Abstract
High doses of salicylate, the anti-inflammatory component of aspirin, induce transient tinnitus and hearing loss. Systemic injection of 250 mg/kg of salicylate, a dose that reliably induces tinnitus in rats, significantly reduced the sound evoked output of the rat cochlea. Paradoxically, salicylate significantly increased the amplitude of the sound-evoked field potential from the auditory cortex (AC) of conscious rats, but not the inferior colliculus (IC). When rats were anesthetized with isoflurane, which increases GABA-mediated inhibition, the salicylate-induced AC amplitude enhancement was abolished, whereas ketamine, which blocks N-methyl-d-aspartate receptors, further increased the salicylate-induced AC amplitude enhancement. Direct application of salicylate to the cochlea, however, reduced the response amplitude of the cochlea, IC and AC, suggesting the AC amplitude enhancement induced by systemic injection of salicylate does not originate from the cochlea. To identify a behavioral correlate of the salicylate-induced AC enhancement, the acoustic startle response was measured before and after salicylate treatment. Salicylate significantly increased the amplitude of the startle response. Collectively, these results suggest that high doses of salicylate increase the gain of the central auditory system, presumably by down-regulating GABA-mediated inhibition, leading to an exaggerated acoustic startle response. The enhanced startle response may be the behavioral correlate of hyperacusis that often accompanies tinnitus and hearing loss. Published by Elsevier Ltd on behalf of IBRO.
Keywords: salicylate, tinnitus, hyperacusis, auditory cortex, inferior colliculus, GABA
A challenging question for tinnitus research is to identify the neural generator(s) in the cochlea and/or central auditory system (CAS) of tinnitus. Although the phantom sound of tinnitus is commonly induced by noise exposure or ototoxic drugs, increasing evidence suggests that noise and drug-induced cochlear damage that reduces the output of the cochlea induces a plethora of functional changes in the CAS. In many cases of cochlear damage, the CAS appears to increase its gain to compensate for the reduced sensorineural input from the cochlea. An excessive increase in central gain may give rise to the phantom sound of tinnitus under quiet conditions, as well as an intolerance to loud sounds (hyperacusis) (Gerken, 1996; Salvi et al., 2000; Eggermont and Roberts, 2004). While many studies in animals have focused on tinnitus resulting from salicylate intoxication, few have investigated the phenomenon of hyperacusis which has been linked to chronic tinnitus in humans (Goldstein and Shulman, 1996; Nelson and Chen, 2004). Chronic tinnitus and hyperacusis in humans are most often associated with cochlear damage induced by aging, noise or ototoxic drugs. The salicylate-induced pathology provides a reversible and highly reliable method to investigate how the CAS adjusts due to insult as well as the possible neural correlates of tinnitus and hyperacusis.
Previous studies have shown that acoustic trauma which damages hair cells in the cochlea causes an enhancement in sound-evoked activity in the cochlear nucleus (CN) (Kaltenbach and Afman, 2000; Brozoski and Bauer, 2005), the inferior colliculus (IC) (Salvi et al., 1996) and the auditory cortex (AC) (Syka and Rybalko, 2000). Hyperactivity in the AC also occurs in chinchillas with carboplatin-induced inner hair cell lesions (Salvi et al., 2000). The mechanisms that give rise to an increase in central gain are not clear. Synaptic sprouting has been implicated (Illing et al., 1999); however, since some gain changes occur within 1 hour of cochlear trauma, it is unlikely that the formation of new synapses plays a major role. An alternative hypothesis is that the CAS has an intrinsic neural circuit that can rapidly modulate the amount of neural activity flowing from the cochlea to the central auditory pathway (Salvi et al., 1996). Neurotransmitters such as GABA and glutamate and their receptors can respond rapidly to changing inputs or patterns of activity (Garraghty et al., 2006) and could play a major role in modulating the gain of the CAS.
About 50% of patients who regularly take large amounts of aspirin to treat arthritis report tinnitus and hearing loss (Grigor et al., 1987; Halla and Hardin, 1988). High-doses of salicylate reliably induce transient tinnitus in humans (Myers and Bernstein, 1965; McFadden et al., 1984; Day et al., 1989; Brien, 1993) and several different behavioral paradigms have identified doses of salicylate that reliably induced tinnitus in animal models (Jastreboff et al., 1988; Guitton et al., 2003; Lobarinas et al., 2004). Animal studies have shown that salicylate reduces the sensitivity of cochlea by blocking prestin-based electromotility of outer hair cells, reducing the neural output of the cochlea (Muller et al., 2003; Ermilov et al., 2005; Drexl et al., 2008). However, the effects of salicylate are not confined to the cochlea. Whole cell patch clamp recordings on AC brain slices showed that high doses of salicylate greatly reduced inhibitory postsynaptic currents suggesting a direct effect of salicylate in the CAS (Wang et al., 2006). In our previous studies, we noticed that high doses of salicylate increased sound-evoked activity in the AC (Yang et al., 2007). The salicylate-induced reduction of inhibition noted in brain slice preparations could conceivably contribute to the enhancement of the AC responses seen in our unanesthetized subjects.
The goals of the present study were to: (1) determine if the salicylate-induced enhancement of the AC response originated in the cochlea, IC or AC; (2) determine how anesthetics that target different neurotransmitter receptors would affect the salicylate-induced enhancement of the AC response; (3) determine if the salicylate-induced enhancement of the AC response was correlated with a change in the amplitude of the startle reflex, a potential behavioral correlate of hyperacusis.
EXPERIMENTAL PROCEDURES
Animals
Adult male Sprague–Dawley rats (3–5 months, 200–400 g) were used for the physiological recordings and acoustic startle reflex test. All protocols were approved by the institutional animal care and use committee (IACUC) of the University at Buffalo that conform to the guidelines issued by the National Institutes of Health. This research minimized the number of animals used and their suffering.
Surgery procedure
For AC recording, a chronic electrode was implanted on the right AC using procedures described previously (Yang et al., 2007). Briefly, rats were anesthetized with 1%–2% isoflurane. The AC was surgically exposed using anatomical landmarks on the skull and blood vessels on the surface of the cortex (Horikawa et al., 1988; Sally and Kelly, 1988; Polley et al., 2007). The dura mater was excised and a Teflon-coated silver wire (0.127 mm, A-M Systems, Carlsborg, WA, USA) electrode was gently advanced until it just penetrated the surface of AC. A stainless steel ground electrode was placed on the surface of the skull. The electrodes were attached to connectors that were mounted to the skull using cyanoacrylic glue and dental cement. Six small stainless steel screws were anchored to the skull under the dental cement. A threaded holding bar (1/2″ long with ¼″ diameter) was cemented to hold the skull to maintain the head in a relatively stable position during the electrophysiological tests. The wound was sutured closed around the electrode connector and the animal was allowed to recover for 7–10 days before recording.
For the acute experiment to measure hearing loss induced by salicylate, rats were anesthetized with a mixture of ketamine (50 mg/kg) and acepromazine (0.5 mg/kg). The round window of the left ear (contralateral ear to the AC electrode) was exposed and a silver ball electrode was placed on the round window (Sun et al., 2000). Body temperature was measured and maintained using a heating pad system. The compound action potential (CAP) and AC responses were recorded before and after the delivery of salicylate on the round window or after systematic i.p. injection. Sodium salicylate (S-2679, Sigma, St. Louis, MO, USA) was dissolved in Ringer’s solution (25 mg/ml).
Stimuli and recordings
Stimuli were generated with hardware and software from Tucker-Davis Technologies (TDT, Alachua, FL, USA) and presented through a high frequency speaker (Super Tweeter, Radio Shack, Fort Worth, TX, USA or Fostex FT28D, Fostex, Tokyo, Japan). Tone-bursts centered at 4, 8, 12, 16 and 20 kHz were used to elicit the AC (4 ms duration, 1 ms rise/fall), IC (4 ms duration, 1 ms rise/fall) and CAP (2 ms duration and 1 ms rise/fall) responses at intensities ranging from 20 to 100 dB SPL. Stimuli were calibrated using a sound level meter (824, Larson Davis, Depew, NY, USA) with ½″ microphone (Larson Davis).
The output of the local field potential was connected to a preamplifier (RA16LA, TDT) using a flexible, low noise cable. The output of the preamplifier was delivered to a digital signal processing module (RX5-2, Pentusa Base Station, TDT) connected to a computer. The electrical response was digitized (25 kHz), filtered and averaged. The filter settings used for AC, IC and CAP responses were 3–3000 Hz, 10–3000 Hz and 100–3000 Hz respectively. The sampling windows for AC, IC and CAP responses were 300, 40 and 10 ms respectively.
Acoustical startle reflex test
Adult Sprague–Dawley rats (3–5 months, 200–400 g) were used for the acoustic startle reflex test. Animals were placed in a small, wire mesh cage mounted on a Plexiglas base that rested on a sensitive piezoelectric transducer. The wire mesh cage (7 cm W×5.5 cm, H×L 15–20 cm) restricted the rat’s movement within a calibrated sound field. The output of the piezo transducer was connected to an A/D converter on an RP2 Real-time Processor (TDT). The startle reflex was amplified (1–10×) and filtered (0–1000 Hz) using a low pass filter (LPF-300, World Precision Instruments, Sarasota, FL, USA) and the root mean square (rms) of the waveform from 100 to 200 ms response was measured to estimate the startle amplitude. Sound stimuli were presented by a high frequency speaker (Fostex FT28D) located approximately 28 cm above the rat’s head. Sound signals were generated by an RP2 Real-time Processor (TDT) controlled by custom software. The startle eliciting stimulus consisted of a single broadband noise burst presented at six intensities from 50 to 100 dB SPL (10 trials per intensity in a pseudorandomized order, 10 dB steps). The inter-trial interval was randomized between 20 and 30 s.
Statistical data analysis
The graphs and statistical analyses were performed using Graph-Pad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA) unless otherwise noted. Results are presented as mean±standard error (SE) of the mean.
RESULTS
Salicylate effects on AC response
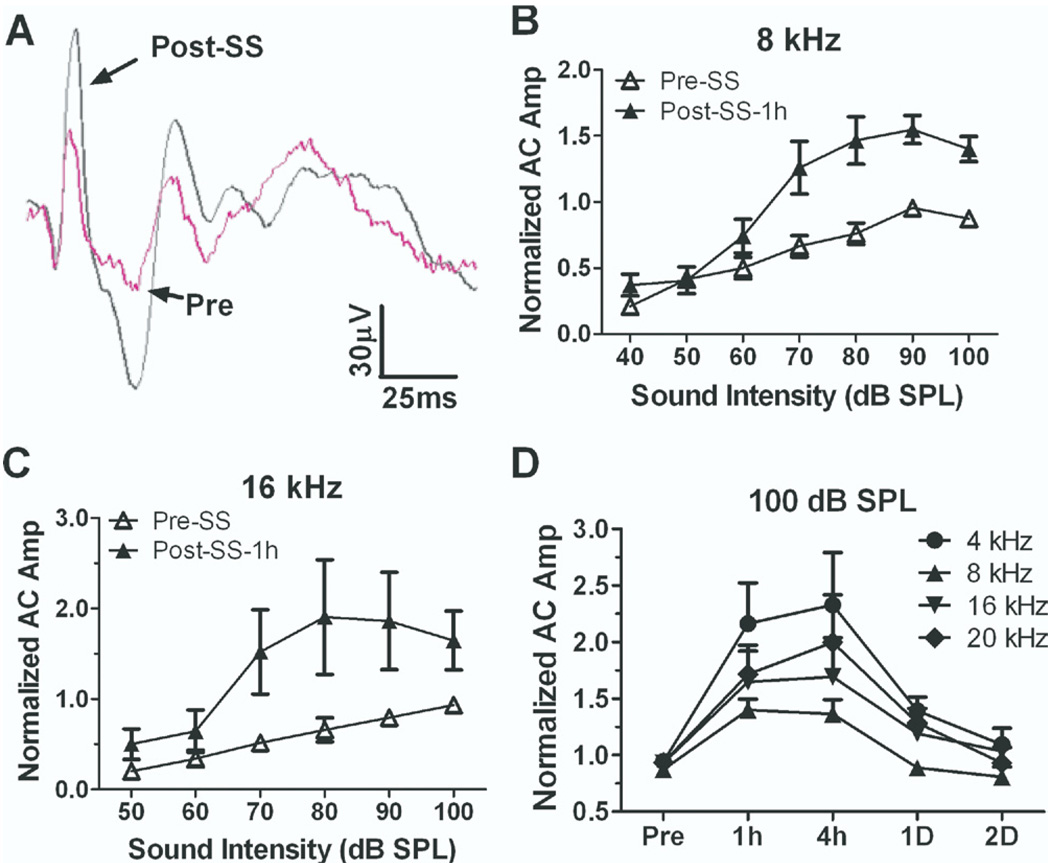
Sound-evoked local field potentials were recorded from chronic silver ball electrodes surgically implanted on the rat AC (Lobarinas et al., 2006). Initial recordings were performed on conscious rats to avoid the confounding effects of anesthetics. Rats were placed in a small testing cage 1–2 hours per day for 5–7 days until they adapted and sat quietly during testing. The rat’s head was maintained in a constant position in a calibrated sound field by connecting a holding post implanted on the head to a stabilizing clamp. Fig. 1A shows typical AC response before and 1 h after salicylate treatment (250 mg/kg i.p.) recorded from a conscious rat. The peak-to-peak amplitude of the AC response was measured before (3–5 baseline measures) and after salicylate treatment (1 h, 4 h, 1 day and 2 days). Each animal’s AC response was normalized to the highest amplitude recorded at each frequency during baseline testing. The averaged normalized AC input/output functions (n=5) at 8 and 16 kHz before and 1 h after salicylate treatment are plotted in Fig. 1B and 1C. The AC response showed a significant enhancement at intensities above 60 dB SPL 1 h after salicylate treatment (two-way ANOVA, main effect of treatment, F(2,50)=26.33, P<0.0001). The average enhancement (±SE) of the AC response from 70 to 100 dB SPL was 80%±21% and 170%±78% at 8 and 16 kHz respectively (n=5). The average change in the AC response at 100 dB SPL after salicylate treatment is plotted in Fig. 1D at 4, 8, 16 and 20 kHz. The AC responses increased significantly at all frequencies 1–4 h post-treatment and partially or fully recovered 1–2 days post-treatment. These data show that salicylate caused a transient increase in the AC field potential.
Fig. 1.
Salicylate induced enhancement of AC response recorded from conscious rats. (A) Examples of AC response before and after salicylate injection (250 mg/kg). The average (n=5) AC response evoked by tone bursts at 8 kHz (B) and 16 kHz (C) at intensities from 50 to 100 dB SPL before and after 1 h after salicylate injection. The average (mean±SE, n=5) AC response increased more than 50% at sound levels higher than 60 dB SPL. (D) Change in normalized AC amplitude at 100 dB SPL from 4 to 20 kHz after salicylate injection. AC amplitudes were significantly enhanced 1 and 4 h after salicylate treatment, but partially or fully recovered 1–2 days post-treatment.
Isoflurane suppresses salicylate-induced AC enhancement
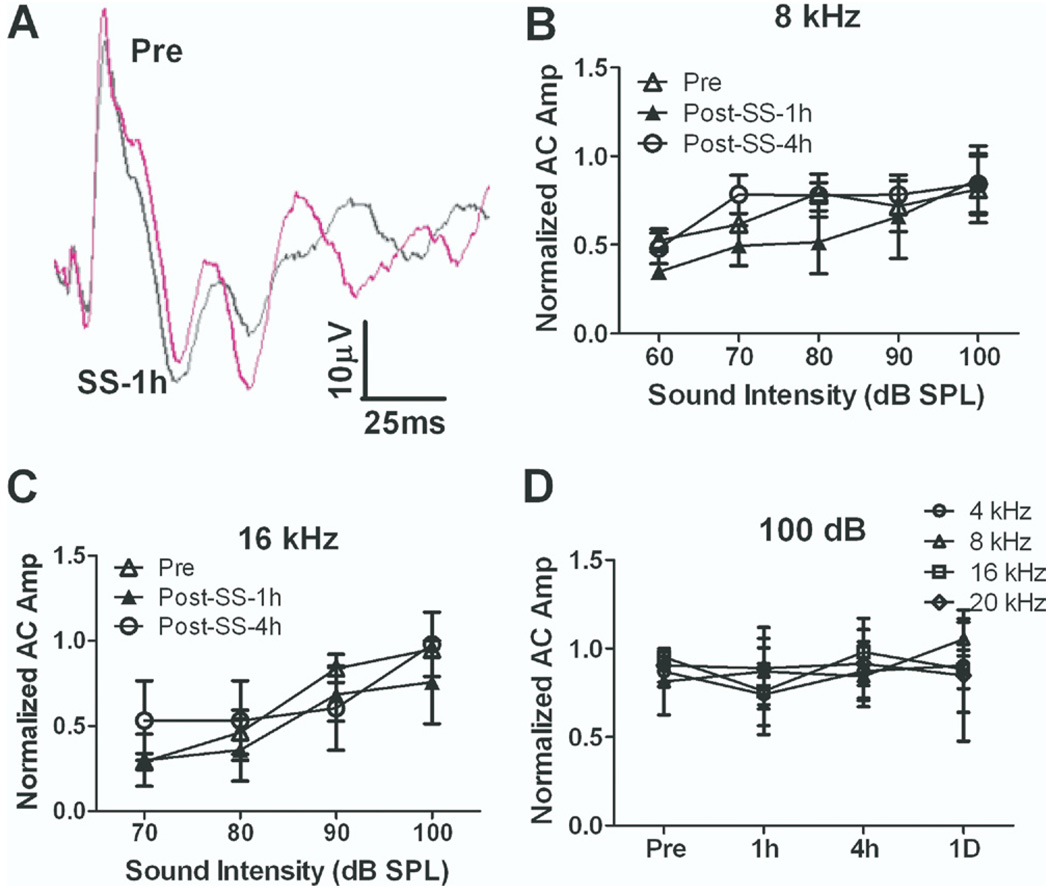
Salicylate’s effects on AC responses were tested under isoflurane (1.5%) gas anesthesia. Fig. 2A shows typical AC responses before and after salicylate injection. AC responses under isoflurane were smaller than those recorded in conscious rats (compare to Fig. 1A). Fig. 2B and 2C shows the average (n=3) normalized AC input/output functions at 8 and 16 kHz pre-treatment and 1 and 4 h post-salicylate treatment. Salicylate treatment failed to cause a significant change in AC response amplitude under isoflurane anesthesia (two-way ANOVA, P>0.05, n=3). Fig. 2D shows the average normalized AC amplitudes at 100 dB SPL at 4, 8, 16 and 20 kHz (n=3) before and after salicylate treatment. Salicylate did not cause a significant change in AC amplitude at any frequency between 4 and 20 kHz under isoflurane (1.5%) from 1 h to 1 day post-treatment (two-way ANOVA, P>0.05). These results indicate that isoflurane suppresses the salicylate-induced enhancement of the AC response seen in conscious rats.
Fig. 2.
Salicylate did not induce an enhancement on AC response from anesthetized rats under isoflurane (1.5%). (A) Examples of AC response recorded before and after salicylate injection under isoflurane anesthesia. AC responses recorded before and after salicylate application overlapped. The average AC responses evoked by tone burst at 8 kHz (B) and 16 kHz (C) from 50 to 100 dB SPL before and after 1 h and 4 h salicylate injection (250 mg/kg i.p.). The average AC response did not show a significant change. (D) Time course of AC response change. AC did not show a significant change at 1 h, 4 h and 1 day after salicylate injection.
Ketamine increased salicylate-induced AC enhancement
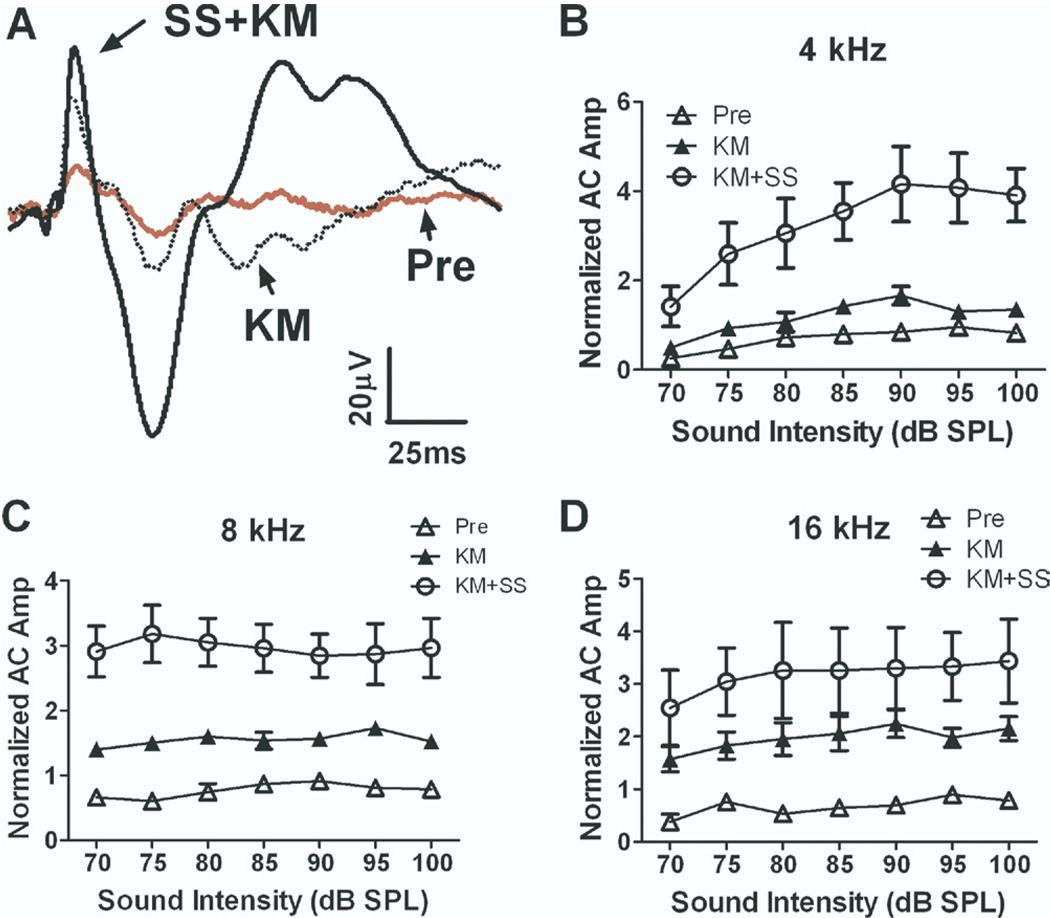
Rats were anesthetized with ketamine (136 mg/kg i.p.) to determine its effect on the salicylate-induced enhancement of the AC response. Interestingly, ketamine alone caused a significant increase of the AC response. When salicylate (250 mg/kg i.p.) was administered under ketamine anesthesia, the AC response increased dramatically (Fig. 3A). Fig. 3B–D shows the average normalized AC input/output functions at 4, 8 and 16 kHz recorded under three conditions: (1) pre-treatment conscious, (2) ketamine-anesthesia alone, and (3) ketamine anesthesia and 1 h after salicylate treatment. Ketamine alone caused the AC amplitude to increase by approximately 25% at 4 kHz to roughly 100%–200% at 8 and 16 kHz. An even larger increase occurred when salicylate was administered during ketamine anesthesia. Ketamine plus salicylate treatment caused the average AC amplitude (sound intensity at 70–100 dB) to increase by 285%±104% at 4 kHz, 292%±7% at 8 kHz, 283%±13% at 12 kHz, 246%±28% at 16 kHz and 265%±33% at 20 kHz (mean±SE, n=3) relative to the pre-treatment baseline; these changes were highly significant (two-way ANOVA, F(1,42)=60.25 and P<0.001 at 4 kHz, F(1,42)=81.72 and P<0.0001 at 8 kHz, F(1,42)=15.47 and P=0.0003 at 16 kHz). Thus, ketamine potentiated the salicylate-induced enhancement of the AC response.
Fig. 3.
Ketamine anesthesia (135 mg/kg) increased salicylate induced enhancement of the AC response. (A) Examples of AC responses recorded in a conscious rat (pre), after ketamine (kM) anesthesia and after salicylate injection under ketamine anesthesia (SS+ kM). The AC amplitude evoked by tone bursts at 4 kHz (B), 8 kHz (C) and 16 kHz (D) increased significantly (>300%) after salicylate injection under ketamine anesthesia.
Salicylate reduces cochlear response
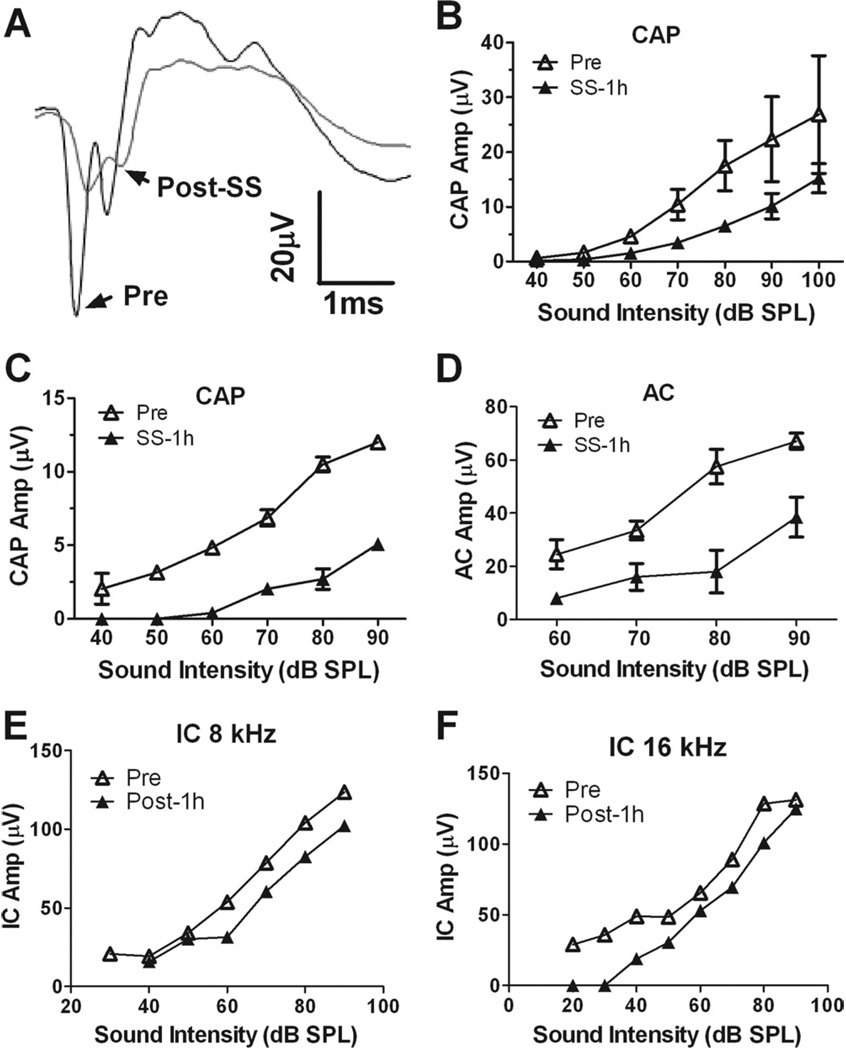
To determine if the salicylate-induced AC enhancement was originating in the cochlea, we recorded the CAP from the round window of the cochlea from rats anesthetized with ketamine (40 mg/kg) and acepromazine (0.5 mg/kg) before and after salicylate treatment (250 mg/kg i.p.). Salicylate treatment caused a large reduction in the N1 and N2 component of the CAP (Fig. 4A). Fig. 4B shows the average CAP input/output function at 8 kHz before and 1 h after salicylate treatment. The average CAP amplitude between 70 and 100 dB decreased 46%±8% (mean±SE) 1 h after salicylate application; this reduction was statistically significantly (two-way ANOVA, F(1,21)=16.2, P=0.0006). The average CAP threshold (lowest intensity that elicited a visible CAP response) elevation was 17±3 dB (mean±SE, n=4) 1 h after salicylate injection.
Fig. 4.
Salicylate’s effects on cochlea, IC and AC (A) examples of CAP recorded from the round window (RW) before and after salicylate injection (i.p.). CAP amplitude decreased significantly after 1 h post–salicylate injection. (B) The input–output function of CAP amplitude before and after salicylate injection. Salicylate-induced CAP amplitude decreased as well as the dynamic range decreased. (C) Salicylate application on RW-induced decrease of CAP amplitude and the elevation of CAP threshold. (D) Salicylate application on RW decreased AC response. (E–F) Typical input–output functions of IC amplitude before and after salicylate application on RW. One hour after salicylate application on RW significantly reduced IC responses recorded at 8 kHz (E) and 16 kHz (F).
Cochlear application of salicylate
To determine if the AC enhancement induced by systemic injection of salicylate was the result of peripheral hearing loss, the AC, IC and CAP responses were recorded before and after the administration of salicylate on the round window of the cochlea. AC and CAP recordings were obtained from two rats with chronically implanted AC electrodes before, 1 and 2 h after salicylate was applied to the round window (~50 µl, 25 mg/ml). The amplitude of both the CAP and AC responses decreased significantly (Fig. 4C, 4D) 1 h after salicylate treatment. In addition, sound-evoked potentials were also recorded from the IC of three rats with chronically implanted IC electrodes before and 1 h after salicylate was applied to the round window (~50 µl, 25 mg/ml). The amplitude of IC responses elicited by 8 and 16 kHz tone bursts (20–100 dB SPL) decreased significantly 1 h after salicylate treatment (Fig. 4E, 4F). Although round window application of salicylate induced a peripheral deficit similar to that seen with systemic drug treatment, only systemic administration of salicylate enhanced the amplitude of the AC response. Thus, the salicylate-induced enhancement of the AC response cannot be solely due to cochlear dysfunction.
IC response
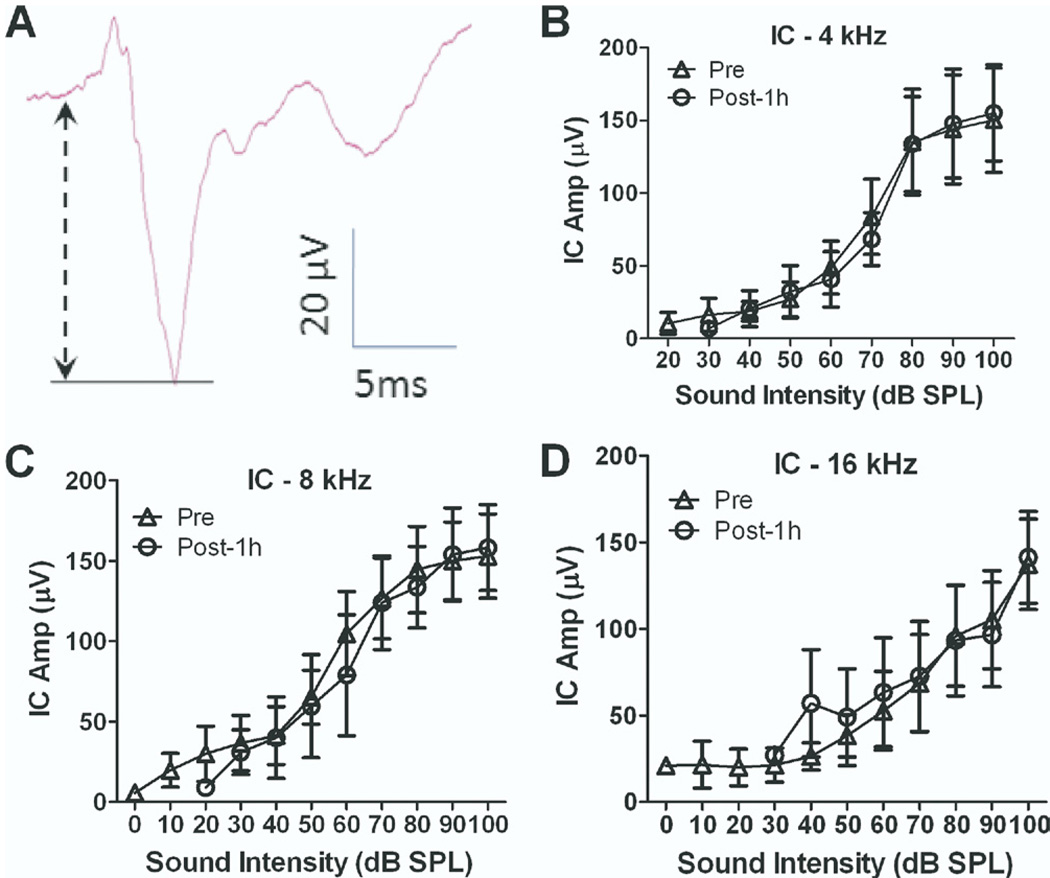
To determine if the salicylate-induced enhancement of the AC was originating at lower levels of the auditory systems, recording electrodes were chronically implanted in both the IC and AC (n=4). Conscious rats were then acclimated to the testing cage for 5–7 days. After acclimation, the local field potential from the IC and AC was evoked with tone-bursts ranging from 20 to 100 dB SPL. The IC response consisted of a positive peak followed by a much larger negative peak (Fig. 5A). The IC input/output functions (Fig. 5B–D) measured before and 1 h after systemic salicylate treatment were largely unchanged (two-way ANOVA, P>0.05); however, the average threshold of the IC response was elevated 20±2.4 dB (mean±SE, n=4). In contrast, the AC responses recorded from the same group of rats were greatly enhanced at high stimulus levels (two-way ANOVA adjusted with matching, for 4 kHz F(1,26)=7.22 and P=0.01, for 8 kHz F(1,35)=11.7 and P=0.002, for 16 kHz F(1,29)=12.4 and P=0.001, n=6). These results suggest that the salicylate-induced enhancement of the AC response is primarily mediated above the level of the IC.
Fig. 5.
Salicylate did not change the amplitude of the IC response. (A) A typical waveform of IC response recorded from a chronic electrode from a conscious rat. (B–D) The average IC responses recorded at 4, 8 and 16 kHz before and 1 h after salicylate injection. No significant difference was detected before and after salicylate treatment.
Acoustic startle response
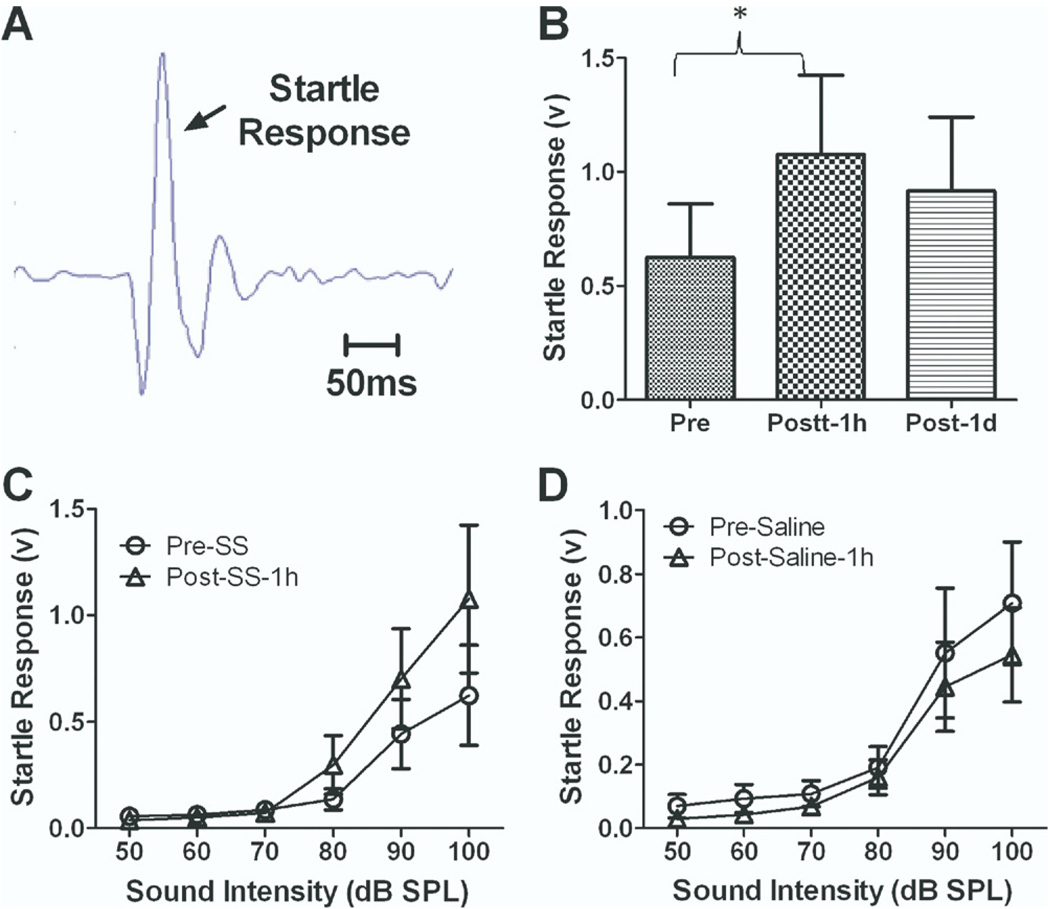
To determine if the salicylate-induced enhancement of the AC response observed at suprathreshold intensities had a behavioral correlate, the amplitude of the acoustic startle response was tested before and after salicylate injection (n=12). The acoustic startle response was evoked using a short duration noise burst (50 ms duration, 50–100 dB SPL, broad-band noise) (Yang et al., 2007) (Fig. 6A). The rms amplitude of the startle response was measured in a 100 ms window starting at the onset of the startle stimuli. Baseline startle amplitudes increased with intensity from 70 to 100 dB SPL (Fig. 6A). Startle amplitudes showed a large increase 1 h following salicylate treatment particularly at higher intensities (Fig. 6C, two-way ANOVA adjusted with matching, F(1,66)=13.5, P=0.0005, n=12). The amplitude of the startle response elicited by a 100 dB SPL noise burst increased by 95%±32% (paired t-test, P=0.02, n=12) (Fig. 6B) 1 h after the salicylate treatment. The amplitude of the startle response recovered, but was slightly elevated 1 day post-treatment; however, the difference was not statistically different from baseline (Fig. 6B). Saline treatment by contrast caused a slight reduction in startle amplitude possibly due to habituation of the response (Fig. 6D, two-way ANOVA adjusted with matching, F(1,66)=7.3, P=0.009, n=12). These results indicate that salicylate treatment enhances the amplitude of the startle response over the same range of intensities and time as the AC amplitude enhancement.
Fig. 6.
Salicylate (250 mg/kg i.p.) injection induced an enhancement of the acoustic startle response. (A) A typical startle response recorded from a rat. (B) The amplitude of the acoustic startle response evoked by 100 dB SPL sound stimuli significantly increased 1 h after the salicylate injection and partially recovered on the second day. (C) The average startle amplitude evoked by sound stimuli from 50 to 100 dB SPL recorded before and 1 h after salicylate injection. One hour after salicylate injection, startle amplitude at high sound intensity (70–100 dB SPL) increased significantly. (D) Startle amplitude recorded before and 1 h after saline injection. The startle amplitude showed a slight decrease 1 h after saline injection.
DISCUSSION
Aspirin, with its active ingredient sodium salicylate, is one of the most widely used analgesic, anti-inflammatory and antipyretic drugs. High doses of sodium salicylate have long been known to cause transient hearing loss and tinnitus (Cazals, 2000). These perceptual changes have generally been attributed to functional changes in the cochlea such as the down-regulation of the electromotile response of outer hair cells and the decrease in neural output of the cochlea (Brownell, 1990; Tunstall et al., 1995; Muller et al., 2003; Ermilov et al., 2005; Zhi et al., 2007; Ruel et al., 2008). Our results confirm earlier reports showing that high doses of salicylate decrease the sound evoked neural output of the cochlea (Cazals, 2000; Muller et al., 2003). However, the two novel and unexpected findings that emerged from this study were that high systemic doses of salicylate (1) significantly enhanced the amplitude of the local field potential from the AC of conscious animals, and (2) significantly enhanced the amplitude of the startle reflex.
Mechanisms of salicylate-induced AC enhancement
Salicylate administered systemically or directly to the round window of the cochlea significantly reduced the amplitude of the CAP thereby greatly reducing the neural input to the central auditory pathway. These results indicate that both local and systemic administration of salicylate disrupt cochlear function, consistent with previous results showing that salicylate reduces the amplitude of distortion product otoacoustic emissions and the CAP and increases the threshold of the CAP and single auditory nerve fibers (Fitzgerald et al., 1993; Guitton et al., 2003; Muller et al., 2003; Peng et al., 2003; Ruel et al., 2008). The salicylate-induced changes in the CAP could be blocked by cochlear administration of N-methyl-d-aspartate (NMDA) antagonists. However, our results indicate that the effects of systemic administration of salicylate on CAS function were strikingly different from cochlear drug delivery. Cochlear application of salicylate reduced the amplitude of the AC sound-evoked potential whereas systemic treatment significantly increased the amplitude. Thus, the enhanced AC response induced by systemic treatment of salicylate is unlikely caused by salicylate’s effects on NMDA receptors of spiral ganglion neurons in the cochlea (Peng et al., 2003; Ruel et al., 2008). The increase in the AC response implies that the gain of the central auditory pathway increased after systemic salicylate treatment. Given that the IC response was essentially normal after systemic injection of salicylate despite the large reduction in CAP amplitude, implies that part of the increase in system gain probably occurs between the cochlea and the IC during systemic salicylate administration. Since the round window application of salicylate caused a significant reduction of the IC amplitude, salicylate-induced cochlear dysfunction does not appear to alter the system gain between the cochlea and the IC.
The effects on neuronal response properties due to salicylate administration have been studied in other neural systems. In hippocampal brain slices, sodium salicylate has been found to significantly enhance the excitation of neurons by reducing inhibitory neurotransmission mediated by GABA (Gong et al., 2008). A study on salicylate’s effects on isolated spinal dorsal horn neurons reported a reversible and dose-dependent reduction in GABA-A-mediated post-synaptic currents suggesting that GABA-A receptors are pharmacological targets of salicylate (Xu et al., 2005). Moreover, whole cell patch clamp recordings in AC brain slices showed that high doses of salicylate greatly reduced inhibitory postsynaptic currents (Wang et al., 2006). The loss of cortically mediated inhibition induced by systemic salicylate administration could be a contributing factor to the enhancement of the AC responses seen in unanesthetized rats (Fig. 2). The failure of systemically administered salicylate to enhance AC response under isoflurane anesthesia most likely occurred because isoflurane enhances GABA-mediated neurotransmission while suppressing glutamatergic synaptic activity (Neumahr et al., 2000; Ranft et al., 2004). The isoflurane enhancement of GABA neurotransmission in the AC presumably suppressed the salicylate-induced enhancement of the AC response (Fig. 2).
Salicylate also affects earlier sites in the auditory pathway. In brain slices from the IC, 5-HT normally enhances GABA-mediated spontaneous postsynaptic currents. However, this effect was blocked by high dose of salicylate thereby reducing the level of GABA-mediated inhibition in the IC (Wang et al., 2008). Other in vivo studies report that long term administration of salicylate decreases the number of GABA-immunolabeled neurons in the IC while increasing the number of glutamate-immunolabeled neurons. Furthermore, long term systemic administration of salicylate increases the expression of glutamic acid decarboxylase (GAD), the enzyme that catalyzes the conversion of glutamate to GABA, in the IC, but decreases the number of GABA-A receptor binding sites (Bauer et al., 2000). Taken together, these results suggest that salicylate reduces GABA-mediated inhibition and increases excitability at the AC and IC thereby increasing the gain of the CAS.
Ketamine effects
Ketamine, a non-competitive NMDA receptor antagonist, greatly enhanced AC response amplitude. This increase is consistent with human studies showing that ketamine increased the amplitude of the auditory steady state and middle latency responses that originate in the AC (Plourde et al., 1997). In contrast, ketamine had little effect on the amplitude of the more peripherally generated auditory brainstem evoked response (Bobbin et al., 1979). The ability of ketamine to enhance AC amplitude may be due to a unique glutamatergic-GABAergic interneuron circuit in the cortex. Cortical, parvalbumin-positive, GABA-releasing interneurons receive more glutamatergic inputs than other GABAergic neurons in the cortex (Gulyas et al., 1999). These GABAergic interneurons receive glutamatergic inputs that activate post-synaptic NMDA receptors. Consequently, these interneurons are strongly inhibited by NMDA antagonists (Jones and Buhl, 1993). Therefore, we hypothesize that the ketamine-induced increase in AC amplitude occurs because ketamine greatly suppresses the NMDA-mediated excitatory inputs to these GABA releasing interneurons. This disinhibition results in enhanced sound-evoked activity in the AC during ketamine treatment. When ketamine was combined with salicylate, AC response amplitude was substantially greater than with salicylate or ketamine alone. We propose that this potentiating effect is the result of two factors: (1) ketamine’s suppression of NMDA synaptic inputs to GABA releasing interneurons and (2) salicylate’s suppression of inhibitory post-synaptic currents mediated by GABA-A receptors (Jones and Buhl, 1993; Xu et al., 2005; Wang et al., 2006).
Startle amplitude and hyperacusis
Previous studies have reported an increase in startle amplitude to low frequency stimuli in mice with high-frequency age-related hearing loss. An increase in startle amplitude has been suggested as a behavioral correlate of hyperacusis (Ison et al., 2007). High doses of salicylate also caused an increase in startle amplitude and enhanced the neural response from the AC (Fig. 6 and Fig. 1, respectively). Both of these measures could conceivably be interpreted as evidence of hyperacusis. Since high doses of salicylate induce a hearing loss with sensorineural-like features, it seems likely that the salicylate-induced hearing loss would be accompanied by loudness recruitment. In human studies, some volunteers reported hyperacusis after taking a high dose of salicylate, but no formal measurement of loudness intolerance was obtained (Myers et al., 1965). These informal observations suggest that high doses of salicylate may induce hyperacusis in humans. However, additional studies are needed in order to determine if humans and/or animals develop hyperacusis after treatment with high doses of salicylate. Local administration of NMDA antagonists has been shown to block behavioral evidence of salicylate-induced tinnitus (Guitton et al., 2003) whereas systemic administration of NMDA antagonists did not prevent salicylate-induced tinnitus (Lobarinas et al., 2006). It is not yet clear what effect local or systemic application of NMDA antagonists has on salicylate-induced hyperacusis or the enhancement of the startle reflex amplitude.
Additional studies are also needed to determine if salicylate-induced hyperacusis and tinnitus share a common mechanism with noise-induced or other ototoxic drug-induced, tinnitus and hyperacusis.
Acknowledgments
This work was supported in part by grants from NIH (R01DC009091 and R01DC009219), the Tinnitus Research Consortium, the American Tinnitus Association and the Royal National Institute for Deaf People.
Abbreviations
- AC
auditory cortex
- CAP
compound action potential
- CAS
central auditory system
- IC
inferior colliculus
- NMDA
N-methyl-d-aspartate
- TDT
Tucker-Davis Technologies
REFERENCES
- Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res. 2000;147:175–182. doi: 10.1016/s0378-5955(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, May JG, Lemoine RL. Effects of pentobarbital and ketamine on brain stem auditory potentials. Latency and amplitude intensity functions after intraperitoneal administration. Arch Otolaryngol. 1979;105:467–470. doi: 10.1001/archotol.1979.00790200029006. [DOI] [PubMed] [Google Scholar]
- Brien JA. Ototoxicity associated with salicylates. A brief review. Drug Saf. 1993;9:143–148. doi: 10.2165/00002018-199309020-00006. [DOI] [PubMed] [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206:227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000;62:583–631. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Day RO, Graham GG, Bieri D, Brown M, Cairns D, Harris G, Hounsell J, Platt-Hepworth S, Reeve R, Sambrook PN, et al. Concentration–response relationships for salicylate-induced ototoxicity in normal volunteers. Br J Clin Pharmacol. 1989;28:695–702. doi: 10.1111/j.1365-2125.1989.tb03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexl M, Lagarde MM, Zuo J, Lukashkin AN, Russell IJ. The role of prestin in the generation of electrically evoked otoacoustic emissions in mice. J Neurophysiol. 2008;99:1607–1615. doi: 10.1152/jn.01216.2007. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Ermilov SA, Murdock DR, El-Daye D, Brownell WE, Anvari B. Effects of salicylate on plasma membrane mechanics. J Neurophysiol. 2005;94:2105–2110. doi: 10.1152/jn.00414.2005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JJ, Robertson D, Johnstone BM. Effects of intracochlear perfusion of salicylates on cochlear microphonic and other auditory responses in the guinea pig. Hear Res. 1993;67:147–156. doi: 10.1016/0378-5955(93)90242-s. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Arnold LL, Wellman CL, Mowery TM. Receptor autoradiographic correlates of deafferentation-induced reorganization in adult primate somatosensory cortex. J Comp Neurol. 2006;497:636–645. doi: 10.1002/cne.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken GM. Central tinnitus and lateral inhibition: an auditory brainstem model. Hear Res. 1996;96:75–83. [PubMed] [Google Scholar]
- Goldstein B, Shulman A. Tinnitus—hyperacusis and the loudness discomfort level test—A preliminary report. Int Tinnitus J. 1996;2:83–89. [PubMed] [Google Scholar]
- Gong N, Zhang M, Zhang XB, Chen L, Sun GC, Xu TL. The aspirin metabolite salicylate enhances neuronal excitation in rat hippocampal CA1 area through reducing GABAergic inhibition. Neuropharmacology. 2008;54:454–463. doi: 10.1016/j.neuropharm.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Grigor RR, Spitz PW, Furst DE. Salicylate toxicity in elderly patients with rheumatoid arthritis. J Rheumatol. 1987;14:60–66. [PubMed] [Google Scholar]
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23:3944–3952. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single inter-neurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halla JT, Hardin JG. Salicylate ototoxicity in patients with rheumatoid arthritis: a controlled study. Ann Rheum Dis. 1988;47:134–137. doi: 10.1136/ard.47.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa J, Ito S, Hosokawa Y, Homma T, Murata K. Tonotopic representation in the rat auditory cortex. Proc Jpn Acad B. 1988;64:260–263. [Google Scholar]
- Illing RB, Cao QL, Forster CR, Laszig R. Auditory brainstem: development and plasticity of GAP-43 mRNA expression in the rat. J Comp Neurol. 1999;412:353–372. doi: 10.1002/(sici)1096-9861(19990920)412:2<353::aid-cne12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O’Neill WE. Age-related hearing loss in C57BL/6 J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J Assoc Res Otolaryngol. 2007;8:539–550. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98:280–286. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- Jones RS, Buhl EH. Basket-like interneurones in layer II of the entorhinal cortex exhibit a powerful NMDA-mediated synaptic excitation. Neurosci Lett. 1993;149:35–39. doi: 10.1016/0304-3940(93)90341-h. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning. SIP-AC. Hear Res. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, Hilczmayer E, Fitzgerald S, Zhang L, Salvi R. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl. 2006:13–19. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS, Pasanen EG. Aspirin-induced hearing loss as a model of sensorineural hearing loss. Hear Res. 1984;16:251–260. doi: 10.1016/0378-5955(84)90114-x. [DOI] [PubMed] [Google Scholar]
- Muller M, Klinke R, Arnold W, Oestreicher E. Auditory nerve fibre responses to salicylate revisited. Hear Res. 2003;183:37–43. doi: 10.1016/s0378-5955(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Myers EN, Bernstein JM, Fostiropolous G. Salicylate ototoxicity: a clinical study. N Engl J Med. 1965;273:587–590. doi: 10.1056/NEJM196509092731104. [DOI] [PubMed] [Google Scholar]
- Myers EN, Bernstein JM. Salicylate ototoxicity: a clinical and experimental study. Arch Otolaryngol. 1965;82:483–493. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- Nelson JJ, Chen K. The relationship of tinnitus, hyperacusis, and hearing loss. Ear Nose Throat J. 2004;83:472–476. [PubMed] [Google Scholar]
- Neumahr S, Hapfelmeier G, Scheller M, Schneck H, Franke C, Kochs E. Dual action of isoflurane on the gamma-aminobutyric acid (GABA)-mediated currents through recombinant alpha(1)beta(2) gamma(2 L)-GABAA-receptor channels. Anesth Analg. 2000;90:1184–1190. doi: 10.1097/00000539-200005000-00035. [DOI] [PubMed] [Google Scholar]
- Peng BG, Chen S, Lin X. Aspirin selectively augmented N-methyl-d-aspartate types of glutamate responses in cultured spiral ganglion neurons of mice. Neurosci Lett. 2003;343:21–24. doi: 10.1016/s0304-3940(03)00296-9. [DOI] [PubMed] [Google Scholar]
- Plourde G, Baribeau J, Bonhomme V. Ketamine increases the amplitude of the 40-Hz auditory steady-state response in humans. Br J Anaesth. 1997;78:524–529. doi: 10.1093/bja/78.5.524. [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Ranft A, Kurz J, Deuringer M, Haseneder R, Dodt HU, Zieglgansberger W, Kochs E, Eder M, Hapfelmeier G. Isoflurane modulates glutamatergic and GABAergic neurotransmission in the amygdala. Eur J Neurosci. 2004;20:1276–1280. doi: 10.1111/j.1460-9568.2004.03603.x. [DOI] [PubMed] [Google Scholar]
- Ruel J, Chabbert C, Nouvian R, Bendris R, Eybalin M, Leger CL, Bourien J, Mersel M, Puel JL. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J Neurosci. 2008;28:7313–7323. doi: 10.1523/JNEUROSCI.5335-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. J Neurophysiol. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Powers N. Rapid functional reorganization in the inferior colliculus and cochlear nucleus after acute cochlear damage. In: Salvi RJ, et al., editors. Auditory plasticity and regeneration. New York: Thieme Medical Publishers; 1996. pp. 275–296. [Google Scholar]
- Sun W, Ding D, Reyes S, Salvi RJ. Effects of AC and DC stimulation on chinchilla SOAE amplitude and frequency. Hear Res. 2000;150:137–148. doi: 10.1016/s0378-5955(00)00195-7. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N. Threshold shifts and enhancement of cortical evoked responses after noise exposure in rats. Hear Res. 2000;139:59–68. doi: 10.1016/s0378-5955(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Gale JE, Ashmore JF. Action of salicylate on membrane capacitance of outer hair cells from the guinea-pig cochlea. J Physiol Lond. 1995;485:739–752. doi: 10.1113/jphysiol.1995.sp020765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Zhou KQ, Xu TL, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res. 2006;215:77–83. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Xu H, Gong N, Chen L, Xu TL. Sodium salicylate reduces gamma aminobutyric acid-induced current in rat spinal dorsal horn neurons. Neuroreport. 2005;16:813–816. doi: 10.1097/00001756-200505310-00007. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Zhi M, Ratnanather JT, Ceyhan E, Popel AS, Brownell WE. Hypotonic swelling of salicylate-treated cochlear outer hair cells. Hear Res. 2007;228:95–104. doi: 10.1016/j.heares.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]