Abstract
The E. coli Min system contributes to spatial regulation of cytokinesis by preventing assembly of the Z ring away from midcell. MinC is a cell division inhibitor whose activity is spatially regulated by MinD and MinE. MinC has two functional domains of similar size, both of which have division inhibitory activity in the proper context. However, the molecular mechanism of the inhibitory action of either domain is not very clear. Here, we report that the septal localization and division inhibitory activity of MinCC/MinD requires the conserved C-terminal tail of FtsZ. This tail also mediates interaction with two essential division proteins, ZipA and FtsA, to link FtsZ polymers to the membrane. Overproduction of MinCC/MinD displaces FtsA from the Z ring and eventually disrupts the Z ring, probably because it also displaces ZipA. These results support a model for the division inhibitory action of MinC/MinD. MinC/MinD binds to ZipA and FtsA decorated FtsZ polymers located at the membrane through the MinCC/MinD-FtsZ interaction. This binding displaces FtsA and/or ZipA, and more importantly, positions MinCN near the FtsZ polymers making it a more effective inhibitor.
Introduction
Cell division in E.coli is mediated by a cytokinetic ring, formed by the ordered assembly of at least a dozen division proteins (Lutkenhaus, 2007). The assembly of this ring is initiated by the formation of a membrane-associated structure called the Z ring (Bi & Lutkenhaus, 1991), which consists of FtsZ polymers attached to the membrane by ZipA and FtsA, two essential membrane-associated division proteins (Pichoff & Lutkenhaus, 2002, Lutkenhaus, 2007, Hale & de Boer, 1997, Pichoff & Lutkenhaus, 2005). ZapA and ZapB are also part of the Z ring, but are not essential (Gueiros-Filho & Losick, 2002, Ebersbach et al., 2008). The Z ring functions as a scaffold for the recruitment of all other cell division proteins to make a mature cytokinetic ring capable of carrying out cytokinesis (Pichoff & Lutkenhaus, 2002, Dajkovic et al., 2008a).
Both FtsA and ZipA bind to the conserved, C-terminal tail of FtsZ, which consists of approximately 15 amino acids at the extreme C-terminus (Haney et al., 2001, Huang et al., 1996, Hale & de Boer, 1997, Hale & de Boer, 1999, Liu et al., 1999, Hale et al., 2000, Ma & Margolin, 1999, Wang et al., 1997). Although a Z ring can form with the aid of just ZipA or FtsA, such a ring is nonfunctional, as other division proteins are not recruited unless both are present (Pichoff & Lutkenhaus, 2002, Hale & de Boer, 1999).
The Z ring must be accurately placed at the middle of the cell to ensure the production of equally-sized progeny cells. There are two known negative regulatory systems involved in the spatial regulation of the Z ring positioning (Rothfield et al., 2005, Lutkenhaus, 2007). One system, called Noc (nucleoid occlusion), inhibits Z ring formation over the nucleoid. This activity restricts Z ring formation to the poles and mid-cell, which are not occupied by the nucleoid (Mulder & Woldringh, 1989, Yu & Margolin, 1999). SlmA in E. coli contributes to nucleoid occlusion by interacting with DNA and FtsZ directly but a detailed mechanism is not clear (Bernhardt & de Boer, 2005). The other system, called Min, blocks Z ring formation at the cell poles (de Boer et al., 1989). It consists of three components (MinC, MinD and MinE) and has been extensively studied (de Boer et al., 1989), however, some aspects of its function are still not known. The combination of Min and Noc restricts Z ring formation to the middle of the cell.
The effector of the Min system is MinC, which blocks cell division by preventing Z ring formation (de Boer et al., 1989, Hu et al., 1999, de Boer et al., 1990, Bi & Lutkenhaus, 1993). MinC requires MinD for full activity; in part, because MinD recruits MinC to the membrane (Hu & Lutkenhaus, 2001, Hu et al., 2003, Lackner et al., 2003). MinD is a membrane-associated ATPase which plays a central role in the Min system. When bound to ATP, MinD dimerizes and binds to the membrane (Lackner et al., 2003, Hu et al., 2003). The subsequent recruitment of MinC leads to a cell division inhibitory complex (MinC/MinD) that is evenly distributed on the membrane. The activity of the MinC/MinD complex is spatially regulated by MinE, which restricts the MinC/MinD complex to the poles of the cell (de Boer et al., 1989). MinE does this by stimulating the pole to pole oscillation of MinC/MinD through its ability to stimulate the ATPase activity of MinD and thus, the release of MinD from the membrane (Raskin & de Boer, 1999b, Raskin & de Boer, 1999a, Fu et al., 2001, Hale et al., 2001, Hu & Lutkenhaus, 2001, Hu et al., 2002, Hu & Lutkenhaus, 1999, Shih et al., 2003). Such dynamic behavior of the Min proteins results in a time-averaged concentration of the MinC/MinD division inhibitor that is highest at cell poles and lowest at mid-cell where the Z ring forms (Meinhardt & de Boer, 2001).
Structural, sequence and functional analyses of MinC reveal that it has two domains of approximately equal size (Cordell et al., 2001, Hu & Lutkenhaus, 2000). Both domains are essential for MinC to spatially regulate division since mutations mapping in either domain lead to minicell formation. The min operon on a single copy plasmid containing the minC-G10D (located in the N-terminal domain (MinC1–115 [MinCN])) or minC-R172A mutation (located in the C-terminal domain (MinC116–231 [MinCC])) has lost the ability to complement a min deletion strain (unpublished data, Wissel and Lutkenhaus and (Zhou & Lutkenhaus, 2005).
The two domains of MinC have been separated to determine the basis for their activity. Overproduction of a MalE-MinCN fusion blocks Z ring formation in vivo and the purified protein antagonizes FtsZ polymer assembly in vitro without affecting the GTPase activity of FtsZ (Hu & Lutkenhaus, 2000). The C-terminal domain of MinC mediates homodimerization and interaction with MinD. Overproduction of MinCC alone does not affect cell division, but it has inhibitory activity in the presence of MinD (Shiomi & Margolin, 2007). In vitro MinCC also limits the bundling of FtsZ filaments (Dajkovic et al., 2008a).
At low expression levels, GFP-MinCC/MinD localizes to the Z ring without disrupting it (Johnson et al., 2002). This localization is dependent upon FtsZ but not other early division proteins such as FtsA, ZipA and ZapA (Johnson et al., 2002, Johnson et al., 2004, Zhou & Lutkenhaus, 2005), suggesting that MinCC/MinD interacts with FtsZ directly. An interaction between FtsZ and MinCC/MinD was observed in an in vitro assay (Dajkovic et al., 2008a), which strongly supports this idea. In this paper we further investigate the mechanism by which MinCC/MinD antagonizes Z ring formation. We isolated FtsZ mutants that are resistant to MinCC/MinD and then used these mutants to study the MinCC/MinD-FtsZ interaction and the basis of the toxicity associated with MinCC/MinD.
Results
Isolation of FtsZ mutants resistant to MinCC/MinD
Overproduction of MinCC/MinD disrupts the Z ring and causes filamentation and therefore lethality (Shiomi & Margolin, 2007). There are several lines of evidence suggesting that MinCC/MinD binds FtsZ (Dajkovic et al., 2008a, Johnson et al., 2004, Johnson et al., 2002). To further study the inhibitory mechanism of MinCC/MinD, we exploited the above phenotype in a screen for FtsZ mutants that are resistant to MinCC/MinD. To this end, we performed PCR random mutagenesis over the coding region of ftsZ and screened for mutants that still support division and can suppress the toxicity of MinCC/MinD overexpression.
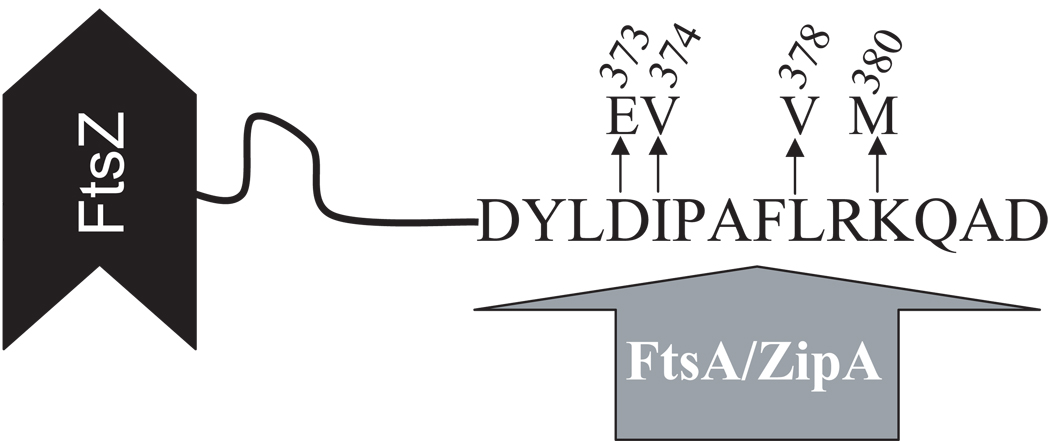
Using the strategy described in Experimental Procedures, we isolated four FtsZ mutants (each was obtained multiple times) which are resistant to MinCC/MinD. Each of these mutants contained a single mutation in ftsZ – either D373E, I374V, L378V or K380M (Fig. 1 and Fig. S1). Interestingly, each of these mutations result in an amino acid substitution in the C-terminal conserved tail of FtsZ that is also involved in interaction with the essential division proteins FtsA and ZipA. All four mutations, when introduced into pBANG112, do not affect its ability to complement the ftsZ depletion strain (S7 [ftsZo recA∷Tn10 min∷kan]/pKD3C (ftsZ+)) at 42°C or 37°C, although the presence of the ftsZ-L378V mutation causes the cells to grow a little slower than the others (data not shown). Since these mutations do not affect complementation of the ftsZ depletion strain, they must not disrupt the essential activities of FtsZ required for cell division (see below). Among the four mutants, FtsZ-I374V afforded the greatest resistance to MinCC/MinD (Fig. S1 and data not shown) and was therefore chosen for subsequent studies. It is likely that the resistance to MinCC/MinD afforded by all four mutations is due to the same mechanism since they map to the same small region of ftsZ.
Fig. 1.
Diagram of FtsZ. A cartoon of FtsZ showing the sequence of the conserved C-terminal tail involved in binding ZipA and FtsA and the location of the amino acid substitutions due to the mutations isolated in this study.
Characterization of FtsZ-I374V mutant
E.coli cells are sensitive to the FtsZ protein level since they have different morphologies with different ftsZ expression levels. To get a better idea about the effect of this mutation (I374V) on FtsZ function we used the lambda RED recombineering system (Datsenko & Wanner, 2000) to put the ftsZ-I374V mutation to the chromosome at its native locus. The resultant strain (BSZ374 [ftsZ-I374V]) does not show any significant difference to the control strain (S3 [ftsZ-WT]) in terms of growth rate and morphology. The only effect of the mutation was observed when the entire min locus was deleted from these strains to give BSM374 (BSZ374 min∷kan) and S4 (S3 min∷kan). BSM374 grew similarly to S4 at 30°C and 37°C but failed to form isolated colonies at room temperature (20 °C). S4 grew at room temperature but the cells were filamentous.
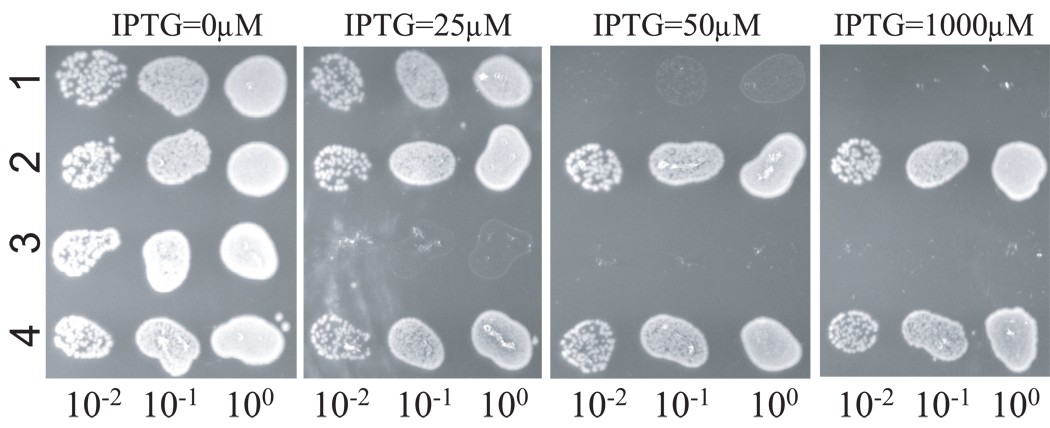
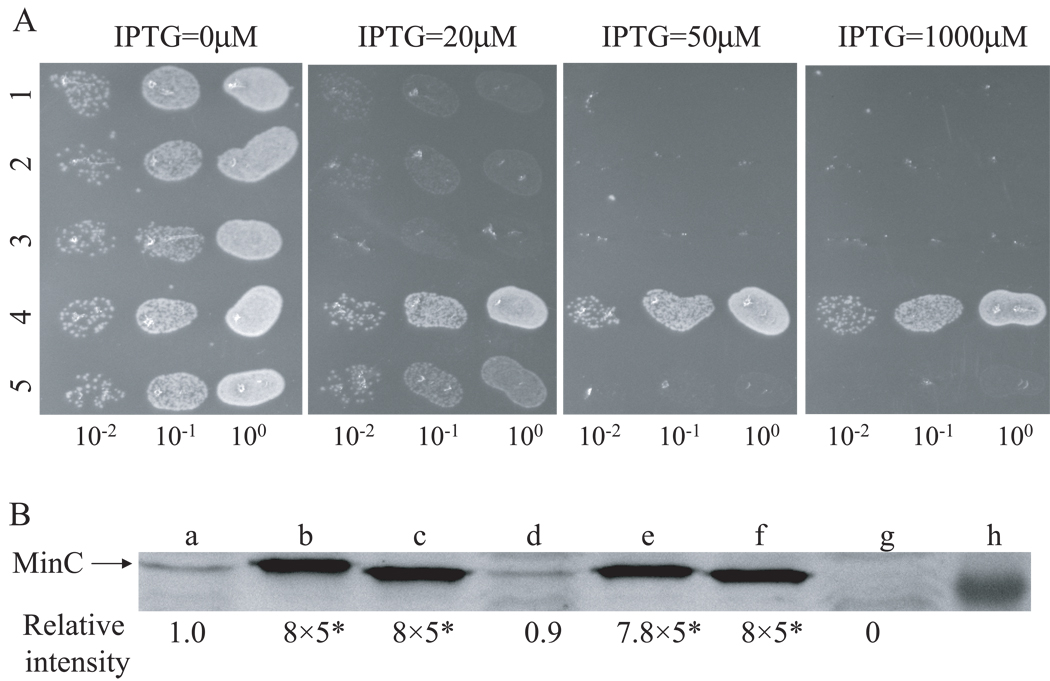
BSM374 (ftsZ-I374V, min∷kan) is resistant to MinCC/MinD and also displays resistance to MinC/MinD. As shown in the spot test in Fig. 2, the control strain S4 (min∷kan) containing plasmids expressing MinC/MinD (pBANG59/Ptac∷minCD) or MinCC/MinD (pBANG75/Plac∷minCCD)under control of IPTG-inducible promoters did not grow at or above 50 µM IPTG (Fig. 2, row 1) and 25 µM IPTG (Fig. 2, row 3) respectively. Even though pBANG75 requires less IPTG to prevent the growth of the S4 strain, it is a higher copy number plasmid than pBANG59 (around 10 for pBANG75 and 1–2 for pBANG59) and has a stronger ribosome binding site for MinCC translation (compared to MinC). As will be shown later, the minimal amount of protein required to prevent the growth of this strain is actually higher for MinCC/MinD than for MinC/MinD. In contrast to S4 containing these plasmids, BSM374/pBANG59 (ftsZ-I374V, min∷kan/Ptac∷minCD) and BSM374/pBANG75 (ftsZ-I374V, min∷kan/Plac∷minCCD) could grow with IPTG as high as 1000 µM (Fig 2, rows 2 and 4) and the cells were not filamentous. Since FtsZ-I374V confers resistance to MinC/MinD in addition to MinCC/MinD, it is surprising to note that the BSZ374 cells have WT morphology and produce very few minicells. We expected it might produce minicells at a frequency comparable to a min null strain since FtsZ-I374V displays some resistance to MinC/MinD. Deletion of the min locus in BSZ374 did result in a minicell phenotype indicating that FtsZ-I374V could produce polar rings and lead to polar divisions when Min was absent. These observations suggest that FtsZ-I374V still responds to MinC/MinD to some extent and the polar Z rings made from FtsZ-I374V are still susceptible to MinC/MinD (discussed later).
Fig. 2.
FtsZ-I374V is resistant to both MinCC/MinD and MinC/MinD. One colony of each strain was resuspended in 900 µl of LB medium and serially diluted by 10. Then 3µl culture from each dilution was spotted on plates (with Spc) with or without IPTG (as indicated) and incubated overnight at 37°C. 1, S4/pBANG59 [ftsZ-WT, min∷kan/Ptac∷minCD]; 2, BSM374/pBANG59 [ftsZ-I374V, min∷kan/Ptac∷minCD]; 3, S4/pBANG75 [ftsZ-WT, min∷kan/Plac∷minCCD]; 4, BSM374/pBANG75 [ftsZ-I374V, min∷kan/Plac∷minCCD].
To rule out the possibility that the MinC/MinD resistance of the BSZ374 (ftsZ-I374V) is due to an increased steady state protein level or reduced GTPase of the mutant protein, we checked the SulA sensitivity of this strain. SulA is another cell division inhibitor (Bi & Lutkenhaus, 1993) and it is well documented that increased FtsZ or FtsZ mutants with decreased GTPase activity confer resistance to SulA (Dai et al., 1994, Dajkovic et al., 2008b, Lutkenhaus et al., 1986). A careful spot test revealed that the SulA sensitivity of the BSZ374 (ftsZ-I374V) strain was identical to that of the WT strain S3 (data not shown). This indicates that the protein level and GTPase of this mutant FtsZ are not significantly affected by the mutation.
Interaction of FtsZ I374V with FtsA and ZipA
Previously, a yeast-two-hybrid assay showed that the conserved extreme C-terminus of FtsZ containing the I374 residue was involved in the FtsZ-FtsA and FtsZ-ZipA interactions (Haney et al., 2001). Here, we asked whether the ftsZ-I374V mutation affected these interactions. Using the yeast-two-hybrid system (YTH) to look at these interactions, we found that FtsZ-I374V interacted with ZipA similarly to WT FtsZ but failed to interact with FtsA (Table S1). The loss of the interaction between FtsZ-I374V and FtsA in this assay is somewhat surprising since FtsZ-I374V can fully complement the ftsZ depletion strain and support cell division. We therefore examined the effect of the ftsZ-I374V mutation on the recruitment of FtsA to the septum by immunofluorescent microscopy.
Immuno-staining of cells from exponentially growing cultures revealed that Z rings were present at similar frequencies in BSZ374 (ftsZ-I374V) and the wild type strain S3 (>80% of cells had a Z ring at midcell; no polar rings were observed). FtsA and ZipA were both efficiently recruited to the Z ring in BSZ374 (ftsZ-I374V) since FtsA rings and ZipA rings were observed at similar frequencies (> 80%) to Z rings (Table S2). The ability of ZipA and FtsA to localize to Z rings in the FtsZ-I374V mutant strain was also investigated by using green fluorescent protein (GFP) fusions as reported previously (Pichoff & Lutkenhaus, 2001, Pichoff & Lutkenhaus, 2007). Although these fusions cause filamentation, at appropriate induction levels both ZipA-GFP and GFP-FtsA formed ring structures in FtsZ-I374V cells that were similar to those in WT cells (Fig S2), indicating that both of these fusions localized to the Z ring. The results of the FtsA localization appear inconsistent with the YTH result. It is possible, however, that there is an interaction between FtsZ-I374V and FtsA that is sufficient to recruit FtsA to the Z ring even though it can not be detected by YTH.
FtsZ-I374V is unable to recruit MinCC/MinD in vivo
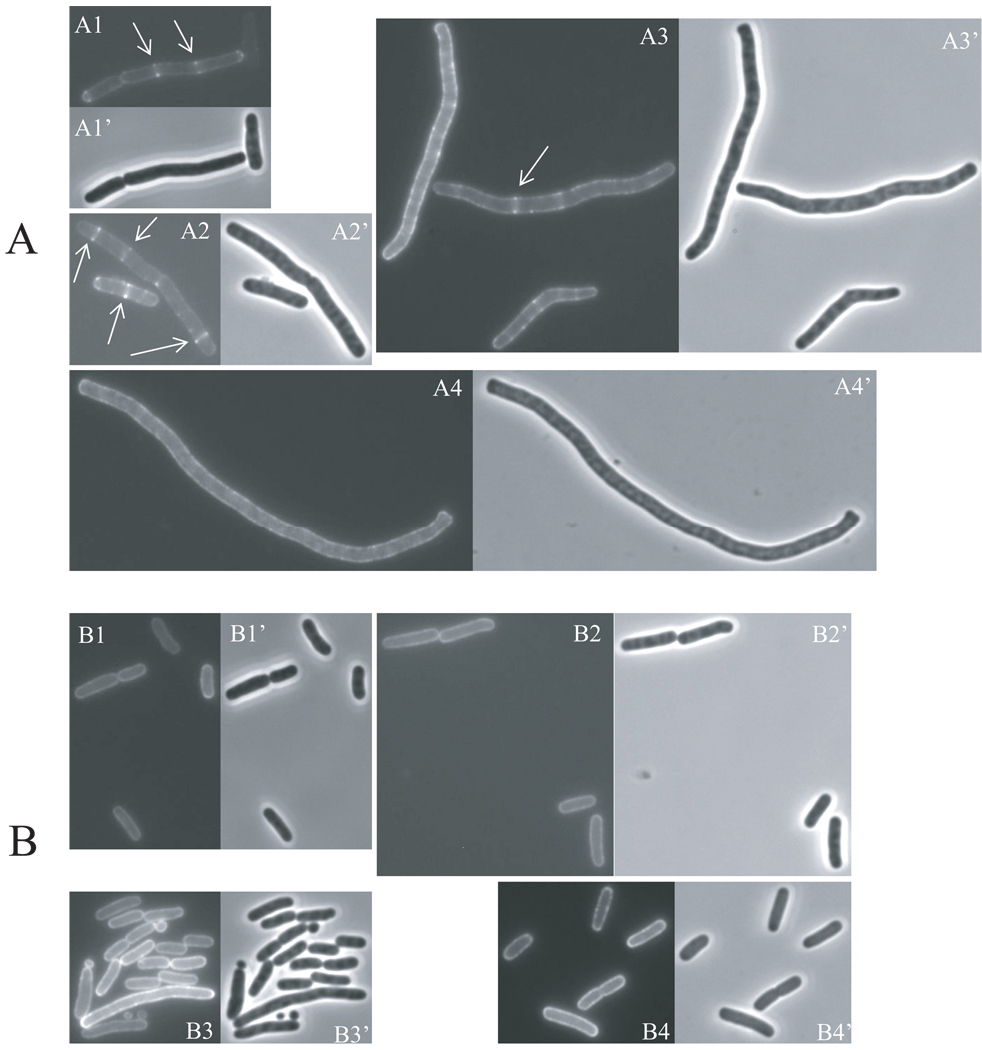
MinCC/MinD has been shown to localize to the Z ring as revealed by GFP-tagging of MinCC (Johnson et al., 2002, Zhou & Lutkenhaus, 2005). Several lines of evidence suggest that MinCC/MinD interacts with FtsZ directly to achieve this localization (Johnson et al., 2002, Johnson et al., 2004, Dajkovic et al., 2008a). Our results are consistent with this idea since we isolated FtsZ mutants that are resistant to MinCC/MinD. We speculated that the MinCC/MinD resistance of these mutants is due to the loss of interaction with MinCC/MinD. To test this hypothesis, we first checked the ability of GFP-MinCC/MinD to localize to the Z ring. We introduced a plasmid (pHJZ109) expressing GFP-MinCC/MinD under an IPTG-inducible promoter control into FtsZ-WT and FtsZ-I374V strains [the GFP fusion used here is distinct from the GFP-MinC122–231 construct described in (Shiomi & Margolin, 2007) since it does not interfere with the activity of MinCC]. GFP-MinCC/MinD expression was induced with IPTG and fluorescence was monitored over time. As shown in Fig. 3, the localization of GFP-MinCC/MinD to the Z ring in the S4 (ftsZ-WT, min∷kan) strain was already observed before induction (Fig. 3, A1), indicating the basal expression was sufficient to allow visualization of fluorescent rings. The GFP-MinCC/MinD rings became brighter (Fig. 3, A2) after 40 minutes of induction, however, as the induction time increased further, the GFP-MinCC/MinD rings gradually disappeared indicating that the Z rings were being disrupted. At 70 minutes after induction, cells were longer and only a small fraction still possessed GFP-MinCC/MinD rings (Fig. 3, A3); in most filaments the GFP-MinCC/MinD was primarily on the membrane but was also in spiral-like structures in many cells. At 100 minutes of induction, all cells became filamentous and no GFP-MinCC/MinD rings were observed (Fig. 3, A4). These results are consistent with previously reported observations that GFP-MinCC/MinD is able to localize to the Z ring but that high levels disrupt the Z ring causing filamentation (Johnson et al., 2002, Shiomi & Margolin, 2007). In contrast, when GFP-MinCC/MinD was induced in the FtsZ mutant strain BSM374 (ftsZ-I374V min∷kan), cells never became filamentous and GFP-MinCC/MinD never localized as a ring but was evenly distributed on the membrane at both early and late times after induction (Fig. 3B). These observations strongly indicate that FtsZ-I374V does not interact with MinCC/MinD.
Fig. 3.
GFP-MinCC/MinD does not localize to Z rings composed of FtsZ-I374V. Expression of GFP-MinCC/MinD was induced with IPTG in S4/pHJZ109 [ftsZ-WT, min∷kan/P∷gfp-minCCD] (A) and BSM374/pHJZ109 [ftsZ-I374V, min∷kan/Plac∷gfp-minCCD] (B) and samples at various times were subjected to fluorescent microscopy. Fluorescence micrographs (A1-A4, B1-B4) and the corresponding phase contrast images (A1’-A4’, B1’- B4’) showing the localization of GFP-MinCC/MinD over time in these two strains are presented. Cells were grown in the presence of 50 µM IPTG at 37°C for 0 min (A1, B1), 40 min (A2, B2), 70 min (A3, B3) and 100 min (A4, B4). Arrows in panel A indicate GFP-MinCC/MinD localized to rings. The contrast and brightness of these pictures are adjusted unequally for better viewing.
FtsZ-I374V does not bind MinCC/MinD in vitro
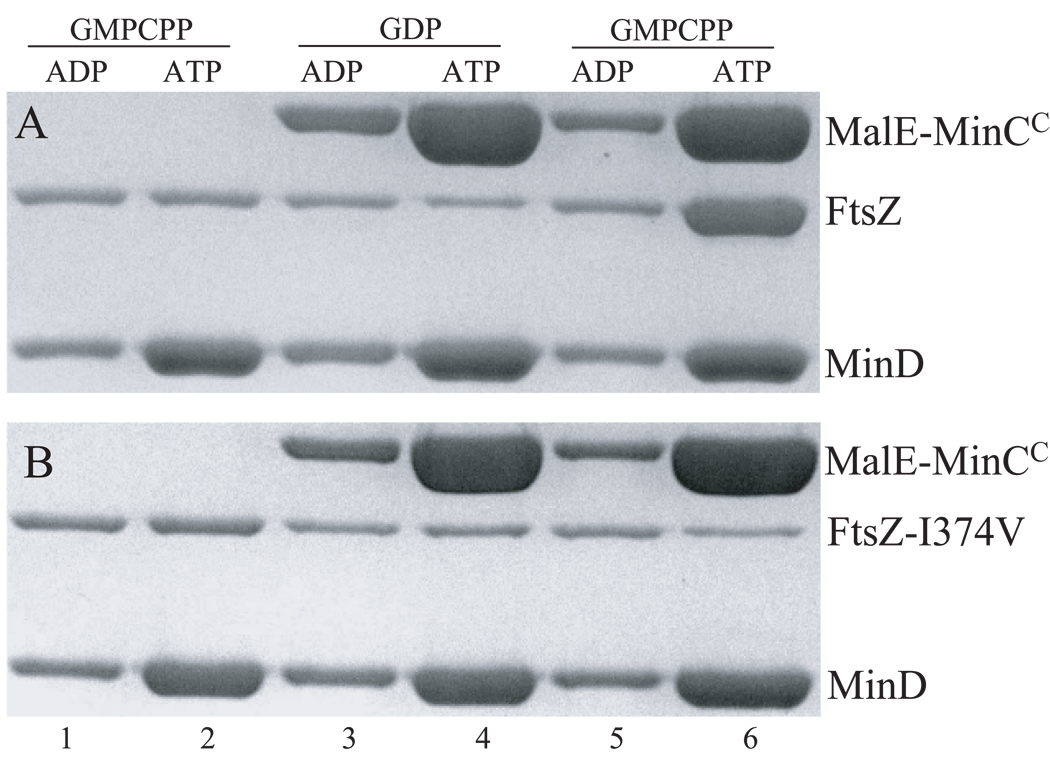
To confirm the loss of interaction between FtsZ-I374V and MinCC/MinD, we employed an in vitro recruitment assay described previously (Dajkovic et al., 2008a) to assess the interaction between FtsZ and MinCC/MinD. In this assay FtsZ-WT polymers that are assembled in the presence of GMPCPP are efficiently recruited to vesicles to which MinD and MinCC are bound. MinD binds to phospholipid vesicles (MLVs) in an ATP-dependent manner and this MinD-MLV complex is readily sedimented in a tabletop centrifuge. If MinCC is added to the reaction, it is recruited to the MinD-MLV complex due to the interaction between MinCC and MinD. If WT FtsZ is polymerized with GMPCPP and included in the reaction with MinD+ATP, MinCC and MLVs, the FtsZ polymers are recruited to the vesicle-Min complex (Fig 4A, lane 6). This recruitment requires MinCC as FtsZ polymers are not sedimented when MinCC is not included (Fig. 4A, lane 2). Also, unpolymerized FtsZ is not recruited to the MinCC/D-vesicle complex (Fig. 4A, lane 4). These results demonstrate that the centrifugation forces used here are insufficient to pellet FtsZ polymers not bound to vesicles and that there is a direct interaction between the MinCC/MinD complex and FtsZ. We then purified the FtsZ-I374V protein and tested its ability to bind MinCC/MinD using the above recruitment assay. Preliminary results revealed that the FtsZ-I374V protein displayed identical polymerization properties to WT FtsZ; both polymerized with GTP and GMPCPP and not GDP (Fig S3). When used in the recruitment assay, FtsZ-I374V polymers did not bind to the MinCC/MinD-phospholipid vesicle complex (Fig 4B, lane 6), confirming that FtsZ-I374V does not interact with MinCC/MinD.
Fig. 4.
FtsZ-I374V does not bind MinCC/MinD in vitro. The interaction between FtsZ and MinCC/MinD was determined by a sedimentation assay in which the recruitment of FtsZ polymers to a phospholipid vesicle-associated MinCC/MinD complex was assessed. Reactions containing mutilamellar phospholipid vesicles (MLV, 400 µg/mL), MinD (6µM), WT FtsZ (A) or FtsZ-I374V (B) at 6 µM, GMPCPP or GDP (200 µM) with (lane 3 to 6) or without (lane 1 and 2) MalE-MinCC (6 µM) were incubated at room temperature for 5 min in ATPase buffer (25 mM Tris-HCl [pH 7.5], 50mM KCl, and 5mM MgCl2). ATP or ADP (1 mM) were then added to the reactions and incubated for another 5 min. The reactions were then centrifuged at 10,000×g for 2 min. The pellets were solubilized and analyzed by SDS-PAGE.
FtsZ-I374V is still sensitive the N-terminus of MinC
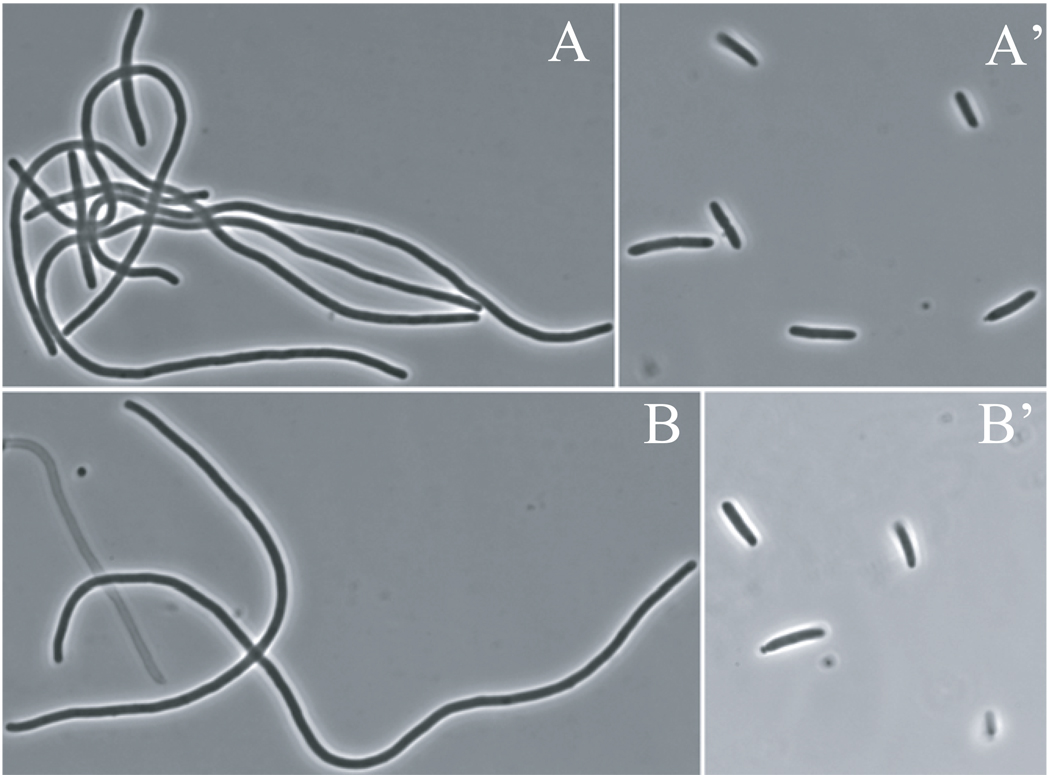
MinC has two functional domains and each domain affects FtsZ function but the mechanism of action is quite different (Hu & Lutkenhaus, 2000, Shiomi & Margolin, 2007, Hu et al., 1999, Dajkovic et al., 2008a). Although FtsZ-I374V confers resistance to MinC/MinD we speculated that it only suppresses the action of MinCC and is still sensitive to MinCN. To test this we introduced a plasmid (pZH111) expressing a MalE-MinCN fusion under arabinose promoter control into the S4 (min∷kan) and BSM374 (ftsZ-I374V min∷kan) strains to determine their sensitivity to arabinose. Neither S4 nor BSM374 containing the plasmid pZH111 (Para∷malE-minCN) formed colonies on plates containing 0.2% arabinose (data not shown). Colony formation was rescued by introduction of a point mutation (G10D) in MinCN [this mutation disrupts the activity of MinCN (Hu et al., 1999)] confirming that MinCN was responsible for the toxicity. At low arabinose concentrations, pZH111 caused filamentation in both strains and no difference in arabinose sensitivity was detected. When grown on plates with 0.05% arabinose for 5 hours, both S4/pZH111 and BSM374/pZH111 became extremely filamentous (Fig. 5, A and B). This filamentation phenotype was not observed if the G10D substitution was present in MinCN (Fig. 5, A’ and B’). These observations indicate that the FtsZ-I374V mutant is as sensitive to MinCN as the WT strain.
Fig. 5.
The FtsZ-I374V mutant strain is sensitive to MinCN. (A) BSM374 (ftsZ-I374V, min∷kan) and(B) S4 (min∷kan) harboring the plasmid pZH111 (Para∷malE-minCN) were grown on plates containing 0.05% arabinose at 37°C. The morphology of these cells was then checked at 5 hours by phase contrast microscopy. As a control, a point mutation (G10D) that inactivates MinCN was introduced into pZH111 and the mutant protein was expressed in BSM374 (A’) and S4 (B’) with the above conditions.
In the above assay MalE-MinCN is not on the membrane, although it is still toxic if sufficiently overexpressed. To test the MinCN sensitivity of the WT and FtsZ-I374V mutant strains under more physiological conditions, we compared the sensitivity of these two strains to MinC-R172A/MinD. The division inhibitory activity of this mutant form of MinC/MinD is from the N-terminal domain of MinC as the inhibitory activity of the C terminal domain (and its ability to localize to the Z ring) is abolished by the R172A mutation (Zhou & Lutkenhaus, 2005). As shown in Fig 6A rows 2 and 5, the growth of both S4 (min∷kan) and BSM374 (ftsZ-I374V, min∷kan) bearing the plasmid (pBANG78-R172A) expressing MinC-R172A/MinD under lac promoter control was blocked at similar IPTG concentrations (20 µM for S4 and 30 µM for BSM374, data not shown). A Western blot showed that a similar level of the MinC-R172A protein (with MinD) was required to cause uniform filamentation in these two strains in liquid cultures (Fig 6B, lane c and f), suggesting similar sensitivity of both strains. We also tested the sensitivity of FtsZ-WT and FtsZ-I374V to MinCN in vitro. Sedimentation of both WT FtsZ and FtsZ-I374V in the presence of GTP was prevented by MalE-MinCN in a concentration dependent manner (data not shown). Together, these results demonstrate that FtsZ-I374V and FtsZ-WT have similar sensitivity to MinCN. Therefore, we conclude that the MinC/MinD resistance of FtsZ-I374V is due to the resistance to MinCC/MinD.
Fig. 6.
Comparison of the killing efficiency of MinC/MinD and its mutants in FtsZ-WT and FtsZ-I374V strains. A) Plasmids pBANG78 (Plac∷minCD), pBANG78-G10D (minC-G10D on pBANG78) or pBANG78-R172A (minC-R172A on pBANG78) was transformed into S4 (min∷kan) or BSM374 (ftsZ-I374V, min∷kan). A spot test was done following the protocol described in Fig. 2 with the following samples: Row 1: S4/pBANG78-G10D; 2: S4/pBANG78-R172A; 3: BSM374/pBANG78; 4: BSM374/pBANG78-G10D; 5: BSM374/pBANG78-R172A. B) The minimal amount of MinC or MinC mutant proteins to cause uniform filamentation in S4 and BSM374 strains. Plasmid pBANG78 (pBANG59/Ptac∷minCD in the case of sample d) and its derivatives containing the minC-G10D or minC-R172A mutations were induced with minimal concentrations of IPTG to cause uniform filamentation in the indicated strains in liquid culture. The MinC protein level was determined by immunoblot with the WT strain S3 and Δmin strain S4 as controls: a: S3; b: S4/pBANG78-G10D; c: S4/pBANG78-R172A; d: S4/pBANG59; e: BSM374/pBANG78; f: BSM374/pBANG78-R172A; g: S4 and h: Marker at 28.8KD. The relative protein levels were indicated. *Loading volume for samples a, d and g was 5 fold more than that of samples b, c, e and f (see Experimental Procedures).
Concentration dependent effect of MinCC/MinD on Z rings
Overproduction of MinCC in the presence of MinD inhibits assembly of Z rings (Shiomi & Margolin, 2007) even though MinCC does not affect the polymerization of FtsZ in vitro (Hu & Lutkenhaus, 2000). MinCC was shown to block the lateral association of FtsZ polymers in vitro and this activity may be responsible for the disruption of the Z ring in vivo (Dajkovic et al., 2008a). However, the importance of lateral interactions between FtsZ polymers in Z-ring assembly remains enigmatic because of the relatively short length of the FtsZ protofilaments and a failure to observe multistranded polymers at division sites by electron cryotomography (Chen & Erickson, 2005, Li et al., 2007). Our finding that the septal localization and division inhibitory action of MinCC/MinD require the region of FtsZ that is also involved in the interaction with FtsA and ZipA raised the possibility that MinCC/MinD could compete with FtsA and ZipA. Such competition could disrupt the Z ring by dislodging FtsZ polymers from the membrane.
There are at least two discrete steps in the inhibition of cell division by MinCC/MinD. We had already observed that limited induction of GFP-MinCC/MinD caused filamentation, but the fluorescence was present in ring structures (Fig. S4, A1). This result indicated that Z rings were present but they did not support division: the limited expression of GFP-MinCC/MinD prevented Z-ring function without disrupting its formation. Induction of GFP-MinCC/MinD at a higher level resulted in filamentation and initial localization to Z rings (Fig. S4, B1) which eventually disappeared (Fig. S4, B2). We reasoned that one possible mechanism by which MinCC/MinD could block Z-ring function is to preferentially displace either FtsA or ZipA from the Z ring. If MinCC/MinD competes more efficiently with one of the two (FtsA or ZipA) for interaction with FtsZ, then overproduction of MinCC/MinD at some level could displace just one of them and not disrupt the ring since the Z ring can form with either FtsA or ZipA (Pichoff & Lutkenhaus, 2002).
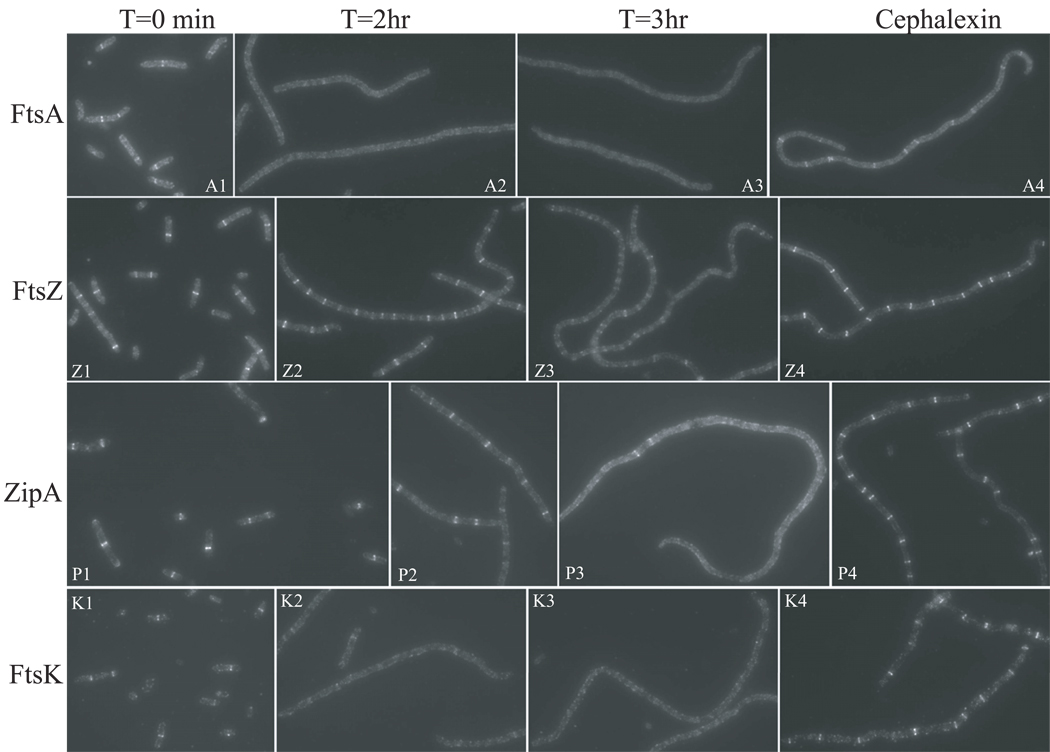
To test this, we did immuno-staining to examine the localization of FtsZ, FtsA, ZipA and FtsK proteins in cells over-expressing MinCC/MinD. The localization pattern of FtsK serves as an indicator of the integrity of the Z ring since its septal localization requires FtsZ, FtsA and ZipA (Wang & Lutkenhaus, 1998, Pichoff & Lutkenhaus, 2002). MinCC/MinD was induced in strain S4/pBANG75 (min∷kan /Plac∷MinCCD) with 100 µM IPTG, which is sufficient to cause filamentation and prevent colony formation. At different time points samples were taken and stained using antisera against FtsZ, FtsA, ZipA and FtsK.
Before IPTG induction (Fig. 7, T=0 min), most cells had FtsZ and ZipA rings and slightly less contained FtsA and FtsK rings. After one hour induction, most cells still had FtsZ and ZipA rings but only a small portion of the cells had FtsA and FtsK rings (data not shown). Two hours after induction, cells were filamentous but the vast majority contained FtsZ and ZipA rings throughout their length (Fig. 7, Z2 and P2). In contrast, FtsA and FtsK rings were only rarely observed (Fig. 7, A2 and K2). By three hours, ring localization of all 4 proteins was lost (Fig 7, T=3hr) although FtsZ and ZipA rings were sporadically observed (Fig. 7, Z3 and P3). Interestingly at this time point, FtsZ was present in spiral and ladder-like structures instead of rings. These spiral structures were also observed in other experiments using either GFP-MinCC/MinD or FtsZ-GFP in the presence of overexpressed MinCC/MinD, but only at specific time points. We think these structures are due to the spiraling-out of FtsZ polymers (possibly attached to the membrane through interaction with MinCC/MinD) as Z rings are disrupted.
Fig. 7.
Effect of MinCC/MinD induction on the localization of FtsZ, FtsA, ZipA and FtsK. MinCC/MinD was induced with 100 µM IPTG in S4/pBANG75 [ftsZ-WT, min∷kan/Plac∷minCCD]. Cells were removed at indicated time points and analyzed by immunofluorescent microscopy using antisera against FtsA (A1-A3), FtsZ (Z1-Z3), ZipA (P1-P3) and FtsK (K1-K3). As a control, S4/pBANG75 cells were treated with cephalexin at 20 µg/mL for 2 hours to generate filamentous cells. The localization of FtsA (A4), FtsZ(Z4), ZipA(P4) and FtsK(K4) in these filaments was also checked by immuno-staining.
The change in the localization pattern of these proteins upon MinCC/MinD induction is not due to filamentation per se, as filamentous cells of S4/pBANG75 generated by cephalexin treatment contained all four proteins in ring structures (Fig. 7, cephalexin) although FtsA and FtsK rings were present at a slightly lower number per filament. As another control MinCC/MinD was induced in BSM374 (ftsZ-I374V, min∷kan/ pBANG75). The cells did not filament and FtsZ, FtsA, ZipA and FtsK formed rings at similar frequencies at all time points (data not shown). This failure of MinCC/MinD to cause filamentation or affect the frequency of these rings in this strain is consistent with the failure of MinCC/MinD to bind to FtsZ-I374V and compete with FtsA and ZipA.
These results clearly demonstrate that overproduction of MinCC/MinD initially displaces FtsA, and therefore FtsK (and presumably other downstream proteins), from the WT Z ring. Later, this overproduction eventually disrupts the Z ring, probably because it also displaces ZipA. This result provides an explanation for the earlier observation that at a low induction level GFP-MinCC/MinD prevented division but still localized to rings (Fig. S4, A1); FtsA was displaced but Z rings still formed with the aid of ZipA. Also, the reduced frequency of FtsA rings (and FtsK rings) in S4/pBANG75 at T=0 min (Fig. 7, T=0 min) and in cephalexin treated cells (Fig. 7, cephalexin) is likely due to the basal expression of MinCC/MinD from pBANG75, since S4 and BSM374 contained FtsA rings at a similar frequency in the absence of this plasmid (Fig. S2 and data not shown).
Relative division inhibitory activity of the two domains of MinC
In the proper context both the N- and the C-terminal domains of MinC have division inhibitory activity, however, the relative efficiency of the two domains and their contribution to the activity of full length MinC/MinD have not been determined. Previous studies have shown that when MinD is not present, MalE-MinCN is a more potent division inhibitor than MalE-MinCC (Hu & Lutkenhaus, 2000). The division inhibitory activity of MinCC is only observed in the presence of MinD (Shiomi & Margolin, 2007). To compare the activity of the two domains of MinC in the same context and under more physiological conditions, we took advantage of the two MinC mutants (MinC-G10D and MinC-R172A) described previously (Hu et al., 1999, Zhou & Lutkenhaus, 2005). The minC-G10D and minC-R172A mutations significantly reduce the activity of MinCN and MinCC respectively, so that the division inhibitory activity of MinC-G10D/MinD is mainly from the MinCC domain and that of MinC-R172A/MinD is mainly from the MinCN domain. As shown in Fig 6A (rows 1 and 2), the two MinC mutants displayed very similar ability to prevent the growth of the FtsZ-WT µmin strain (S4) when expressed with MinD under lac promoter; no growth at or above 20 µM IPTG. Western blot analysis further confirmed that the same amount of the mutant MinC proteins was required to cause uniform filamentation in liquid culture (Fig 6B, b&c).
The killing efficiency of the two MinC mutants must be significantly lower than WT MinC since WT MinC/MinD on the same vector (pBANG78/Plac∷minCD) could not be transformed into the S4 strain (data not shown). However, another plasmid (pBANG59/Ptac∷minCD) with lower expression of MinC/MinD could be transformed into S4 (Fig 2, row 1). Western analysis of this strain (S4/pBANG59) indicated that the minimal MinC level required to cause uniform filamentation was slightly less than the MinC level expressed from the chromosome of a wild type strain (Fig 6B, lane d and a, respectively), consistent with what was reported before (Zhou & Lutkenhaus, 2005). Comparison of the protein levels also indicates that about 40 times more mutant MinC (MinC-G10D or R-172A) was required to cause uniform filamentation in S4 (µmin) strain than WT MinC (Fig 6B, b, c&d and data not shown), suggesting that each domain of MinC is at least 40 fold less active than full length MinC in blocking division.
We also wanted to compare the effect of the ftsZ-I374V mutation to that of the minC-R172A mutation to see whether they are equivalent in affecting the responsiveness of FtsZ to MinC/MinD. Both mutations eliminate the toxicity of MinCC/MinD and therefore should have a similar effect. To do this, under the control of an IPTG-inducible promoter, wild type MinC/MinD was expressed in BSM374 (ftsZ-I374V, min∷kan) and MinC-R172A/MinD was expressed in S4 (ftsZ-WT, min∷kan) and the IPTG sensitivity of these two strains was compared. As shown in Fig 6A rows 2 and 3, growth of both strains was inhibited at the same IPTG concentration (20 µM). Subsequent analysis showed that very similar amount of MinC protein was required for wild type MinC/MinD to cause filamentation in BSM374 (Fig 6B, lane e) as for MinC-R172A/MinD to cause filamentation in the S4 strain (Fig 6B, lane c). Thus, WT MinC/MinD has the same toxicity in the FtsZ-I374V strain as MinC-R172A/MinD has in FtsZ-WT strain. This result confirms that FtsZ-I374V is resistant to MinCC/MinD. Consistent with this conclusion, MinC-R172A/MinD is as toxic (Fig 6A, row 5; 6B, lane f) as wild type MinC/MinD in the FtsZ-I374V strain. Also, MinC-G10D/MinD did not show any detectable toxicity in BSM374 (ftsZ-I374V, min∷kan) (Fig 6A, row 4). This latter result was expected since the inhibitory activity of both domains of MinC would be abolished.
We also used GFP-MinC/MinD and the corresponding mutants (GFP-MinC-G10D and GFP-MinC-R172A) in JS964 (Δmin) strain to assess the relative toxicity of the two domains of MinC and to compare them to WT MinC. The results were similar to what was shown above with untagged proteins---the two mutant forms of MinC/MinD have very similar division inhibitory activity and are 40-fold less active than WT MinC/MinD (data not shown).
Discussion
In E.coli and many other bacteria, the Min system contributes to the spatial regulation of cell division by inhibiting Z ring formation near the poles of the cell (de Boer et al., 1989). The Min system has been studied for almost 20 years and many fascinating aspects of it have been described, however, a detailed mechanism of its function is not known. The effector of the Min system is MinC, an inhibitor of Z ring formation and, therefore cytokinesis (de Boer et al., 1989, Bi & Lutkenhaus, 1993, de Boer et al., 1990, Hu et al., 1999). MinC is composed of two domains with distinct functions and both domains have division inhibitory activity when overexpressed: MinCN blocks Z ring formation in vivo and disrupts FtsZ polymer assembly in vitro as assayed by sedimentation (Hu & Lutkenhaus, 2000); MinCC does not affect FtsZ sedimentation but decreases filament bundling and can localize to Z rings and block cell division when MinD is present (Hu & Lutkenhaus, 2000, Shiomi & Margolin, 2007). The detailed mechanism of the inhibitory activity of each domain is not fully understood. In the present study, we found that localization of MinCC/MinD to the Z ring requires the C-terminal conserved tail of FtsZ, an unstructured region which also interacts with FtsA and ZipA (Liu et al., 1999, Ma & Margolin, 1999, Haney et al., 2001). MinCC/MinD efficiently displaces FtsA on the Z ring and can therefore block the function of the Z ring; further overproduction of MinCC/MinD eventually disrupts the Z ring, probably because it also displaces ZipA.
Localization of MinCC/MinD to the Z ring
MinCC/MinD has been shown to localize to the Z ring and this localization depends on MinCC since a point mutation (R172A) in MinCC prevents localization of GFP-MinCC/MinD (Zhou & Lutkenhaus, 2005, Johnson et al., 2002). However the component(s) of the Z ring that is directly involved in recruiting MinCC/MinD to the septum was not clearly demonstrated even though there are several lines of evidence suggesting that it is FtsZ: a) localization of MinCC/MinD does not require other known components of the Z ring (FtsA, ZapA or ZipA) (Johnson et al., 2004); and b) direct interaction between FtsZ and MinCC has been detected in several assays (Dajkovic et al., 2008a). Our results strongly support that FtsZ directly recruits MinCC/MinD to the septum because we isolated FtsZ mutants that are resistant to MinCC/MinD, the one we studied in detail---FtsZ-I374V fails to recruit MinCC/MinD to the septum, and does not interact with it in vitro.
The role of MinD is still not clear as the targeting of MinCC/MinD to the Z ring is generally thought to be mediated by the FtsZ-MinCC interaction (Dajkovic et al., 2008a). However, MinCC is not targeted, nor does it have toxicity in the absence of MinD (Johnson et al., 2002, Shiomi & Margolin, 2007) and there is no evidence to show that MinD contacts any septal components directly. This is in contrast to DicB, a phage-encoded protein that can also target MinCC to the Z ring, but does so via an interaction with ZipA. In this case the localization is thought to be a bipartite signal involving both DicB and MinCC making contacts with ZipA and FtsZ, respectively (Johnson et al., 2004). We examined the targeting of MinCC/DicB to the Z ring in BSM374 (ftsZ-I374V, min∷kan) strain by GFP tagging of MinCC and found that GFP-MinCC/DicB was still targeted to the Z rings. Consistent with this, and unlike the resistance to MinC/MinD (Fig 2), BSM374 does not show significant resistance to MinC/DicB (data not shown). This is perhaps not very surprising because of the difference in targeting of MinCC to the Z ring by MinD and DicB as discussed above. In addition, FtsZ is targeted by MinCC/MinD or MinCC/DicB complexes and not MinCC alone. In this sense, it is not surprising if the requirements for FtsZ to recognize these two complexes are not the same since the complexes are different. This may explain why FtsZ-I374V blocks the targeting of MinCC/MinD but not MinCC/DicB.
One role for MinD is to recruit and concentrate MinCC on the membrane but there is more, since MinD greatly enhances the toxicity of a version of MinCC (MinCC-MTS) which is targeted to the membrane artificially by addition of a membrane targeting sequence (unpublished data). MinCC-MTS does not show detectable toxicity in the absence of MinD but becomes a potent division inhibitor when MinD is present. This observation is similar to what was observed previously with full length MinC (Johnson et al., 2002). Also, MinDΔ10, which lacks the membrane targeting sequence, increases the toxicity of MinC, just not as well as the full length MinD (Hu & Lutkenhaus, 2001). These results are consistent with MinD enhancing the affinity of MinCC for FtsZ, and/or performing some other function of which we are unaware.
The last 15–20 residues of FtsZ---a busy region for protein interactions
FtsZ is one of the most conserved proteins in bacteria, consistent with its critical role in cell division. It consists of a main body (FtsZ1–320 in E.coli), which is structurally homologous to tubulin, and an unstructured tail (FtsZ321–383 in E.coli), which is less conserved and shows significant variation in length and sequence (Ma & Margolin, 1999, Lowe & Amos, 1998) (Fig. 1). However the last ~15 residues of this tail are highly conserved in FtsZ from bacteria, suggesting a conserved function for this region. Indeed, this tail is involved in interaction with FtsA and ZipA in E.coli and EzrA and SepF (YlmF) in B.subtilis (Haney et al., 2001, Singh et al., 2007, Ishikawa et al., 2006). Here, we show that it is also involved in interacting with MinCC/MinD and therefore MinC/MinD. The interaction with FtsA and ZipA is required for Z ring formation, whereas the interaction with EzrA or MinC/MinD has regulatory roles. Because this region is mediating interaction with many proteins, most of the mutants we isolated (D373E, I374V, L378V) result in small biochemical changes; the mutant proteins must still interact with other proteins (FtsA and ZipA) to function in division. Nonetheless, these subtle changes result in significant resistance to MinC/MinD and are deficient in MinCC/MinD binding. The ftsZ-I374V mutation did not affect the interaction between FtsZ and ZipA, however, the interaction with FtsA was possibly reduced. Nonetheless, recruitment of FtsA to the Z ring appeared normal suggesting that there is still a strong enough interaction.
MinCC/MinD disrupts the Z ring in two stages
It was shown previously that MalE-MinCC inhibits the lateral association of FtsZ polymers in vitro and this activity was proposed to be the basis for the inhibitory activity of MinCC/MinD in vivo (Dajkovic et al., 2008a). Here we show that MinCC/MinD blocks division in two stages. In the first stage MinCC/MinD displaces FtsA from the Z ring. This requires less MinCC/MinD but prevents the recruitment of downstream proteins such as FtsK. As a result the Z ring is nonfunctional for division. At the second stage, requiring more MinCC/MinD, the Z ring is disrupted probably because ZipA is also displaced. The fact that MinCC/MinD displaces FtsA more readily than ZipA could mean that FtsA binds the tail of FtsZ with lower affinity than ZipA or could simply be due to the Z ring containing less FtsA molecules than ZipA (ZipA/FtsA=4/1). We don’t have direct evidence to demonstrate that the eventual disintegration of the Z ring by MinCC/MinD is due to the displacement of ZipA. It is possible that inhibition of the lateral association of FtsZ polymers by MinCC/MinD is also a contributing factor.
The observation that the FtsA is displaced from the Z ring more readily than ZipA explains why the Z rings fail to function upon MinCC/MinD induction. The Z rings persist with the aid of ZipA but do not function since FtsA, FtsK and presumably other cell division proteins are absent. A previous study (Justice et al., 2000), concluded that MinC/MinD prevented the recruitment of FtsA to the Z ring instead of disrupting the Z ring to inhibit division. However, there are several studies showing that MinC/MinD does indeed disrupt the Z ring (Johnson et al., 2002, Pichoff & Lutkenhaus, 2001, Hu et al., 1999) and we find that the integrity of Z rings are much more sensitive to intact MinC/MinD than MinCC/MinD. We can not explain the difference between our results and their observations although they obtained different results depending upon the fixation conditions. It’s possible that they had a mutation that inactivated the N terminus of MinC in their MinC/MinD construct, making it more or less like MinCC/MinD. However, they also reported that overexpression of FtsA reduced the filamentation caused by their MinC/MinD construct but we were unable to find conditions where overexpression of FtsA (despite trying many different expression levels) reduced the filamentation caused by overexpression of MinCC/MinD. This is perhaps not so surprising since expression of MinCC/MinD at the minimal level required to cause uniform filamentation is unlikely to only displace FtsA but also starts to affect ZipA. Also, overexpression of FtsA would place more FtsA on the Z ring but this can be detrimental since the ratio of these proteins (FtsZ, FtsA , ZipA) must be within a certain window for the normal functioning of the Z ring (Hale & de Boer, 1997, Dai & Lutkenhaus, 1992).
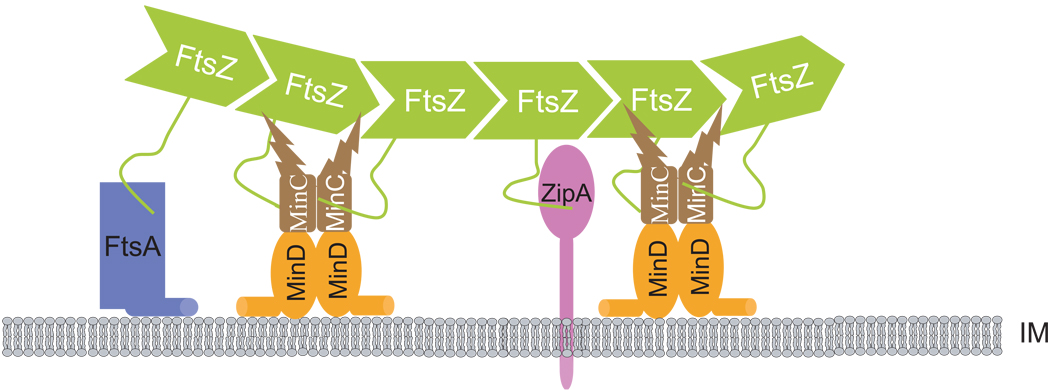
Model for MinC/MinD on Z ring formation
With the results obtained here and previous studies, we provide details that support a model for the mode of action of MinC/MinD in preventing formation of Z rings (Johnson et al., 2004). As shown in Fig. 8, MinC/MinD localizes to membrane-associated FtsZ polymers through MinCC/MinD interacting with the conserved C-terminal tail of FtsZ. By directly contacting FtsZ, MinC/MinD prevents Z ring formation in at least two ways: first, MinCC/MinD disrupts the function of the Z ring by interfering with the recruitment of FtsA and possible reducing polymer bundling; second, this targeting of MinC/MinD to the Z ring brings MinCN in close proximity to FtsZ polymers, so that it is near its target. The combination of these two activities makes MinC/MinD a potent division inhibitor.
Fig. 8.
Model for the inhibitory action of MinC/MinD on Z ring assembly. Under physiological conditions polar Z ring formation is prevented by MinC/MinD concentrated at the poles through the Min oscillation. MinC/MinD in the polar zone binds to ZipA and FtsA decorated FtsZ polymers located at the membrane before a complete Z ring is assembled through the MinCC/MinD-FtsZ interaction. This binding may displace FtsA and/or ZipA from FtsZ polymers. More importantly the binding of MinC/MinD to FtsZ brings MinCN close to FtsZ polymers, resulting in disruption of FtsZ polymers. The combination of these activities ensures that no polar Z rings are assembled. IM: inner membrane.
The resistance of the FtsZ I374V mutant to MinC/MinD suggests that the targeting of MinC/MinD to the Z ring (through MinCC/MinD) is very important for its activity; on the other hand the observation that the presence of the ftsZ I374V allele (BSZ374 strain) in a wild type background (min+) results in very few minicells almost argues against this. Comparison of the division inhibitory activity of the two domains of MinC (by employing mutations that inactivate either domain in the context of MinC/MinD), revealed that they have the same efficiency in blocking division and preventing colony formation. Each domain, however, is much less efficient than full length MinC/MinD (Fig 6), suggesting that the two parts of MinC work synergistically to achieve maximum activity. The importance of the two domains of MinC is further highlighted by the observation that the min operon containing the minC-G10D or the minC-R172A mutations on a single copy plasmid can not prevent minicell formation (Zhou & Lutkenhaus, 2005).
Why the FtsZ-I374V mutant does not make minicells is not clear. We think one possible reason is that, as we have shown, FtsZ-I374V is still sensitive to MinCN. The presence of MinE in the cell concentrates MinC/MinD at the poles through the oscillation and the high polar concentration of MinCN (as part of MinC) may disrupt the polar Z rings. Consistent with FtsZ-I374V being sensitive to MinCN, we have shown that expression of MinC/MinD at a higher level prevents the growth of BSM374 (Fig 6, row 3). However, this can not be the only reason as we have shown in Fig. 6 that BSM374 (ftsZ-I374V, min∷kan) has the same sensitivity to WT MinC/MinD as S4 (ftsZ-WT, min∷kan) has to MinC-R172A/MinD. This result indicates that the two mutations (ftsZ-I374V and minC-R172A) are similar in affecting the responsiveness of FtsZ to MinC/MinD. Nevertheless, MinC-R172A can not block minicell formation in FtsZ-WT strain (Zhou & Lutkenhaus, 2005) whereas WT MinC efficiently prevents minicell formation in FtsZ-I374V strain. We do not know what the basis is for the difference between these two cases. However, the fact that BSZ374 strain is not making minicells does not necessarily mean that the targeting of MinC/MinD to the Z ring is not required for its function at physiological levels (can be deduced from the effect of MinC-R172A). At overexpressed levels the two domains of MinC can function separately but combining them results in an inhibitor that works synergistically to ensure the disruption of polar Z rings. In this sense, it will be interesting to know whether minicells are formed if a MinCN resistant ftsZ allele is present on the chromosome.
Experimental procedures
Bacterial strains, plasmids and growth conditions
Strains and plasmids used in this study are listed in table S3. Cells were grown in LB medium at 37°C unless otherwise indicated. When needed, antibiotics were used at the following concentrations: ampicillin=100 µg/ml; spectinomycin=25 µg/ml; kanamycin=25 µg/ml, tetracycline=10 µg/ml, cephalexin=20 µg/ml and chloramphenicol=20 µg/ml.
Strain S7 (ftsZ0 recA∷Tn10)/ pKD3C was constructed in two steps. First the ftsZ0 allele was introduced into strain S4 (leu∷Tn10 ftsZ+ min∷kan)/pKD3C (ftsZ+) with P1 phage grown on PB143 (leu+ ftsZ0 recA∷Tn10) by selecting Leu+ at 30°C on M9 minimal medium. The resultant transductants were checked for temperature and tetracycline sensitivity. The desired transductants (S6/pKD3C) should have the genotype of leu+, ftsZ0, min∷kan with the temperature sensitive plasmid pKD3C supplying FtsZ. In a second step, the recA∷Tn10. allele from PB143 was transduced into S6/pKD3C by selecting tetracycline resistance on LB plates at 30°C. The resultant cells S7 (ftsZ0 recA∷Tn10)/pKD3C) were checked for UV sensitivity to confirm recA inactivation.
The strain BSZ374 (ftsZ-I374V) was generated by replacing the ftsZ84 allele (TS) on the chromosome of strain PS106 with ftsZ-I374V through recombineering using the lambda RED system (Datsenko & Wanner, 2000). The PCR product of ftsZ containing the I374V mutation was electroporated into PS106 (ftsZ84)/pKD46 induced with arabinose=0.04% for 3 h at 30oC and recombinants were selected on LB plates with no salt at 42°C. To determine if the ftsZ-I374V mutation was present, 10 colonies were randomly selected and checked for MinC/MinD resistance (about 25% of the randomly streaked colonies showed MinC/MinD resistance). We then did PCR to amplify the ftsA-ftsZ region from the chromosome of the MinC/MinD resistant strains and confirmed that ftsZ I374V. was present and that ftsZ84 was absent. P1 transduction was then used to move the min∷kan allele into BSZ374 to give BSM374.
The plasmid pBANG112 was constructed by inserting a fragment containing ftsZ into a pACYC184 based vector. The ftsZ gene was obtained by XmaI and PstI digestion of pKD4 (Dai & Lutkenhaus, 1991). To make pBANG59, minC/minD was PCR amplified from pSC104CD using primers (restriction sites underlined): 5’-MinC-SstI: GGAGCTCGCTAATTGAGTAAGGCCAGGATG and 3’-MinD- HindIII: CATGTCCTGCAGAAGCTTGCATTAAAATCTAGCGAGGGC. digested with SstI+HindIII and inserted to a Spcr version of pEXT22. pBANG75 and pBANG78 were made by replacing gfp-MinCC/minD in pHJZ109 (Zhou & Lutkenhaus, 2005) with the properly digested minCC/minD and minC/minD PCR product respectively. Both minC/minD and minCC/minD were amplified from pCS104CD (Zhou & Lutkenhaus, 2005) using the following primers (restriction sites underlined): 5’-MinC*-SstI: CGAGCTCTTTAAGAAGGAGATATACGGATGTCAAACACGCCAATCG; 5’-MinCC116-SstI:
CGAGCTCTAAGGAGGTTATAAATAATGGCGCAAAATACAACGCCGGTC and 3’-MinD- HindIII: CATGTCCTGCAGAAGCTTGCATTAAAATCTAGCGAGGGC. Both pBANG75 and pBANG78 contain artificially conserved ribosome binding site (RBS) for MinCC and MinC translation respectively.
PCR random mutagenesis of ftsZ
Using pBANG112 as the template and the primers: 5’- GCCTCAGGCGACAGGCACAAATCGGAGAG and 5’-GCTGCAGATATTCGATATCACGCATGAAAC, ftsZ was PCR amplified using the GeneMorph II Random Mutagenesis kit from Stratagene with a mutagenesis rate of 1–4 bases/Kb. The PCR fragments were then digested with EcoRI + EagI and ligated into EcoRI + EagI digested pBANG112. The ligation product was then electroporated into S7/pKD3C and transformants were selected at 42°C on plates with ampicillin. All colonies that grew up were pooled to give a library of FtsZ mutants that can still complement the ftsZ depletion strain and support cell division. Then pBANG75 was transformed into these cells and colonies resistant to MinCC/MinD were selected with IPTG=200 µM at 42°C on plates containing Amp and Spc. Plasmids were isolated from colonies that grew and the ftsZ gene was sequenced to identify the mutations.
Analysis of GFP-MinCC/MinD localization
Overnight cultures of S4, BSM374 or JS964 (min∷kan) containing the plasmid pHJZ109 (gfp-MinCC minD) were diluted 1000 fold into LB+Spc and grown at 37 °C until OD600≈0.3. IPTG was then added at the indicated concentrations and the cultures were diluted every 0.5–1 h to keep the OD600<0.4. Samples were taken at different time points and checked by microscopy as previously described (Zhou & Lutkenhaus, 2005).
Yeast two hybrid assay
To detect FtsZ-FtsA and FtsZ-ZipA interactions, the appropriate plasmids were transformed into the reporter strain SFY526 as described (Huang et al., 1996). The colonies obtained were analyzed for β-galactosidase production by the colony lift assay described in the CLONTECH manual.
Protein purification and FtsZ recruitment assay
WT FtsZ and FtsZ I374V were expressed and purified from W3110/pKD126 (ftsZ) and BSZ374/pKD126-1(ftsZ-I374V) respectively according to the method described previously (Mukherjee & Lutkenhaus, 1998). A slight modification was that after ammonium sulfate precipitation, the pellet was dissolved and dialyzed in Buffer A and further purified by chromatography on a Resource Q column (GE healthcare) eluting with 50 mM Tris-HCl (pH 7.9), 1 mM EDTA, 10% glycerol, and a gradient of 50–500 mM KCl. The FtsZ fractions (eluting at 200–300 mmM KCl) were pooled and dialyzed against 50 mM HEPES-NaOH (pH 7.2), 0.1mM EDTA and 10% glycerol, aliquoted and stored at −80°C. The quality of the purified proteins was checked by SDS-PAGE following the standard FtsZ polymerization assay (Dajkovic et al., 2008a). The purification of MalE-MinCC and MinD and the FtsZ recruitment assay were performed as previously described (Dajkovic et al., 2008a).
Immunofluorescent microscopy
Overnight cultures of S4/pBANG75 and BSM374/pBANG75 were diluted 1000-fold in LB+Spc and grown at 37 °C. At OD600≈0.3, samples were taken and fixed with paraformaldehyde + glutaraldehyde. At the same time the cultures were diluted 10 times to fresh LB+Spc and induced with IPTG=100µM. Samples were taken and fixed every hour and cultures were diluted every hour to maintain the exponential phase. As a control, an exponentially growing culture of S4/pBANG75 was treated with 20 µg/ml cephalexin for 2 hours and then cells were fixed with paraformaldehyde + glutaraldehyde. Fixation of the cells, preparation for immuno-staining and photography of samples were done as described before (Pichoff & Lutkenhaus, 2002). The antisera were used at following concentrations: FtsZ (1/5000), FtsA (1/5000), ZipA (1/4000) and FtsK (1/1000).
Western blot
Strains grown in LB medium (supplemented with Spc if necessary and IPTG at indicated concentrations) included S3, S4, S4/pBANG59 (with IPTG=40 µM), S4/pBANG78-G10D (with IPTG=30 µM), S4 /pBANG78-R172A (with IPTG=30 µM), BSM374/pBANG78 (with IPTG=25 µM) and BSM374/pBANG78-R172A (with IPTG=40 µM) at 37°C for about 3 hours to reach OD600=0.4. Cells are then collected and resuspended in sodium dodecyl sulfate (SDS) sample buffer, boiled for 10 min, and subjected to SDS polyacrylamide gel electrophoresis (PAGE). Subsequent immunoblotting was done as previously described (Zhou & Lutkenhaus, 2005). For a better comparison, the loading volume for these samples is adjusted as folows: for S3, S4 and S4/pBANG59, the loading volume is equivalent to 300 µl of OD600=0.4 cells; for the others it is 60 µl of OD600=0.4 cells.
Acknowledgements
We thank Piet de Boer for sending strains. This work was supported by National Institutes of Health Grant GM029764.
References
- Bernhardt TG, de Boer PA. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over Chromosomes in E. coli. Mol Cell. 2005;18:555–564. doi: 10.1016/j.molcel.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Erickson HP. Rapid in vitro assembly dynamics and subunit turnover of FtsZ demonstrated by fluorescence resonance energy transfer. J Biol Chem. 2005;280:22549–22554. doi: 10.1074/jbc.M500895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell SC, Anderson RE, Lowe J. Crystal structure of the bacterial cell division inhibitor MinC. EMBO J. 2001;20:2454–2461. doi: 10.1093/emboj/20.10.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Lutkenhaus J. ftsZ is an essential cell division gene in Escherichia coli. J Bacteriol. 1991;173:3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Lutkenhaus J. The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J Bacteriol. 1992;174:6145–6151. doi: 10.1128/jb.174.19.6145-6151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Mukherjee A, Xu Y, Lutkenhaus J. Mutations in ftsZ that confer resistance to SulA affect the interaction of FtsZ with GTP. J Bacteriol. 1994;176:130–136. doi: 10.1128/jb.176.1.130-136.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajkovic A, Lan G, Sun SX, Wirtz D, Lutkenhaus J. MinC spatially controls bacterial cytokinesis by antagonizing the scaffolding function of FtsZ. Curr Biol. 2008a;18:235–244. doi: 10.1016/j.cub.2008.01.042. [DOI] [PubMed] [Google Scholar]
- Dajkovic A, Mukherjee A, Lutkenhaus J. Investigation of regulation of FtsZ assembly by SulA and development of a model for FtsZ polymerization. J Bacteriol. 2008b;190:2513–2526. doi: 10.1128/JB.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer PA, Crossley RE, Rothfield LI. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- de Boer PA, Crossley RE, Rothfield LI. Central role for the Escherichia coli minC gene product in two different cell division-inhibition systems. Proc Natl Acad Sci U S A. 1990;87:1129–1133. doi: 10.1073/pnas.87.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersbach G, Galli E, Moller-Jensen J, Lowe J, Gerdes K. Novel coiled-coil cell division factor ZapB stimulates Z ring assembly and cell division. Mol Microbiol. 2008;68:720–735. doi: 10.1111/j.1365-2958.2008.06190.x. [DOI] [PubMed] [Google Scholar]
- Fu X, Shih YL, Zhang Y, Rothfield LI. The MinE ring required for proper placement of the division site is a mobile structure that changes its cellular location during the Escherichia coli division cycle. Proc Natl Acad Sci U S A. 2001;98:980–985. doi: 10.1073/pnas.031549298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueiros-Filho FJ, Losick R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 2002;16:2544–2556. doi: 10.1101/gad.1014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, de Boer PA. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- Hale CA, de Boer PA. Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J Bacteriol. 1999;181:167–176. doi: 10.1128/jb.181.1.167-176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, Meinhardt H, de Boer PA. Dynamic localization cycle of the cell division regulator MinE in Escherichia coli. EMBO J. 2001;20:1563–1572. doi: 10.1093/emboj/20.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, Rhee AC, de Boer PA. ZipA-induced bundling of FtsZ polymers mediated by an interaction between C-terminal domains. J Bacteriol. 2000;182:5153–5166. doi: 10.1128/jb.182.18.5153-5166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney SA, Glasfeld E, Hale C, Keeney D, He Z, de Boer P. Genetic analysis of the Escherichia coli FtsZ.ZipA interaction in the yeast two-hybrid system. Characterization of FtsZ residues essential for the interactions with ZipA and with FtsA. J Biol Chem. 2001;276:11980–11987. doi: 10.1074/jbc.M009810200. [DOI] [PubMed] [Google Scholar]
- Hu Z, Gogol EP, Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc Natl Acad Sci U S A. 2002;99:6761–6766. doi: 10.1073/pnas.102059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol. 1999;34:82–90. doi: 10.1046/j.1365-2958.1999.01575.x. [DOI] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. Analysis of MinC reveals two independent domains involved in interaction with MinD and FtsZ. J Bacteriol. 2000;182:3965–3971. doi: 10.1128/jb.182.14.3965-3971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. Topological regulation of cell division in E. coli. spatiotemporal oscillation of MinD requires stimulation of its ATPase by MinE and phospholipid. Mol Cell. 2001;7:1337–1343. doi: 10.1016/s1097-2765(01)00273-8. [DOI] [PubMed] [Google Scholar]
- Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci U S A. 1999;96:14819–14824. doi: 10.1073/pnas.96.26.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Saez C, Lutkenhaus J. Recruitment of MinC, an inhibitor of Z-ring formation, to the membrane in Escherichia coli: role of MinD and MinE. J Bacteriol. 2003;185:196–203. doi: 10.1128/JB.185.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Cao C, Lutkenhaus J. Interaction between FtsZ and inhibitors of cell division. J Bacteriol. 1996;178:5080–5085. doi: 10.1128/jb.178.17.5080-5085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Kawai Y, Hiramatsu K, Kuwano M, Ogasawara N. A new FtsZ-interacting protein, YlmF, complements the activity of FtsA during progression of cell division in Bacillus subtilis. Mol Microbiol. 2006;60:1364–1380. doi: 10.1111/j.1365-2958.2006.05184.x. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Lackner LL, de Boer PA. Targeting of (D)MinC/MinD and (D)MinC/DicB complexes to septal rings in Escherichia coli suggests a multistep mechanism for MinC-mediated destruction of nascent FtsZ rings. J Bacteriol. 2002;184:2951–2962. doi: 10.1128/JB.184.11.2951-2962.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Lackner LL, Hale CA, de Boer PA. ZipA is required for targeting of DMinC/DicB, but not DMinC/MinD, complexes to septal ring assemblies in Escherichia coli. J Bacteriol. 2004;186:2418–2429. doi: 10.1128/JB.186.8.2418-2429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice SS, Garcia-Lara J, Rothfield LI. Cell division inhibitors SulA and MinC/MinD block septum formation at different steps in the assembly of the Escherichia coli division machinery. Mol Microbiol. 2000;37:410–423. doi: 10.1046/j.1365-2958.2000.02007.x. [DOI] [PubMed] [Google Scholar]
- Lackner LL, Raskin DM, de Boer PA. ATP-dependent interactions between Escherichia coli Min proteins and the phospholipid membrane in vitro. J Bacteriol. 2003;185:735–749. doi: 10.1128/JB.185.3.735-749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Trimble MJ, Brun YV, Jensen GJ. The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J. 2007;26:4694–4708. doi: 10.1038/sj.emboj.7601895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Mukherjee A, Lutkenhaus J. Recruitment of ZipA to the division site by interaction with FtsZ. Mol Microbiol. 1999;31:1853–1861. doi: 10.1046/j.1365-2958.1999.01322.x. [DOI] [PubMed] [Google Scholar]
- Lowe J, Amos LA. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J, Sanjanwala B, Lowe M. Overproduction of FtsZ suppresses sensitivity of lon mutants to division inhibition. J Bacteriol. 1986;166:756–762. doi: 10.1128/jb.166.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Margolin W. Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J Bacteriol. 1999;181:7531–7544. doi: 10.1128/jb.181.24.7531-7544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H, de Boer PA. Pattern formation in Escherichia coli: a model for the pole-to-pole oscillations of Min proteins and the localization of the division site. Proc Natl Acad Sci U S A. 2001;98:14202–14207. doi: 10.1073/pnas.251216598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Lutkenhaus J. Purification, assembly, and localization of FtsZ. Methods Enzymol. 1998;298:296–305. doi: 10.1016/s0076-6879(98)98026-0. [DOI] [PubMed] [Google Scholar]
- Mulder E, Woldringh CL. Actively replicating nucleoids influence positioning of division sites in Escherichia coli filaments forming cells lacking DNA. J Bacteriol. 1989;171:4303–4314. doi: 10.1128/jb.171.8.4303-4314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Escherichia coli division inhibitor MinCD blocks septation by preventing Z-ring formation. J Bacteriol. 2001;183:6630–6635. doi: 10.1128/JB.183.22.6630-6635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J. 2002;21:685–693. doi: 10.1093/emboj/21.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol Microbiol. 2005;55:1722–1734. doi: 10.1111/j.1365-2958.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Identification of a region of FtsA required for interaction with FtsZ. Mol Microbiol. 2007;64:1129–1138. doi: 10.1111/j.1365-2958.2007.05735.x. [DOI] [PubMed] [Google Scholar]
- Raskin DM, de Boer PA. The MinE ring: an FtsZ-independent cell structure required for selection of the correct division site in E. coli. Cell. 1997;91:685–694. doi: 10.1016/s0092-8674(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Raskin DM, de Boer PA. MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. J Bacteriol. 1999a;181:6419–6424. doi: 10.1128/jb.181.20.6419-6424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin DM, de Boer PA. Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc Natl Acad Sci U S A. 1999b;96:4971–4976. doi: 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L, Taghbalout A, Shih YL. Spatial control of bacterial division-site placement. Nat Rev Microbiol. 2005;3:959–968. doi: 10.1038/nrmicro1290. [DOI] [PubMed] [Google Scholar]
- Shih YL, Le T, Rothfield L. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc Natl Acad Sci U S A. 2003;100:7865–7870. doi: 10.1073/pnas.1232225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi D, Margolin W. The C-terminal domain of MinC inhibits assembly of the Z ring in Escherichia coli. J Bacteriol. 2007;189:236–243. doi: 10.1128/JB.00666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JK, Makde RD, Kumar V, Panda D. A membrane protein, EzrA, regulates assembly dynamics of FtsZ by interacting with the C-terminal tail of FtsZ. Biochemistry. 2007;46:11013–11022. doi: 10.1021/bi700710j. [DOI] [PubMed] [Google Scholar]
- Wang L, Lutkenhaus J. FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol Microbiol. 1998;29:731–740. doi: 10.1046/j.1365-2958.1998.00958.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Huang J, Mukherjee A, Cao C, Lutkenhaus J. Analysis of the interaction of FtsZ with itself, GTP, and FtsA. J Bacteriol. 1997;179:5551–5559. doi: 10.1128/jb.179.17.5551-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XC, Margolin W. FtsZ ring clusters in min and partition mutants: role of both the Min system and the nucleoid in regulating FtsZ ring localization. Mol Microbiol. 1999;32:315–326. doi: 10.1046/j.1365-2958.1999.01351.x. [DOI] [PubMed] [Google Scholar]
- Zhou H, Lutkenhaus J. MinC mutants deficient in MinD- and DicB-mediated cell division inhibition due to loss of interaction with MinD, DicB, or a septal component. J Bacteriol. 2005;187:2846–2857. doi: 10.1128/JB.187.8.2846-2857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]