Abstract
It is well established that peroxisomes play a crucial role in de novo bile acid synthesis. Studies in patients with a peroxisomal disorder have been indispensable for the elucidation of the precise role of peroxisomes. Several peroxisomal disorders are associated with distinct bile acid abnormalities and each disorder has a characteristic pattern of abnormal bile acids that accumulate, which is often used for diagnostic purposes. The patients have also been important for determining the pathophysiological consequences of defects in bile acid biosynthesis. In this review, we will discuss all the peroxisomal steps involved in bile acid synthesis and the bile acid abnormalities in patients with peroxisomal disorders. We will show the results of bile acid measurements in several tissues from patients, including brain, and we will discuss the toxicity and the pathological effects of the abnormal bile acids.
Keywords: dihydroxycholestanoic acid, trihydroxy-cholestanoic acid, peroxisome deficiency disorders, D-bifunctional protein deficiency, α-methylacyl-CoA racemase deficiency
THE BILE ACID BIOSYNTHESIS PATHWAY
The primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are formed from cholesterol by a sequence of enzymatic modifications involving several enzymes, multiple subcellular compartments, and two main pathways (see Ref. 1 for a detailed review on the bile acid biosynthesis pathway). The most important difference between the classic and alternative pathway is the order of reactions and the subcellular compartment where the first reaction takes place (endoplasmic reticulum vs. mitochondrion, respectively). The enzymes involved in the two pathways are the same except for those responsible for the hydroxylation of the steroid nucleus at the C-7 position. In the classic pathway, this reaction is performed by cholesterol 7α-hydroxylase (CYP7A1), whereas oxysterol 7α-hydroxylase (CYP7B1) is responsible for this hydroxylation in the alternative pathway. The biosynthesis pathway basically consists of modification of the steroid nucleus, oxidation of the sterol side chain, cleavage of the side chain, and finally, conjugation with an amino acid, either taurine or glycine. Microsomal and cytosolic enzymes modify the steroid nucleus. Oxidation of the sterol side chain occurs in the mitochondrion, and finally, both the cleavage of the side chain and subsequent conjugation take place in the peroxisome.
Oxidation of the sterol side chain results in formation of the C27-bile acid intermediates 3α,7α-dihydroxycholestanoic acid (DHCA) and 3α,7α,12α-trihydroxycholestanoic acid (THCA). DHCA is the precursor of the primary C24-bile acid CDCA and, when hydroxylated at C-12, the precursor of CA. THCA is the precursor of CA. The C24-bile acids are formed from the C27-bile acid intermediates by peroxisomal β-oxidation of the side chain (Fig. 1). Before this side chain can be shortened by β-oxidation, it has to be activated to a CoA-thioester and the C27-bile acyl-CoA has to be transported into the peroxisome. To date, two enzymes have been identified that are capable of activating the C27-bile acid intermediates. The first enzyme is the bile acyl-CoA synthetase (BACS, also called bile acid CoA ligase), which is liver-specific and located at the endoplasmic reticulum. It accepts both C27- and C24-bile acids as substrates (2, 3). The second enzyme that has been shown to be capable of handling the C27-bile acid intermediates in expression studies is the very long-chain acyl-CoA synthetase (2). It is expressed primarily in liver and kidney and is located in both the endoplasmic reticulum and in the peroxisome. In addition to the C27-bile acid intermediates, the enzyme has been shown to handle C24:0, C16:0, phytanic and pristanic acid as substrates, but is not capable of activating the primary C24-bile acids (2, 4). To date, formation of DHC-CoA and THC-CoA has only been demonstrated at the endoplasmic reticulum and not in peroxisomes (5, 6). The kinetic properties of the THC-CoA synthetase and choloyl-CoA synthetase activities at the endoplasmic reticulum have been shown to be different (6), which suggests that both BACS and very long-chain acyl-CoA synthetase are involved in the activation of the C27- and C24-bile acids at the endoplasmic reticulum. In agreement with a microsomal, and not peroxisomal, activation of the C27-bile acid intermediates is the demonstration of ATP-dependent transport of THC-CoA into the peroxisome (7). THCA and THC-CoA were incubated with isolated rat liver peroxisomes and the products of peroxisomal β-oxidation were measured (7). The results of these experiments showed that THC-CoA is the preferred substrate for transport. However, it must be noted that peroxisomes isolated by differential centrifugation are leaky, which makes them not optimal for these kinds of experiments. For this reason, transport should ideally be studied by reconstitution of the transporter or peroxisomal membrane proteins in liposomes. The identity of the transporter for the C27-bile acyl-CoA's is at this moment still unknown.
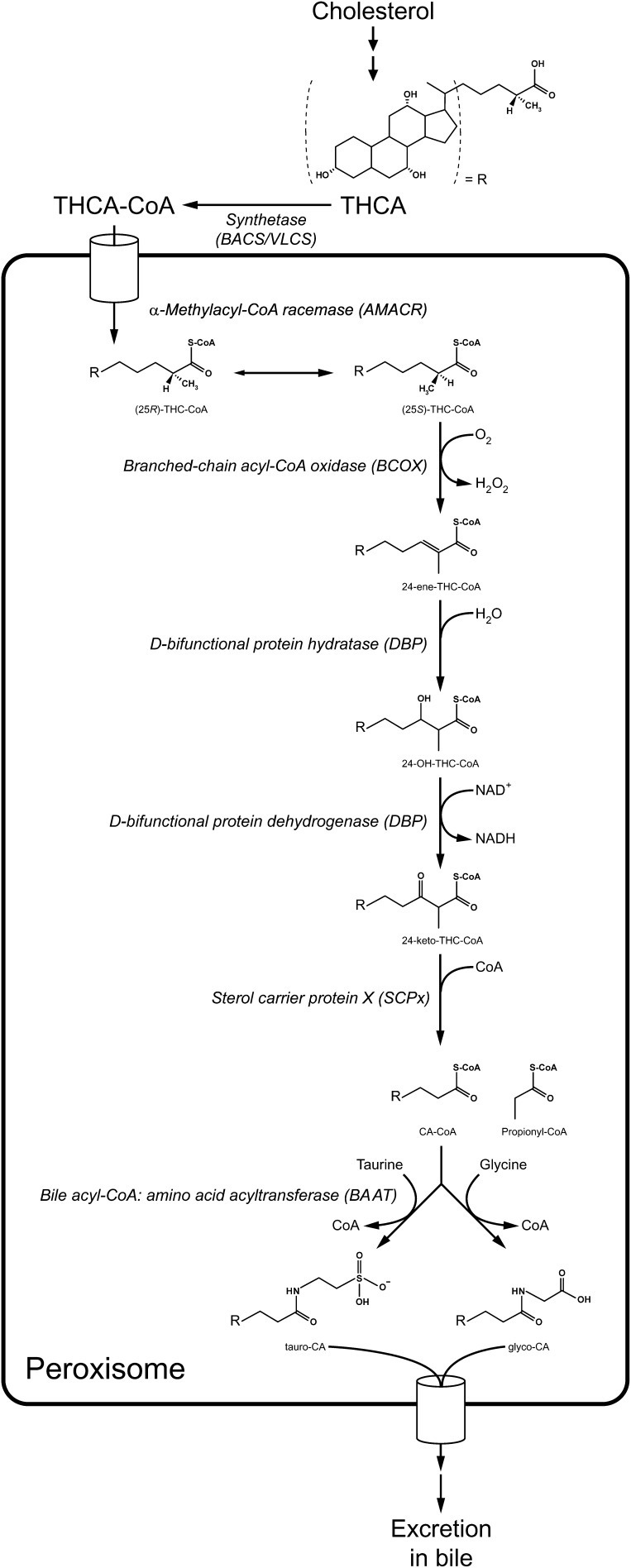
Fig. 1.
Schematic representation of the peroxisomal steps involved in bile acid biosynthesis.
Once inside the peroxisome, the side chain of the C27-bile acid intermediates will be shortened via β-oxidation (Fig. 1). First racemisation of the methyl-group at C-25 will occur by the enzyme α-methylacyl-CoA racemase (AMACR) (8, 9). This is necessary because the first enzyme of the β-oxidation system, branched-chain acyl-CoA oxidase (BCOX), only accepts (S)-isomers as substrate (10, 11), whereas cholesterol 27-hydroxylase (CYP27A1), which forms the carboxyl-group at the side chain in the mitochondrion, only produces the (25R)-isomers of the C27-bile acid intermediates (12, 13). After racemisation, BCOX forms the 24-enoyl-CoA of THCA and DHCA (14). This is followed by a subsequent hydration and dehydrogenation reaction, both performed by D-bifunctional protein (DBP). DBP is also a stereospecific enzyme and only forms (24R)-OH (25R), -isomers, which it subsequently dehydrogenates into 24-keto-CoA-esters (15–18). Finally, sterol carrier protein X (SCPx) is responsible for the thiolytic cleavage into propionyl-CoA and CA-CoA/CDC-CoA (19–21). A detailed description of the different β-oxidation steps has been reviewed previously (22).
The final step in de novo bile acid synthesis is the conjugation/amidation of the C24-bile acids to glycine or taurine. This reaction is performed by the enzyme bile acyl-CoA: amino acid N-acyltransferase (BAAT), which converts the bile acyl-CoAs into tauro- or glyco-bile acids, thereby releasing free CoA (23–25). This reaction occurs in the peroxisome, after which the conjugated C24-bile acids are transported out of the peroxisome and subsequently out of the hepatocyte and into the bile. Recently, bile acid transport activity has been detected and characterized by reconstitution of peroxisomal membrane proteins into liposomes; however, the identity of the transporter remains unknown (26). Conjugation of bile acids not only involves newly synthesized bile acids but also bile acids that have been deconjugated in the intestine and recycled via enterohepatic circulation (2). The deconjugated primary and secondary bile acids are reactivated to CoA thioesters at the cytosolic side of the endoplasmic reticulum by BACS (2); however, the question remains as to where they are reconjugated. By now, the peroxisomal localization of BAAT has been firmly established (25, 27, 28) but there is a longstanding dispute in the literature about a possible dual localization of BAAT, both in the peroxisome and in the cytosol (29–32). A cytosolic localization would negate the need for recycled bile acids to be transported back into the peroxisome just to undergo conjugation. For this reason, further research is needed to firmly establish the precise localization of BAAT.
BILE ACID ABNORMALITIES IN PATIENTS WITH PEROXISOMAL DISORDERS
Investigation of the bile acid abnormalities in the various peroxisomal disorders has been of great value, not only for establishing that functional peroxisomes are necessary for bile acid biosynthesis, but also for determining which peroxisomal enzymes are involved in this pathway. Analysis of plasma from patients with a peroxisome deficiency disorder (i.e., Zellweger syndrome, neonatal adrenoleukodystrophy, and infantile Refsum disease) reveals the presence of C27-bile acid intermediates but also a C29- dicarboxylic acid (33, 34). This dicarboxylic acid (3α,7α,12α-trihydroxy-27-carboxymethyl-5β-cholestane-26- oic acid) is thought to be formed by chain-elongation of THC-CoA (35, 36). The extent of the accumulation of bile acid intermediates has been shown to vary greatly and correlates with the extent to which peroxisomes are deficient (34). In urine of patients with a peroxisome deficiency disorder, the C27-bile acid intermediates are also present, but predominantly as tetrahydroxy-5β-cholestanoic acid and sometimes even pentahydroxy-5β-cholestanoic acid (37–39). Hydroxylation of THCA (of the steroid nucleus mainly at C-1 and C-6 but also of the side chain at C-24, C-25 or C-26) is an important mechanism for increasing the polarity and thereby, the urinary excretion of the C27-bile acid intermediates. In addition, bile alcohol glucuronides are present in urine, which are formed via an alternative pathway for bile acid biosynthesis not requiring the participation of peroxisomes, i.e., the microsomal 25-hydroxylase pathway. The C29-dicarboxylic acid is poorly excreted and for this reason it is not always present in urine.
The most striking bile acid abnormality in AMACR deficiency is the presence of only the (25R)-isomer of the C27-bile acid intermediates (40, 41). In contrast, in other peroxisomal disorders with a defect in bile acid biosynthesis, both the (25R)- and (25S)-isomers accumulate. Just like in patients with a peroxisome deficiency disorder, many polyhydroxylated C27-bile acids are present in urine (41).
To date, no patients with a deficiency of BCOX have been identified but the bile acid abnormalities will most likely resemble those of peroxisome deficiency disorders and AMACR deficiency, although the main accumulating isomer will be the (25S)-isomer in BCOX deficiency. The role of BCOX in the oxidation of bile acids has been established by in vitro studies with the purified enzyme (42, 43) and by the fact that patients with a deficiency of the other peroxisomal acyl-CoA oxidase (straight-chain acyl-CoA oxidase) do not have a bile acid biosynthesis defect (44).
The bile acid abnormalities in DBP deficiency are more complex because DBP displays two catalytic activities, resulting in multiple types of DBP deficiency. In addition, the other peroxisomal bifunctional enzyme, L-bifunctional protein, also displays activity with certain isomers of the C27-bile acid intermediates. In all types of DBP deficiency, DHCA, THCA, and the direct substrate of DBP, the 24-enoyl of the C27-bile acid intermediates are present in plasma (45, 46). When the hydratase activity is deficient (DBP type I and II deficiency, i.e., combined deficiency of the hydratase and dehydrogenase activity and isolated hydratase deficiency, respectively) (24S)-OH (25S), -THC-CoA is also present, being formed by L-bifunctional protein. This isomer is not a substrate for any of the two peroxisomal dehydrogenases and cannot be metabolized further. When only the dehydrogenase activity is deficient (DBP type III deficiency), (24S), -OH (25S), -THC-CoA is also present, but the main accumulating metabolite is (24R)-OH (25R), -THC-CoA, which is formed by the hydratase unit of DBP (45, 46). A small portion is converted into (24R)-OH (25S), -THC-CoA by AMACR, which cannot be metabolized further (47). In urine, the presence of the taurine conjugate of hydroxylated 24-ene-THCA and dihydroxylated 24-ene-THCA is characteristic for patients with DBP deficiency (22). As for peroxisome deficiency disorders, the extent of the accumulation of the C27-bile acid intermediates varies between patients and this seems to correlate with the extent of the deficiency of DBP. Bile acid abnormalities are not found in plasma in 26% of DBP-deficient patients (48).
In plasma of the only SCPx-deficient patient identified to date, only trace amounts of DHCA and THCA were present (49). However, large amounts of bile alcohol glucuronides were identified in urine. In addition, products formed by hydroxylation and decarboxylation of the SCPx substrate 3α,7α,12α-trihydroxy-24-keto-cholestanoyl-CoA were present, namely pentahydroxy-27-nor-5β-cholestane-24-one (m/z 611) and hexahydroxy-27-nor-5β-cholestane-24-one glucuronides (m/z 627).
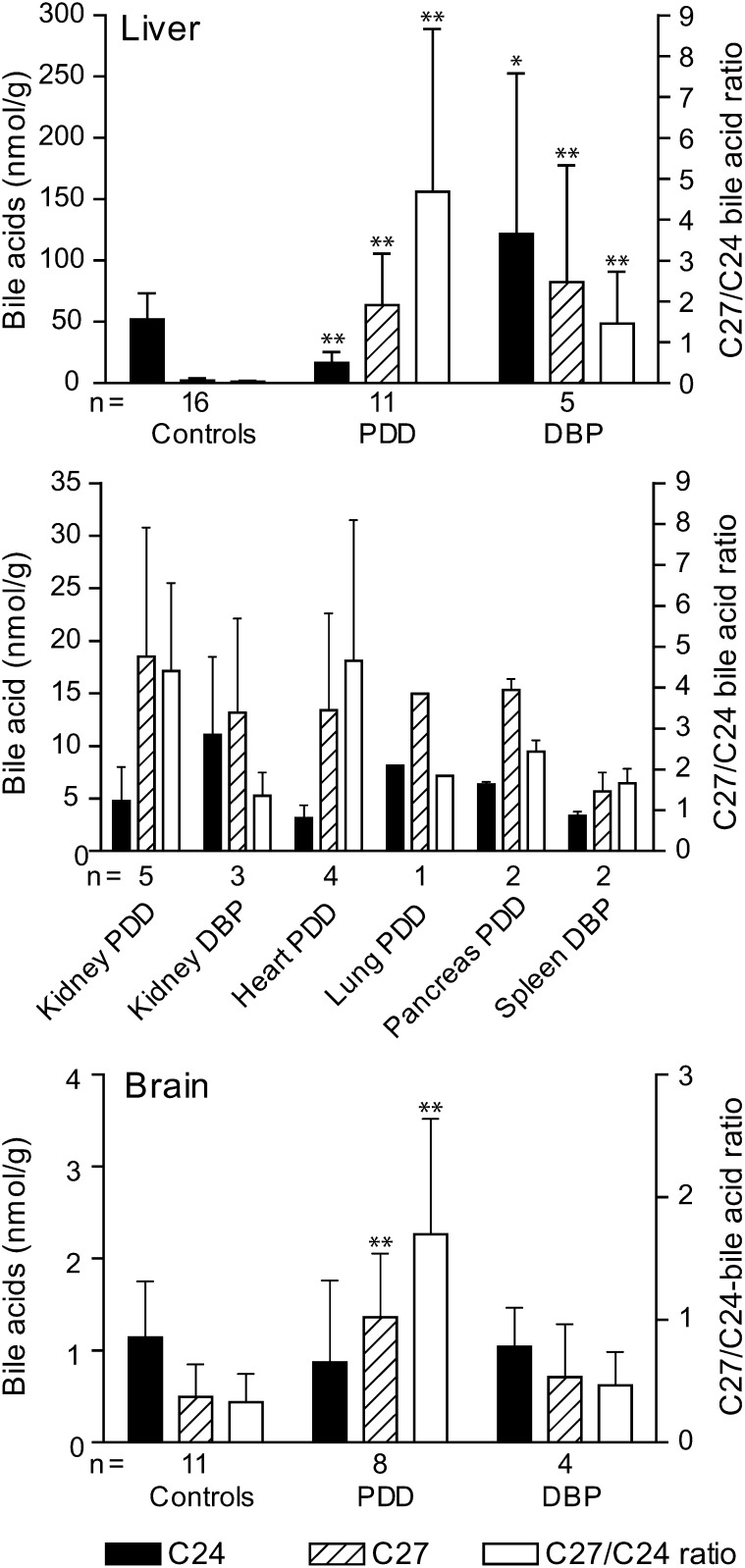
Although the bile acid abnormalities in plasma and urine of patients with a peroxisomal fatty acid oxidation disorder have been well characterized and can be used as diagnostic markers, bile acids in tissues of these patients have not been well studied. It is important to gain insight into the accumulation of bile acids and their intermediates in tissues of patients because bile acid toxicity is postulated to play an important role in the underlying pathological mechanisms of these disorders, not only in liver but also in other organs such as the brain. For this reason, we analyzed bile acids by HPLC-negative ion electrospray tandem mass spectrometry (50, 51) in several tissues of patients with different peroxisomal disorders. The results of these analyses have not been published previously. We studied liver, brain, kidney, heart, spleen, lung, pancreas, and bile of patients with a peroxisome deficiency disorder (including patients with the severe Zellweger phenotype and patients with the milder neonatal adrenoleukodystrophy phenotype), patients with a deficiency of DBP (including patients with a DBP deficiency type I and type III) and one patient with an AMACR deficiency. The results of the bile acid measurements in liver are presented in Table 1 and Fig. 2A. The main accumulating bile acids in the liver of patients with a peroxisome deficiency disorder were t-THCA and THCA. Total C27-bile acid intermediates were significantly increased and total C24-bile acids significantly decreased compared with control livers. This resulted in clearly elevated C27/C24-bile acid ratios in all patient livers. The percentage of conjugated C27-bile acid intermediates was only 51.5% compared with 96.4% for the C24-bile acids. This shows that C27-bile acid intermediates are poor substrates for the conjugating enzyme BAAT. The fact that the C24-bile acids were almost fully conjugated (96.4% compared with 99% in control livers) suggests that BAAT could very well have a dual localization, not only in peroxisomes but also in the cytosol, or at least that BAAT is normally active and stable in the cytosol when peroxisomes are deficient. Both in the livers with a peroxisome deficiency and in the AMACR-deficient liver, the C29-dicarboxylic acid (3α,7α,12α-trihydroxy-27-carboxymethyl-5β-cholestane-26-oic acid) was present, whereas it was not detected in DBP-deficient livers. In livers of patients with a DBP deficiency, the main accumulating C27-bile acid intermediates were OH-THCA, t-THCA:1, and THCA, whereas those in the liver from the AMACR-deficient patient were t-THCA and THCA. The bile acid measurements showed that two DBP-deficient livers and the AMACR-deficient liver were cholestatic but this did not change the fact that the C27/C24-bile acid ratios were elevated in all patient livers. As in the peroxisome-deficient livers, the C27-bile acid intermediates were partly conjugated, confirming that BAAT is poorly active with bile acids with an increased side chain length compared with C24-bile acids (29, 52).
TABLE 1.
Bile acids in liver of control subjects, patients with a peroxisome deficiency disorder (PDD), and patients with a D-bifunctional protein (DBP) deficiency
| Controls |
PDD |
DBP |
AMACR |
|||||
|---|---|---|---|---|---|---|---|---|
| n = 16 |
n = 11 |
n = 5 |
n = 1 |
|||||
| m/z | Mean | Range | Mean | Range | Mean | Range | ||
| C29DCA | 507 | 0.00 | 0.00-0.02 | 1.71** | 0.37-4.66 | 0.00 | 0.00-0.00 | 0.19 |
| t-OH-THCA | 572 | 0.03 | 0.00-0.14 | 2.99** | 0.54-9.78 | 1.48* | 0.00-5.35 | 1.28 |
| t-OH-THCA:1 | 570 | 0.03 | 0.00-0.53 | 0.06 | 0.00-0.29 | 3.45** | 0.02-8.78 | 0.37 |
| t-THCA | 556 | 0.36 | 0.05-2.26 | 26.51** | 4.54-95.43 | 4.80* | 0.38-19.61 | 74.14 |
| t-THCA:1 | 554 | 0.01 | 0.00-0.11 | 0.74** | 0.06-2.04 | 10.85** | 0.82-32.68 | 13.85 |
| t-DHCA | 540 | 0.02 | 0.00-0.08 | 3.40** | 0.39-8.87 | 0.53* | 0.05-2.22 | 4.66 |
| t-DHCA:1 | 538 | 0.01 | 0.00-0.17 | 0.10* | 0.00-0.47 | 1.32** | 0.19-4.56 | 0.13 |
| t-CA | 514 | 9.97 | 1.66-33.00 | 4.89 | 0.41-9.30 | 37.38* | 0.91-100.18 | 21.05 |
| t-UDCA | 498 | 0.30 | 0.00-1.17 | 0.04* | 0.00-0.15 | 0.92* | 0.00-1.90 | 1.90 |
| t- CDCA/DCA | 498 | 11.64 | 3.16-24.41 | 3.26** | 0.94-4.98 | 14.23 | 1.27-30.81 | 44.43 |
| g-THCA | 506 | 0.15 | 0.00-0.33 | 0.80** | 0.04-2.45 | 0.47* | 0.02-1.42 | 9.93 |
| g-CA | 464 | 10.08 | 1.45-41.30 | 3.67 | 0.39-10.01 | 37.08* | 2.31-96.25 | 52.96 |
| g-UDCA | 448 | 0.65 | 0.00-2.23 | 0.14* | 0.00-0.81 | 1.40 | 0.02-3.77 | 6.34 |
| g-CDCA/DCA | 448 | 18.47 | 2.00-48.50 | 4.26** | 0.86-18.07 | 20.62 | 1.26-48.68 | 74.16 |
| OH-THCA | 465 | 0.31 | 0.00-1.89 | 6.99** | 1.20-19.73 | 35.06* | 8.41-126.43 | 68.41 |
| OH-THCA:1 | 463 | 0.49 | 0.14-1.71 | 1.30** | 0.60-2.63 | 5.58** | 2.13-16.01 | 22.24 |
| THCA | 449 | 0.09 | 0.00-0.95 | 14.56** | 5.69-37.11 | 9.00** | 5.13-12.27 | 88.82 |
| THCA:1 | 447 | 0.36 | 0.00-0.62 | 1.76** | 0.70-4.04 | 6.13** | 2.76-13.87 | 10.54 |
| DHCA | 433 | 0.03 | 0.00-0.12 | 3.74** | 0.46-8.15 | 3.15** | 1.25-7.66 | 0.43 |
| DHCA:1 | 431 | 0.15 | 0.01-0.55 | 0.57** | 0.15-1.16 | 0.54* | 0.21-1.29 | 0.06 |
| CA | 407 | 0.41 | 0.07-2.22 | 0.29 | 0.14-0.75 | 1.99 | 0.31-7.78 | 4.89 |
| UDCA | 391 | 0.06 | 0.01-0.20 | 0.07 | 0.00-0.31 | 0.36 | 0.00-1.74 | 0.00 |
| CDCA/DCA | 391 | 0.07 | 0.01-0.21 | 0.12 | 0.05-0.30 | 0.93 | 0.03-4.09 | 9.07 |
| Total C27-BA | 2.04 | 0.53-7.63 | 63.52** | 18.54-178.36 | 82.36** | 32.37-252.15 | 294.86 | |
| Total C24-BA | 51.97 | 15.46-80.62 | 16.74** | 6.38-34.40 | 121.66* | 9.89-317.55 | 216.46 | |
| Total BA | 54.01 | 15.98-88.30 | 80.25** | 24.91-190.01 | 204.02** | 42.97-444.28 | 444.28 | |
| C27/C24-BA ratio | 0.04 | 0.01-0.1 | 4.69** | 1.57-15.31 | 1.45** | 0.16-3.35 | 1.36 | |
Bile acids are expressed as nmol/g wet weight. BA, bile acids. CDCA and DCA cannot be distinguished with the method used for bile acid measurement. * P < 0.05; ** P < 0.005 (t-test).
Fig. 2.
Bile acids measured in tissues of control subjects, patients with a peroxisome deficiency disorder (PDD), and patients with a D-bifunctional protein (DBP) deficiency. C24-bile acids are the sum of conjugated and unconjugated CA, CDCA, ursodeoxycholic acid, and deoxycholic acid. C27-bile acids are the sum of conjugated and unconjugated THCA, THCA:1, OH-THCA, OH-THCA:1, DHCA, DHCA:1, and C29DCA. Data represent the mean ± SD, all measurements were performed in duplicate. The number (n) of patients analyzed per tissue is indicated. *, P < 0.05; ** P < 0.005 (t-test).
Bile of one patient with a peroxisome deficiency and the AMACR-deficient patient was analyzed in addition to the liver sample. For both patients, the C27/C24-bile acid ratio was higher in liver than in bile (2.58 vs. 1.66 in the peroxisome-deficient patient) and the percentage of conjugated C27-bile acid intermediates was higher in bile than in liver (81% vs. 66% in the same patient). This suggests that C27-bile acid intermediates are less efficiently transported across the canalicular membrane and that conjugated C27-bile acid intermediates are the preferred substrates for this transport. At the moment, it is not known which transporters in the canalicular membrane are specifically involved in this process.
In the analyzed peripheral tissues (kidney, heart, spleen, lung, pancreas) of the different peroxisomal patients, the C27-bile acid intermediates clearly accumulated and the C27/C24-bile acid ratio was comparable to the one found in liver (Fig. 2B). The pattern of accumulating metabolites was also comparable to liver except that the main accumulating C27-bile acid intermediates were mostly unconjugated (resulting in a percentage of conjugated C27-bile acid intermediates of around 20-25% in the different tissues), which again suggests that the conjugated C27-bile acid intermediates are more efficiently excreted into the bile.
In brains of patients with a peroxisome deficiency disorder, there were clear bile acid abnormalities (Table 2 and Fig. 2C). C27-bile acid intermediates were increased compared with control brains and C24-bile acids were decreased, resulting in a significantly increased C27/C24-bile acid ratio. The accumulating C27-bile acid intermediates were THCA, DHCA, and OH-THCA, all unconjugated, consistent with a less efficient passage of conjugated C27-bile acid intermediates of the blood-brain barrier. The C29-dicarboxylic acid was also detectable in brain. The brains of DBP-deficient patients were much harder to distinguish from control brains based on the bile acid measurements. There were no significant differences for total C27-bile acid intermediates or C24-bile acids levels. The only bile acids that were elevated were OH-THCA and DHCA. For two patients with a peroxisome deficiency disorder, two DBP-deficient patients, and four control subjects, tissue from different brain areas was analyzed, including frontal white and gray matter, parietal white and gray matter, and extra frontal and parietal cortex. No significant difference between the bile acid content in the different brain areas was detected.
TABLE 2.
Bile acids in brain of control subjects, patients with a peroxisome deficiency disorder (PDD) and patients with a D-bifunctional protein (DBP) deficiency
| Controls |
PBD |
DBP |
|||||
|---|---|---|---|---|---|---|---|
| n = 11 |
n = 8 |
n = 4 |
|||||
| m/z | Mean | Range | Mean | Range | Mean | Range | |
| C29DCA | 507 | 0.01 | 0.00-0.03 | 0.18** | 0.01-0.38 | 0.00 | 0.00-0.00 |
| t-CA | 514 | 0.25 | 0.07-1.18 | 0.22 | 0.05-1.04 | 0.15 | 0.08-0.19 |
| t- CDCA/DCA | 498 | 0.18 | 0.07-0.50 | 0.11 | 0.02-0.21 | 0.15 | 0.08-0.19 |
| g-CA | 464 | 0.16 | 0.03-0.36 | 0.18 | 0.00-0.83 | 0.22 | 0.09-0.37 |
| g-CDCA/DCA | 448 | 0.26 | 0.04-0.43 | 0.14* | 0.00-0.18 | 0.26 | 0.10-0.39 |
| OH-THCA | 465 | 0.05 | 0.00-0.22 | 0.24** | 0.11-0.43 | 0.13 | 0.06-0.22 |
| OH-THCA:1 | 463 | 0.15 | 0.00-0.40 | 0.14 | 0.03-0.38 | 0.15 | 0.00-0.38 |
| THCA | 449 | 0.05 | 0.00-0.23 | 0.50** | 0.07-1.29 | 0.06 | 0.00-0.16 |
| DHCA | 433 | 0.02 | 0.00-0.10 | 0.20* | 0.00-0.53 | 0.06* | 0.04-0.09 |
| CA | 407 | 0.12 | 0.06-0.25 | 0.10 | 0.05-0.27 | 0.11 | 0.08-0.18 |
| CDCA/DCA | 391 | 0.14 | 0.03-0.28 | 0.04** | 0.01-0.09 | 0.09 | 0.05-0.14 |
| Total C27-BA | 0.51 | 0.04-0.98 | 1.36** | 0.40-2.49 | 0.71 | 0.12-1.40 | |
| Total C24-BA | 1.20 | 0.39-2.77 | 0.87 | 0.34-3.06 | 1.04 | 0.49-1.51 | |
| Total BA | 1.71 | 0.78-3.75 | 2.23 | 1.35-4.67 | 1.75 | 0.61-3.02 | |
| C27/C24-BA ratio | 0.44 | 0.05-1.00 | 2.27** | 0.47-3.83 | 0.62 | 0.27-0.94 | |
Bile acids are expressed as nmol/g wet weight. BA, bile acids. CDCA and DCA cannot be distinguished with the method used for bile acid measurement. * P < 0.05; ** P < 0.005 (t-test).
TOXICITY OF C27-BILE ACID INTERMEDIATES AND THE PATHOLOGICAL CONSEQUENCES OF A BILE ACID BIOSYNTHESIS DEFECT AT THE LEVEL OF THE PEROXISOME
The bile acid abnormalities in patients with a peroxisomal disorder have been shown to contribute to the liver disease associated with these disorders (53, 54) and also have been hypothesized to have an effect on the developing nervous system (55). The C27-bile acid intermediates are more hydrophobic than their C24-products, and as shown by the bile acid measurements in tissues of patients described above, they are less efficiently conjugated (augmenting to their hydrophobicity) and excreted into the bile. These factors will contribute to the retention of these intermediates in the liver, cholestasis, and, consequently, to hepatic injury in these patients. In addition, the primary bile acid deficiency decreases the intestinal dietary lipid and lipid soluble vitamin absorption. Furthermore, we have shown that in brains of peroxisome deficiency patients, C27-bile acid intermediates accumulate and that these were almost exclusively unconjugated, increasing their potential toxic effect. However, because peroxisomes are involved in multiple metabolic pathways, interpretation of the pathological consequences of the bile acid synthesis defect in patients is complex. For this reason, we have recently performed an in vitro study on the toxicity of the conjugated and unconjugated C27-bile acid intermediates. The effects on cell viability, mitochondrial respiratory chain function, and the production of oxygen radicals were examined in a rat hepatoma cell line and compared with the effect on these parameters of the mature C24-bile acids (56). These studies showed that C27-bile acids intermediates are more cytotoxic than mature C24-bile acids, with DHCA being clearly the most cytotoxic bile acid. In addition to the membrane-disruptive effects of bile acids due to their detergent properties, other mechanisms, like the induction of mitochondrial dysfunction, have been shown to be involved in the toxicity of bile acids (57). For this reason, the effect on the mitochondrial respiratory chain function was studied (56). It was shown that the different bile acids caused a dose-dependent decrease in ATP synthesis by isolated mitochondria oxidizing malate and glutamate (the order of potency of inhibition was: DHCA>THCA>CDCA>g-THCA>g-CDCA>t-THCA>CA). The unconjugated bile acids acted predominantly as uncouplers of the oxidative phophorylation whereas the conjugated bile acids (t-THCA, g-THCA and g-CDCA) primarily inhibited the respiratory chain. By inhibiting the respiratory chain, the conjugated bile acids t-THCA, g-THCA, and g-CDCA enhanced reactive oxygen species (ROS) production at the level of complex III. Increased ROS generation leads to depletion of the antioxidant defenses and can eventually lead to cell death. Interestingly, in the very first report describing Zellweger syndrome, brain and liver mitochondria from a patient showed strongly reduced respiration with malate and succinate as substrates (58). In addition, in a mouse model with hepatocyte-selective elimination of peroxisomes, severely reduced activities of complex I, III, and V were measured (59) and in a Pex5 knockout mouse, a model for Zellweger syndrome, reduction in the activities of complex I and V were observed (60), supporting the physiological relevance of the in vitro findings. The reduction of the activities of the respiratory chain complexes was accompanied by alterations of the mitochondrial inner membrane structure, which has also been observed in Zellweger patients (58, 61, 62).
Because the C27-bile acid intermediates are especially cytotoxic, reducing their levels could be beneficial not only for the liver disease in peroxisomal patients but also to prevent potential injury to other organs by circulating C27-bile acid intermediates. C27-bile acid levels can be reduced by treatment with C24-bile acids, which downregulate bile acid biosynthesis via activation of the nuclear receptor farnesoid X receptor (FXR), resulting in reduced transcriptional activation of CYP7A1, the rate-limiting enzyme in bile acid biosynthesis. In addition, bile acid therapy will increase bile flow by replenishing the decreased levels of C24-bile acids and it will increase the intraluminal bile acid concentration, thereby facilitating the absorption of fats and fat soluble vitamins. Oral bile acid therapy has been tried with variable success in peroxisomal disorders (41, 63–65). The first report of such a bile acid treatment was in a 6-month-old Zellweger patient who showed significant improvement of biochemical indices of liver function and a histological improvement in the extent of inflammation and bile duct proliferation and disappearance of canalicular plugs (64). Steatorrhea improved and was accompanied by an improvement in growth. Plasma and urinary C27-bile acid intermediates decreased with treatment. Despite the observed improvements, the patient died at one year of age. Also, in subsequent studies it has been shown that patients with Zellweger syndrome are too severely affected by the multiple metabolic derangements for bile acid therapy to have a positive effect on the outcome of the disease (63, 65). However, in the Pex2 knockout mouse, another model for Zellweger syndrome, positive effects of bile acid therapy were observed (55, 66). Pex2 knockout mice were fed a mixture of CA and ursodeoxycholic acid (UDCA), which resulted in a significant improvement in postnatal survival. Increased body fat deposition was observed and improvement of the intestinal malabsorption. Compared with untreated mutant mice, bile acid treated Pex2 knockout mice were more active and less hypotonic but problems with balance and spasticity persisted. Interestingly, there was a significant improvement in the extent of dendritic arborization of cerebellar Purkinje cells (55). Thus, bile acid therapy may improve some aspects of central nervous system development. In the liver, bile acid treatment alleviated intrahepatic cholestasis and prevented the development of severe steatohepatitis. However, the therapy exacerbated the degree of hepatic steatosis and worsened the already severe mitochondrial and cellular damage in the liver (66). These studies showed that peroxisome-deficient hepatocytes are particularly sensitive to bile acid toxicity, indicating that bile acid therapy will be more beneficial in patients with a relatively mild peroxisome biogenesis disorder (neonatal adrenoleukodystrophy or infantile Refsum disease) or patients with a single enzyme defect (AMACR, DBP, SCPx deficiency). Indeed, a child with AMACR deficiency has been reported to respond well to oral bile acid therapy for 7 years (41). On treatment, the C27-bile acids intermediates are virtually absent in urine, her liver enzymes have remained normal, and her fat-soluble vitamins have remained normal without supplementation. As an alternative to oral bile acid therapy, treatment with an artificial FXR ligand should be considered. These artificial FXR ligands are not harmful for the hepatocyte but they will downregulate the synthesis of the toxic C27-bile acid intermediates (67).
CONCLUDING REMARKS
The importance of proper peroxisomal function for the biosynthesis of bile acids has been firmly established. The peroxisomal enzymes involved in bile acid biosynthesis have all been identified. Only the transporters required for the transport of the C27-bile acid intermediates into the peroxisome and for the conjugated C24-bile acids out of the peroxisome remain unidentified. In addition, it is not clear yet whether deconjugated and recycled C24-bile acids need to reenter the peroxisome for reconjugation or whether this occurs in the cytosol. The bile acid abnormalities in patients with peroxisomal disorders have been well characterized and evidently play a role in the pathology of these disorders. The C27-bile acid intermediates have been shown to be more cytotoxic than the C24-bile acids, and the finding of primarily unconjugated C27-bile acid intermediates in brains from children with Zellweger syndrome suggests that the bile acid synthesis defect in these patients could also play a role in central nervous system pathology. Unfortunately, the clinical presentation of children with classical Zellweger syndrome is too severe due to the multiple metabolic derangements for a true positive effect of oral bile acid therapy in these patients but patients with a mild presentation (i.e., infantile Refsum disease) or a single enzyme deficiency could very well benefit from such a therapy or treatment with an FXR ligand to reduce the toxic C27-bile acid intermediates.
Acknowledgments
The authors thank Dr. Carl E. Clarke for providing patient material. Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders under contracts N01-HD-4-3368 and N01-HD-4-3383. We thank Henk Overmars for technical assistance.
Footnotes
Abbreviations:
- AMACR
- α-methylacyl-CoA racemase
- BACS
- bile acyl-CoA synthetase
- BAAT
- bile acyl-CoA: amino acid N-acyltransferase
- BCOX
- branched-chain acyl-CoA oxidase
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- DBP
- D-bifunctional protein
- DCA
- deoxycholic acid
- DHCA
- dihydroxycholestanoic acid
- FXR
- farnesoid X receptor
- SCPx
- sterol carrier protein X
- THCA
- trihydroxycholestanoic acid
- UDCA
- ursodeoxycholic acid
This work was supported by the Netherlands Organisation for Scientific Research (NWO grant 916.46.109) and by the National Institutes of Health (Grant NS050602). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Russell D. W. 2003. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72: 137–174. [DOI] [PubMed] [Google Scholar]
- 2.Mihalik S. J., Steinberg S. J., Pei Z., Park J., Kim D. G., Heinzer A. K., Dacremont G., Wanders R. J., Cuebas D. A., Smith K. D., Watkins P. A. 2002. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J. Biol. Chem. 277: 24771–24779. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg S. J., Mihalik S. J., Kim D. G., Cuebas D. A., Watkins P. A. 2000. The human liver-specific homolog of very long-chain acyl-CoA synthetase is cholate:CoA ligase. J. Biol. Chem. 275: 15605–15608. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg S. J., Wang S. J., Kim D. G., Mihalik S. J., Watkins P. A. 1999. Human very-long-chain acyl-CoA synthetase: cloning, topography, and relevance to branched-chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 257: 615–621. [DOI] [PubMed] [Google Scholar]
- 5.Prydz K., Kase B. F., Bjorkhem I., Pedersen J. I. 1988. Subcellular localization of 3 alpha, 7 alpha-dihydroxy- and 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoyl-coenzyme A ligase(s) in rat liver. J. Lipid Res. 29: 997–1004. [PubMed] [Google Scholar]
- 6.Schepers L., Casteels M., Verheyden K., Parmentier G., Asselberghs S., Eyssen H. J., Mannaerts G. P. 1989. Subcellular distribution and characteristics of trihydroxycoprostanoyl-CoA synthetase in rat liver. Biochem. J. 257: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Une M., Iguchi Y., Sakamoto T., Tomita T., Suzuki Y., Morita M., Imanaka T. 2003. ATP-dependent transport of bile acid intermediates across rat liver peroxisomal membranes. J. Biochem. 134: 225–230. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz W., Albers C., Fingerhut R., Conzelmann E. 1995. Purification and characterization of an alpha-methylacyl-CoA racemase from human liver. Eur. J. Biochem. 231: 815–822. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz W., Fingerhut R., Conzelmann E. 1994. Purification and properties of an alpha-methylacyl-CoA racemase from rat liver. Eur. J. Biochem. 222: 313–323. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen J. I., Veggan T., Bjorkhem I. 1996. Substrate stereospecificity in oxidation of (25S)-3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoyl-CoA by peroxisomal trihydroxy-5 beta-cholestanoyl-CoA oxidase. Biochem. Biophys. Res. Commun. 224: 37–42. [DOI] [PubMed] [Google Scholar]
- 11.van Veldhoven P. P., Croes K., Asselberghs S., Herdewijn P., Mannaerts G. P. 1996. Peroxisomal beta-oxidation of 2-methyl-branched acyl-CoA esters: stereospecific recognition of the 2S-methyl compounds by trihydroxycoprostanoyl-CoA oxidase and pristanoyl-CoA oxidase. FEBS Lett. 388: 80–84. [DOI] [PubMed] [Google Scholar]
- 12.Batta A. K., Salen G., Shefer S., Dayal B., Tint G. S. 1983. Configuration at C-25 in 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta- cholestan-26-oic acid isolated from human bile. J. Lipid Res. 24: 94–96. [PubMed] [Google Scholar]
- 13.Shefer S., Cheng F. W., Batta A. K., Dayal B., Tint G. S., Salen G., Mosbach E. H. 1978. Stereospecific side chain hydroxylations in the biosynthesis of chenodeoxycholic acid. J. Biol. Chem. 253: 6386–6392. [PubMed] [Google Scholar]
- 14.Vanhove G. F., Van Veldhoven P. P., Fransen M., Denis S., Eyssen H. J., Wanders R. J., Mannaerts G. P. 1993. The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. J. Biol. Chem. 268: 10335–10344. [PubMed] [Google Scholar]
- 15.Jiang L. L., Kurosawa T., Sato M., Suzuki Y., Hashimoto T. 1997. Physiological role of D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J. Biochem. (Tokyo). 121: 506–513. [DOI] [PubMed] [Google Scholar]
- 16.Kurosawa T., Sato M., Yoshimura T., Jiang L. L., Hashimoto T., Tohma M. 1997. Stereospecific formation of (24R,25R)-3 alpha,7 alpha,12 alpha,24-tetrahydroxy-5 beta-cholestan-26-oic acid catalyzed with a peroxisomal bifunctional D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase. Biol. Pharm. Bull. 20: 295–297. [DOI] [PubMed] [Google Scholar]
- 17.Novikov D., Dieuaide-Noubhani M., Vermeesch J. R., Fournier B., Mannaerts G. P., Van Veldhoven P. P. 1997. The human peroxisomal multifunctional protein involved in bile acid synthesis: activity measurement, deficiency in Zellweger syndrome and chromosome mapping. Biochim. Biophys. Acta. 1360: 229–240. [DOI] [PubMed] [Google Scholar]
- 18.Qin Y. M., Haapalainen A. M., Conry D., Cuebas D. A., Hiltunen J. K., Novikov D. K. 1997. Recombinant 2-enoyl-CoA hydratase derived from rat peroxisomal multifunctional enzyme 2: role of the hydratase reaction in bile acid synthesis. Biochem. J. 328: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonenkov V. D., Van Veldhoven P. P., Waelkens E., Mannaerts G. P. 1997. Substrate specificities of 3-oxoacyl-CoA thiolase A and sterol carrier protein 2/3-oxoacyl-CoA thiolase purified from normal rat liver peroxisomes. Sterol carrier protein 2/3-oxoacyl-CoA thiolase is involved in the metabolism of 2-methyl-branched fatty acids and bile acid intermediates. J. Biol. Chem. 272: 26023–26031. [DOI] [PubMed] [Google Scholar]
- 20.Bun-ya M., Maebuchi M., Kamiryo T., Kurosawa T., Sato M., Tohma M., Jiang L. L., Hashimoto T. 1998. Thiolase involved in bile acid formation. J. Biochem. (Tokyo). 123: 347–352. [DOI] [PubMed] [Google Scholar]
- 21.Wanders R. J., Denis S., van Berkel E., Wouters F., Wirtz K. W., Seedorf U. 1998. Identification of the newly discovered 58 kDa peroxisomal thiolase SCPx as the main thiolase involved in both pristanic acid and trihydroxycholestanoic acid oxidation: implications for peroxisomal beta-oxidation disorders. J. Inherit. Metab. Dis. 21: 302–305. [DOI] [PubMed] [Google Scholar]
- 22.Ferdinandusse S., Houten S. M. 2006. Peroxisomes and bile acid biosynthesis. Biochim. Biophys. Acta. 1763: 1427–1440. [DOI] [PubMed] [Google Scholar]
- 23.Falany C. N., Fortinberry H., Leiter E. H., Barnes S. 1997. Cloning, expression, and chromosomal localization of mouse liver bile acid CoA:amino acid N-acyltransferase. J. Lipid Res. 38: 1139–1148. [PubMed] [Google Scholar]
- 24.Falany C. N., Johnson M. R., Barnes S., Diasio R. B. 1994. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 269: 19375–19379. [PubMed] [Google Scholar]
- 25.He D., Barnes S., Falany C. N. 2003. Rat liver bile acid CoA:amino acid N-acyltransferase: expression, characterization, and peroxisomal localization. J. Lipid Res. 44: 2242–2249. [DOI] [PubMed] [Google Scholar]
- 26.Visser W. F., van Roermund C. W., Ijlst L., Waterham H. R., Wanders R. J. 2007. Demonstration of bile acid transport across the mammalian peroxisomal membrane. Biochem. Biophys. Res. Commun. 357: 335–340. [DOI] [PubMed] [Google Scholar]
- 27.Kase B. F., Bjorkhem I. 1989. Peroxisomal bile acid-CoA:amino-acid N-acyltransferase in rat liver. J. Biol. Chem. 264: 9220–9223. [PubMed] [Google Scholar]
- 28.Pellicoro A., van den Heuvel F. A., Geuken M., Moshage H., Jansen P. L., Faber K. N. 2007. Human and rat bile acid-CoA:amino acid N-acyltransferase are liver-specific peroxisomal enzymes: implications for intracellular bile salt transport. Hepatology. 45: 340–348. [DOI] [PubMed] [Google Scholar]
- 29.O'Byrne J., Hunt M. C., Rai D. K., Saeki M., Alexson S. E. 2003. The human bile acid-CoA:amino acid N-acyltransferase functions in the conjugation of fatty acids to glycine. J. Biol. Chem. 278: 34237–34244. [DOI] [PubMed] [Google Scholar]
- 30.Solaas K., Kase B. F., Pham V., Bamberg K., Hunt M. C., Alexson S. E. 2004. Differential regulation of cytosolic and peroxisomal bile acid amidation by PPAR alpha activation favors the formation of unconjugated bile acids. J. Lipid Res. 45: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 31.Solaas K., Ulvestad A., Soreide O., Kase B. F. 2000. Subcellular organization of bile acid amidation in human liver: a key issue in regulating the biosynthesis of bile salts. J. Lipid Res. 41: 1154–1162. [PubMed] [Google Scholar]
- 32.Styles N. A., Falany J. L., Barnes S., Falany C. N. 2007. Quantification and regulation of the subcellular distribution of bile acid coenzyme A:amino acid N-acyltransferase activity in rat liver. J. Lipid Res. 48: 1305–1315. [DOI] [PubMed] [Google Scholar]
- 33.Clayton P. T. 1991. Inborn errors of bile acid metabolism. J. Inherit. Metab. Dis. 14: 478–496. [DOI] [PubMed] [Google Scholar]
- 34.van Eldere J. R., Parmentier G. G., Eyssen H. J., Wanders R. J., Schutgens R. B., Vamecq J., Van Hoof F., Poll-The B. T., Saudubray J. M. 1987. Bile acids in peroxisomal disorders. Eur. J. Clin. Invest. 17: 386–390. [DOI] [PubMed] [Google Scholar]
- 35.Janssen G., Toppet S., Parmentier G. 1982. Structure of the side chain of the C29 dicarboxylic bile acid occurring in infants with coprostanic acidemia. J. Lipid Res. 23: 456–465. [PubMed] [Google Scholar]
- 36.Parmentier G. G., Janssen G. A., Eggermont E. A., Eyssen H. J. 1979. C27 bile acids in infants with coprostanic acidemia and occurrence of a 3 alpha,7 alpha,12 alpha-tridhydroxy-5 beta-C29 dicarboxylic bile acid as a major component in their serum. Eur. J. Biochem. 102: 173–183. [DOI] [PubMed] [Google Scholar]
- 37.Lawson A. M., Madigan M. J., Shortland D., Clayton P. T. 1986. Rapid diagnosis of Zellweger syndrome and infantile Refsum's disease by fast atom bombardment–mass spectrometry of urine bile salts. Clin. Chim. Acta. 161: 221–231. [DOI] [PubMed] [Google Scholar]
- 38.Une M., Tazawa Y., Tada K., Hoshita T. 1987. Occurrence of both (25R)- and (25S)-3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acids in urine from an infant with Zellweger's syndrome. J. Biochem. (Tokyo). 102: 1525–1530. [DOI] [PubMed] [Google Scholar]
- 39.Yousef I. M., Perwaiz S., Lamireau T., Tuchweber B. 2003. Urinary bile acid profile in children with inborn errors of bile acid metabolism and chronic cholestasis; screening technique using electrospray tandem mass-spectrometry (ES/MS/MS). Med. Sci. Monit. 9: MT21–MT31. [PubMed] [Google Scholar]
- 40.Ferdinandusse S., Overmars H., Denis S., Waterham H. R., Wanders R. J. A., Vreken P. 2001. Plasma analysis of di- and trihydroxycholestanoic acid diastereoisomers in peroxisomal alpha-methylacyl-CoA racemase deficiency. J. Lipid Res. 42: 137–141. [PubMed] [Google Scholar]
- 41.Setchell K. D., Heubi J. E., Bove K. E., O'Connell N. C., Brewsaugh T., Steinberg S. J., Moser A., Squires R. H., Jr 2003. Liver disease caused by failure to racemize trihydroxycholestanoic acid: gene mutation and effect of bile acid therapy. Gastroenterology. 124: 217–232. [DOI] [PubMed] [Google Scholar]
- 42.van Veldhoven P. P., Vanhove G., Assselberghs S., Eyssen H. J., Mannaerts G. P. 1992. Substrate specificities of rat liver peroxisomal acyl-CoA oxidases: palmitoyl-CoA oxidase (inducible acyl-CoA oxidase), pristanoyl-CoA oxidase (non-inducible acyl-CoA oxidase), and trihydroxycoprostanoyl-CoA oxidase. J. Biol. Chem. 267: 20065–20074. [PubMed] [Google Scholar]
- 43.Wanders R. J., Denis S. W., Dacremont G. 1993. Studies on the substrate specificity of the inducible and non-inducible acyl-CoA oxidases from rat kidney peroxisomes. J. Biochem. (Tokyo). 113: 577–582. [DOI] [PubMed] [Google Scholar]
- 44.Ferdinandusse S., Denis S., Hogenhout E. M., Koster J., van Roermund C. W., IJlst L., Moser A. B., Wanders R. J., Waterham H. R. 2007. Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Hum. Mutat. 28: 904–912. [DOI] [PubMed] [Google Scholar]
- 45.Une M., Konishi M., Suzuki Y., Akaboshi S., Yoshii M., Kuramoto T., Fujimura K. 1997. Bile acid profiles in a peroxisomal D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein deficiency. J. Biochem. (Tokyo). 122: 655–658. [DOI] [PubMed] [Google Scholar]
- 46.Vreken P., van Rooij A., Denis S., van Grunsven E. G., Cuebas D. A., Wanders R. J. 1998. Sensitive analysis of serum 3alpha, 7alpha, 12alpha,24-tetrahydroxy- 5beta-cholestan-26-oic acid diastereomers using gas chromatography-mass spectrometry and its application in peroxisomal D-bifunctional protein deficiency. J. Lipid Res. 39: 2452–2458. [PubMed] [Google Scholar]
- 47.Cuebas D. A., Phillips C., Schmitz W., Conzelmann E., Novikov D. K. 2002. The role of alpha-methylacyl-CoA racemase in bile acid synthesis. Biochem. J. 363: 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferdinandusse S., Denis S., Mooyer P. A., Dekker C., Duran M., Soorani-Lunsing R. J., Boltshauser E., Macaya A., Gartner J., Majoie C. B., et al. 2006. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann. Neurol. 59: 92–104. [DOI] [PubMed] [Google Scholar]
- 49.Ferdinandusse S., Kostopoulos P., Denis S., Rusch H., Overmars H., Dillmann U., Reith W., Haas D., Wanders R. J. A., Duran M., et al. 2006. Mutations in the gene encoding peroxisomal sterol carrier protein X (SCPx) cause leukencephalopathy with dystonia and motor neuropathy. Am. J. Hum. Genet. 78: 1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bootsma A. H., Overmars H., van Rooij A., van Lint A. E., Wanders R. J., van Gennip A. H., Vreken P. 1999. Rapid analysis of conjugated bile acids in plasma using electrospray tandem mass spectrometry: application for selective screening of peroxisomal disorders. J. Inherit. Metab. Dis. 22: 307–310. [DOI] [PubMed] [Google Scholar]
- 51.Ferdinandusse S., Denis S., Overmars H., Van Eeckhoudt L., Van Veldhoven P. P., Duran M., Wanders R. J., Baes M. 2005. Developmental changes of bile acid composition and conjugation in L- and D-bifunctional protein single and double knockout mice. J. Biol. Chem. 280: 18658–18666. [DOI] [PubMed] [Google Scholar]
- 52.Czuba B., Vessey D. A. 1982. The effect of bile acid structure on the activity of bile acid-CoA:glycine/taurine-N-acetyltransferase. J. Biol. Chem. 257: 8761–8765. [PubMed] [Google Scholar]
- 53.Bove K. E., Daugherty C. C., Tyson W., Mierau G., Heubi J. E., Balistreri W. F., Setchell K. D. 2000. Bile acid synthetic defects and liver disease. Pediatr. Dev. Pathol. 3: 1–16. [DOI] [PubMed] [Google Scholar]
- 54.Sundaram S. S., Bove K. E., Lovell M. A., Sokol R. J. 2008. Mechanisms of disease: inborn errors of bile acid synthesis. Nat. Clin. Pract. Gastroenterol. Hepatol. 5: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faust P. L., Banka D., Siriratsivawong R., Ng V. G., Wikander T. M. 2005. Peroxisome biogenesis disorders: the role of peroxisomes and metabolic dysfunction in developing brain. J. Inherit. Metab. Dis. 28: 369–383. [DOI] [PubMed] [Google Scholar]
- 56.Ferdinandusse S., Denis S., Dacremont G., Wanders R. J. 2009. Toxicity of peroxisomal C27-bile acid intermediates. Mol. Genet. Metab. 96: 121–128. [DOI] [PubMed] [Google Scholar]
- 57.Palmeira C. M., Rolo A. P. 2004. Mitochondrially-mediated toxicity of bile acids. Toxicology. 203: 1–15. [DOI] [PubMed] [Google Scholar]
- 58.Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. 1973. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 182: 62–64. [DOI] [PubMed] [Google Scholar]
- 59.Dirkx R., Vanhorebeek I., Martens K., Schad A., Grabenbauer M., Fahimi D., Declercq P., Van Veldhoven P. P., Baes M. 2005. Absence of peroxisomes in mouse hepatocytes causes mitochondrial and ER abnormalities. Hepatology. 41: 868–878. [DOI] [PubMed] [Google Scholar]
- 60.Baumgart E., Vanhorebeek I., Grabenbauer M., Borgers M., Declercq P. E., Fahimi H. D., Baes M. 2001. Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse). Am. J. Pathol. 159: 1477–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hughes J. L., Poulos A., Robertson E., Chow C. W., Sheffield L. J., Christodoulou J., Carter R. F. 1990. Pathology of hepatic peroxisomes and mitochondria in patients with peroxisomal disorders. Virchows Arch. A Pathol. Anat. Histopathol. 416: 255–264. [DOI] [PubMed] [Google Scholar]
- 62.Mathis R. K., Watkins J. B., Szczepanik-Van Leeuwen P., Lott I. T. 1980. Liver in the cerebro-hepato-renal syndrome: defective bile acid synthesis and abnormal mitochondria. Gastroenterology. 79: 1311–1317. [PubMed] [Google Scholar]
- 63.Maeda K., Kimura A., Yamato Y., Nittono H., Takei H., Sato T., Mitsubuchi H., Murai T., Kurosawa T. 2002. Oral bile acid treatment in two Japanese patients with Zellweger syndrome. J. Pediatr. Gastroenterol. Nutr. 35: 227–230. [DOI] [PubMed] [Google Scholar]
- 64.Setchell K. D., Bragetti P., Zimmer-Nechemias L., Daugherty C., Pelli M. A., Vaccaro R., Gentili G., Distrutti E., Dozzini G., Morelli A., et al. 1992. Oral bile acid treatment and the patient with Zellweger syndrome. Hepatology. 15: 198–207. [DOI] [PubMed] [Google Scholar]
- 65.Setchell K. D., Heubi J. E. 2006. Defects in bile acid biosynthesis–diagnosis and treatment. J. Pediatr. Gastroenterol. Nutr. 43(Suppl 1): S17–S22. [DOI] [PubMed] [Google Scholar]
- 66.Keane M. H., Overmars H., Wikander T. M., Ferdinandusse S., Duran M., Wanders R. J., Faust P. L. 2007. Bile acid treatment alters hepatic disease and bile acid transport in peroxisome-deficient PEX2 Zellweger mice. Hepatology. 45: 982–997. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Binz J., Numerick M. J., Dennis S., Luo G., Desai B., MacKenzie K. I., Mansfield T. A., Kliewer S. A., Goodwin B., et al. 2003. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Invest. 112: 1678–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]