Abstract
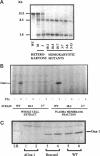
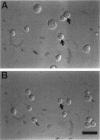
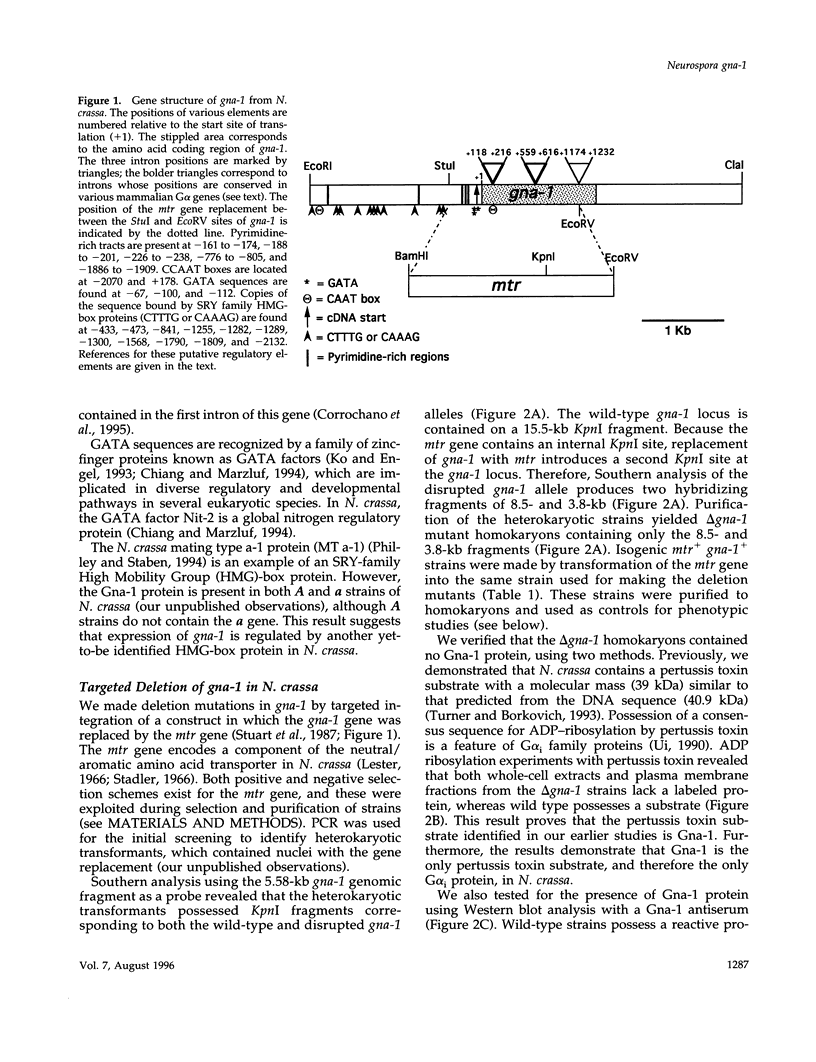
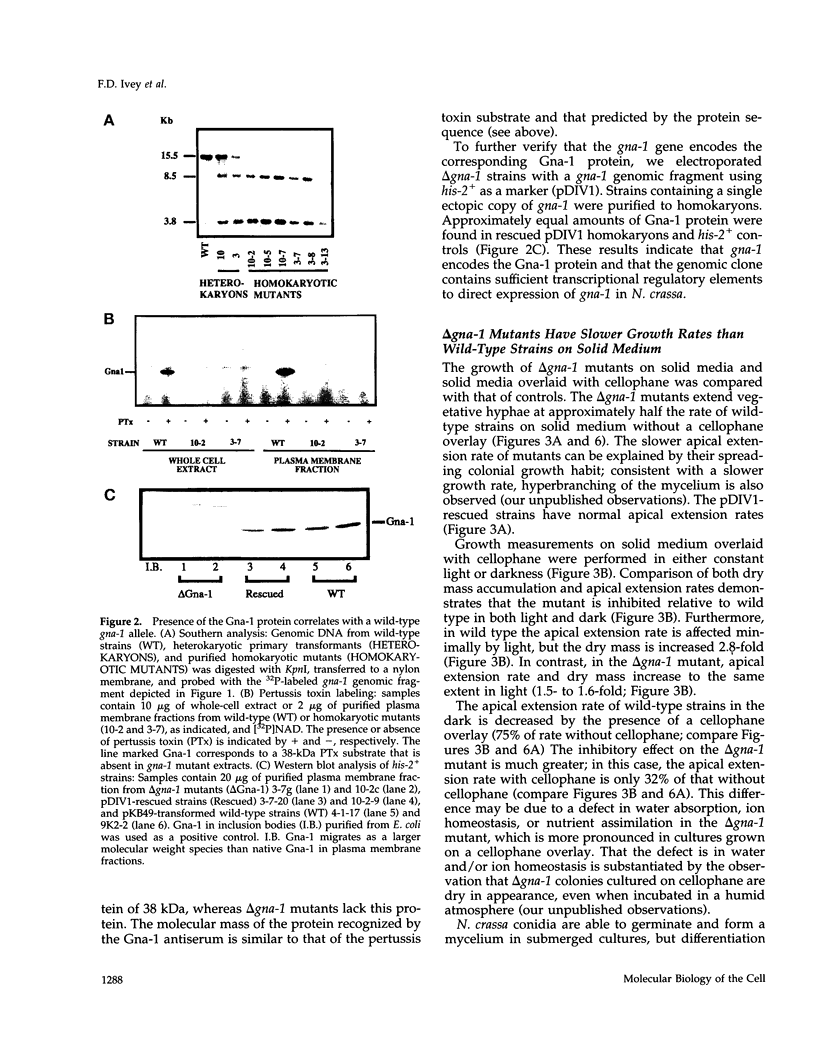
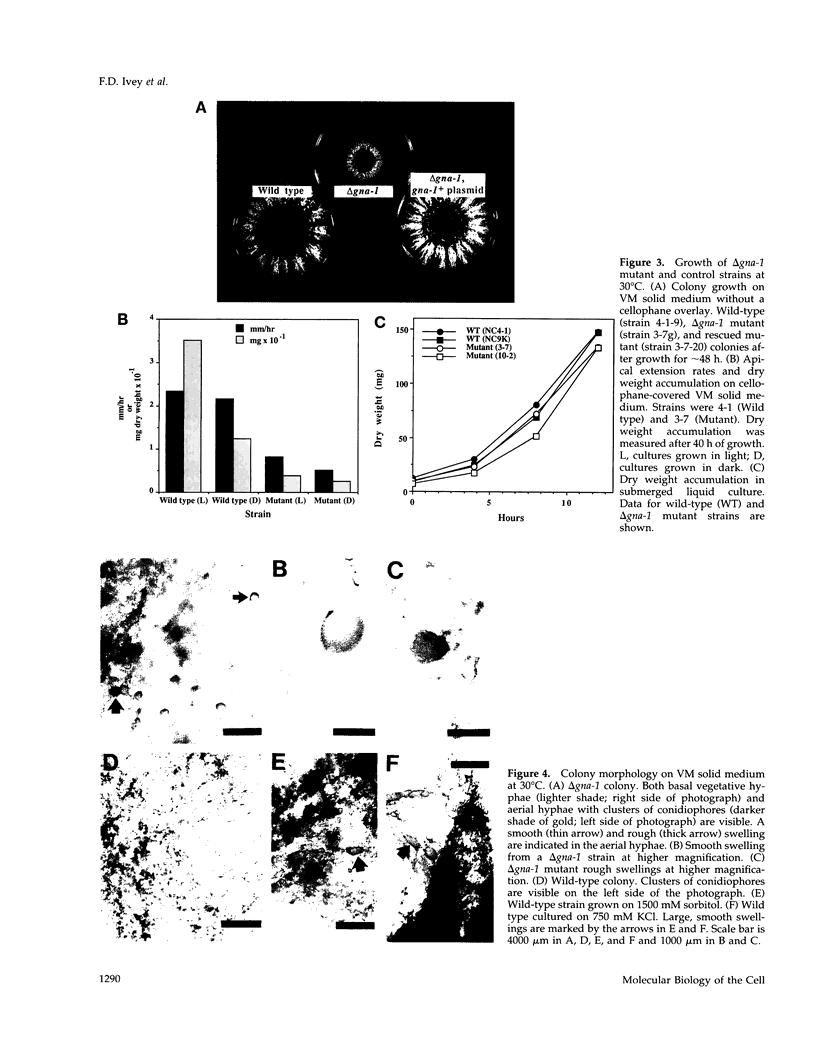
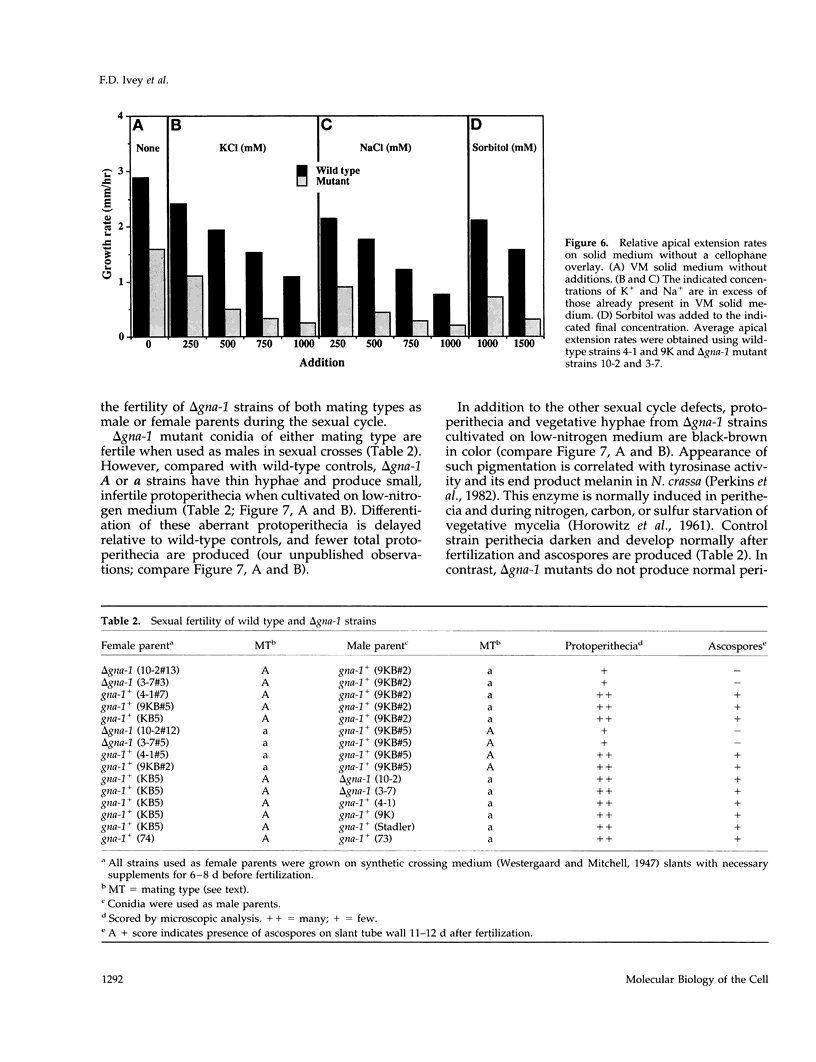
Heterotrimeric G proteins are components of principal signaling pathways in eukaryotes. In higher organisms, alpha subunits of G proteins have been divided into four families, Gi, Gs, Gq, and G12. We previously identified a G alpha i homologue gna-1 in the filamentous fungus Neurospora crassa. Now we report that deletion of gna-1 leads to multiple phenotypes during the vegetative and sexual cycles in N. crassa. On solid medium, delta gna-1 strains have a slower rate of hyphal apical extension than wild type, a rate that is more pronounced under hyperosmotic conditions or in the presence of a cellophane overlay. delta gna-1 mutants accumulate less mass than wild-type strains, and their mass accumulation is not affected in the same way by exposure to light. delta gna-1 strains are defective in macroconidiation, possessing aerial hyphae that are shorter, contain abnormal swellings, and differentiate adherent macroconidia. During the sexual cycle, delta gna-1 strains are fertile as males. However, the mutants are female-sterile, producing small, aberrant female reproductive structures. After fertilization, delta gna-1 female structures do not enlarge and develop normally, and no sexual spores are produced. Thus, mutation of gna-1 results in sex-specific loss of fertility.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson B. D., Lindgren K. M., Dunlap J. C., Loros J. J. An efficient method for gene disruption in Neurospora crassa. Mol Gen Genet. 1994 Feb;242(4):490–494. doi: 10.1007/BF00281802. [DOI] [PubMed] [Google Scholar]
- Baxevanis A. D., Landsman D. The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Res. 1995 May 11;23(9):1604–1613. doi: 10.1093/nar/23.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B. R., Christie P. J. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994 Jun;176(12):3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiello H. F., Patenaude C. R., Ausiello D. A. G protein subunit, alpha i-3, activates a pertussis toxin-sensitive Na+ channel from the epithelial cell line, A6. J Biol Chem. 1989 Dec 15;264(35):20867–20870. [PubMed] [Google Scholar]
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T. Y., Marzluf G. A. DNA recognition by the NIT2 nitrogen regulatory protein: importance of the number, spacing, and orientation of GATA core elements and their flanking sequences upon NIT2 binding. Biochemistry. 1994 Jan 18;33(2):576–582. doi: 10.1021/bi00168a024. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Chen B., Nuss D. L. Virus-mediated or transgenic suppression of a G-protein alpha subunit and attenuation of fungal virulence. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):305–309. doi: 10.1073/pnas.92.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrochano L. M., Lauter F. R., Ebbole D. J., Yanofsky C. Light and developmental regulation of the gene con-10 of Neurospora crassa. Dev Biol. 1995 Jan;167(1):190–200. doi: 10.1006/dbio.1995.1016. [DOI] [PubMed] [Google Scholar]
- Degli-Innocenti F., Chambers J. A., Russo V. E. Conidia induce the formation of protoperithecia in Neurospora crassa: further characterization of white collar mutants. J Bacteriol. 1984 Aug;159(2):808–810. doi: 10.1128/jb.159.2.808-810.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Flick J. S., Thorner J. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Sep;13(9):5861–5876. doi: 10.1128/mcb.13.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994 Aug 5;265(5173):806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Guarente L., Lalonde B., Gifford P., Alani E. Distinctly regulated tandem upstream activation sites mediate catabolite repression of the CYC1 gene of S. cerevisiae. Cell. 1984 Feb;36(2):503–511. doi: 10.1016/0092-8674(84)90243-5. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Johnson G. L. Mitogenic pathways regulated by G protein oncogenes. Mol Biol Cell. 1992 Jan;3(1):123–128. doi: 10.1091/mbc.3.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Lowndes J. M., Pleiman C. M., Sable C., Eisfelder B. J., Johnson G. L. Analysis of the fibroblast transformation potential of GTPase-deficient gip2 oncogenes. Mol Cell Biol. 1992 Jan;12(1):190–197. doi: 10.1128/mcb.12.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ N. H., FLING M., MACLEOD H., WATANABE Y. Structural and regulative genes controlling tyrosinase synthesis in Neurospora. Cold Spring Harb Symp Quant Biol. 1961;26:233–238. doi: 10.1101/sqb.1961.026.01.028. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J. D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hong K., Driscoll M. A transmembrane domain of the putative channel subunit MEC-4 influences mechanotransduction and neurodegeneration in C. elegans. Nature. 1994 Feb 3;367(6462):470–473. doi: 10.1038/367470a0. [DOI] [PubMed] [Google Scholar]
- Howe H. B., Jr, Prakash V. A regulatory system controlling inhibition in the sexual cycle of Neurospora. Can J Genet Cytol. 1969 Sep;11(3):689–705. doi: 10.1139/g69-082. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Cogan M. G., Cragoe E. J., Jr, Ives H. E. Thrombin activation of the Na+/H+ exchanger in vascular smooth muscle cells. Evidence for a kinase C-independent pathway which is Ca2+-dependent and pertussis toxin-sensitive. J Biol Chem. 1987 Oct 15;262(29):14134–14140. [PubMed] [Google Scholar]
- Huang M., Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature. 1994 Feb 3;367(6462):467–470. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- Itoh H., Toyama R., Kozasa T., Tsukamoto T., Matsuoka M., Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988 May 15;263(14):6656–6664. [PubMed] [Google Scholar]
- Johnson G. L., Gardner A. M., Lange-Carter C., Qian N. X., Russell M., Winitz S. How does the G protein, Gi2, transduce mitogenic signals? J Cell Biochem. 1994 Apr;54(4):415–422. doi: 10.1002/jcb.240540408. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Toyama R., Tsukamoto T., Matsuoka M., Nakafuku M., Obara T., Takagi T., Hernandez R. Structures of the genes coding for G-protein alpha subunits from mammalian and yeast cells. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):209–220. doi: 10.1101/sqb.1988.053.01.027. [DOI] [PubMed] [Google Scholar]
- Ko L. J., Engel J. D. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol. 1993 Jul;13(7):4011–4022. doi: 10.1128/mcb.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo K., Stuart W. D. Sequence and structure of mtr, an amino acid transport gene of Neurospora crassa. Genome. 1991 Aug;34(4):644–651. doi: 10.1139/g91-098. [DOI] [PubMed] [Google Scholar]
- Kozasa T., Itoh H., Tsukamoto T., Kaziro Y. Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2081–2085. doi: 10.1073/pnas.85.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kurjan J. Pheromone response in yeast. Annu Rev Biochem. 1992;61:1097–1129. doi: 10.1146/annurev.bi.61.070192.005313. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lester G. Genetic control of amino acid permeability in Neurospora crassa. J Bacteriol. 1966 Feb;91(2):677–684. doi: 10.1128/jb.91.2.677-684.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Errede B. The proliferation of MAP kinase signaling pathways in yeast. Curr Opin Cell Biol. 1995 Apr;7(2):197–202. doi: 10.1016/0955-0674(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Lochrie M. A., Mendel J. E., Sternberg P. W., Simon M. I. Homologous and unique G protein alpha subunits in the nematode Caenorhabditis elegans. Cell Regul. 1991 Feb;2(2):135–154. doi: 10.1091/mbc.2.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Maeda T., Takekawa M., Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995 Jul 28;269(5223):554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Mendel J. E., Korswagen H. C., Liu K. S., Hajdu-Cronin Y. M., Simon M. I., Plasterk R. H., Sternberg P. W. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science. 1995 Mar 17;267(5204):1652–1655. doi: 10.1126/science.7886455. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Neer E. J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995 Jan 27;80(2):249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Parks S., Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991 Jan 25;64(2):447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D. The use of duplication-generating rearrangements for studying heterokaryon incompatibility genes in Neurospora. Genetics. 1975 May;80(1):87–105. doi: 10.1093/genetics/80.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philley M. L., Staben C. Functional analyses of the Neurospora crassa MT a-1 mating type polypeptide. Genetics. 1994 Jul;137(3):715–722. doi: 10.1093/genetics/137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raport C. J., Dere B., Hurley J. B. Characterization of the mouse rod transducin alpha subunit gene. J Biol Chem. 1989 May 5;264(13):7122–7128. [PubMed] [Google Scholar]
- Reissig J. L., Kinney S. G. Calcium as a branching signal in Neurospora crassa. J Bacteriol. 1983 Jun;154(3):1397–1402. doi: 10.1128/jb.154.3.1397-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G., Pall M. L. Properties of two cyclic nucleotide-deficient mutants of Neurospora crassa. J Bacteriol. 1979 Mar;137(3):1140–1144. doi: 10.1128/jb.137.3.1140-1144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A. Adenosine 3':5'-cyclic monophosphate deficiency in Neurospora crassa. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2995–2999. doi: 10.1073/pnas.73.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Springer M. L., Yanofsky C. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 1989 Apr;3(4):559–571. doi: 10.1101/gad.3.4.559. [DOI] [PubMed] [Google Scholar]
- Stadler D. R. Genetic control of the uptake of amino acids in Neurospora. Genetics. 1966 Aug;54(2):677–685. doi: 10.1093/genetics/54.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart W. D., Koo K., Vollmer S. J. Cloning of mtr, an amino acid transport gene of Neurospora crassa. Genome. 1988 Apr;30(2):198–203. doi: 10.1139/g88-034. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Ségalat L., Elkes D. A., Kaplan J. M. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science. 1995 Mar 17;267(5204):1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Adenylyl cyclases. Cell. 1992 Sep 18;70(6):869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- Turner G. E., Borkovich K. A. Identification of a G protein alpha subunit from Neurospora crassa that is a member of the Gi family. J Biol Chem. 1993 Jul 15;268(20):14805–14811. [PubMed] [Google Scholar]
- Vigfusson N. V., Cano R. J. Artificial induction of the sexual cycle of Neurospora crassa. Nature. 1974 May 24;249(455):383–385. doi: 10.1038/249383a0. [DOI] [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya T., Conklin B. R., Gilbert R. L., Hooley R., Bourne H. R., Barber D. L. G alpha 13 stimulates Na-H exchange. J Biol Chem. 1994 Feb 18;269(7):4721–4724. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weinstein L. S., Spiegel A. M., Carter A. D. Cloning and characterization of the human gene for the alpha-subunit of Gi2, a GTP-binding signal transduction protein. FEBS Lett. 1988 May 23;232(2):333–340. doi: 10.1016/0014-5793(88)80764-6. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]