Abstract
The effect of endothelin-1 and its receptors EDNRA and EDNRB in migraine with aura (MA) susceptibility is not established yet. We studied the association between the MA end-diagnosis and three migraine trait components and 32 single nucleotide polymorphisms (SNPs) capturing the variation of endothelin genes in 850 Finnish migraine patients and 890 non-migrainous individuals. The SNPs showing evidence of association were further studied in 648 German migraine patients and 651 non-migrainous individuals. No significant association was detected. However, the homozygous minor genotype (5% in cases) of the EDNRA SNP rs2048894 showed nominal association with MA both in the Finnish sample (P = 0.015) and in the pooled sample [odds ratio (OR) 1.61, 95% confidence interval (CI) 1.12-2.32, P = 0.010] when adjusted for gender and sample origin. The trait age of onset < 20 years was also associated with rs2048894 (OR 1.69, 95% CI 1.13-2.54, P = 0.011) in the pooled sample. To confirm this finding studies on even larger samples are required.
Keywords: Endothelin-1, endothelin receptors A and B, migraine with aura, single nucleotide polymorphism
Introduction
There are two main hypotheses concerning the pathophysiology of migraine aura. The ‘vascular theory of Wolff’ postulates (1) that migraine starts in the walls of blood vessels where cerebral vasoconstriction causes the aura and prompts also vasodilation during migraine headache. On the other hand, the neuronal theory disagrees and emphasizes neuronal cortical mechanisms and downgrades the vascular changes to secondary symptoms. Mechanisms that connect vessels and neurons could be the key to migraine. Cortical spreading depression (CSD) of Leão is one such mechanism (2). CSD is characterized by a slowly propagating depolarization wave along the cortex, which closely resembles the gradual spread of the typical visual migraine aura (3). The origin of CSD is in the neuron and glia, but it provokes changes in vascular tone as it moves along the cortex (4, 5).
Endothelin-1, coded by EDN1 on chromosome 6p24 (6), is one of the most potent vasoconstrictors known, but also has neuronal effects. EDN1 can provoke CSD in rodents (7), and in humans elevated levels of EDN1 have been measured during migraine attacks (8-11), although a contradictory study exists (12). In mammals EDN1 acts via two distinct receptors, A and B, coded by EDNRA on chromosome 4q31 and EDNRB on chromosome 13q22, respectively, both widely expressed in the brain. EDN1 is able to trigger vasoconstriction and pain through its actions on EDNRA (13). Furthermore, EDNRB mediates vasodilation, probably leading to migrainous headache (14). Interestingly, a single nucleotide polymorphism (SNP) in the ENDRA gene has been associated with migraine (15) and migraine families have shown linkage to the 4q28-4q31 region adjacent to the EDNRA locus (16). Thus, the EDN1 with its receptors has potential to be the link between neural, vascular and pain-related aspects of migraine. Based on these facts, we analysed EDN1 and its receptors EDNRA and EDNRB as candidate genes for migraine with aura (MA) in a large Finnish case–control sample using a dense set of SNP markers. The best associated SNPs were further analysed in a German replication sample. The SNP selection was based on the HapMap data to capture information on the whole genes (17). Because our previous trait component analysis showed suggestive linkage to the migraine traits phonophobia and photo- and phonophobia (on 4q31), and age of onset < 20 years (on 4q28) (16), associations of these traits were also analysed in our study.
Materials and methods
Subjects
The Finnish sample consisted of 850 Finnish migraine patients and 890 non-migrainous subjects. The Finnish migraine patients were recruited from four headache clinics across Finland. Most of the patients were drawn from the Finnish migraine family sample that has been primarily ascertained for MA. The German sample consisted of 648 German migraine patients and 651 individuals without migraine recruited from a single clinical centre. All participants gave informed consent, and approval to conduct the research was obtained from the Helsinki University Central Hospital Ethics Committee for the Finnish study (approval no. 622/E0/02) and from the Bonn University Hospital Ethics Committee (approval no. 184/00) for the German sample.
End-diagnosis
The end-diagnosis was based on the International Headache Society (IHS) criteria in both the Finnish and German migraine patients (18). All Finnish migraine patients completed the validated Finnish Migraine-Specific Questionnaire for Family Studies (19). The selection of the study population is described in detail by Kaunisto et al. (20). After careful re-evaluation of the phenotypes, 708 patients fulfilled the IHS criteria for typical aura with migraine headache (MA; diagnostic criteria 1.2.1) (18). Diagnosis of the German MA patients was made by experienced physicians from a pain clinic in Kiel, Germany. All individuals were of Caucasian origin and completed a questionnaire enabling the identification of migraine-suspicious individuals. A detailed description of the German sample is published by Netzer et al. (21).
Trait components
In addition to the end-diagnosis, we also analysed four other phenotypes: any visual aura, phonophobia, photo- and phonophobia and age of onset < 20 years. One hundred and forty-two patients had visual aura features that did not fulfil IHS criteria. Combined with the 708 MA patients fulfilling the IHS criteria of MA, these patients form the visual aura group in our analyses (n = 850). Of these 850 Finnish migraine patients, 601 had phonophobia and 462 had both photo- and phonophobia. The age of onset was < 20 years in 403 patients. However, for these traits information was not available on 19, 294 and 295 patients, respectively. Table 1 summarizes the different phenotypes and gender distribution in this study.
Table 1.
Number of subjects in the migraine with aura (MA) end-diagnosis, trait and control groups by sex and country
| Finnish sample |
German sample |
|||
|---|---|---|---|---|
| Phenotype | n | % female | n | % female |
| MA | 708 | 79 | 648 | 77 |
| Visual aura | 850* | 79 | 607 | 76 |
| Phonophobia | 601 | 84 | 568 | 79 |
| Photo- and phonophobia | 462 | 83 | 548 | 79 |
| Age of onset (< 20 years) | 403 | 82 | 377 | 75 |
| Controls | 890 | 75 | 651 | 76 |
One hundred and forty-two patients do not fulfil the International Headache Society criteria for MA.
Genotyping
We selected 33 SNPs for genotyping based on the information provided by the HapMap project (dbSNP Build 125) (17) on the Caucasian population. The SNPs that captured most of the genetic information (tagging SNPs) in the studied gene regions were selected using the Haploview 3.2. program (22). If the primer designing (AssayDesign 2.0.7.0 software; Sequenom®, San Diego, CA, USA) for a tagging SNP failed, the nearest possible SNP in as high linkage disequilibrium (LD) as possible based on r2 values was selected for genotyping. Some additional SNPs were selected from the UCSC database (http://genome.ucsc.edu/) (23) based on their position on the exonic or 5′- and 3′-flanking areas.
DNA was extracted from peripheral blood by the standard phenol-chloroform extraction procedure (24) or by using the Autopure LS automated DNA purification instrument (Gentra Systems, Minneapolis, MN, USA) or by salting out procedures. Genotyping was performed using the homogeneous MassExtend MassARRAY system or iPlex (Sequenom®) (25). The Typer 2.0 software (Sequenom®) was used to verify the genotype calls and the in-house developed KariOTyper software was used to handle the genotype data from the Sequenom database (26).
We evaluated the power of our study sample with the Case–Control Genetic Power Calculator for Discrete Traits (http://statgen.iop.kcl.ac.uk/gpc/cc2.html) (27). The power calculation was based on the guidelines provided by Zondervan and Cardon (28): we assumed that the disease-associated allele has a minor allele frequency of 0.20 and carrying this allele increases the risk of migraine to 1.5-fold. Furthermore, we assumed that the studied SNP is not necessarily the causative one but has an allele frequency of 20% and is in a ≥ 80% LD (D') with the predisposing allele. Our sample reached the power of 91% to reject the null hypothesis (no association) at the P-value of 0.05.
Statistical analyses
Genotype distributions in the whole sample and for cases and the non-migrainous controls separately were tested for Hardy–Weinberg equilibrium (HWE) applying the χ2 method in the PLINK 1.0 program (29). The Haploview 4.0 program was used to determine the pair-wise LD between the SNPs (r2 value) within studied gene regions and to identify tagging SNPs and haploblock structures using the confidence interval algorithm implemented in the Haploview program (22, 30). The PLINK 1.0 program was used to calculate allele and genotype frequency-based association statistics (29). As the exclusion threshold, individuals or SNPs with missing > 10% of genotypes were excluded from the analyses. For allelic association the Cochran-Armitage (trend) test was used. The uncorrected allelic or genotypic P-value of ≤ 0.05 was considered as an indication of possible association, and for those genotypes the recessive and dominant gene action tests of the PLINK program for the minor alleles were applied. In the recessive test the genotypes homozygous for the minor allele were tested against all other genotypes between cases and controls, and in the dominant test genotypes either homo- or heterozygous for the minor allele were tested against the homozygous major allele genotypes, respectively. The permutation procedure implemented in the PLINK program was used to elucidate the significance of a single test adjusted for a number of SNPs (the global EMP2 P-value) with 1000 replications (29, 31). The haplotype association analysis for the haplotypes containing the best associated SNPs was performed using the PLINK program. Furthermore, logistic regression analyses of the pooled sample were performed using the spss (version 16; SPSS Inc., Chicago, IL, USA) and Stata (version 9.2; StataCorp LP, College Station, TX, USA) software to determine the effects of the sample origin and gender for SNPs genotyped in the German sample. All given P-values are uncorrected for multiple testing unless otherwise stated.
Results
Genotyping
Altogether, 33 SNPs were genotyped to be analysed for association between the EDN1, EDNRA and EDNRB genes and migraine among Finnish patients. The best observed associations were verified in the German sample of MA patients and non-migrainous individuals. Of the 33 SNPs, one EDN1 SNP (rs6413478) was monomorphic and thus excluded from analyses. Figure 1 presents the location of the studied SNPs for each gene and the confidence interval method-based LD block structures in the Finnish control subjects. The average success rate of genotyping was 99%. The heterozygosity ratio varied between 0.04 and 0.49. Four SNPs showed deviation from HWE (P = 0.004-0.04) in cases, controls or both, but manual re-checking of these genotypes did not change the genotype calls. The success rates, minor allele frequencies and HWE values are presented in Supplementary Table S1.
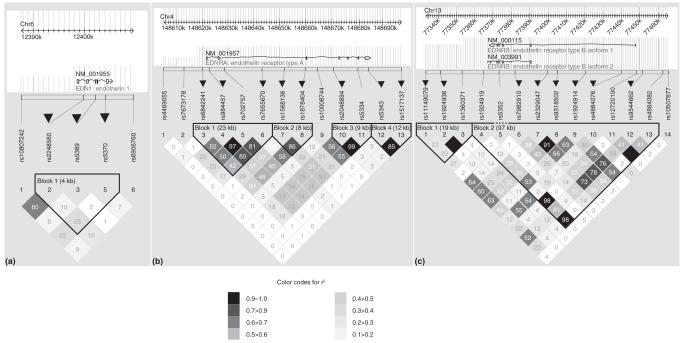
Figure 1.
Linkage disequilibrium (LD) plots of EDN1 (a), EDNRA (b) and EDNRB (c) genes defined by single nucleotide polymorphisms (SNPs) genotyped in the Finnish control subjects. Chromosomal locations of these genes with their coding regions are shown above the LD plots. The pair-wise LD adjusted for allele frequencies (r2 value) is shown as different shadings of grey. The haploblocks were identified with the confidence interval algorithm implemented in the Haploview program that uses the D’ LD statistics. In the figure the haploblocks are circled with a black line. Tagging SNPs that capture the variation within haploblocks are marked with black triangles above the SNP identifiers.
Association results based on the MA end-diagnosis
We found no statistically significant association between EDN1, EDNRA and EDNRB SNPs and the MA end-diagnosis. The corresponding allele and genotype frequencies are summarized in Supplementary Table S2 along with χ2 and P-values. Furthermore, allelic odds ratio (OR) values with a confidence interval (CI) of 95% are shown. However, two EDNRA SNPs showed nominally suggestive association. A genotype-based P-value of 0.028 was detected with the SNP rs5334, while the SNP rs2048894 in a high LD (r2 approximately 1.0) with rs5334 showed a P-value of 0.046. However, when adjusting the tests for the 32 analysed SNPs no globally significant P-value (P < 0.05) was gained after permutation. These results did not change in the gender-specific analyses either. On the other hand, gender-specific analysis improved the allelic P-value of the EDNRB SNP rs2329047 to 0.035 (OR 1.20, 95% CI 1.01-1.42) when only females were treated as affected. Furthermore, the haplotype analysis for the haplotypes containing the SNPs showing the smallest P-values did not provide any evidence of association either in the whole sample or in females (data not shown).
The nominally suggestive findings were further analysed under the recessive and dominant model of the PLINK program and these association values are shown in Supplementary Table S3. The genotypic association of the EDNRA SNP rs2048894 was due to minor homozygous genotypes, 5% in cases and 3% in controls (recessive P-value of 0.015). This P-value did not change in the gender-specific analysis. The association detected between the EDNRB SNP rs2329047 and females with MA was also due to the minor homozygous genotype (P-value of 0.037; 16% in patients and 12% in control individuals, Supplementary Table S3).
We selected two tagging SNPs providing intriguing association values (P < 0.05), the EDNRA SNP rs2048894 and the EDNRB SNP rs2329047, to be tested in the German MA sample. No significant association was seen either in the whole German sample or in the females-only analyses (Supplementary Table S3). However, since similar overrepresentation of homozygous minor allele genotype was seen in cases compared with controls in the German sample (7% in MA cases vs. 5% in controls) as in the Finnish sample (5% in MA cases vs. 3% in controls) an association test in the combined sample was performed. To be able to conduct association analysis of the pooled sample the effect of the risk genotype should be similar in both samples. This was tested using the logistic regression model with an interaction term (genotype by country). Since the effect was the same, we performed analysis in the pooled sample adjusted for the sample origin and gender. The results indicated that the homozygous minor genotype of rs2048894 was associated with increased risk of MA (OR 1.61, 95% CI 1.21-2.32, P = 0.010). In the corresponding analysis of the EDNRB SNP rs2329047, no association was detected (data not shown).
Association results based on traits
In our sample 850 individuals had visual aura symptoms (according to the IHS or not meeting all the criteria preceding their headache phase). No association between the visual aura symptoms and the three genes studied was detected (Supplementary Fig. S1 shows the −log P-values for these genes). However, in the gender-specific analysis three EDNRB SNPs, rs1924917, rs12720100 and rs4884082, showed nominal allelic associations of 0.024, 0.019 and 0.021 [minor allele frequency (MAF) of 12% in cases and 18% in controls for each SNP] in the males, respectively. These SNPs are in high LD (r2 > 0.96) in the male control subjects. The number of affected males (n = 177) studied is, however, small and associations did not survive from the permutation procedures (permutated P-value > 0.05).
EDNRA was tested as a candidate gene for traits phonophobia and photo- and phonophobia, and age of onset < 20 years. No significant association with phonophobia or photo- and phonophobia was detected in the whole sample (Supplementary Fig. S2). However, in the gender-specific analysis nominal association between phonophobia and the EDNRA SNP rs7655670 was detected in females (genotypic P-value of 0.028), but neither the dominant nor the recessive tests produced further evidence of association (data not shown).
The trait age of onset < 20 years showed nominal association with the same two SNPs rs2048894 and rs5334, which showed association with the MA end-diagnosis [P-values of 0.020 (−log P = 1.70) and 0.012 (−log P = 1.94)]. In the gender-specific analyses association with the trait age of onset < 20 years improved slightly in the female sample: with rs2048894 the genotypic P-value of 0.013 and recessive P-value of 0.0081 were detected (Supplementary Table S3). When this result was adjusted for the number of tested SNPs no association was detected (EMP2 P-value of 0.28). Analysis of the EDNRA SNP rs2048894 in the German sample was not significant. However, in the pooled sample a genotypic P-value of 0.023 was obtained and a P-value of 0.011 (OR 1.69, 95% CI 1.13-2.54) was gained for a minor homozygous genotype.
Discussion
END1 and its receptors A and B are good candidates in migraine pathophysiology due to their role in controlling both neuronal and vascular homeostasis (4, 7). To clarify the role of the genes encoding these proteins, we collected a large set of MA patients and carefully selected control subjects. Our strategy was to study the association between endothelin genes and migraine using the information provided by the HapMap project (17). Unlike in the two previous studies (15, 32), with this haploblock-based approach we could choose SNPs that capture the common variation (minor allele frequency of > 10%) within the genes and 5′- and 3′-untranslated regions that may have importance in the regulation of gene transcription. In addition to 19 tagging SNPs, we also studied 13 other SNPs including some amino acid changing ones. A German replication sample was used to study association of the SNPs giving the most promising results.
No significant evidence of association was detected between any of the SNPs and migraine. However, nominal associations (P = 0.02-0.05) were seen between two ENDRA SNPs, rs2048894 and rs5334, and the MA end-diagnosis. The functional significance of these two SNPs, the former being intronic and the latter a synonymous exonic SNP, is unknown. Overrepresentation of the same alleles or genotypes was seen both in the Finnish and German patients. For example, in the Finnish sample the frequency of the minor homozygous genotype of rs2048894 was 5% in MA patients and 3% in the controls. In the German sample the corresponding values were 7% and 5%. Thus, when the analyses were performed in the pooled sample adjusted for gender and sample origin, a P-value of 0.010 was obtained. Interestingly, according to the HapMap data (17), the EDNRA SNP rs2048894 is not in LD (r2 = 0.078) with the variant showing association with migraine in the study by Tzourio et al. (15). A nominal (P = 0.035) association of the EDNRB SNP rs2329047 with the MA end-diagnosis was seen when females were treated as affected. However, this finding did not remain significant in the German or pooled sample. When using the less strict visual aura phenotype including migraine cases with aura symptoms not fulfilling the IHS aura criteria, no evidence of association was detected.
We also applied the trait component analysis strategy (TCA) (16, 33) to study associations between EDNRA and the traits phonophobia and photo- and phonophobia and age of onset < 20 years, which had shown suggestive evidence of linkage adjacent to the ENDRA locus in our previous linkage analysis of 50 Finnish MA families (16). TCA uses single migraine symptoms, which may vary even between the members of a single family, as phenotypes. In other words, the symptoms may sum up to the same end-diagnosis although the underlying components differ from each other. The best uncorrected P-value reached using this strategy was 0.0081 for the EDNRA SNP rs2048894. It was detected in females using a recessive model and the trait age of onset < 20 years as a phenotype. Similar to the association with the MA enddiagnosis, this association also is due to a rare homozygous genotype being slightly more common (5%) in patients than in control subjects (3%).
There are some limitations to our study. We can obviously not exclude the possibility of variants with a modest effect in the current sample size. Our study is potent to detect common variants with the relative risk of ≥ 1.5. On the other hand, recent genome-wide association analyses on different complex diseases have indicated that associated common SNPs (MAF ≥ 10%) have often modest risk of < 1.5 in the population (34). Thus, our gender-specific analyses with trait components lacked power to assign conclusive evidence of low-effect susceptibility variants. It is also possible that the EDN1, EDNRA and EDNRB genes may have a greater functional significance in the pathophysiology of migraine without aura (MoA) than MA. In the study of Tzourio and co-workers (15) the association between the EDNRA SNP and migraine was detected in a study population where 77% of the patients were suffering from MoA. Furthermore, studies measuring plasma EDN1 levels have been performed mainly with MoA patients (8-12).
Our results do not remain significant when the correction for multiple testing is taken into account. However, we can not totally exclude the role of the EDNRA gene in MA susceptibility, since (i) the SNPs rs2048894 and rs5334 showed nominal association with two different phenotypes in the Finnish sample (the MA end-diagnosis and the trait age of onset < 20 years); (ii) the difference in the frequency of the rare risk genotype of the rs2048894 SNP between the cases and controls in both the Finnish and German samples was similar; (iii) analyses in the pooled sample also showed nominal association between the rs2048894 SNP and the MA end-diagnosis with relative risk of 1.6. Since the homozygous minor genotype of this SNP is relatively rare (5% in Finnish migraine patients), even larger samples are required to confirm this finding. For a more conclusive study both functional studies and quantitative association analyses based on plasma endothelin-1 levels should be performed in both MA and MoA samples.
Supplementary Material
Results of genotypic and allelic trend tests for the EDN1 (a), EDNRA (b) and EDNRB (c) single nucleotide polymorphisms (SNPs) using the visual aura trait. Marker position (Mb) is plotted along the x-axis while the −log P-values are plotted along the y-axis.
Results of genotypic and allelic trend tests of the EDNRA single nucleotide polymorphisms (SNPs) on the traits phonophobia, phono- and photophobia and age of onset < 20 years. Marker position (Mb) is plotted along the x-axis while the −log P-values are plotted along the y-axis. The dotted line represents the −log P-value threshold value of 1.3 corresponding to a P-value of 0.05.
Success rates, heterozygosity values, Hardy–Weinberg equilibrium P-values and minor allele frequencies of 32 genotyped SNPs in the Finnish sample. SNP, single nucleotide polymorphism; Nt, nucleotide; Aa, amino acid; Het, heterozygosity value; HWE, Hardy–Weinberg equilibrium; MAF, minor allele frequency; kb, kilobase; chr, chromosome
Table S2
Allele and genotype frequencies of the studied SNPs with χ2 and P-values and allelic OR values with a 95% CI. SNP, single nucleotide polymorphism; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval; chr, chromosome.
Table S3
Detailed genotype association data for the SNPs in the Finnish and German sample (P-values < 0.05 are in bold). *In the genotypic association test genotype frequencies (11/12/22), allelic association test allele frequencies (trend-test, 1/2), dominant association test genotypes with minor allele (11, 12) vs. genotypes homozygous for major allele (22) and recessive association test genotypes homozygous for minor allele (11) vs. genotypes with major allele (12, 22) are given. SNP, single nucleotide polymorphism; Aff, affected; Unaff, unaffected; DF, degree of freedom; geno, genotypic association test; trend, allelic association Cochran–Armitage test; dom, dominant gene action test; rec, recessive gene action test.
Acknowledgements
This study was supported by the Sigrid Juselius Foundation, the Academy of Finland (200923 to A.P.; 00213 to M.W.), the Helsinki University Central Hospital, the EuroHead (LSHM-CT-2004-504837), the GenomEUtwin project (QLG2-CT-2002-01254), the Oxnard Foundation, the Helsinki Biomedical Graduate School and Biomedicum Helsinki Foundation (to P.T-K.), the Nordic Centre of Excellence for Disease Genetics, Centre of Excellence for Complex Disease Genetics of the Academy of Finland, and the National Institutes of Health (RO1 NS37675 to A.P.). The German study was supported by the Deutsche Forschungsgemeinschaft (DFG, FOR423), National Genome Network (NGFN) by the Bundesministerium für Bildung und Forschung (BMBF), Centre for Molecular Medicine (ZMMK), University of Cologne. We thank Mrs I. Goebel for technical assistance. Finally, we wish to thank the Finnish and German migraine patients for their invaluable participation in this study.
References
- 1.Wolff HG. Headache and other head pain. Oxford University Press; London: 1963. [Google Scholar]
- 2.Leão AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–90. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 3.Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA. 2001;98:4687–92. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreier JP, Kleeberg J, Petzold G, Priller J, Windmüller O, Orzechowski HD, et al. Endothelin-1 potently induces Leão’s cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura? Brain. 2002;125:102–12. doi: 10.1093/brain/awf007. [DOI] [PubMed] [Google Scholar]
- 5.Brennan KC, Beltrán-Parrazal L, López-Valdés HE, Theriot J, Toga AW, Charles AC. Distinct vascular conduction with cortical spreading depression. J Neurophysiol. 2007;97:4143–51. doi: 10.1152/jn.00028.2007. [DOI] [PubMed] [Google Scholar]
- 6.Yanagisawa M, Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989;10:374–8. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]
- 7.Kleeberg J, Petzold GC, Major S, Dirnagl U, Dreier JP. ET-1 induces cortical spreading depression of the ET A receptor/phospholipase C pathway in vivo. AJP - Heart. 2004;286:H1339–46. doi: 10.1152/ajpheart.00227.2003. [DOI] [PubMed] [Google Scholar]
- 8.Färkkilä M, Palo J, Saijonmaa O, Fyhrquist F. Raised plasma endothelin during acute migraine attack. Cephalalgia. 1992;12:383–4. doi: 10.1111/j.1468-2982.1992.00383.x. [DOI] [PubMed] [Google Scholar]
- 9.Gallai V, Sarchielli P, Firenze C, Trequattrini A, Paciaroni M, Usai F, Palumbo R. Endothelin 1 in migraine and tension-type headache. Acta Neurol Scand. 1994;89:47–55. doi: 10.1111/j.1600-0404.1994.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 10.Kallela M, Färkkilä M, Saijonmaa O, Fyhrquist F. Endothelin in migraine patients. Cephalalgia. 1998;18:329–32. doi: 10.1046/j.1468-2982.1998.1806329.x. [DOI] [PubMed] [Google Scholar]
- 11.Hasselblatt M, Köhler J, Volles E, Ehrenreich H. Simultaneous monitoring of endothelin-1 and vasopressin plasma levels in migraine. Neuroreport. 1999;10:423–5. doi: 10.1097/00001756-199902050-00039. [DOI] [PubMed] [Google Scholar]
- 12.Nattero G, Mengozzi G, Inconis T, Paradisi L. Nitric oxide, endothelin-1, and transcranial Doppler in migraine. Findings in interictal conditions and during migraine attack. Headache. 1996;36:307–11. doi: 10.1046/j.1526-4610.1996.3605307.x. [DOI] [PubMed] [Google Scholar]
- 13.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–61. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 14.Takayanagi R, Kitazumi K, Takasaki C, Ohnaka K, Aimoto S, Tasaka K, et al. Presence of non-selective type of endothelin receptor on vascular endothelium and its linkage to vasodilation. FEBS Lett. 1991;282:103–6. doi: 10.1016/0014-5793(91)80454-b. [DOI] [PubMed] [Google Scholar]
- 15.Tzourio C, El Amrani M, Poirier O, Nicaud V, Bousser M-G, Alpérovitch A. Association between migraine and endothelin type A receptor (ETA-231 A/G) gene polymorphism. Neurology. 2001;56:1273–7. doi: 10.1212/wnl.56.10.1273. [DOI] [PubMed] [Google Scholar]
- 16.Anttila V, Kallela M, Oswell G, Kaunisto MA, Nyholt DR, Hämäläinen E, et al. Trait components provide tools to dissect the genetic susceptibility of migraine. Am J Hum Genet. 2006;79:85–99. doi: 10.1086/504814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The International Haplotype Map Consortium The International HapMap Project. Nature. 2003;426:789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 18.Headache Classification Committee of the International Headache Society The international classification of headache disorders, 2nd edition. Cephalalgia. 2004;24(Suppl. 1):1–96. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 19.Kallela M, Wessman M, Färkkilä M. Validation of a migraine specific questionnaire for use in family studies. Eur J Neurol. 2001;8:61–6. doi: 10.1046/j.1468-1331.2001.00165.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaunisto MA, Kallela M, Hämäläinen E, Kilpikari R, Havanka H, Harno H, et al. Testing of variants of the MTHFR and ESR1 genes in 1798 Finnish individuals fails to confirm the association with migraine with aura. Cephalalgia. 2006;26:1462–72. doi: 10.1111/j.1468-2982.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- 21.Netzer C, Freudenberg J, Toliat MR, Heinze A, Heinze-Kuhn K, Thiele H, et al. Genetic association studies of the chromosome 15 GABA-A receptor cluster in migraine with aura. Am J Med Genet B Neuropsychatr Genet. 2008;147B:37–41. doi: 10.1002/ajmg.b.30560. [DOI] [PubMed] [Google Scholar]
- 22.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 23.Karolchik D, Baertsch R, Diekhans M, Furey TS, Hinrichs A, Lu YT, et al. The UCSC genome browser database. Nucleic Acids Res. 2003;31:51–4. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976;3:2303–8. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leushner J, Chiu NH. Automated mass spectrometry: a revolutionary technology for clinical diagnostics. Mol Diagn. 2000;5:341–8. doi: 10.1007/BF03262095. [DOI] [PubMed] [Google Scholar]
- 26.Tikka-Kleemola P, Hämäläinen E, Tuomainen K, Suvela M, Artma A, Kahre O, et al. The enhancement of homogenous mass extension reaction: comparison of two enzymes. Mol Cell Probes. 2007;21:216–21. doi: 10.1016/j.mcp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Purcell S, Cherny SS, Sham PC. Genetic power calculator: Design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–50. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 28.Zondervan KT, Cardon LR. Designing candidate gene and genome-wide case–control association studies. Nat Protoc. 2007;2:2492–501. doi: 10.1038/nprot.2007.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 31.Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genet. 1994;138:963–71. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisi V, Garbo G, Battistella P, Miccichè F, Stecca A, Terrazzino S, et al. Endothelin receptor A-231G>A polymorphism: no linkage to primary pediatric headache. Headache. 2006;46:486–91. doi: 10.1111/j.1526-4610.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 33.Anttila V, Nyholt DR, Kallela M, Artto V, Vepsäläinen S, Jakkula E, et al. Consistently replicating locus linked to migraine on 10q22-23. Am J Hum Genet. 2008;82:1051–63. doi: 10.1016/j.ajhg.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altshuler D, Daly M. Guilt beyond a reasonable doubt. Nat Genets. 2007;39:813–15. doi: 10.1038/ng0707-813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of genotypic and allelic trend tests for the EDN1 (a), EDNRA (b) and EDNRB (c) single nucleotide polymorphisms (SNPs) using the visual aura trait. Marker position (Mb) is plotted along the x-axis while the −log P-values are plotted along the y-axis.
Results of genotypic and allelic trend tests of the EDNRA single nucleotide polymorphisms (SNPs) on the traits phonophobia, phono- and photophobia and age of onset < 20 years. Marker position (Mb) is plotted along the x-axis while the −log P-values are plotted along the y-axis. The dotted line represents the −log P-value threshold value of 1.3 corresponding to a P-value of 0.05.
Success rates, heterozygosity values, Hardy–Weinberg equilibrium P-values and minor allele frequencies of 32 genotyped SNPs in the Finnish sample. SNP, single nucleotide polymorphism; Nt, nucleotide; Aa, amino acid; Het, heterozygosity value; HWE, Hardy–Weinberg equilibrium; MAF, minor allele frequency; kb, kilobase; chr, chromosome
Table S2
Allele and genotype frequencies of the studied SNPs with χ2 and P-values and allelic OR values with a 95% CI. SNP, single nucleotide polymorphism; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval; chr, chromosome.
Table S3
Detailed genotype association data for the SNPs in the Finnish and German sample (P-values < 0.05 are in bold). *In the genotypic association test genotype frequencies (11/12/22), allelic association test allele frequencies (trend-test, 1/2), dominant association test genotypes with minor allele (11, 12) vs. genotypes homozygous for major allele (22) and recessive association test genotypes homozygous for minor allele (11) vs. genotypes with major allele (12, 22) are given. SNP, single nucleotide polymorphism; Aff, affected; Unaff, unaffected; DF, degree of freedom; geno, genotypic association test; trend, allelic association Cochran–Armitage test; dom, dominant gene action test; rec, recessive gene action test.