Abstract
Objectives. We assessed the potential health and economic benefits of reducing common risk factors in older Americans.
Methods. A dynamic simulation model tracked a national cohort of persons 51 and 52 years of age to project their health and medical spending in prevention scenarios for diabetes, hypertension, obesity, and smoking.
Results. The gain in life span from successful treatment of a person aged 51 or 52 years for obesity would be 0.85 years; for hypertension, 2.05 years; and for diabetes, 3.17 years. A 51- or 52-year-old person who quit smoking would gain 3.44 years. Despite living longer, those successfully treated for obesity, hypertension, or diabetes would have lower lifetime medical spending, exclusive of prevention costs. Smoking cessation would lead to increased lifetime spending. We used traditional valuations for a life-year to calculate that successful treatments would be worth, per capita, $198 018 (diabetes), $137 964 (hypertension), $118 946 (smoking), and $51 750 (obesity).
Conclusions. Effective prevention could substantially improve the health of older Americans, and—despite increases in longevity—such benefits could be achieved with little or no additional lifetime medical spending.
The rise in life expectancy and improvements in the health status of middle-aged and older Americans during the past half century are indisputable products of investments in medical technologies and successful public health efforts to encourage primary and secondary prevention.1,2 But with a growing elderly population, and a large baby boom generation approaching retirement, the prevalence of chronic diseases will rise. If current trends continue, health care costs will consume an ever-increasing share of national income. The future liability of the Medicare program alone is estimated to be $24 trillion over the next 75 years, absent any policy changes.3
With this renewed national focus on health care costs, policymakers have begun to consider investing more health care dollars in prevention programs and in research to identify better treatment strategies. Medical or public health interventions in the form of primary prevention should, in theory, slow or reduce the rising prevalence of chronic disease and simultaneously attenuate the downstream spending associated with it. Thus, if a solution to the problem of rising health care costs exists now, it may lie in the basic improvements in population health that can be achieved by existing treatments. We modeled the potential health benefits and medical cost savings of successfully treating cardiovascular risk factors (obesity, diabetes, hypertension, and smoking) among middle-aged and older Americans under several hypothetical scenarios.
METHODS
We examined whether prevention will consume more health care resources, and if it does, how much value it generates for society. We focused our cost–benefit analysis on the potential benefits successful prevention strategies might generate. The health benefits of prevention are difficult to measure because treatment simultaneously extends life and changes the relative prevalence of fatal and nonfatal disabling diseases, thereby yielding complicated spending patterns. As a way to understand these competing risks, we developed a dynamic microsimulation model (the Future Elderly Model [FEM]) to track cohorts over time to project their health status and economic outcomes under various prevention scenarios. The FEM has been used to assess the financial risk from new medical technologies for Medicare,4 the costs of obesity in older Americans,5 trends in disability,6 the future costs of cancer,7 and the health and economic value of preventing disease after age 65 years.8
Data and Outcomes
We extended the model to a much larger cohort from the Health and Retirement Study (HRS),9 a biennial survey of Americans aged 51 years and older that began in 1992. We supplemented the HRS with data from the Medicare Current Beneficiary Survey10 and Medical Expenditure Panel Survey11 to model medical spending and quality of life. (A detailed description of the model is available in Appendix A as a supplement to the online version of this article at http://www.ajph.org; here we describe the most salient details.)
The core of the FEM was a health module designed to predict the future health and functional status of each individual from his or her current state, accounting for a broad set of risk factors. Health conditions were derived from survey questions about heart disease (acute myocardial infarction, coronary heart disease, angina, congestive heart failure, or other heart problems), cancer (except skin cancer), chronic bronchitis or emphysema, diabetes, high blood pressure or hypertension, and stroke or transient ischemic attack. Functional status was measured by limitations in activities of daily living (ADLs), instrumental activities of daily living (IADLs), and nursing home residency. The ADLs measure was based on a battery of questions assessing difficulty dressing, eating, bathing or showering; getting into and out of a chair; and walking. For IADLs, respondents were asked if they had any difficulty using the phone, managing money, and taking medicine. From the responses to these questions, we constructed a hierarchical measure of physical functioning: no limitations, limited in at least 1 IADL, limited in 1 or 2 ADLs, and limited in 3 or more ADLs. Whether a person lived in a nursing home was included as a binary measure.
Both functional status and the likelihood of developing a health condition depended on several key risk factors: age, gender, education, race/ethnicity, obesity (body mass index [BMI; defined as weight in kilograms divided by height in meters squared] ≥ 30 kg/m2), overweight (BMI = 25–29 kg/m2), ever smoking, current smoking, and functional status and health conditions. All health conditions were treated as absorbing; that is, once a person had an illness, he or she was assumed to have it forever, consistent with the way the survey questions were asked. Transitions into and out of functional states and obesity status were allowed. We modeled all health conditions, functional states, and risk factors with first-order Markov processes that controlled for baseline unobserved factors in a battery of baseline health variables. These were effective controls, according to goodness-of-fit tests (see Appendix A, available as an online supplement).
A cost module linked a person's current state—demographics, economic status, current health, risk factors, and functional status—to medical spending. These estimates were based on pooled weighted least squares regressions of total health care spending on risk factors, self-reported conditions, and functional status, with spending inflated to constant 2004 dollars derived from the medical component of the consumer price index published by the US Bureau of Labor Statistics.12 We used the 2002 to 2004 Medical Expenditure Panel Survey11 (n = 13 942) for regressions for persons younger than 65 years, and the 2002 to 2004 Medicare Current Beneficiary Survey10 (n = 29 523) for regressions for those aged 65 years and older. In the baseline scenario, this spending estimate could be interpreted as the resources consumed by the individual according to the way medicine is currently practiced in the United States.
To value the health benefits of prevention to society, we predicted quality-adjusted life-years (QALYs) with the EQ-5D, a widely used health-related quality-of-life index. The EQ-5D instrument included 5 questions regarding the extent of problems in mobility, self-care, daily activities, pain, and anxiety and depression; it has been widely used in both Europe and the United States.13,14 We used the 2001 Medical Expenditure Panel Survey11 to estimate a linear model fitting EQ-5D scores as a function of 6 chronic conditions and functional status (details available on request). We used this model to predict a QALY measure for all persons in the simulation in every year by their simulated health and functional status.
With the FEM, we simulated outcomes for a representative cohort of respondents aged 51 or 52 years from the 2004 HRS (n = 1028). In each year, the spending module predicted medical expenditures over the next 2 years by each individual's current state. We then used the health module to predict who would survive to 2006 and to predict the obesity status, disease, and functional state of the surviving population, as well as a QALY for that year. The spending module was then used to predict that period's health care resource use. We repeated the simulation until everyone in the 2004 cohort would have died. For each scenario, we conducted the simulation 10 times and averaged the outcomes. We implemented scenarios by changing the transition probabilities in the health module and then rerunning the model. Primary outcomes were life expectancy, quality-adjusted life expectancy, and lifetime medical spending. All costs and QALYs were discounted by a 3% annual discount rate as suggested by Gold et al.15
To estimate the net benefits, we valued the health improvement (measured by QALY-adjusted life expectancy) minus additional lifetime medical spending. For these calculations, we compared outcomes to the status quo and assumed that each QALY was worth $100 000.
Scenarios
We considered 4 types of interventions: treatment for diabetes, hypertension, obesity, and smoking (Table A in Appendix B, available as a supplement to the online version of this article at http://www.ajph.org). The scenarios were designed to estimate the potential benefits from the development of an efficacious prevention regimen. We modeled these as lifetime prevention of diabetes, hypertension, obesity, or smoking at various efficacy levels. A 10% scenario assumed prevention would be successful for 10% of the at-risk population; other scenarios considered 25%, 50%, and 100% efficacy.
The 100% scenario—although certainly not feasible—was useful for predicting the maximum health and longevity benefit that might be achieved by independently eliminating smoking, hypertension, diabetes, and obesity. This percentage is of policy significance as society grapples with issues such as which diseases would be most valuable to eradicate. All interventions applied only to the population aged 51 years and older because of data limitations.
RESULTS
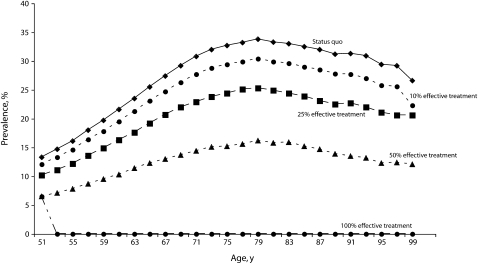
Figure 1 presents the effect of 4 scenarios on diabetes prevalence over the remaining lifetime of the cohort of 51- and 52-year-old respondents. Under the status quo, diabetes prevalence was expected to rise as this population ages, peaking at 33.8% at 79 years of age. In the 10%, 25%, and 50% scenarios, peak diabetes prevalence would be reduced to 30.4%, 25.3%, and 16.2%, respectively. The 100% simulation was the best-case scenario, in which all diabetes would effectively be cured, affecting 43.0% of the cohort who would have developed it in their lifetime.
FIGURE 1.
Prevalence of diabetes in a national cohort of adults aged 51 years, by status quo and 4 intervention scenarios.
We also modeled the effect of 4 hypertension scenarios (Figure A in Appendix B, available as an online supplement). With increasing age, hypertension prevalence in the cohort would rise, reaching 91% at 97 years of age. Under the 10%, 25% and 50% scenarios, peak hypertension prevalence was reduced to 78.9%, 64.5%, and 41.0%, respectively. In the 100% scenario, all hypertension would be cured, affecting the 84.8% of the cohort who would have developed it over their lifetime.
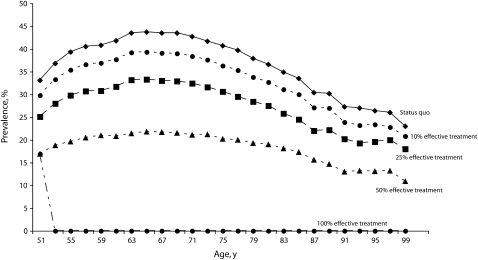
Figure 2 shows the effect of weight reduction in this population. Under the status quo, the prevalence of obesity would continue to rise until 65 years of age, peaking at 43.7%. Under the 10%, 25%, and 50% scenarios, peak obesity prevalence would be reduced to 39.3%, 33.3%, and 21.8%, respectively. Over the cohort's lifetime, 71.3% of the population would become obese, but all of these persons would be successfully treated immediately in the 100%, best-case scenario.
FIGURE 2.
Prevalence of obesity (BMI ≥ 30 kg/m2 ) in a national cohort of adults aged 51 years, by status quo and 4 intervention scenarios.
Smoking prevalence would decrease with age because of the excessive mortality risk of smokers, as well as smoking cessation at older ages (Figure B in Appendix B, available as an online supplement). The prevalence would be reduced to half at 65 years of age. In the 25% scenario, the prevalence would be reduced to half at 61 years of age. In the 50% scenario, the prevalence of smoking would be approximately half of the prevalence in the status quo. Over the cohort's lifetime, 28% of the population would smoke under the status quo, but they would all quit in the 100%, best-case scenario.
Table 1 and Table 2 summarize the health and cost implications of the 4 scenarios, relative to the status quo. Results averaged over the entire population are shown in Table 1. Under the status quo, a person aged 51 or 52 years in 2004 could expect to live 30.19 more years, corresponding to 15.53 discounted QALYs, and would incur $170 013 in medical expenses (2004 dollars). A 10% effective treatment of diabetes control would increase average life expectancy for the entire population to 30.32 years (15.60 QALYs), a gain of 0.07 QALYs, and would reduce lifetime medical spending to $168 836. A 25% effective treatment of diabetes would increase average life expectancy for the entire population to 30.53 years (15.71 QALYs), a gain of 0.18 QALYs, and would reduce lifetime medical spending to $166 298. The maximum life-extending benefit of diabetes prevention would come from a cure, which, when averaged over the entire cohort, would add 1.36 years, or 0.70 QALYs.
TABLE 1.
Per Capita Effects of Prevention of Cardiovascular Risk Factors on Health and Economic Outcomes of Americans Aged 51 or 52 Years
| Hypothetical Scenario | % of Population Successfully Treated | Remaining Life Expectancy in Years | QALY-Adjusted Life Expectancy in Years | Lifetime Medical Spending,ab $ | Value of Treatment,b $ |
| Status Quo | 30.19 | 15.53 | 170 013 | ||
| Treatment effective for 10% of at-risk population | |||||
| Diabetes | 4.0 | 30.32 | 15.60 | 168 836 | 7 957 |
| Hypertension | 8.0 | 30.33 | 15.62 | 168 868 | 10 341 |
| Obesity | 6.8 | 30.25 | 15.56 | 169 511 | 3 673 |
| Smoking | 2.5 | 30.30 | 15.58 | 170 643 | 3 854 |
| Treatment effective for 25% of at-risk population | |||||
| Diabetes | 10.8 | 30.53 | 15.71 | 166 298 | 21 305 |
| Hypertension | 20.8 | 30.58 | 15.78 | 166 953 | 27 614 |
| Obesity | 18.1 | 30.35 | 15.62 | 168 613 | 9 817 |
| Smoking | 7.0 | 30.44 | 15.63 | 171 214 | 8 447 |
| Treatment effective for 50% of at-risk population | |||||
| Diabetes | 21.8 | 30.89 | 15.89 | 162 504 | 43 664 |
| Hypertension | 43.0 | 31.07 | 16.07 | 163 919 | 60 045 |
| Obesity | 35.2 | 30.52 | 15.70 | 167 708 | 18 820 |
| Smoking | 14.8 | 30.72 | 15.74 | 172 517 | 18 306 |
| Treatment effective for 100% of at-risk population | |||||
| Diabetes | 43.0 | 31.55 | 16.23 | 155 190 | 85 120 |
| Hypertension | 84.8 | 31.93 | 16.58 | 158 392 | 117 015 |
| Obesity | 71.3 | 30.79 | 15.85 | 164 905 | 36 878 |
| Smoking | 28.0 | 31.15 | 15.91 | 174 479 | 33 287 |
Note. QALY = quality-adjusted life-year. Lifetime medical spending and QALYs were discounted at 3%. Value of treatment was computed relative to the status quo and calculated as the benefit from additional QALYs ($100 000 per year) minus the change in medical spending.
Derived from the medical component of the consumer price index published by the US Bureau of Labor Statistics.
Spending inflated to constant 2004 dollars.
TABLE 2.
Per Capita Effects of Prevention of Cardiovascular Risk Factors for Successfully Treated Americans Aged 51 or 52 Years in 2004
| Treatment Effective for 100% of At-Risk Population | Additional Life Years | Additional QALYs | Additional Medical Spending,a $ | Value of Treatment,a $ |
| Diabetes | 3.17 | 1.64 | −34 483 | 198 018 |
| Hypertension | 2.05 | 1.24 | −13 702 | 137 964 |
| Obesity | 0.85 | 0.45 | −7168 | 51 750 |
| Smoking | 3.44 | 1.35 | 15 959 | 118 946 |
Note. QALY = quality-adjusted life-year. Lifetime medical spending and QALYs were discounted at 3%. Value of treatment was computed relative to the status quo and calculated as the benefit from additional QALYs ($100 000 per year) minus the change in medical spending.
Spending inflated to constant 2004 dollars.
The population averages shown in Table 1, however, do not reveal the much bigger gains in persons who are successfully treated. Table 2 shows the effect of prevention for each successfully treated individual in the 100% effective treatment scenario (results were similar for the other scenarios). For example, we found that a person aged 51 or 52 years who was successfully treated for obesity would live 0.85 additional years; for hypertension, 2.05 years; and for diabetes, 3.17 years; quitting smoking would add 3.44 years. Despite living longer, those successfully treated would still accumulate less health care spending over their lifetime for obesity (savings of $7168), hypertension (savings of $13 702), and diabetes (savings of $34 483). Smoking cessation, on the other hand, would cost an additional $15 959 over an individual's lifetime.
Thus, for persons treated with diabetes, hypertension, and obesity interventions, life extension could be achieved without increasing average lifetime medical spending. Although these estimates do not include the cost of implementing interventions, they are useful for calibrating the benefits of preventing and treating disease. For example, a 100% effective hypertension treatment would add 1.24 QALYs and would reduce lifetime medical spending by $13 702. Valuing the life-year conservatively at $100 000, we calculated an economic gain of approximately $137 964 per treated 51- or 52-year-old person. On a population basis, this hypertension treatment would be worth $117 015 per capita. A diabetes cure would be worth $85 120. The benefits for obesity and smoking cures would be $36 878 and $33 287, respectively.
DISCUSSION
Research suggests that anticipated treatments for cardiovascular disease, neurologic disorders, and cancer16 could make us live longer but could carry a substantial price tag.4 Our data indicate that primary prevention could improve the health and longevity of future cohorts of elderly persons in the United States at a relatively low cost.
These improvements in health and quality of life would be even larger if the interventions we evaluated were adopted at earlier ages (before 51 years), a scenario we were unable to model because of data limitations. Although the complete elimination of the risk factors we studied is not realistically achievable, our results suggest that even partial success would extend life expectancy as much as would be gained by elimination of major fatal diseases such as cancer and ischemic heart disease.
We focused only on the benefits to society from intervention and did not consider the costs of implementing our 10%, 25%, 50%, and 100% scenarios. Rather, we proposed a threshold for how costly and efficacious a prevention intervention should be to justify its implementation. Still, universal cures would generate large economic gains, ranging from $33 287 per person (smoking) to $117 015 (hypertension) for each person aged 51 or 52 years, according to a conservative valuation of $100 000 per QALY. Prevention costs would reduce these gains, but the interventions are likely to be worthwhile if costs are not prohibitive. Another interpretation is that if lifetime prevention costs were less than $117 015 per person, society would benefit from intervening to prevent hypertension in a person aged 51 or 52 years. Diabetes prevention, in particular, would be a highly valuable intervention for both patients and society.
How feasible are these prevention scenarios? Some may be attainable with existing prevention methods. A recent meta-analysis found that several efficacious treatments can prevent or delay the onset of type 2 diabetes.17 The pooled hazard ratios were 0.51 for lifestyle interventions (diet, exercise, or both) versus standard advice; 0.70 for oral diabetes drugs versus control; and 0.44 for Orlistat versus control. For hypertension, the Trials of Hypertension Prevention found that a 3-year program of group meetings and individual counseling focused on dietary change, physical activity, and social support reduced the incidence of hypertension among middle-aged participants. The risk ratio for developing hypertension after 6 months was 0.58 relative to usual care.18 For smoking reduction, the latest guideline by the US Department of Health and Human Services affirms that pharmacotherapy, physician advice, and psychosocial treatments (self-help, individual counseling, group counseling, and telephone counseling) have all been shown to be effective in treating tobacco use in adults aged 50 years and older; the combination of counseling and medication was found to be most effective.19
For obesity, however, existing interventions are not as promising. Clinical trials of low-calorie diets (1000–1200 kilocalories/day) resulted in an 8% weight loss over 3 to 12 months.20 Increases in physical activity, primarily aerobic exercise, lowered weight by approximately 3%, and combinations of caloric reduction and increased physical activity were more effective than either intervention alone.20 Data from the HRS show that among individuals aged 50 years and older in 2004, a 5% weight loss among obese individuals would reduce the prevalence of obesity by 27%.9
Limitations
Because of the complexity of our model, we assumed that health conditions followed a Markovian process. That is, the last period's risk factors and health conditions were sufficient to determine future health, mortality, and functional outcomes. Although the model fit well in our sample for simulations during 1992 to 2004, it is unclear whether this would be sustained over a different and longer period. Furthermore, we were limited to 2-year incidence rates and summary measures of each condition (diagnosis). These might have limited our ability to accurately describe disease dynamics. Further research on the duration dependence of diseases, and how they affect our findings, is surely warranted but beyond the scope of this article.
Although the microsimulation was designed to control for competing risks, the model did not adjust for differential spending near the end of life. Each individual in our model was tracked until death, and we observed increases in the prevalence of other diseases when we controlled for cardiovascular risk factors. Thus, a person was allowed to develop diseases and disabilities that might be more or less expensive than the conditions for which we were intervening and to live longer with them. For example, reducing hypertension in our model led to an increase in the prevalence of cancer in our model and the costs associated with it. However, because data on the cause of death were not available—and distinguishing which disease is driving spending at the end of life is difficult even with such data—we modeled end-of-life spending with a binary indicator for the year of death.
Reductions in other cardiovascular risk factors, including cholesterol, blood pressure, and physical activity, have been shown to have contributed to recent declines in cardiovascular mortality.21 Because of data limitations (the HRS does not collect biometric data and the physical activity questions have changed over time), we were unable to include them. Behavioral change has economic costs usually not accounted for in public health discussions. Restrictions on diet and smoking and compliance with prevention regimens would result in some loss of utility, and we did not account for these. D. C. estimated the cost of behavior change to be half the value of prevention (data not shown).
Conclusions
Our findings indicate that primary prevention could generate significant health and longevity benefits among existing cohorts, perhaps at considerably lower cost than for the disease-specific interventions now being pursued. Greater attention should be paid to the development and implementation of more effective prevention strategies as a way to immediately address demographic shifts that are about to transform the landscape of human health and longevity.
Acknowledgments
This work was funded by the National Institute on Aging through its support of the RAND Roybal Center for Health Policy Simulation (grant P30AG024968), the Bing Center for Health Economics, the MacArthur Foundation's National Research Network on an Aging Society, and Steven Lazarus.
Note. The sponsors had no control over the design and conduct of the study, the analysis of the data, or the conclusions drawn.
Human Participant Protection
This research was reviewed by the RAND Corporation's human subjects protection committee and was found to be exempt.
References
- 1.Cutler DM, Rosen AB, Vijan S. The value of medical spending in the United States, 1960–2000. N Engl J Med 2006;355:920–927 [DOI] [PubMed] [Google Scholar]
- 2.Murphy KM, Topel RH. The value of health and longevity. J Polit Econ 2006;114:871–904 [Google Scholar]
- 3.Government Accountability Office Do T, ed. Fiscal year 2006 financial report of the United States government. Available at: http://www.gao.gov/financial/fy2006financialreport.html. Accessed October 1, 2008
- 4.Goldman DP, Shang B, Bhattacharya J, et al. Consequences of health trends and medical innovation for the future elderly. Health Aff (Millwood) 2005;24(Suppl 2):W5R5–W5R17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakdawalla DN, Goldman DP, Shang B. The health and cost consequences of obesity among the future elderly. Health Aff (Millwood) 2005;24(Suppl 2):W5R30–W5R41 [DOI] [PubMed] [Google Scholar]
- 6.Chernew ME, Goldman DP, Pan F, Shang B. Disability and health care spending among Medicare beneficiaries. Health Aff (Millwood) 2005;24(Suppl 2):W5R42–W5R52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya J, Shang B, Su CK, Goldman DP. Technological advances in cancer and future spending by the elderly. Health Aff (Millwood) 2005;24(Suppl 2):W5R53–W5R66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman DP, Cutler DM, Shang B, Joyce GF. The Value of Elderly Disease Prevention. Forum Health Econ Policy 2006;9:article1. Available at: http://www.bepress.com/fhep/biomedical_research/1. Accessed October 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour 1995;30(special issue):S7–S56 [Google Scholar]
- 10.Adler G. A profile of the Medicare Current Beneficiary Survey. Health Care Financ Rev 1994;15:153–163 [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JW, Monheit AC, Beauregard KM, et al. The Medical Expenditure Panel Survey: a national health information resource. Inquiry 1996;33:373–389 [PubMed] [Google Scholar]
- 12.Bureau of Labor Statistics Consumer Price Index, medical component, 2004. Available at: http://www.bls.gov/cpi. Accessed May 1, 2007
- 13.Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–1108 [DOI] [PubMed] [Google Scholar]
- 14.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–220 [DOI] [PubMed] [Google Scholar]
- 15.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine New York, NY: Oxford University Press; 1996 [Google Scholar]
- 16.Shekelle PG, Ortiz E, Newberry SJ, et al. Identifying potential health care innovations for the future elderly. Health Aff (Millwood) 2005;24(Suppl 2):W5R67–W5R76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001;134:1–11 [DOI] [PubMed] [Google Scholar]
- 19.Fiore M, Jaén C, Baker T. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline Rockville, MD: Public Health Service; 2008 [Google Scholar]
- 20.National Heart, Lung, and Blood Institute Obesity Education Initiative Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report Bethesda, MD: National Institutes of Health; 1998. Report 98-4083 [Google Scholar]
- 21.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in US deaths from coronary disease, 1980–2000. N Engl J Med 2007;356:2388–2398 [DOI] [PubMed] [Google Scholar]