Abstract
Rationale
Dopamine (DA) receptor stimulation in the nucleus accumbens (NAc) plays an important role in regulating cocaine–seeking behavior. Adenosine receptors antagonize the effects of DA receptor stimulation on intracellular signaling, neuronal output and behavior.
Objectives
The goal of the present study is to determine the effects of adenosine A2A receptor stimulation on reinstatement of cocaine-seeking behavior in rats.
Methods
Rats were trained to lever press for cocaine in daily self-administration sessions on a fixed-ratio 1 schedule for 3 weeks. After one week of abstinence, lever pressing was extinguished in 6 daily extinction sessions. We subsequently assessed the effects of the adenosine A2A receptor agonist, CGS 21680, on cocaine-, quinpirole (D2 agonist)- and cue-induced reinstatement to cocaine seeking. We also assessed the effects of CGS 21680 on sucrose seeking in rats extinguished from sucrose self-administration.
Results
Pretreatment of CGS 21680 dose-dependently blunted cocaine-induced reinstatement (15 mg/kg, i.p.). Pretreatment with CGS 21680 (0.03 mg/kg, i.p.) also attenuated quinpirole- and cue-induced reinstatement. A minimally effective dose of CGS 21680 failed to alter cocaine-induced locomotor activity or sucrose seeking.
Conclusions
Stimulation of adenosine A2A receptors antagonizes reinstatement of cocaine seeking elicited by cocaine, DA D2-receptor stimulation and cocaine-conditioned cues. These findings suggest that adenosine A2A receptor stimulation may oppose DA D2 receptor signaling in the NAc that mediates cocaine relapse.
Keywords: A2A receptor, D2 receptor, self-administration, craving, relapse, reinstatement, reward, incentive motivation
Introduction
Relapse to drug seeking is induced by exposure to drug-associated cues and pharmacological stimuli that activate the mesolimbic dopamine (DA) system (Shalev et al. 2002). The mesolimbic DA system is composed of DA cells in the ventral tegmental area (VTA) that terminate in forebrain regions such as the nucleus accumbens (NAc). DA release in the NAc targets two major classes of DA receptors that are differentiated by their opposing effects on intracellular signaling cascades. DA D1 receptors increase adenylyl cyclase activity, while DA D2 receptors decrease activity of this enzyme (Lachowicz and Sibley 1997). These DA receptor subtypes are also distinguished by differential expression patterns on distinct subtypes of NAc neurons. Although there is evidence for co-localization in the same neurons (Aizman et al. 2000; Schwartz et al. 1998), D1 receptors exist primarily on neurons expressing substance P and dynorphin, whereas D2 receptors exist primarily on neurons expressing enkephalin. These two distinct populations of NAc neurons differ in their projection targets and reflect the direct and indirect striatal output pathways, respectively (Aubert et al. 2000; Lu et al. 1998; Steiner and Gerfen 1998).
Chronic cocaine self-administration increases behavioral responses mediated by DA D2 receptors. Thus, repeated cocaine administration produces cross-sensitization with DA D2 receptor agonists (Ujike et al. 1990) and animals with high self-regulated cocaine intake patterns display greater D2 receptor-induced locomotion (Edwards et al. 2007). In addition, reinstatement of cocaine seeking is elicited by systemic and intra-NAc stimulation of D2 receptors (Bachtell et al. 2005; De Vries et al. 1999; Dias et al. 2004; Khroyan et al. 2000; Schmidt et al. 2006; Schmidt and Pierce 2006; Self et al. 1996). Thus, amplification of D2-mediated behaviors following chronic cocaine administration may enhance relapse elicited by cocaine-conditioned cues and pharmacological stimuli by targeting D2 receptors (Cervo et al. 2003; Gal and Gyertyan 2006). Tempering enhancements in D2 receptor-mediated behaviors following cocaine administration may provide effective treatments for curbing relapse vulnerability.
Adenosine functions as a neuromodulator of dopamine neurotransmission and recent studies suggest that stimulation of adenosine A2A receptors oppose many behavioral effects of cocaine. Thus, stimulation of A2A receptors reduces both the development and expression of cocaine sensitization (Filip et al. 2006) and impairs the initiation of cocaine self-administration (Knapp et al. 2001). Adenosine A2A receptor antagonists, on the other hand, exacerbate cocaine sensitization (Filip et al. 2006) and enhance cocaine-evoked discriminative stimulus effects (Justinova et al. 2003). While the exact mechanisms of these A2A receptor effects are not known, they may involve reciprocal regulation of DA D2 receptors through receptor heteromerization and/or opposing intracellular signaling cascades (Fuxe et al. 2003).
Adenosine A2A receptors are primarily localized to striatal regions in the brain where they are co-expressed with DA D2 receptors on enkephalin-containing neurons (Dixon et al. 1996; Fink et al. 1992; Svenningsson et al. 1998). Interestingly, A2A and D2 receptors exert a number of effects that oppose one another on intracellular signaling cascades, cellular functioning and behavioral responses. Stimulation of A2A receptors decreases the affinity of D2 receptors for dopamine (Ferre et al. 1991b), counteracts D2 receptor-mediated signal transduction (Yang et al. 1995), opposes the effects of DA receptor stimulation on immediate early gene expression in the striatum (Morelli et al. 1994; Svenningsson et al. 1999a) and reverses D2-induced inhibition of GABA output to the pallidum (Ferre et al. 1993). Consequently, adenosine A2A receptor stimulation exerts behavioral effects that are functionally similar to DA receptor antagonists (Barraco et al. 1993; Brown et al. 1991; Heffner et al. 1989; Rimondini et al. 1997; Zarrindast et al. 1993).
Based on the aforementioned relationship between A2A and D2 receptors, stimulation of A2A receptors would decrease DA neurotransmission and consequently relapse behaviors mediated by D2 receptor signaling. In the present set of experiments, we tested the hypothesis that stimulation of adenosine A2A receptors will blunt cocaine seeking using an animal model of relapse. We trained animals to self-administer cocaine and tested the effects of adenosine A2A receptor stimulation on cocaine seeking following extinction training. The effects of adenosine A2A receptor stimulation were evaluated on cocaine seeking elicited by cocaine, the D2 receptor agonist, quinpirole, and cocaine-associated cues. We used the A2A agonist, CGS 21680, to stimulate A2A adenosine receptors since the specificity of CGS 21680 binding to A2A receptors in nucleus accumbens is abolished in adenosine A2A but not A1 receptor knock-out mice (Halldner et al. 2004), and is widely used as a ligand for stimulating striatal adenosine A2A receptors (Cunha et al. 1996; Jarvis et al. 1989). In addition, recent work has demonstrated that motor depression induced by the A2A agonist, CGS 21680, but not the A1 agonist, N6-cyclopentyladenosine, was specifically blocked by the A2A antagonist, MSX-3, and not an A1-specific antagonist (Karcz-Kubicha et al. 2003).
Materials and Methods
Animals and housing conditions
Male Sprague–Dawley rats initially weighing 275–325 g (Charles River Laboratories, Kingston, NY) were individually housed in wire cages with food and water available ad libitum. Experiments were conducted during the light cycle of a 12:12-h light:dark cycle (lights on at 0700 hours) in accordance with guidelines established by the National Institute of Health and the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Cocaine self-administration procedure
Self-administration and reinstatement testing were performed in operant conditioning chambers (Med-Associates, St. Albans, VT) equipped with two response levers and an infusion pump system as previously described (Edwards et al. 2007). Animals were initially trained to lever press for sucrose pellets to facilitate acquisition of cocaine self-administration. These rats were food-restricted to prevent weight gain, and trained to lever-press for sucrose pellets on a fixed ratio 1 (FR1) reinforcement schedule until acquisition criteria had been achieved (100 sucrose pellets in two consecutive sessions). After lever-press training, animals were fed ad libitum for at least 1 day prior to surgical implantation with a chronic intrajugular catheter as previously described (Self et al. 1998).
Following at least 5-7 days recovery from surgery, animals were allowed to self-administer intravenous cocaine (0.5 mg/kg/50 μl injection) on a fixed ratio 1 (FR1) reinforcement schedule in daily 4-hr sessions for 5–6 days/week. Cocaine injections were delivered over 2.5 sec concurrent with the illumination of a cue light above the active lever, and followed by an additional 12.5-s time out period (TO 15 sec) when the house light remained off and responding was without consequence. Inactive lever responses produced no consequence throughout testing.
Extinction/reinstatement testing
After a minimum of 15 cocaine self-administration sessions, animals remained in their home cages for 7 days of abstinence. On days 8-13 of abstinence, animals returned to the operant conditioning chambers for extinction training in the absence of cocaine reinforcement in 4-hr test sessions. Responses on the lever that previously delivered cocaine injections during self-administration (drug-paired lever) and on the inactive lever were recorded but had no programmed drug or cue delivery.
Cocaine-primed reinstatement
The effects of adenosine A2A receptor stimulation on cocaine-primed reinstatement was tested over repeated reinstatement sessions to allow for testing of several doses of the A2A agonist (0.01, 0.03, 0.1, and 0.3 mg/kg, i.p.). Each test session was initiated with 3 hrs of extinction conditions followed by a 1 hr reinstatement test period. A pretreatment of the A2A receptor agonist, CGS 21680 (vehicle, 0.01, 0.03, 0.1, 0.3 mg/kg, i.p.), was administered 5 min prior to a priming injection of cocaine (15 mg/kg, ip), which was followed by a 1-hr reinstatement test. Animals received a maximum of 4 treatments in a randomized order although different dose ranges were tested spanning the 5 doses. All animals were tested under the vehicle pretreatment/cocaine reinstatement condition to provide a baseline of cocaine-primed reinstatement. However, all animals did not receive all doses of CGS 21680 due to concerns of residual testing and weakening of reinstatement responding over repeated trials. Responses at both drug-paired and inactive levers were recorded but produced no cue or drug delivery during testing.
Cue-induced reinstatement
In a separate set of animals, we tested the effects of adenosine A2A receptor stimulation on reinstatement elicited by cocaine-associated cues. Cue-induced reinstatement of cocaine-seeking behavior was measured in a 4-hr reinstatement session consisting of 3 hrs of extinction conditions followed by a 1 hr cue-primed reinstatement test period. A pretreatment of vehicle or 0.03 mg/kg CGS 21680 was administered 5 min prior to the cue reinstatement test. This dose was used because it proved most effective in blunting cocaine-induced reinstatement, while having little sedative effects. The cue-induced reinstatement test was initiated with non-contingent (priming) presentation of the cocaine injection cues delivered every 2 min for the first 10 min. During the entire session, responding at the drug-paired lever resulted in response-contingent cue delivery (2.5 sec illumination of cue light and infusion pump, 15 sec termination of house light).
Quinpirole-induced reinstatement
In separate study groups, the effect of adenosine A2A receptor stimulation on dopamine D2 receptor-primed relapse behavior was assessed. Given the longer duration of quinpirole action relative to cocaine, priming injections of quinpirole (0, 0.1, 0.3, and 1.0 mg/kg, s.c.) were given before the final 2 hrs of the session immediately after 2 hrs of extinction conditions. A pretreatment of CGS 21680 (0.03 mg/kg, i.p.) was administered 5 min prior to quinpirole treatment. Quinpirole doses were administered in randomized order across test days. Responses at both levers were recorded, but resulted in no cue or cocaine delivery.
Sucrose reinstatement
Animals were trained to self-administer sucrose pellets on an FR1:TO 15 sec schedule as described above. After 15 daily sessions (100 pellets/session), animals remained in their home cages for 7 days of “abstinence”, and were then subjected to extinction training in six daily 4-hr sessions. Following extinction training, animals were tested for reinstatement of sucrose seeking. A pretreatment of CGS 21680 (0.03 mg/kg, i.p.) was administered 5 min prior to sucrose reinstatement testing. Reinstatement testing was initiated by non-contingent sucrose pellet delivery in a single 1 hr test immediately following 1-hr of extinction conditions. During the reinstatement phase, animals were presented with the non-contingent delivery of a sucrose pellet every two minutes for 1 hr (30 pellets/1 hr). Responding at both levers was recorded, but resulted in no cues or sucrose pellet delivery.
Locomotor Testing
Locomotor activity was recorded in darkened circular test chambers with a 12 cm wide runway, equipped with four pairs of photocells located at 90-degree intervals around the 1.95 m perimeter. All locomotor tests were performed during the light-phase of the light:dark cycle. Five days following cocaine self-administration and reinstatement procedures, animals were habituated to the locomotor testing apparatus for 2 hrs on the day prior to cocaine-induced locomotor activity testing. On the test day, animals were again habituated for 2 hrs, given a pretreatment of vehicle or CGS 21680 (0.03 mg/kg, i.p.) and administered a cocaine challenge (15 mg/kg, i.p.) 5 min later. Locomotor activity was assessed for 2 hrs.
Data analysis
Cocaine-induced reinstatement data (lever presses) were analyzed with a mixed design 2-factor ANOVA with lever (within) and CGS 21680/cocaine treatment (between) as the factors. Quinpirole-induced reinstatement data (lever presses) were analyzed with a 2-factor ANOVA with quinpirole and CGS pretreatment as the factors. Cue- and sucrose-induced reinstatement data (lever presses) were analyzed with a mixed design 2-factor ANOVA with the within factor, reinstatement (extinction vs. cue/sucrose), and the between factor, pretreatment. The effect of CGS 21680 pretreatment on cocaine-induced locomotor activity (beam breaks) was analyzed by an unpaired t-test. All interactive effects of the ANOVAs were followed by simple main effects analyses (one-way ANOVA) and post hoc tests (Bonferroni's comparisons, Dunnett's Test or t-test). Statistical significance was preset at p < 0.05.
Drugs
CGS 21680 [4-[2-[[6-Amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H -purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride] was purchased from Tocris Bioscience (Ellisville, MO). Quinpirole [(-)-Quinpirole hydrochloride] was purchased from Sigma-Aldrich (St. Louis, MO). Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Research Triangle Park, NC). All drugs were dissolved in sterile-filtered physiological saline.
Results
Adenosine A2A receptor stimulation dose-dependently blocks cocaine-induced reinstatement
Figure 1 illustrates that administration of the adenosine A2A agonist CGS 21680 dose-dependently reduced cocaine-induced drug seeking. A significant treatment × lever interaction (F5,50 = 5.47, p < 0.001) and significant main effects of treatment (F5,50 = 5.07, p < 0.001) and lever (F1,50 = 26.32, p < 0.001) were observed. Subsequent analysis of the interaction found that cocaine treatment significantly induced drug-paired lever pressing, which was dose-dependently decreased by pretreatment with CGS 21680 (F5,50 = 5.29, p < 0.001). A statistical trend for reduced inactive lever pressing following the treatments was observed (F5,50 = 2.25, p = 0.06).
Fig. 1.
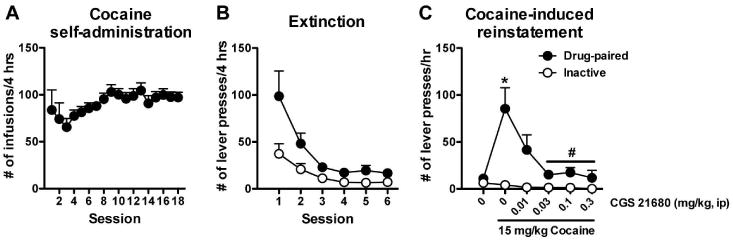
Administration of the adenosine A2A receptor agonist CGS 21680 dose-dependently blocked cocaine-induced reinstatement. A) Number of cocaine infusions in each 4-hour session during the cocaine self-administration phase. B) Extinction training was performed in six daily sessions one week following the last self-administration session. Responses on the previously drug-paired lever were reduced to levels comparable to inactive lever responses. C) Cocaine-induced reinstatement testing conducted across five days following extinction training. Each reinstatement session included an initial extinction phase (2 hrs) that preceded the reinstatement phase. The A2A agonist, CGS 21680, dose-dependently reduced cocaine-induced drug-paired lever responding. The numbers of animals in each treatment group is as follows: 0 CGS/saline = 7, 0 CGS/cocaine = 10, 0.01 CGS/cocaine = 10, 0.03 CGS/cocaine = 14, 0.1 CGS/cocaine = 11, 0.3 CGS/cocaine = 4. * significant from vehicle (p < 0.05, Bonferroni's posttest), # significant from 15 mg/kg cocaine with 0 CGS 21680 pretreatment (p < 0.05, Bonferroni's posttest)2A
Because systemic administration of CGS 21680 produces sedation and reduced sensitized locomotor activity to psychostimulants (Filip et al. 2006; Rimondini et al. 1997), we tested the effects of the minimally effective dose of CGS 21680 (0.03 mg/kg, i.p.) on cocaine-stimulated locomotor activity. These tests were performed in the same animals that had self-administered cocaine and were tested for cocaine-induced reinstatement. As is shown in Figure 2, pretreatment of CGS 21680 had no effect on cocaine-induced locomotion at the same dose (15 mg/kg) used for cocaine priming in reinstatement (t22 < 1, NS).
Fig. 2.
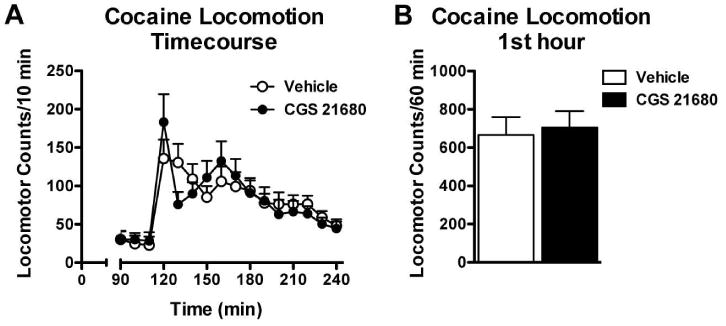
Cocaine-induced locomotor activity was unaltered by a pretreatment with CGS 21680. A) Time-course of locomotor activity illustrating the last 30 minutes of the habituation period (90-110 min) followed by the effects of 15 mg/kg cocaine (i.p.) with and without a pretreatment of 0.03 mg/kg CGS 21680 (i.p.). This dose was chosen since it was the lowest dose that was effective in reducing cocaine-induced reinstatement (Figure 1). B) Cocaine-induced locomotor activity over the first hour in animals pretreated with vehicle or 0.03 mg/kg CGS 21680 (i.p.). No significant changes in locomotor activity were observed, n = 12/group.
Adenosine A2A receptor stimulation blunts quinpirole-induced reinstatement
Systemic administration of DA D2 receptor agonists robustly stimulates reinstatement of cocaine seeking in extinguished animals. Therefore, we tested whether a pretreatment of CGS 21680 (0.03 mg/kg, i.p.) would attenuate quinpirole-induced reinstatement using the minimum dose effective at blocking cocaine priming. Figure 3 illustrates the dose-dependent increase in drug-paired lever pressing resulting from quinpirole administration, which was attenuated by a pretreatment with 0.03 mg/kg CGS 21680. Significant main effects of pretreatment (F1,44 = 8.44, p < 0.006) and quinpirole (F3,44 = 14.29, p < 0.001) were observed, however, the interaction was not significant (F3,44 = 1.68, p = 0.18). No effects of quinpirole or pretreatment were observed in inactive lever pressing (data not shown).
Fig. 3.
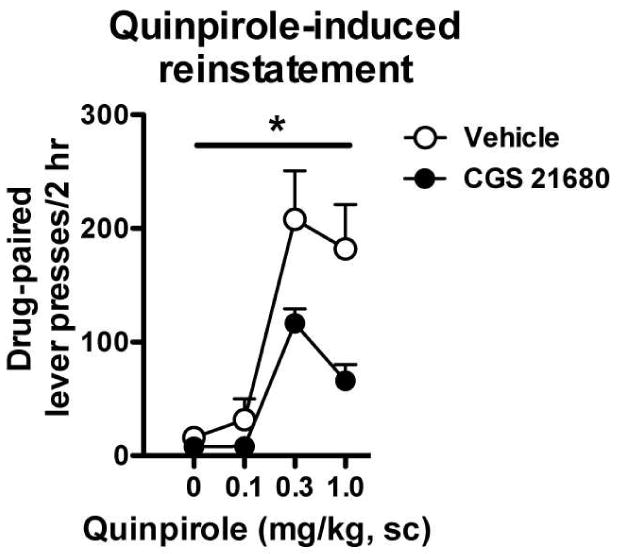
Administration of the adenosine A2A receptor agonist CGS 21680 blunted dopamine D2 receptor-induced reinstatement. Animals were trained to self-administer cocaine in 4-hour sessions over three weeks and extinguished following a week of abstinence. On the subsequent 5 days, animals were tested for D2 agonist (quinpirole)-induced reinstatement. As can be seen, pretreatment with 0.03 mg/kg CGS 21680 (i.p.) blunted quinpirole-induced reinstatement at the two highest doses. * significant main effect of the CGS 21680 pretreatment, n = 5-8/group
Adenosine A2A receptor stimulation reduces cue-induced reinstatement
Presentation of cocaine-associated cues is sufficient to elicit reinstatement of cocaine seeking in extinguished animals. Because this is mediated in part by DA transmission in the striatum, we tested whether a pretreatment of CGS 21680 (0.03 mg/kg, i.p.) would block cue-induced reinstatement. Figure 4 illustrates that CGS 21680 pretreatment significantly blunts cue-induced reinstatement. While both pretreatment groups displayed significant increases in drug-paired lever pressing during the cue presentations compared to the preceding hour of extinction (Cue: F1,15 = 40.61, p < 0.001), the CGS 21680 group responded at significantly lower levels (Pretreatment: F1,15 = 7.49, p < 0.02; Cue × Pretreatment: F1,15 = 8.28, p < 0.02) No effects of the cue presentation or CGS 21680 pretreatment were observed on inactive lever responding (data not shown).
Fig. 4.
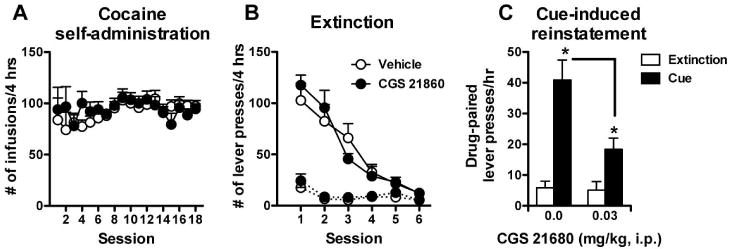
Administration of the adenosine A2A receptor agonist CGS 21680 blunted reinstatement induced by cocaine-associated cues. A) Number of cocaine infusions in each 4-hour session during the cocaine self-administration phase. B) Extinction training was performed in six daily sessions one week following the last self-administration session. Responses on the previously drug-paired lever (solid lines) were reduced to levels comparable to inactive lever responses (dotted lines). C) Cue-induced reinstatement testing was conducted in a 4-hr reinstatement session that included an initial extinction phase (3 hrs) that preceded the reinstatement phase (1 hr). Shown in the figure is the third hour of the extinction phase and the following hour of cue testing. The A 2A agonist, CGS 21680, significantly reduced cue-induced drug-paired lever responding. * significant from extinction (p < 0.05, Bonferroni posttest), Bar- significant from 0 CGS 21680 pretreatment (t15 = 3.12, p < 0.01), Vehicle N = 8; CGS 21680 N = 9
Adenosine A2A receptor stimulation has no effect on sucrose seeking
Finally, we incorporated a procedural control to account for any generalized performance effects of CGS 21680 (0.03 mg/kg, i.p.) on reinstatement using non-contingent delivery of sucrose pellets in animals trained to self-administer sucrose pellets. Figure 5 illustrates that a pretreatment of CGS 21680 had no effect on sucrose reinstatement (Pretreatment: F1,13 < 1, NS; Sucrose × Pretreatment: F1,13 < 1, NS), despite the significant levels of sucrose-induced lever pressing (Sucrose: F1,13 = 11.97, p < 0.005), which was comparable to cue-induced reinstatement following cocaine self-administration.
Fig. 5.
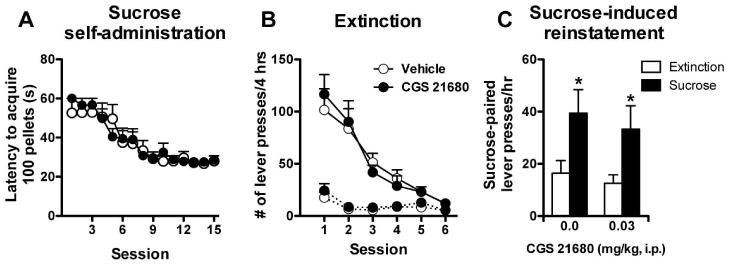
Administration of the adenosine A 2A receptor agonist CGS 21680 had no effect on sucrose seeking. A) Sucrose self-administration was conducted over three weeks. B) Extinction training was performed in six daily sessions one week following the last self-administration session. Responses on the previously sucrose-paired lever (solid line) were reduced to levels comparable to inactive lever responses (dotted line). C) Sucrose reinstatement testing was conducted in a 2-hr reinstatement session that included an initial extinction phase (1 hr) that preceded the reinstatement phase (1 hr). The A 2A agonist, CGS 21680, failed to alter sucrose seeking despite significant responding on the lever previously paired with sucrose delivery. * significant from extinction (p < 0.05, Dunnett's Test), Vehicle N = 7, CGS 21680 N = 8
Discussion
These findings demonstrate for the first time that pharmacological activation of adenosine A2A receptors attenuates cocaine-seeking behavior. We show that A2A receptor activation reduces cocaine seeking induced by pharmacological stimuli such as cocaine and quinpirole and also by discrete cocaine-associated cues. These findings agree with previous work demonstrating that A2A stimulation attenuated the development and expression of behavioral sensitization to cocaine and methamphetamine (Filip et al. 2006; Shimazoe et al. 2000), the expression of cocaine place conditioning (Poleszak and Malec 2002), and the initiation of cocaine self-administration (Knapp et al. 2001). Other complementary work utilizing pharmacological blockade of adenosine A2A receptors also supports an antagonistic effect of A2A receptors on cocaine-mediated behaviors. Thus, antagonists of A2A receptors enhanced the acute locomotor effects of cocaine, the development and the subsequent expression of cocaine sensitization (Filip et al. 2006). Previous studies also showed a reversal in intracranial self-stimulation current threshold impairments observed during cocaine withdrawal, further suggesting that A2A receptor antagonism alters behavioral indices of withdrawal observed during abstinence (Baldo et al. 1999; Filip et al. 2006)). Together, these findings suggest that adenosine A2A receptor stimulation oppose the effects of cocaine and cocaine-associated cues. Our findings that CGS 21680 completely abolished drug-paired lever pressing establish the potential beneficial effects of adenosine A2A receptor stimulation on cocaine-induced cocaine-seeking behavior, although cocaine seeking elicited by the D2 agonist, quinpirole or cocaine-associated cues was only partially attenuated.
Other work utilizing genetic deletion of the adenosine A2A receptor report conflicting evidence and paradoxically display behavioral effects similar to those utilizing pharmacological stimulation. Thus, mice lacking the A2A receptor display attenuated locomotor responses to cocaine, impaired development of amphetamine sensitization, and reductions in the reinforcing efficacy of self-administered cocaine (Chen et al. 2000; Chen et al. 2003; Soria et al. 2006). These findings may result from compensatory changes during development or may reflect the lack of neuroanatomical specificity of A2A receptor knockdown in neural circuits regulating these behaviors. Recent work supports the latter since extra-striatal (forebrain) knockdown of A2A receptors reduced psychostimulant-induced locomotion, while striatal-specific knockdown of A2A receptors enhanced psychostimulant-induced locomotion akin to pharmacological antagonism (Shen et al. 2008). These findings further suggest an inhibitory role for adenosine A2A receptors specifically in the striatum, however further study will be required to ascertain the site of action for reducing cocaine seeking.
Stimulation of the adenosine A2A receptors is known to activate dopamine D2- and enkephalin-containing neurons in the striatum that form the indirect pathway (Karcz-Kubicha et al. 2006). Thus, local stimulation of A2A receptors in the dorsal and ventral striatum enhances GABA input to the globus pallidus and ventral pallidum, respectively (Mingote et al. 2008; Ochi et al. 2000). It is plausible that A2A receptor stimulation antagonizes the heightened sensitivity of dopamine D2 receptors in the striatum following long-term cocaine administration. In this manner, A2A receptor stimulation acts similarly to a D2 receptor antagonist in blocking the functional inhibition of indirect striatal GABA output mediated by D2 receptors.
Not only are A2A and D2 receptors co-localized on the enkephalin-containing neurons as previously described, the receptors play an antagonistic and reciprocal role in modulating cellular function (Ferre 1997; Ferre et al. 1991a). The inhibitory role of adenosine A2A receptor stimulation on dopamine D2 receptors may reflect opposing intracellular signaling cascades mediated by A2A and D2 receptors acting independently, or may involve the formation of A2A/D2 heteromers. Stimulation of A2A receptors counteracts D2 receptor-mediated signal transduction (Yang et al. 1995) and opposes the effects of DA receptor stimulation on immediate early gene expression in the striatum (Morelli et al. 1994; Svenningsson et al. 1999a). Thus, A2A receptor-induced activation of stimulatory G proteins and increases in cAMP production would consequently increases neuron excitability and effectively offset D2 receptor effects mediated by inhibitory G proteins (Colwell and Levine 1995; Schiffmann et al. 2007; Svenningsson et al. 1999a; Tozzi et al. 2007). Some of the opposing effects also may be dictated by the formation of heteromeric receptor complexes between postsynaptic A2A and D2 receptors (Canals et al. 2003; Fuxe et al. 2003; Hillion et al. 2002). The formation of A2A/D2 receptor complexes provides inhibitory regulation over dopamine D2 receptor binding and inhibitory G-protein coupling (Ferre et al. 1991a; Fuxe et al. 1998; Hillion et al. 2002; Torvinen et al. 2005). The relative contribution of heteromerized and non-heteromerized A2A and D2 receptors to counteract D2 mediated signaling remains unclear, especially in the context of addiction.
Alternatively, it is possible that presynaptic A2A receptors located on glutamate terminals in striatum indiscriminately alter the striatal neuronal function since local injections of CGS 21680 have been shown to increase striatal glutamate release (Corsi et al. 1999; Rodrigues et al. 2005). However, previous work has demonstrated that stimulation of AMPA glutamate receptors in the NAc induces drug seeking (Cornish et al. 1999) while blockade of AMPA receptors attenuates cocaine- and cue-induced drug seeking (Backstrom and Hyytia 2007; Cornish et al. 1999). It therefore does not seem likely that presynaptic A2A receptor stimulation resulting in striatal glutamate release would mediate a reduction in cocaine seeking.
It is also possible that the effects of systemic CGS 21680 administration on the reinstatement behavior shown here are mediated by A2A receptors located outside of the striatum. While adenosine A2A receptors are most abundant in striatal regions, low to moderate levels of A2A receptors are found in other regions such as the medial prefrontal cortex and the amygdala (Svenningsson et al. 1999b). These two regions also receive dopaminergic innervation that is known to be involved in cocaine seeking (McFarland and Kalivas 2001), and A2A receptor stimulation within these structures may similarly modulate dopamine transmission. Stimulation of A2A receptors in the prefrontal cortex decreased sedation time and electroencephalographic activity, both measures of increased arousal (Van Dort et al. 2009), which were not observed in the present study with the most effective dose of CGS 21680. The effect of A2A receptor stimulation in the amygdala is unclear. Given the amygdala's involvement in cue-induced cocaine seeking, one may predict that stimulation of A2A receptors in the amygdala would blunt cue-induced cocaine seeking perhaps through similar antagonism of D2 receptors as found in the striatum. Future studies will be necessary to fully elucidate the involvement of A2A receptors in specific brain circuits regulating drug seeking.
In conclusion, our findings suggest an important antagonistic role for adenosine A2A receptor stimulation in mediating cocaine relapse behaviors. We demonstrate that stimulation of A2A receptors blunts cocaine seeking induced by pharmacological and conditioned stimuli in an animal model of relapse. These findings support the notion that this effect involves negative interaction between adenosine A2A receptors and relapse mediated by D2 receptor stimulation. Together, these findings suggest that enhancing the inhibitory regulation of dopamine D2 receptors may provide an effective pharmacological treatment strategy.
Acknowledgments
This work was supported by United States Public Health Services Grants DA 10460 (D.W.S.), DA 08227 (D.W.S.), DA 18481 (R.K.B.) and the Wesley Gilliland Professorship in Biomedical Research.
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nature Neuroscience. 2000;3:226–30. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. Journal of Comparative Neurology. 2000;418:22–32. [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Koob GF, Markou A. Role of adenosine A2 receptors in brain stimulation reward under baseline conditions and during cocaine withdrawal in rats. J Neurosci. 1999;19:11017–26. doi: 10.1523/JNEUROSCI.19-24-11017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraco RA, Martens KA, Parizon M, Normile HJ. Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull. 1993;31:397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Gill R, Evenden JL, Iversen SD, Richardson PJ. Striatal A2 receptor regulates apomorphine-induced turning in rats with unilateral dopamine denervation. Psychopharmacology (Berl) 1991;103:78–82. doi: 10.1007/BF02244078. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, Ferre S, Lluis C, Bouvier M, Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–9. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–9. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, Aloyo VJ, Fink JS, Schwarzschild MA. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Yu L, Martin AB, Xu K, Bastia E, Hackett E, Alberti I, Schwarzschild MA. Inactivation of adenosine A2A receptors selectively attenuates amphetamine-induced behavioral sensitization. Neuropsychopharmacology. 2003;28:1086–95. doi: 10.1038/sj.npp.1300152. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cyclic AMP-dependent mechanisms. J Neurosci. 1995;15:1704–13. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Corsi C, Melani A, Bianchi L, Pepeu G, Pedata F. Striatal A2A adenosine receptors differentially regulate spontaneous and K+-evoked glutamate release in vivo in young and aged rats. Neuroreport. 1999;10:687–91. doi: 10.1097/00001756-199903170-00005. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, Constantino MD, Sebastiao AM, Fredholm BB. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:261–71. doi: 10.1007/BF00168627. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Vanderschuren LJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 1999;143:254–60. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- Dias C, Lachize S, Boilet V, Huitelec E, Cador M. Differential effects of dopaminergic agents on locomotor sensitisation and on the reinstatement of cocaine-seeking and food-seeking behaviour. Psychopharmacology. 2004;175:105–115. doi: 10.1007/s00213-004-1839-1. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–8. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–66. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Ferre S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology (Berl) 1997;133:107–20. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferre S, Herrera-Marschitz M, Grabowska-Anden M, Ungerstedt U, Casas M, Anden NE. Postsynaptic dopamine/adenosine interaction: I. Adenosine analogues inhibit dopamine D2-mediated behaviour in short-term reserpinized mice. Eur J Pharmacol. 1991a;192:25–30. doi: 10.1016/0014-2999(91)90064-w. [DOI] [PubMed] [Google Scholar]
- Ferre S, O'Connor WT, Fuxe K, Ungerstedt U. The striopallidal neuron: a main locus for adenosine-dopamine interactions in the brain. J Neurosci. 1993;13:5402–6. doi: 10.1523/JNEUROSCI.13-12-05402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A. 1991b;88:7238–41. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegalinski E, Muller CE, Agnati L, Franco R, Roberts DC, Fuxe K. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 2006;1077:67–80. doi: 10.1016/j.brainres.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–95. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferre S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson's disease. Neurology. 2003;61:S19–23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Brain Res Rev. 1998;26:258–73. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Halldner L, Lopes LV, Dare E, Lindstrom K, Johansson B, Ledent C, Cunha RA, Fredholm BB. Binding of adenosine receptor ligands to brain of adenosine receptor knock-out mice: evidence that CGS 21680 binds to A1 receptors in hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:270–8. doi: 10.1007/s00210-004-0970-1. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Wiley JN, Williams AE, Bruns RF, Coughenour LL, Downs DA. Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology (Berl) 1989;98:31–7. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–7. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther. 1989;251:888–93. [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, Antoniou K, Solinas M, Pappas LA, Highkin JL, Hockemeyer J, Munzar P, Goldberg SR. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–86. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Muller CE, Fuxe K, Goldberg SR, Popoli P, Ferre S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–91. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Ferre S, Diaz-Ruiz O, Quiroz-Molina C, Goldberg SR, Hope BT, Morales M. Stimulation of adenosine receptors selectively activates gene expression in striatal enkephalinergic neurons. Neuropsychopharmacology. 2006;31:2173–9. doi: 10.1038/sj.npp.1301035. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: Effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Cottam N, Ciraulo DA, Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol Biochem Behav. 2001;68:797–803. doi: 10.1016/s0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Lachowicz JE, Sibley DR. Molecular characteristics of mammalian dopamine receptors. Pharmacology & Toxicology. 1997;81:105–13. doi: 10.1111/j.1600-0773.1997.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–80. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008;28:9037–46. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Pinna A, Di Chiara G. Adenosine A2 receptors interact negatively with dopamine D1 and D2 receptors in unilaterally 6-hydroxydopamine-lesioned rats. Eur J Pharmacol. 1994;251:21–5. doi: 10.1016/0014-2999(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Ochi M, Koga K, Kurokawa M, Kase H, Nakamura J, Kuwana Y. Systemic administration of adenosine A(2A) receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience. 2000;100:53–62. doi: 10.1016/s0306-4522(00)00250-5. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Malec D. Adenosine receptor ligands and cocaine in conditioned place preference (CPP) test in rats. Pol J Pharmacol. 2002;54:119–26. [PubMed] [Google Scholar]
- Rimondini R, Ferre S, Ogren SO, Fuxe K. Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology. 1997;17:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–41. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–92. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–28. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–61. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P. Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Research - Brain Research Reviews. 1998;26:236–42. doi: 10.1016/s0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova L, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–5. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazoe T, Yoshimatsu A, Kawashimo A, Watanabe S. Roles of adenosine A(1) and A(2A) receptors in the expression and development of methamphetamine-induced sensitization. Eur J Pharmacol. 2000;388:249–54. doi: 10.1016/s0014-2999(99)00899-7. [DOI] [PubMed] [Google Scholar]
- Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2006;31:978–87. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Experimental Brain Research. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Fourreau L, Bloch B, Fredholm BB, Gonon F, Le Moine C. Opposite tonic modulation of dopamine and adenosine on c-fos gene expression in striatopallidal neurons. Neuroscience. 1999a;89:827–37. doi: 10.1016/s0306-4522(98)00403-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Aubert I, Burbaud P, Fredholm BB, Bloch B. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. J Comp Neurol. 1998;399:229–40. doi: 10.1002/(sici)1096-9861(19980921)399:2<229::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999b;59:355–96. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Torvinen M, Torri C, Tombesi A, Marcellino D, Watson S, Lluis C, Franco R, Fuxe K, Agnati LF. Trafficking of adenosine A2A and dopamine D2 receptors. J Mol Neurosci. 2005;25:191–200. doi: 10.1385/JMN:25:2:191. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Tscherter A, Belcastro V, Tantucci M, Costa C, Picconi B, Centonze D, Calabresi P, Borsini F. Interaction of A2A adenosine and D2 dopamine receptors modulates corticostriatal glutamatergic transmission. Neuropharmacology. 2007;53:783–9. doi: 10.1016/j.neuropharm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Ujike H, Akiyama K, Otsuki S. D-2 but not D-1 dopamine agonists produce augmented behavioral response in rats after subchronic treatment with methamphetamine or cocaine. Psychopharmacology (Berl) 1990;102:459–64. doi: 10.1007/BF02247125. [DOI] [PubMed] [Google Scholar]
- Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–81. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Dasgupta S, Lledo PM, Vincent JD, Fuxe K. Reduction of dopamine D2 receptor transduction by activation of adenosine A2a receptors in stably A2a/D2 (long-form) receptor co-transfected mouse fibroblast cell lines: studies on intracellular calcium levels. Neuroscience. 1995;68:729–36. doi: 10.1016/0306-4522(95)00171-e. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Modabber M, Sabetkasai M. Influences of different adenosine receptor subtypes on catalepsy in mice. Psychopharmacology (Berl) 1993;113:257–61. doi: 10.1007/BF02245707. [DOI] [PubMed] [Google Scholar]


