, for example, that non-native proteins are bound by an open ring, typically of an asymmetric GroEL/GroES/ADP “bullet” complex [11; see Fig. 1 panels a, b], via hydrophobic contacts. Yet whether such binding mediates polypeptide unfolding, effectively taking a misfolded protein back to the top of its energy landscape, has been unclear. In the subsequent step of the reaction, GroES binding to the same ring as polypeptide and ATP releases substrate from the cavity wall into a now encapsulated hydrophilic chamber [12–14; Fig. 1c]. The fate of substrate during this sequence of ATP-mediated freeing of the apical domains, GroES collision, and large forceful rigid body movements to produce the domed end-state, has also been under study. Finally, protein folding proceeds within the GroEL/GroES/ATP cis folding chamber, the longest-lived state in the reaction cycle (Fig. 1c). Does the GroEL cavity wall actively direct or modify this reaction, or does it simply passively contain the folding polypeptide? The first questions, concerning GroEL actions on polypeptide during the steps of polypeptide binding and cis complex formation, are beginning to be resolved, and we review current understanding of them at length elsewhere. The present discussion focuses on the last question concerning the mechanism by which the cis GroEL/GroES folding chamber, a unique encapsulated hydrophilic cavity, supports productive folding.
Our thesis, derived from recent experiments coupled with consideration of past observations, is that the cis chamber is, as John Ellis termed it in 1993, a passive “Anfinsen folding cage”, where a non-native polypeptide chain is isolated as a monomer and employs the information intrinsic to its primary structure, in the absence of external information, to fold to its energetic minimum, the native state [15]. The polypeptide may be subject to kinetic errors during this process, particularly at physiologic temperature, taking it off the productive pathway, but its confinement as a monomer protects it from multimolecular aggregation, enabling kinetically unproductive monomeric states to ultimately redirect themselves, through the energetic action of thermal fluctuations, onto a productive pathway to the native state. The major action of the chamber is thus to prevent protein aggregation, which comprises, when reversible, a set of off-pathway diversions that slows productive folding, and when irreversible, an off-pathway end-state that diminishes yield and produces potentially harmful structures.
In support of the foregoing conclusion about the cis chamber, we summarize below a number of key observations concerning the GroEL/GroES reaction. We focus first on those derived from studying the machine’s action under so-called nonpermissive conditions, where polypeptide substrate cannot reach the native state without the presence of the complete GroEL/GroES/ATP system, then on studies under permissive conditions, where the same protein substrates can reach the native state either while inside the GroEL/GroES cavity or while folding free in solution.
Nonpermissive conditions
Requirement for the GroES “lid”
The first in vitro reconstitution of the chaperonin reaction showed that both GroES and ATP had to be added to a binary complex of GroEL and R. rubrum Rubisco in order to recover native active Rubisco enzyme [16]. Notably that first experiment, and many that have followed, was carried out under so-called non-permissive conditions, in particular involving a concentration of substrate protein (in that case ~100 nM) and temperature (25°C), where, in the absence of the GroEL/GroES system, the substrate protein quantitatively aggregated, and where, for any recovery of native protein, the complete chaperonin system was required. In this latter regard, although GroEL alone at stoichiometric or greater concentration could forestall aggregation, recovery of the native state required GroES, whose binding to GroEL was known to be ATP-dependent.
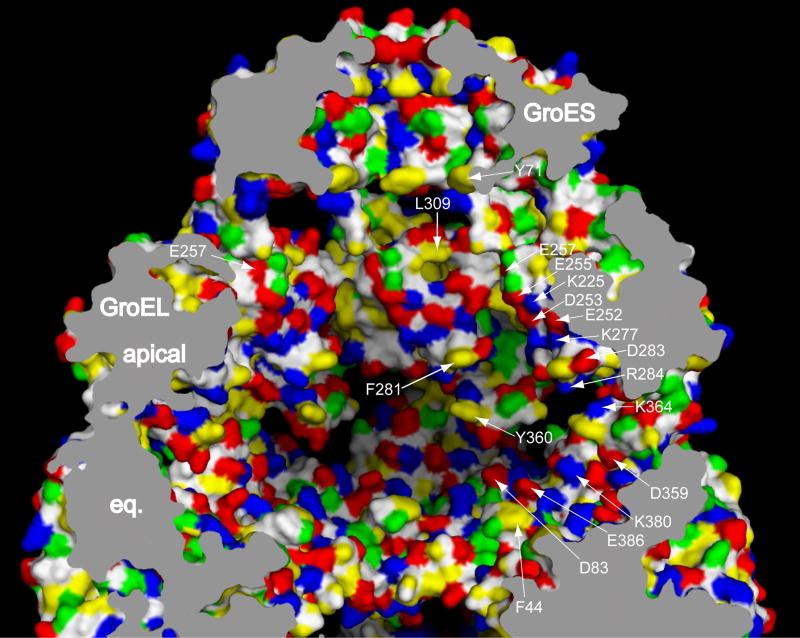
The role of GroES as an encapsulating agent was deduced from EM and biochemical studies [5,6], and it was established that substrate proteins as large as the R. rubrum Rubisco subunit (51 kDa) could be refolded in the cis chamber formed when GroES bound to GroEL [8,12]. One obvious well-commented role of this chamber, which measures ~65 Å in both height and diameter (Fig. 2), was to provide a space where proteins of ~20–60 kDa, the typical size of authentic cis substrates, could be confined as single molecules and would be unable to aggregate [10]. In addition, it seemed unlikely that exposed hydrophobic surfaces of these non-native states, surfaces that originally recruited them to the hydrophobic lining of an open ring, would interact with the cis cavity wall, because the wall of the cis chamber was observed by X-ray studies to have been switched by rigid body movements, which occur during ATP/GroES binding, to a polar character, exposing 315 electrostatic side chains and only 14 hydrophobic ones [10,17].
Fig. 2. Cis GroEL/GroES folding chamber.
The domed chamber formed by GroES binding to a nucleotide-bound GroEL ring is shown in a cutaway view of a space-filling model, with the wall character revealed by coloring of amino acid side chains: red, acidic; blue, basic; yellow, hydrophobic; green, polar; white, main chain. Parts of three subunits are visible. A number of residues are identified by the arrowing. Note that there is considerable electrostatic character to the wall (see text). eq., equatorial domain. Figure adapted from ref.22.
Single round refolding mediated by SR1-GroES
The importance of the cis chamber to productive folding is highlighted by the observation that a single ring version of GroEL, called SR1, which binds GroES in the presence of ATP but then does not release it, is fully productive, with kinetics and extent of native state recovery in this stable folding chamber that are virtually identical to those of the cycling GroEL/GroES reaction, where GroES and substrate polypeptide are discharged from GroEL approximately every 10 seconds [8,12]. The cis chamber of SR1/GroES is stable because the normal allosteric signal for discharge of the cis ligands, ATP binding to the opposite (trans) GroEL ring, cannot occur – there is no trans ring. Importantly, the length of time required for full recovery of the native state of many substrate proteins, both by the cycling reaction and by SR1/GroES, is greater than 15 minutes. In the case of the SR1/GroES-mediated reaction, this implies that a significant fraction of the non-native states inside the cis chamber of SR1/GroES can spend a relatively large amount of time exploring their folding free energy landscapes without becoming irreversibly trapped. They clearly do not have any requirement to be re-bound by an open ring during this time, because they continue to form the native state at the same rate as in a cycling reaction and ultimately, as in the cycling reaction, they yield the native form with high efficiency, approaching 90–100% for many substrates.
Notably, there is just one round of ATP turnover by an SR1/GroES complex, occurring in the first 10 seconds after its formation, after which the complex is stable as an ADP-SR1/GroES complex [6]. Thus there is no ongoing involvement of ATP in the folding process inside this complex, and the polypeptide chain has to rely on the energy of thermal fluctuations to drive conformational changes, essentially as it would if free in solution. The difference is that, in this chamber, it cannot aggregate. It can remain unfolded or misfolded as a monomer and thus is free of the complications of an additional set of kinetic mis-steps involving multimolecular associations.
But how can the kinetics of recovering the native state in SR1/GroES be the same as in a cycling reaction, where a nonnative polypeptide spends time free in solution, subject to aggregation? It appears that this is a function of the rapid rebinding of non-native forms during a cycling reaction, relative to the much longer period of time these states spend folding inside the cis chamber [8,18]. In studies employing mutant GroEL’s that function as polypeptide traps, it has been observed that non-native forms released from a GroEL ring can be re-bound by another chaperonin molecule in well under a second [19]. A more accurate estimate for the rate of rebinding comes from kinetic measurements of fluorescently labeled substrate binding. For example, the bimolecular rate constant for MDH binding to GroEL has been measured as 2.4 × 106 M−1sec−1, enabling an estimate of the binding rate at a physiologic GroEL concentration (1 μM) of 2.4 sec−1, which equates to a half-time for binding of 0.3 sec [18]. This can be compared to the lifetime of a cis ternary folding complex of approximately 10 seconds [8,12,18). Thus a non-native MDH polypeptide in a cycling reaction spends no more than ~3% of its time in free solution, apparently insufficient time to produce an effect on either the rate or extent of recovery of the native state.
Do proteins in the cis cavity explore a conformational landscape, as they would in free solution, or are they somehow behaving differently? Studies using a secretory protein, bovine trypsinogen (TG), to report on topology during folding indicate that conformations both on and off-pathway are being explored [20]. While TG is normally folded in the ER compartment (absent a chaperonin system), when it is diluted in vitro from denaturant and reductant into a solution containing GroEL or SR1, it becomes efficiently bound to the chaperonin, forestalling otherwise rapid and irreversible aggregation. Upon addition of ATP, GroES, and a redox buffer (GSH/GSSG), the protein proceeds to the native state with a half-time of ~45 minutes. The 12 cysteines of this protein, forming 6 disulfide bonds in the native state, were used to report on three-dimensional topology during folding by halting the reaction at various times with alkylation and then recovering the TG to determine by proteolysis and mass spectrometry what disulfide bonds had been formed. At early times following initiation of folding, a collective of short-range disulfide bonds, both native and non-native, were formed. Subsequently, three middle-range non-native bonds as well as a long-range native bond were also detected. Both the non-native and native bonds were detectable throughout the course of the reaction, only resolving to all native at late times when most of the molecules had reached native form. These data imply that non-native off-pathway states do become populated along with native ones during folding in the cis chamber. Whether the non-native states are identical to the conformers that would be formed in solution is not resolvable for this substrate, because it has not been possible to refold TG in free solution. Even at 10 nM concentration and low temperature (what would be permissive conditions for many proteins, see below), it quantitatively aggregated upon dilution from denaturant on the timescale of a few seconds (E.S. Park, unpublished observations). This latter behavior, however, further reflects the benefits of solitary confinement in the cis chamber as a means of preventing off-pathway multimolecular associations.
Blocking polypeptide rebinding during a cycling reaction halts recovery of the native state
If the cis cavity prevents aggregation during a folding reaction, then blocking return to the cavity following release into free solution should lead to a halt or diminution of further recovery of the native state associated with the occurrence of aggregation, essentially a repartitioning from monomeric chain folding in the cavity to multimolecular aggregation in free solution. This was exactly what was observed when, during a cycling reaction, polypeptide substrate was blocked from returning to GroEL [21]. The study involved engineering a programmable obstruction of an open substrate-accepting GroEL ring by placement of an innocuous biotin modification at the cavity-facing aspect of the unliganded GroEL ring. This enabled precipitous obstruction of the open cavity by addition of streptavidin. During a folding reaction, such addition produced a rapid halt to productive refolding of the substrates Rubisco and rhodanese. For Rubisco, there was an immediate and complete halt of recovery of activity upon prevention of its rebinding, and the Rubisco present in free solution was reported to form aggregates that were observable by centrifugation. Thus, released non-native Rubisco confined to free solution proceeded immediately to irreversible multimolecular aggregation. For rhodanese, a small percentage of additional native protein was recovered over a period of a few minutes after addition of streptavidin. In this case, a slower aggregation process apparently enabled a successful kinetic competition by a small fraction of the released monomers for folding into the native state.
Role of the cis cavity wall in folding – mutational studies
If the GroEL cis cavity wall were actively influencing the refolding of substrate proteins, it might be expected that amino acid substitutions affecting the wall would produce substantial effects on rates of refolding or extent of recovery of the native state. While a final tally of such mutations is not in as yet, it seems that, in general, single substitutions are entirely benign and a number of multiple mutations are likewise benign [22,23]. Although several multiple mutants have shown effects in the overall reaction [23], the specifics of the effects as they relate to the cis folding cavity have not been fully evaluated, leaving open whether they are directly affecting cis folding or some other aspect of the reaction cycle (e.g., substrate binding or ATP hydrolysis).
No effects when hydrophilic residues are changed to hydrophobic Character
As noted above, it was observed with the first X-ray structure of a GroEL-GroES complex, the asymmetric GroEL-GroES-ADP7 complex, that the cis cavity lining was hydrophilic in character, largely devoid of hydrophobic side chains [10; Fig. 2]. This raised the issue of whether mutations of hydrophilic cavity-facing residues back to hydrophobic character could affect productive folding. A variety of single, double, and triple substitutions changing acidic or basic residues to valine or alanine were designed, and all were without effect when tested in vivo by examining whether such substituted GroEL’s could rescue a GroEL-deficient strain; that is, transformation with plasmids encoding the various substitutions efficiently rescued cell growth [22].
Negative side-chains changed to polar but neutral character
An additional study measured effects of mutating acidic residues facing the cavity. In a cis cavity, there are 189 such negatively charged side chains (27 per GroEL/GroES unit) and 126 positively charged side chains (18 per unit) facing the cis cavity (Fig. 2). Here, simultaneous mutation of three of the negative side-chains to uncharged polar character (2 aspartates to asparagine and 1 glutamate to glutamine: a loss of 21 total negative charges per ring) was without effect; transformation of plasmids encoding these substitutions produced efficient rescue of cell growth [23].
Reversal of charge, negative to positive
When the same residues were changed to lysine or when three additional negatively charged residues were changed to uncharged polar character, in both cases associated with a net loss of 42 negative charges per ring, there was a strong reduction of rescue efficiency [23]. Whether the growth defects of such mutants, however, lie strictly at the level of the character of the cis cavity wall, or lie instead at the level of polypeptide encapsulation or ATP turnover, has not been addressed. The in vitro studies of these mutants to date have been limited to determination of rates and extents of recovery inside the corresponding stable mutant SR1/GroES complexes, revealing variable levels of recovery of the native state depending on the substrate and mutant examined [24]. Although these results were interpreted to indicate a specific role of the cis cavity in directing the trajectory or efficiency of folding, the effects of such substitutions on other aspects of the chaperonin reaction were not evaluated. It remains possible, however, that negatively charged residues have a role in the cis cavity wall, potentially providing local repulsion to substrates exposing negative charges during refolding. How or whether a net negative charge of the wall, amounting to ~20 % of the total of electrostatics, could have a general effect seems unclear, albeit that there is a predominance of acidic proteins in E.coli. It seems possible that such charge contributes to a “no stick” action of the cis cavity lining, which keeps collapsed folding conformers from interacting with the cavity wall, remanding them to their own primary structure for directing the refolding reaction. On the other hand, this neglects the fact that there are also basic proteins that are assisted by the chaperonin system; for example, mitochondrial malate dehydrogenase (MDH; from pig heart), a commonly used GroEL/GroES substrate, has a predicted isoelectric point of ~8.2.
Indeed, any conclusion from studies employing six simultaneous substitutions per GroEL subunit (42 per ring) must also be tempered by its reliance on investigation of a heavily mutagenized local region. Our experience with multiply substituted GroEL’s has been that, while they may be able to assemble into tetradecamers, they are often affected in multiple steps of the reaction cycle, making attribution of observed defects to a single step difficult. This multiplicity of effects is in part a function of residues playing different structural and functional roles in the two major states. For example, rigid body movements of the machine bring a host of apical domain residues lying away from the central cavity in an open binding-proficient GroEL ring, where they support the subunit-subunit interface, directly into the cis cavity wall in the GroES-bound folding-active state. In addition, within a single state, allosteric properties of the machine can be at play, where, for example, a substitution in the apical domain can substantially affect the rate of ATP turnover in the equatorial domain [25]. Finally, multiple local substitutions can perturb local secondary structure, making it difficult in the described experiments to know whether there is simply a charge effect as opposed to an additional effect, for example, on local structure of the cavity.
Optimizing GroEL/GroES for GFP folding does not affect the GroEL cavity wall but combines ATPase stimulating mutations of GroEL with alteration of a cavity-facing GroES residue
Using the observation that the aggregation-prone behavior of GFP expressed in E.coli can be countered to some extent by overexpression of GroEL/GroES, increasing the recovery of green fluorescence, a mutagenesis was carried out on GroEL/GroES aimed at further optimizing the efficiency of GFP folding and recovery of fluorescence [26]. Strikingly, none of the mutations improving fluorescence mapped to the GroEL cavity lining. Instead, combinations of mutations were obtained, simultaneously affecting both GroEL and GroES. The mutations affecting GroEL mapped to the equatorial and intermediate domains and, when studied in vitro, all of them accelerated the GroEL ATPase (and enhanced the suppression of ATPase activity by GroES). The effective mutations in GroES mapped to a single site, tyrosine 71, whose side chain projects into the cavity at its top aspect (Fig. 2), the collective of seven forming a hydrophobic ridge in what is otherwise a hydrophilic, net negatively charged portion of the cis cavity contributed by GroES. Here the residue was changed to either arginine or histidine. At first glance, it would seem that the GroES substitutions might be acting by changing the lining contiguous to refolding GFP, influencing the kinetics of its cis folding, but, when examined alone in vivo, only a small effect of the GroES substitutions to improve GFP fluorescence was observed (increase of 50–100%). Instead, the major effect (increase of 500–600%) was contributed by the GroEL substitutions, affecting the ATPase. Given the observations of Horovitz and coworkers that mutations in GroES can strongly affect the GroEL ATPase [27], an effect on ATPase activity by the Y71 changes remains an alternative possibility to the conclusion that local effects on cavity character had improved recovery of fluorescent GFP. Given the altered ATPase behavior of the substituted GroEL’s in the presence of wild-type GroES, it is attractive to consider that the Tyr71 substitutions may be further modulating such behavior. Notably, while the ATPase activity of the substituted GroEL’s was reported, the effects on ATPase activity of the substituted GroES’s were not presented. Significantly, the improvement of GFP folding, presumably largely through adjustment of the ATPase cycle timing, came at the price of reduced folding of other substrate proteins, as indicated by loss of growth at heat shock temperature, inability to support λphage growth, and reduced ability to fold a substrate, HrcA. Finally, the action of the various substitutions on GFP folding could not be fully resolved because the substituted chaperonins produced little or no effect on the rate or extent of GFP refolding in vitro, leaving uncertain whether the effects observed in vivo were related at all to the function of the cis cavity as a folding chamber.
Summary concerning effects of mutations in the cavity wall
In summary concerning mutations of the cis cavity wall, it has to be concluded that single substitutions (7 per ring) in general do not have the profound effects on the step of productive substrate folding in the cavity that a host of single cavity-facing substitutions had on the step of substrate polypeptide binding by the open GroEL ring. In that case, changes of any one of ten individual cavity-facing hydrophobic residues to hydrophilic character (7 per ring) abolished polypeptide binding and completely abolished rescue of growth of a GroEL-deficient strain in vivo [28]. In the case of the cis cavity electrostatic studies, two triple mutations were without effect, and while both a third mutation carrying out triple charge reversal and a sextuple negative charge-to-polar mutant exerted a variable effect on folding in vitro, there was still a degree of rescue in vivo. Thus, it seems difficult to interpret such results as evidence that the cavity wall is actively directing the folding reaction, as opposed to simply comprising a hydrophilic “non-stick” surface. That is, it seems more likely that overall wall character is involved here, as a non-stick barrier, as opposed to providing direct interactions between individual wall residues and specific residues of a folding polypeptide chain. It appears that evolution has optimized a non-stick wall for the folding chamber, not specific sets of interactions between the wall and each of dozens of refolding substrate proteins. Consider at the extreme the cross-kingdom-mediated folding by bacterial GroEL of proteins from eukaryotic mitochondria –there is a very large evolutionary separation involved, yet folding in the cis cavity proceeds efficiently. The lack of residue-specific interactions is further supported by additional experimental data reviewed below that conclude that the “intrinsic” rates of reaching the native state inside the cavity and outside in solution are the same if one removes the complication of multimolecular aggregation occurring in solution. This enforces the notion that the cavity wall has evolved to be inert relative to monomeric chain folding.
Folding to native form under permissive conditions in the cis cavity vs free in solution - the rate is the same but folding free in solution is kinetically complicated by reversible multimolecular aggregation
Early after their reconstitution of GroEL/GroES-mediated refolding of R. rubrum Rubisco, the Lorimer group observed that, if the temperature of the refolding reaction was lowered from 25°C to 15°C or below, Rubisco could now spontaneously and quantitatively refold to its native form in solution [29]. While there was a virtually complete recovery of the native state, the rate of recovery was only approximately one tenth that of the GroEL/GroES/ATP reaction carried out under the same conditions. The authors commented concerning this difference: “Presumably, intermolecular aggregation is suppressed at lower temperatures, enabling proper intramolecular folding reactions to predominate …At higher temperatures, restrictive for spontaneous folding, the formation of aggregates is largely avoided by a stable interaction…with the…’double donut’ [GroEL] …We propose that cpn60 is the biological equivalent of…insoluble matrices, permitting unfolded polypeptides to undergo folding rather than aggregation.” At that point in history, both this study and another [30] could only speculate that cpn10 (GroES) functioned as a “coupling factor”, although this anticipated the understanding that GroES functions as a physically encapsulating “lid”. Clearly spelled out, however, was the notion that permissive conditions simply shifted kinetic partitioning of the non-native monomer in solution from multimolecular aggregation toward productive folding. Likewise clear was the proposal that acceleration of folding by the GroEL/GroES system under these conditions represented an even more extensive partitioning away from aggregation.
A more recent study likewise observed the acceleration of Rubisco folding by the GroEL/GroES system under permissive conditions, but it concluded that this represented not an effect on multimolecular association but rather the elimination of off-pathway misfolding of Rubisco monomer under these conditions by “close confinement” in the cis cavity [21]. This conveys the idea that such confinement of a relatively large substrate would entropically favor more collapsed states, potentially favoring formation of the native state. Such an idea further implies that the cis cavity is playing an active role in folding this substrate. Notably, however, this study of Rubisco refolding, like the earlier study from the Lorimer group, added 1 mg/ml BSA to the reaction mixtures. One would assume that such an addition was, albeit unstated, critical to a partitioning of the Rubisco in the solution that favored folding over aggregation or prevented its loss on the tube walls. This leaves open to consideration whether the solution reaction might contain Rubisco species that were other than monomeric and that contributed to the difference in folding kinetics.
To directly address the behavior of Rubisco under these conditions, Adrian Apetri of our laboratory has recently carried out a number of tests [31]. First, he examined the effect of omission of BSA on recovery of Rubisco under permissive refolding conditions either free in solution or in the GroEL/GroES reaction. Strikingly, he observed in the case of the solution reaction that, upon omission of BSA, there was essentially no recovery of native Rubisco. By contrast, the rapid rate of refolding by the GroEL/GroES system remained unchanged in the absence of BSA. Second, he assessed whether Rubisco aggregation could occur while free in solution even in the presence of BSA, using both dynamic light scattering and gel filtration chromatography, observing by both analyses that aggregation indeed occurred. In the first test, light scattering increased rapidly following dilution of Rubisco from denaturant into the BSA-containing buffer used for refolding under permissive conditions (observable at the earliest time of measurement after mixing, within seconds). In gel filtration using radiolabeled Rubisco, the formation of a collective of Rubisco species of several hundred kDa was observed within minutes of dilution into refolding buffer. By contrast, no Rubisco monomer was observed. At 75 and 140 min (75 min corresponding to t½for refolding), the species of several hundred kDa were no longer observed, converted approximately equally into 100 kDa native dimeric protein and into larger aggregates at the void volume of the gel filtration column (~10 MDa). Thus, a very substantial fraction of the Rubisco that forms aggregates at early times is able to convert into the native state, presumably because the aggregation is reversible. This offers a probable explanation for the relatively slow rate of refolding of Rubisco free in solution under permissive conditions; as conjectured in the 1990 paper from the Lorimer group [29], the protein is reversibly aggregating.
A further GroEL/GroES dependent substrate whose rate of folding under permissive conditions is 10-fold faster at GroEL/GroES than in free solution, a double mutant form of maltose binding protein (DM-MBP) [32], has also been examined and proposed as a further example of a protein whose folding is actively affected by the cis cavity [24]. Here also, however, as opposed to an effect of the cis cavity to accelerate folding of the monomeric species, it appears that it is reversible multimolecular aggregation in solution that is leading to slowing of the rate of reaching the native state in that setting. For example, the rate of folding of the double mutant protein when free in solution was inversely dependent on concentration, a tell-tale sign of protein aggregation [31]. Further, DM-MBP diluted from denaturant exhibited light scattering whereas wild-type MBP protein did not. Finally, if chloride ions were omitted from the refolding buffer, light scattering during folding in solution did not occur. If aggregation was the source of slowed folding relative to the GroEL/GroES reaction, then chloride omission should also restore the rate of refolding in solution to that inside the chaperonin. This was indeed observed, with the curves of recovery now being indistinguishable between folding free in solution and chaperonin-assisted [31]. This further establishes that it is reversible aggregation under permissive conditions during folding in solution that contributes (entirely, as observed here) to the slower rates of folding in this setting than inside the cis cavity. This leaves the hypothesis of an active role in folding for the cis chamber unsupported.
Finally, Apetri addressed a further experiment toward the function of the cis chamber, asking whether it could have any active role in protecting an already native protein against the consequences of misfolding, here induced by thermal exposure, a condition known to produce protein misfolding and aggregation [31]. Human dihydrofolate reductase (DHFR), refolded to its native state either free in solution or inside the chamber of SR1/GroES formed in ADP-AlFx (a transition state analogue of ATP that further stabilizes the complex against dissociation), was exposed to various elevated temperatures for a period of 10 min, followed by immediate assay at 23°C, asking whether there was any difference in protein inactivation between the two conditions. None was observed, suggesting no particular ability of the cis cavity to protect DHFR against thermal inactivation. In the case of 50°C exposure, the DHFR was completely inactivated. This reaction mixture was downshifted to 23°C for a half hour and then assayed for recovery of DHFR enzyme activity. Whereas the solution reaction failed to exhibit any recovery, having already formed noticeable aggregates observable by light scattering during the thermal treatment, the SR1/GroES mixture exhibited a recovery of ~50% of the input DHFR in active form. The cis chamber had thus apparently protected the thermally misfolded protein from aggregation and had allowed a substantial fraction of the encapsulated monomers to recover the native state. This thus comprises a further demonstration that the cis cavity acts to protect monomeric species against aggregation.
Folding trajectories inside the cis cavity and in solution
If productive non-aggregated monomers folding inside the chaperonin and free in solution under permissive conditions lie on the same energy landscape, then one would predict that probes of the trajectory of such folding should show identical time-dependent changes. Such an experiment was carried out using human DHFR as substrate, analyzing its development of amide proton protection during refolding, either inside the SR1/GroES cavity or free in solution, by hydrogen-deuterium exchange and NMR [33]. The experiment was carried out under permissive conditions at 15°C, where the rates of recovery of native DHFR inside the cis cavity and free in solution were roughly equal, but where the extent of recovery in solution was reduced to ~50% due to formation of aggregates. The equal rates of native state recovery imply that here, in contrast to DM-MBP, there is no significant reversible aggregation in free solution – aggregated DHFR appears to be irrecoverable. As such, however, the aggregated material could be readily removed from the solution reaction by centrifugation, allowing direct comparison of the DHFR folding free in solution with the cis encapsulated DHFR. Refolding was commenced in protic solution by dilution of DHFR from urea denaturant or by addition of ATP/GroES to a SR1-DHFR binary complex, and then, at various times during the reaction, a 10-fold volume excess of D2O was added and the folding reaction simply allowed to proceed to completion in D2O. Native DHFR recovered from either reaction was analyzed by NMR spectroscopy for the relative intensity of 51 amide “probes”, which are from non-surface residues highly protected in the native state. When an amide proton had become incorporated into a hydrogen bond by the time of addition of D2O, it would remain protected against exchange and be NMR visible in analysis of the native protein. When it was not bonded at the time of exchange (or not buried), it would be exchanged for a deuteron and would become NMR invisible. In this way, both the development of protection and the location of protection in DHFR in the two settings could be compared. These comparisons revealed an identical trajectory and pattern of protection in DHFR when folding either free in solution or confined to the cis cavity. In both cases, there was early protection (15 sec) observed in the central parallel β-sheet of the protein, followed by progressive development of protection in surrounding regions including the α-helices. Even at 5 sec, the same general pattern of protection was developing under both conditions (unpublished observations; see also discussion of the initial states from which folding commences in the last section below). Thus the same route to the native state appears to be taken by DHFR monomers in both settings and, likewise, the same kinetics are followed. Strikingly, of course, in free solution the reaction was significantly less efficient, complicated by production of irreversible aggregates. These data further reinforce the idea that the cis cavity of the chaperonin does not influence or otherwise direct the folding of a substrate polypeptide.
Further considerations regarding behavior of the cis cavity toward particular substrate proteins
A number of additional experimental findings are worth considering in relation to the apparently passive behavior of the GroEL/GroES cis cavity as a device for preventing aggregation during folding. These concern its behavior toward a number of specific substrates.
Actin and tubulin
Unfolded forms of these two eukaryotic cytoskeletal proteins are efficiently bound by GroEL upon dilution from denaturant, but upon addition of ATP/GroES, they are fated to continuous cycling by the system without ever reaching native form [34]. Clearly the cis cavity prevents aggregation in this non-permissive context, because if these same substrates are diluted into free solution they quantitatively aggregate. Why they fail to reach native form remains unclear. By contrast, the dedicated eukaryotic cytosolic chaperonin, CCT, efficiently binds and refolds these same proteins [35,36], but, in contrast, does not recognize such GroEL substrate proteins as MDH or rhodanese. Thus, the CCT chaperonin, in contrast with GroEL, may recognize features other than hydrophobicity of non-native states, or may recognize hydrophobicity only in a specific structural context [see ref. 37 for discussion]. In this regard, later-formed conformations than those bound by GroEL may be recognized by CCT. Alternatively, the nature of ATP-mediated release of bound substrate by CCT may be different from that of GroEL, with sequential release occurring from the heterologous subunits [38] as opposed to the concerted release directed by ATP/GroES from the GroEL cavity wall. Sequential release of a characteristically bound substrate could produce a process of folding that is directly ordered by the chaperonin. Potentially, this could be crucial to steps of actin and tubulin folding that will always kinetically “go wrong” in the case of concerted release into the GroEL/GroES cis chamber. Such considerations are, however, in the absence of any experimental data, purely speculative.
T4 gp23
The T4 phage major capsid protein, product of gene 23, was known by 1970 to be the single dominant protein species produced in T4 phage-infected E.coli, and its assembly into phage particles was noticed to require action of the phage’s gene 31 protein, gp31. It was then observed by Georgopoulos and colleagues in 1972 that E.coli strains harboring mutations in the host gene encoding GroEL were also not able to produce phage particles, with the phage capsid protein observed in aggregates, termed “lumps”, associated with the bacterial membrane [34]. Twenty years then elapsed before genetic and biochemical studies by Georgopoulos and coworkers made clear that gp31 is in fact a phage-encoded version of GroES, supporting phage growth in vivo and forming a 7-membered homooligomeric ring that in vitro can both physically associate with GroEL in the presence of ATP and mediate folding of several well-studied GroEL substrate proteins [40]. X-ray studies of gp31 then indicated that its ring structure exhibited a taller inside cavity height than GroES [41], and consistently, it was recognized in in vitro studies that, whereas GroES could not encapsulate GroEL-bound capsid protein, the gp31 component was able to do so, thus allowing for cis folding of the capsid protein [42]. Very recently, the refolded capsid protein enclosed inside a stable GroEL-gp31-ADP/AlFx cage has been resolved as a density inside the cis chamber, using cryoEM [43]. The capsid protein extensively fills the cis chamber and appears to occupy a specific orientation inside of it. But most remarkable in functional terms, however, is the observation from Georgopoulos and coworkers that phage encoded gp31 can completely replace GroES in E.coli to support the essential function of GroES, conferring normal cell growth [44]. This indicates that gp31 can support the productive refolding of all of the natural GroEL-GroES-dependent substrates of the bacterial cell. These substrate proteins have never “seen” T4 gp31, nor ever had to rely on it for productive refolding. This further implies a passive role, here for the cochaperonin lid structure, in cis chamber folding.
Implications of prolonged rotational correlation times of substrate proteins inside the cis cavity relative to free solution
Early measurements of time-resolved fluorescence anisotropy performed with native GFP either inside GroEL/GroES or free in solution suggested that the protein tumbles several times more slowly inside the cis chamber than free in solution [8]. This is not surprising considering that the protein is encapsulated, and as such, must be affected in its rotational and translational movements by collisions with the cavity wall. Yet the measured rotational correlation time, 42 nsec, is considerably faster than that of GroEL itself under similar conditions, which is >200 nsec as measured by NMR studies [45]. This implies that the substrate has considerable freedom within the cis chamber, presumably sufficient to allow the conformational changes of chain folding of substrate polypeptide to proceed without impairment by collisions with the cavity wall.
Difference of initial collapse of non-native forms released from cavity wall vs diluted from denaturant
It seems likely that the ensemble of conformations present immediately after the initial step of collapse of a chaotrope-unfolded polypeptide upon dilution into aqueous buffer, effectively collapse of a random coil, would be different from that produced by the step of release of an already-collapsed globular substrate from the cavity wall of a GroEL ring. This latter step appears to involve a concerted release of the bound polypeptide chain from the apical domains into the cis chamber [46,47], although it has been suggested from a recent fluorescence study that hydrophobic segments may be last to leave the apical domains [14]. Yet beyond the step of initial collapse either in solution or in the cis cavity, which must occur in well under 1 second, it appears that the same ensembles of collapsed non-native states are likely to be populated. This would explain, for example, the virtually identical results from analysis of amide hydrogen-deuterium exchange protection of DHFR folding in cis vs free solution [33].
There remains, however, an observation that refolding of GFP in the two environments shows some difference, with refolding in the complete chaperonin reaction, measured by acquisition of fluorescence, exhibiting a lag of ~3 sec, relative to immediate appearance of fluorescence when refolding either free in solution after dilution from denaturant or upon addition of ATP alone to a GroEL/GFP binary complex [48; GFP generally refolds permissively]. It thus appears that GroES binding is associated with the lag in recovery, raising the question of whether this particular protein becomes transiently kinetically trapped in the states formed after release into the cis chamber. Perhaps an early state with an extended geometry that is a direct route to the native state cannot be formed in the confines of the cis cavity. Alternatively, concerted release of the protein, associated with the large apical movements upon ATP and GroES binding, may beget a particular state that is trapped. By contrast, the smaller apical movements produced by ATP binding alone [49] may not produce such a state. Whatever the case for GFP, its behavior appears exceptional. By contrast, such other substrates as MDH [17, see Suppl. Fig. 1] and MBP (A. Apetri, unpublished observations) exhibit single exponential recovery of the native state, devoid of any such lag phase, indicating that for those substrates productive folding in the cis chamber commences immediately upon release.
Concluding remarks
In sum, then, the experiments and their interpretations presented here all support the conclusion that the GroEL/GroES cavity is indeed a passive “Anfinsen folding cage,” as suggested by Ellis. The hydrophilic, net negatively charged, lining of the cavity provides a non-stick surface that effectively forces a non-native polypeptide to follow its intrinsic folding pathways without providing any general or substrate-specific direction to the process. The observation of identical folding trajectories within the cavity and free in solution confirms that GroEL/GroES-mediated folding conforms to Anfinsen’s principles. Furthermore, it seems clear that the chaperonin’s anti-aggregation actions, both in binding non-native polypeptides and in permitting them to fold in a sequestered environment, are crucial to its essential roles both in folding newly-translated proteins and in protection and recovery of cells from heat shock. The generality of such actions is what allows the chaperonin system to efficiently assist its wide range of protein substrates to reach their native, functional state.
Abbreviations
- R. rubrum
Rhodospirillum rubrum
- DM-MBP
double mutant of maltose binding protein
- GSH/GSSG
reduced and oxidized glutathione, respectively
- TG
trypsinogen
- BSA
bovine serum albumin
- NMR
nuclear magnetic resonance
- DHFR
dihydrofolate reductase
- GFP
green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thirumalai D, Lorimer GH. Chaperonin-mediated protein folding. Annu Rev Biophys Biomol Struct. 2001;30:245–269. doi: 10.1146/annurev.biophys.30.1.245. [DOI] [PubMed] [Google Scholar]
- 2.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 3.Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: Physiology and mechanism. Annu Rev Cell and Dev Biol. 2007;23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555. [DOI] [PubMed] [Google Scholar]
- 4.Braig K, Otwinowski Z, Hegde R, Boisvert D, Joachimiak A, Horwich AL, Sigler PB. Crystal structure of GroEL at 2.8 Å. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Roseman AM, Hunter AS, Wood SP, Burston SG, Ranson NA, Clarke AR, Saibil HR. Location of a folding protein and shape changes in GroEL-GroES complexes imaged by cryo-electron microscopy. Nature. 1994;371:261–264. doi: 10.1038/371261a0. [DOI] [PubMed] [Google Scholar]
- 6.Weissman JS, Hohl CM, Kovalenko O, Chen S, Braig K, Saibil HR, Fenton WA, Horwich AL. Mechanism of GroEL action: Productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83:577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 7.Hunt JF, Weaver AJ, Landry SJ, Gierasch L, Deisenhofer J. The crystal structure of the GroES co-chaperonin at 2.8 Å resolution. Nature. 1996;379:37–45. doi: 10.1038/379037a0. [DOI] [PubMed] [Google Scholar]
- 8.Weissman JS, Rye HS, Fenton WA, Beechem JM, Horwich AL. Characterization of the active intermediate of a GroEL-GroES-mediated folding reaction. Cell. 1996;84:481–490. doi: 10.1016/s0092-8674(00)81293-3. [DOI] [PubMed] [Google Scholar]
- 9.Mayhew M, da Silva ACR, Martin J, Erdjument-Bromage H, Tempst P, Hartl FU. Protein folding in the central cavity of the GroEL-GroES chaperonin complex. Nature. 1996;379:420–426. doi: 10.1038/379420a0. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Horwich AL, Sigler PB. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature. 1997;388:741–751. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- 11.Rye HS, Roseman AM, Furtak K, Fenton WA, Saibil HR, Horwich AL. GroEL-GroES cycling: ATP and non-native polypeptide direct alternation of folding-active rings. Cell. 1999;97:325–338. doi: 10.1016/s0092-8674(00)80742-4. [DOI] [PubMed] [Google Scholar]
- 12.Rye HS, Burston SG, Fenton WA, Beechem JM, Xu Z, Sigler PB, Horwich AL. Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL. Nature. 1997;388:792–798. doi: 10.1038/42047. [DOI] [PubMed] [Google Scholar]
- 13.Motojima F, Chaudhry C, Fenton WA, Farr GW, Horwich AL. Substrate polypeptide presents a load on the apical domains of the chaperonin GroEL. Proc Natl Acad Sci USA. 2004;101:15005–15012. doi: 10.1073/pnas.0406132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S, Chakraborty K, Müller BK, Astola N, Tang YC, Lamb DC, Hayer-Hartl M, Hartl FU. Monitoring protein conformation along the pathway of chaperonin-assisted folding. Cell. 2008;133:142–153. doi: 10.1016/j.cell.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Saibil HR, Zheng D, Roseman AM, Hunter AS, Watson GM, Chen S, Auf Der Mauer A, O’Hara BP, Wood SP, Mann NH, Barnett LK, Ellis RJ. ATP induces large quaternary rearrangements in a cage-like chaperonin structure. Curr Biol. 1993;3:265–273. doi: 10.1016/0960-9822(93)90176-o. [DOI] [PubMed] [Google Scholar]
- 16.Goloubinoff P, Christeller JT, Gatenby AA, Lorimer GH. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfolded state depends on two chaperonin proteins and MgATP. Nature. 1989;342:884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhry C, Farr GW, Todd MJ, Rye HS, Brunger AT, Adams PD, Horwich AL, Sigler PB. Role of the γ-phosphate of ATP in triggering protein folding by GroEL-GroES: Function, structure, and energetics. EMBO J. 2003;22:4877–4887. doi: 10.1093/emboj/cdg477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranson NA, Burston SG, Clarke AR. Binding, encapsulation and ejection: substrate dynamics during a chaperonin-assisted folding reaction. J Mol Biol. 1997;266:656–664. doi: 10.1006/jmbi.1996.0815. [DOI] [PubMed] [Google Scholar]
- 19.Weissman JS, Kashi Y, Fenton WA, Horwich AL. GroEL-mediated protein folding proceeds by multiple rounds of release and rebinding of non-native forms. Cell. 1994;78:693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]
- 20.Park ES, Fenton WA, Horwich AL. Disulfide bond formation as probe of topology during folding in the GroEL-GroES chamber: Correct formation of long-range bonds and editing of incorrect short-range ones. Proc Natl Acad Sci USA. 2007;104:2145–2150. doi: 10.1073/pnas.0610989104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinker A, Pfeifer G, Kerner MJ, Naylor DJ, Hartl FU, Hayer-Hartl M. Dual function of protein confinement in chaperonin-assisted protein folding. Cell. 2001;107:223–233. doi: 10.1016/s0092-8674(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 22.Farr GW, Fenton WA, Horwich AL. Perturbed ATPase activity and not “close confinement” of substrate in the cis cavity affects rates of folding by tail-multiplied GroEL. Proc Natl Acad Sci USA. 2007;104:5342–5347. doi: 10.1073/pnas.0700820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y-C, Chang H-C, Chakraborty K, Hartl FU, Hayer-Hartl M. Essential role of the chaperonin folding compartment in vivo. EMBO J. 2008;27:1458–1468. doi: 10.1038/emboj.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang YC, Chang HC, Roeben A, Wischnewski D, Wischnewski N, Kerner MJ, Hartl FU, Hayer-Hartl M. Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein. Cell. 2006;125:903–914. doi: 10.1016/j.cell.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Danziger O, Shimon L, Horovitz A. Glu257 in GroEL is a sensor involved in coupling polypeptide substrate binding to stimulation of ATP hydrolysis. Protein Sci. 2006;15:1270–1276. doi: 10.1110/ps.062100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JD, Herman C, Tipton KA, Gross CA, Weissman JS. Directed evolution of substrate-optimized GroEL/S chaperonins. Cell. 2002;111:1027–1039. doi: 10.1016/s0092-8674(02)01198-4. [DOI] [PubMed] [Google Scholar]
- 27.Kovalenko O, Yifrach O, Horovitz A. Residue Lysine-34 in GroES modulates allosteric transitions in GroEL. Biochemistry. 1994;33:14974–14978. doi: 10.1021/bi00254a004. [DOI] [PubMed] [Google Scholar]
- 28.Fenton WA, Kashi Y, Furtak K, Horwich AL. Residues in chaperonin GroEL required for polypeptide binding and release. Nature. 1994;371:614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- 29.Viitanen PV, Lubben TH, Reed J, Goloubinoff P, O’Keefe DP, Lorimer GH. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990;29:5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- 30.Martin J, Langer T, Boteva R, Schramel A, Horwich AL, Hartl FU. Chaperonin-mediated protein folding occurs at the surface of GroEL via a molten globule-like intermediate. Nature. 1991;352:36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- 31.Apetri AC, Horwich AL. Chaperonin chamber accelerates protein folding through passive action of preventing aggregation. Proc Natl Acad Sci USA. 2008;105:17351–17355. doi: 10.1073/pnas.0809794105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun SY, Strobel S, Bassford P, Randall LL. Folding of maltose-binding protein. Evidence for the identity of the rate-determining step in vivo and in vitro. J Biol Chem. 1993;268:20855–20862. [PubMed] [Google Scholar]
- 33.Horst R, Fenton WA, Englander SW, Wüthrich K, Horwich AL. Folding trajectories of human dihydrofolate reductase inside the GroEL-GroES chaperonin cavity and free in solution. Proc Natl Acad Sci USA. 2007;104:20788–20792. doi: 10.1073/pnas.0710042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian G, Vainberg IE, Tap WD, Lewis SA, Cowan NJ. Specificity in chaperonin-mediated protein folding. Nature. 1995;375:250–253. doi: 10.1038/375250a0. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Thomas JO, Chow RL, Lee GH, Cowan NJ. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992;69:1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- 36.Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- 37.Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: Physiology and mechanism. Annu Rev Cell Dev Biol. 2007;23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555. [DOI] [PubMed] [Google Scholar]
- 38.Rivenzon-Segal D, Wolf SG, Shimon L, Willison KR, Horovitz A. Sequential ATP-induced allosteric transitions of the cytoplasmic chaperonin containing TCP-1 revealed by EM analysis. Nat Struct Mol Biol. 2005;12:233–237. doi: 10.1038/nsmb901. [DOI] [PubMed] [Google Scholar]
- 39.Georgopoulos CP, Hendrix RW, Kaiser AD, Wood WB. Role of the host cell in bacteriophage morphogenesis: Effects of a bacterial mutation on T4 head assembly. Nature New Biology. 1972;239:38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- 40.Van der Vies SM, Gatenby AA, Georgopoulos C. Bacteriophage T4 encodes a co-chaperonin that can substitute for Escherichia coli GroES in protein folding. Nature. 1994;368:654–656. doi: 10.1038/368654a0. [DOI] [PubMed] [Google Scholar]
- 41.Hunt JF, van der Vies SM, Henry L, Deisenhofer J. Structural adaptations in the specialized bacteriophage T4 co-chaperonin Gp31 expand the size of the Anfinsen cage. Cell. 1997;90:361–371. doi: 10.1016/s0092-8674(00)80343-8. [DOI] [PubMed] [Google Scholar]
- 42.Bakkes PJ, Faber BW, van Heerikhuizen H, van der Vies SM. The T4-encoded cochaperonin, gp31, has unique properties that explain its requirement for the folding of the T4 major capsid protein. Proc Natl Acad Sci USA. 2005;102:8144–8149. doi: 10.1073/pnas.0500048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clare DK, Bakkes PJ, van Heerikhuizen H, van der Vies SM, Saibil HR. Chaperonin complex with a newly folded protein encapsulated in the folding chamber. Nature. 2009;457:107–110. doi: 10.1038/nature07479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keppel F, Rychner M, Georgopoulos C. Bacteriophage-encoded cochaperonins can substitute for Escherichia coli’s essential GroES protein. EMBO Rep. 2002;3:893–898. doi: 10.1093/embo-reports/kvf176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riek R, Fiaux J, Bertelsen EB, Horwich AL, Wüthrich K. Solution NMR techniques for very large molecular and supramolecular structures. J Am Chem Soc. 2002;124:12144–12153. doi: 10.1021/ja026763z. [DOI] [PubMed] [Google Scholar]
- 46.Kipnis Y, Papo N, Haran G, Horovitz A. Concerted ATP-induced allosteric transitions in GroEL facilitate release of protein substrate domains in an all-or-none manner. Proc Natl Acad Sci USA. 2007;104:3119–3124. doi: 10.1073/pnas.0700070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papo N, Kipnis Y, Haran G, Horovitz A. Concerted release of substrate domains from GroEL by ATP is demonstrated with FRET. J Mol Biol. 2008;380:717–725. doi: 10.1016/j.jmb.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno T, Taguchi H, Yoshida M, Funatsu T. GroEL mediates protein holding with a two successive timer mechanism. Mol Cell. 2004;14:423–434. doi: 10.1016/s1097-2765(04)00261-8. [DOI] [PubMed] [Google Scholar]
- 49.Ranson NA, Farr GW, Roseman AM, Gowen B, Fenton WA, Horwich AL, Saibil HR. ATP-bound states of GroEL captured by cryo-electron microscopy. Cell. 2001;107:869–879. doi: 10.1016/s0092-8674(01)00617-1. [DOI] [PubMed] [Google Scholar]