Abstract
Meiosis activating sterol, produced directly by lanosterol 14-α-demethylase (CYP51) during cholesterol biosynthesis, has been shown to promote the initiation of oocyte meiosis. However, the physiological significance of CYP51 action on oocyte meiosis in response to gonadotrophins’ induction remained to be further explored. Herein, we analyzed the role of CYP51 in gonadotrophin-induced in vitro oocyte maturation via RNA interference (RNAi). We showed that although both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) significantly induced meiotic resumption in follicle-enclosed oocytes (FEOs), the effect of LH on oocyte meiosis resumption in FEOs was weaker than FSH. Moreover, both FSH and LH were able to upregulate CYP51 expression in cultured follicular granulosa cells when examined at 8 h or 12 h posttreatments, respectively. Interestingly, whereas knockdown of CYP51 expression via small interference RNA (siRNA) moderately blocked (23% reduction at 24 h) FSH-induced oocyte maturation [43% germinal vesicle breakdown (GVBD) rate in RNAi vs. 66% in control, P < 0.05] in FEOs, similar treatments showed no apparent effects on LH-induced FEO meiotic maturation (58% GVBD rate in RNAi vs. 63% in control, P > 0.05). Moreover, the results in a cumulus-enclosed oocytes (CEOs) model showed that approximately 30% of FSH-induced CEOs’ meiotic resumption was blocked upon CYP51 knockdown by siRNAs. These findings suggest that FSH, partially at least, employs CYP51, and therefore the MAS pathway, to initiate oocyte meiosis.
Keywords: RNA interference, meiotic resumption, oocyte
Mammalian oocytes arrested at the diplotene stage of the first meiotic division are triggered to initiate meiosis by the preovulatory gonadotrophin surge in vivo, especially LH surge (1, 2). It has recently been suggested that certain intermediates of cholesterol biosynthesis, such as meiosis activating sterol (MAS), one of the upstream materials for synthesize of steroid hormones, instruct the oocyte to reinitiate meiosis (3–6). In invertebrate animals, such as xenopus, gonadotrophins induce progesterone secretion and initiate oocyte meiotic resumption. However, in mammals, whether gonadotrophins employ sterol to induce oocyte meiosis is still a pending question. In fact, sterol has the capacity to induce oocyte meiotic resumption. For example, Byskov et al. (7–10) first discovered that fetal ovaries, which were cocultured with fetal testis, promoted sperm meiosis by secreting a meiosis activating substance. Later, Xia et al. (11) reported the substance may be a sterol because it is stable for heat. Byskov et al. (12) purified and identified the sterols from women's follicle fluid and cattle's testis and nominated them as follicular fluid derived-meiosis activating sterol and testis-meiosis activating sterol. Follicular fluid derived-meiosis activating sterol, a direct product of lanosterol 14α-demethylase (CYP51) (13), had a dose-dependent effect on stimulating germinal vesicle breakdown (GVBD) in rodent, bovine, and human meiotically arrested oocytes (3, 4, 14–20).
To better understand the mechanism by which gonadotrophin employs CYP51-mediated sterol as the downstream signal to induce oocytes meiosis, many chemicals, such as MAS (21), MAS analogs (22), MAS metabolizing inhibitor (AY9944-A-7) (23), and CYP51 inhibitors (RS-21745, RS21607, ketokonazole) (17, 18, 24) have been used in different animal models. For example, in mouse, cumulus cell-enclosed oocytes (CEOs) could synthesize cholesterol from mevalonate and accumulate follicular fluid derived-meiosis activating sterol in the presence of AY9944-A-7 (23). FSH and AY9944-A-7, but not human chorionic gonadotrophin (hCG), induced follicle-enclosed oocytes (FEOs) maturation, and RS-21745 attenuated gonadotrophins-induced FEOs meiotic resumption (25). In porcine, both RS21745 and RS21607 inhibit FSH-induced CEOs meiotic resumption in a dose- and time-dependent manner and AY9944-A-7 promoted CEOs meiotic resumption (26). The data suggested that FSH-induced oocytes maturation may be mediated by CYP51 in granulosa cells. However, in rat, Tsafriri et al. (27, 28) reported that LH did not employ CYP51 to accumulate MAS and, thus, to induce FEOs meiotic resumption, because inhibitors of CYP51 did not prevent LH-stimulated oocyte meiosis, and AY-9944-A-7-induced maturation in rat FEOs revealed a marked delay compared with LH. Because oocytes are short of gonadotrophin receptors, it is believed that theca and granulosa cells may mediate gonadotrophin action through the accumulation or activation of oocyte maturation signals (29). Until now, it was still difficult to explain the mechanisms that cause the different effects of FSH and LH on oocytes maturation at the molecular level.
Although the conflicting results from different experimental models and animals make it difficult to judge the role of CYP51 in gonadotrophin-induced oocytes maturation, these studies imply that the response of CYP51 to FSH and LH may be different. However, it is difficult to clarify the conflict by traditional methods because the effects of CYP51 inhibitors used in previous studies were different between each research group. For example, the specificities of the inhibitors and the effective concentrations of the same inhibitors were different at different laboratories (27, 28, 30). Therefore, there is a dearth of direct evidence to prove the ambiguous viewpoints based on gene level. In this study, we focused on elucidating whether CYP51 involved in FSH or LH induced oocytes maturation by RNA interference (RNAi). RNAi is an evolutional conserved surveillance mechanism in cells, which revolutionized current gene function analysis shortly after it was discovered (31). RNAi has been successfully employed to investigate both oocyte meiosis and early embryo development related gene functions (32–34). Inspired by these successes, we assumed that RNAi of CYP51 could be a useful method to find new proofs regarding the CYP51-gonadotrophin relationship through in vitro cultured follicular granulose cells, CEOs, and FEOs models.
MATERIALS AND METHODS
Chemicals and culture media
All chemicals and culture media components were purchased from Sigma-Aldrich, Inc. (St. Louis. MO) unless otherwise indicated. Equine chorionic gonadotrophin (eCG) (Ning Bo Hormorne Product, Zhe Jiang, P. R. China), FSH, and LH were dissolved in PBS (10 mM, pH7.4) and stored at −20°C. Enhance green florescence protein plasmid (EGFP)-siRNAs and Nonsilence-siRNAs (plant cell specific, Table 1) were chemically synthesized by Ambion (Austin, TX). Lipofectamine 2000 was purchased from Invitrogen (Invitrogen, Grand Island, NY).
TABLE 1.
Small interference RNA sequences
| siRNA Sequences |
|||
|---|---|---|---|
| Name | Target | 5′-3′ | 3′-5′ |
| CYP-1 | ORF position 326-344 | CCACAUAUUUACUCUCCAADTDT | DTDTGGUGUAUAAAUGAGAGGUU |
| CYP-mismatched (CYP-mis) | ORF position 647-665 | GCCGUAUGUGCCGACGCGDTDT | DTDTCGGCAUACACGGCUGGCGC |
| Non-silencing (Plant cell derived siRNAs) | Thermotoga maritimia | UUCUCCGAACGUGUCACGUDTDT | DTDTAAGAGGCUUGCACAGUGCA |
| EGFP-siRNAs | EGFP | GCAAGCUGACCCUGAAGUUCDTDT | DTDTCGUUCGACUGGGACUUCAAG |
Small interference RNAs (siRNAs) were acquired by In vitro Transcription T7 kit according to producer's protocol (Jingmei Biotech Co., Ltd, Beijing, China). The sequence for CYP51 was taken from Genebank (gi9910171).
CEO maturation medium was M199 (Invitrogen) supplemented with 4 mM hypoxanthine (HX), 0.3% BSA, 0.23 mM sodium pyruvate, 2 mM L-Glutamine, 100 unit/ml penicillin, and 100 unit/ml streptomycin. Follicular granulosa cell culture medium was D-MEM/F-12 (Invitrogen, CA) supplemented with 0.23 mM sodium pyruvate and 5% fetal bovine serum. FEOs culture medium was MEM (Invitrogen) supplemented with 100 unit/ml penicillin, 100 unit/ml streptomycin, 0.23 mM pyruvate, 0.3% BSA, 2 mM L-glutamine, 5 μg/ml insulin, 5 μg/ml transferin, and 10 mM Hepes. The media were incubated at 37°C in humidified air with 5% CO2 for more than 3 h before use.
Animals
Sexually immature (21–23 days old) outbred strain Kunming female mice were used for all experiments; they were kept under controlled temperature (24–26°C) and light (14 h light and 10 h dark cycle) with food and water ad libitum. Before recovery of ovaries, mice were euthanized by cervical dislocation. The experiments were carried out in accordance with the principles and guidelines for the use of laboratory animals and approved by the Animal Care Committee of China Agricultural University.
Follicular granulosa cells, CEOs, and FEOs isolation and culture
Follicular granulosa cells, CEOs, and FEOs were obtained from 21–23 day old female mice. In brief, mice were euthanized 44–48 h after primed with 5 U eCG. Ovaries were immediately recovered and transferred to M199 medium with 4 mM hypoxanthine (HX-M199 medium). Large antral follicles were punctured with sterile needles. Intact CEOs were collected and transferred to HX-M199 medium, and follicular granulosa cells were separated by centrifuging media at 1000 rpm for 10 min at 25°C and transferred to DMEM/F12 medium and incubated in an atmosphere of 5% CO2 in air at 37°C. FEOs were isolated by careful manual dissecting of ovaries under a microscope using a pair of sterilized 27 gauge needles in 10 ml MEM media. Intact spherical isolated follicles with a diameter of 260–400 μm without apparent sign of necrosis were immediately transferred to 35-mm dishes (Nunclon, Nunc, Roskilde, Denmark) containing 2 ml of MEM and cultured at 37°C, 5% CO2 in air before intra follicle antrum injection of CYP51-siRNAs.
CYP51 siRNAs design, transfection, and intra follicle injection
CYP51-siRNAs were designed with software from Ambion (Austin, TX) and were modified according to the suggestions of Reynolds et al. (35). In brief, three CYP51-siRNAs (21bp) were designed as shown in Table 1. The siRNAs were acquired by in vitro Transcription T7 kit (Jingmei Biotech Co., Ltd, Beijing, P. R. China). We used Non-silencing (Plant cell derived siRNAs) and EGFP-siRNA as parallel negative control for CYP51-mismatched siRNA (CYP-mis). Also, we used EGFP-siRNA to knockdown EGFP expression in transfected follicular granulose cells with EGFP plasmid for pretest RNAi efficiency in follicular granulose cells.
Follicular granulosa cells and CEOs were transfected with siRNAs under the guidelines of the transfection kit (Lipofectamine 2000, Invitrogen). In brief, 3 h before transfection, cells at 70–80% confluence were transferred to fresh DMEM/F12 medium without antibiotics and serum in 24-well plates (Corning, NY). For each well, transfecting media with 0.02 μg CYP51-siRNA (2.5 nM) (6-FAM labeled or not) was supplemented into the well. Similarly, being the reporter plasmid, 1 μg EGFP (pEGFP-N1, BD Biosciences Clontech's Living Colors™, CA ) or 1 μg EGFP plus 0.02 μg EGFP-siRNA were supplemented into individual wells. After 24 h, CYP51 mRNA levels were measured by RT-PCR. CYP51 protein levels were investigated at 0 h, 12 h, 24 h, 36 h, and 48 h after transfection by Western blot assay. In addition, to justify the CYP51 protein expression patterns after gonadotrophin induction in follicular granulosa cells 12 h after follicular granulosa cells culture, the medium was replaced by medium with 10 U/l FSH or 0.5 U/ml LH for culture 0 h, 4 h, 6 h, 8 h, 10 h, and 12 h. Then the cells were collected for Western blot assay. Observations of EGFP transfection were performed with the Leica DMLB (Leica Microsystems, Wetzlar, Germany ) fluorescence microscope and digital photomicrographs were taken with the Leica DFC 320 camera (Leica Microsystems).
CEOs were transfected with 10 nM siRNAs through lipofectamine 2000 kit. Twelve h after CYP51-siRNAs transfection, CEOs were transfered to HX-M199 medium containing 10 U/l FSH and further cultured 12 h for observing oocytes meiosis data. In parallel, 12 h after transfection, 10 U/l FSH were supplemented into HX-M199 media and CEOs were cultured for an additional 8 h, then total proteins of 50 CEOs in each group were extracted for Western blot analysis.
For intra follicle injection of CYP51-siRNAs, an injection pipette with an inner diameter of 2 μm and a holding pipette with an inner diameter of 40 μm were attached to a Leica DMLB (Leica Microsystems,) microinjector. Frozen aliquots of 200 nM siRNAs of CYP51(6-FAM labeled or not), CYP-1(CYP51 siRNA), CYP-mis, and the control buffer were thawed and front-loaded into microinjection pipettes, respectively. Recovered follicles were placed in 50 μl droplets of Hepes-buffered handling medium covered with mineral oil. Injection pipettes were driven through the basement membrane of follicles into the antra. Enough solution was injected such that expansion of the follicle was observed. Follicles that remained intact following injection were allocated to the culture medium. RNAi effects on gonadotrophins induced FEOs maturation were measured by RT-PCR 8, 12, 20, and 28 h post intra antrum injection of CYP51-siRNAs. In brief, about 200 nM CYP51-siRNAs were microinjected into the follicular antrum of individual FEOs until the antrum showed obvious expansion. Then, FEOs were allocated to the culture medium with 10 U/l FSH or 0.5 U/ml LH and cultured for 12 h. After culture, oocytes were released by manual rupture of follicles using a pair of needles under an inverted microscope. After oocytes maturation data were recorded, follicular granulose cells were recovered by centrifuging the media at 1000 rpm for 10 min at 25°C. The synchronous inhibiting efficacy of CYP51-siRNA was further demonstrated by Western blot analysis.
RT-PCR and real time PCR
Mice follicular granulosa cells and FEOs mRNA were isolated with TRIzol Reagent (Invitrogen), and then washed in 75% ethanol, dissolved in RNase-free distilled water. Total RNA of each sample was paralleled to quantify the concentration of the product by using a DU640 spectrophotometer (Beckman Coulter, Inc., Palo Alto, CA). The PCR primer sequences are shown in Table 2.
TABLE 2.
Primer sequences of CYP51 and β-actin for RT-PCR or real time PCR
| Sequence |
|||
|---|---|---|---|
| Name | Product Length (base pair) | 5′-3′ | 5′-3′ |
| CYP51 | 172 | ATACAACAATGATCCACACCCC | TCAGAACCACACTCTTCAACCC |
| β-actin | 185 | GAGGGAAATCGTGCGTGAC | AGAAGGAAGGCTGGAAAA |
Primer sequences of CYP51 and β-actin for RT-PCR or real time PCR. The cDNAs of two genes were taken from Genebank (gi9910171) and (gi6671508), respectively.
For RT-PCR, β-actin was used as the internal standard. PCR cycle conditions for genes were: initial denaturation at 94°C for 5 min followed by 25 cycles of 94°C for 20 s, 52°C for 20 s, and 72°C for 20 s, finished with a final extension at 72°C for 10 min. The PCR products of CYP51 and β-actin were sequenced to confirm that they were identical to their gene sequences. The PCR was performed with a Bio-Rad thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA) and the products were subjected to electrophoresis in a 3% agarose gel, and the signals were quantified by AlphaImager 2200 analysis software.
For real-time PCR, products were amplified in reactions containing 10× SYBR Green I PCR buffer, 25 mM MgCl2, 12.5 mM dNTPs, 10 μM primers, 1 U/uL AmpErase UNG (Applied Biosystems, CA), and 5 U/ul AmpliTaq Gold (Hoffmann-Lar., Roche Ltd., Greezachestrasse, Switzerland). The PCR was performed with ABI prime 7900HT (Applied Biosystems). The data were quantified by using the ABI 7900HT SDS 2.2 analysis software. A standard curve for the CYP51 plasmid was created and the standard deviation was less than 0.005. The results were expressed initially as the number of target molecules/1 μl cDNA. To standardize the results for variability in RNA and cDNA quantity and quality, we quantified the total number of β-actin transcripts in each sample as an internal control. Normalized levels of the CYP51 transcripts were calculated as the ratio of the number of CYP51 transcripts to β-actin.
Western blot analysis
Total proteins from 50 CEOs were extracted with double-strength electrophoresis sample buffer composed of 125 mM Tris, pH 6.8, 4% (w/v) SDS, 20% (w/v) glycerol, 10% (v/v) β-mercaptoethanol, and 0.004% (w/v) bromophenol blue. Total proteins of follicular granulosa cells and FEOs were lysed in MEM-R (Perice Co.) according to manufacturer's protocol, and then measured by BCA procedure (cellchipbj Biotechnology Co., Ltd, Beijing, China) to normalize the quantity of sum proteins.
The cell lysates were heated to 100°C for 5 min. After being cooled down on ice for 20 min and centrifuged at 12,000 rpm for 3 min, samples were frozen at −80°C until used. For FEOs and follicular granulosa cells, the 18 μg total protein of each sample was loaded on SDS-PAGE. Samples were separated by SDS-PAGE with a 4% stacking gel and a 10% separating gel for 60 min at 160 V and electrophoretically transferred onto a piece of Protran nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) for 2 h at 200 mA using a Trans-Blot apparatus (Bio-Rad Laboratories). After blocking for 1 h with 5% fat-free milk powder in 20 mM Tris and 137 mM NaCl (TBS, pH 7.6), the membrane was incubated overnight at 4°C with rabbit anti-CYP51(55 kDa, gift from Dr. Byskov) with 1/400 dilution. After three washes of 5 min each in TBS containing 0.1% Tween-20 (TBST), the membrane was incubated for 1 h at room temperature with HRP-conjugated goat anti-rabbit IgG (Zhongshan Golden Bridge Biotech Co., Beijing, China) diluted 1:5000 in TBST. After three washes in TBST, the membrane was visualized using the ECL detection system (Amersham, Arlington Heights, IL). For every experiment, CYP51 protein was blotted first and stripped 30 min at 50°C with stripping buffer solution (125 mM Tris, pH 6.8, 2% (w/v) SDS, 7% (v/v) β-mercaptoethanol) for second blot ACTIN antibody. After three washes of each 5 min in TBST, the membrane was incubated overnight at 4°C with rabbit anti-ACTIN (43 kDa) antibodies with 1/500 dilution (SantaCruz Co., CA). After three washes of 5 min each in TBST, the membrane was incubated for 1 h at room temperature with HRP-conjugated goat anti-rabbit IgG (Zhongshan Golden Bridge Biotech Co., Beijing, P.R. China) diluted 1:5000 in TBST. After three washes in TBST, the membrane was visualized using the ECL detection system (Amersham, Arlington Heights, IL).
Statistics
The data were given as the mean percentage (± SEM) with each model by three repeats at least. There were 50–60 CEOs or 30–40 FEOs per group within one experiment. Oocytes were isolated from 12–15 mice per experiment. For GVBD, we judged it as disappearing of nucleus within the oocyte. The percentage of GVBD was defined as the number of oocytes undergoing GVBD divided by the total number of undegraded oocytes. The percentage of degeneration was defined as the number of oocytes degenerated derived by the total number of cultured FEOs. For oocyte degeneration, we defined it as oocytes with yellow color and irregular shape. All frequencies of GVBD were subjected to arcsine transformation before analysis. Oocytes GVBD ratio data of CEOs and FEOs and data of CYP51 expression in follicle granulosa cells and FEOs were statistically analyzed by ANOVA using Sigmaplot 9.01 software (SISTAT Software, Inc., Chicago, IL). When a significant F ratio was defined by ANOVA, groups were compared using the Holm-Sidak method test. Differences were considered significant at P < 0.05.
RESULTS
RNAi knocked down EGFP and CYP51 expression in cultured follicular granulosa cells
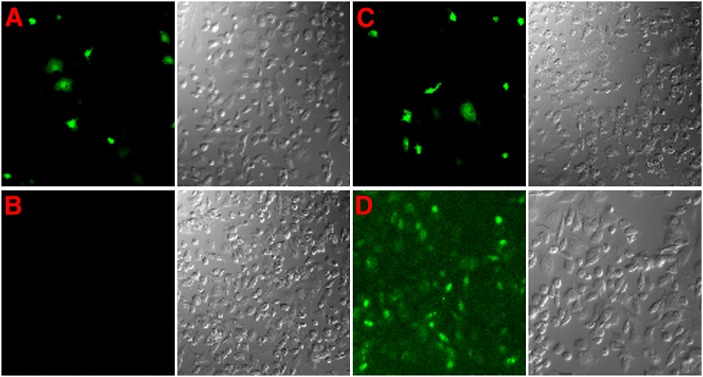
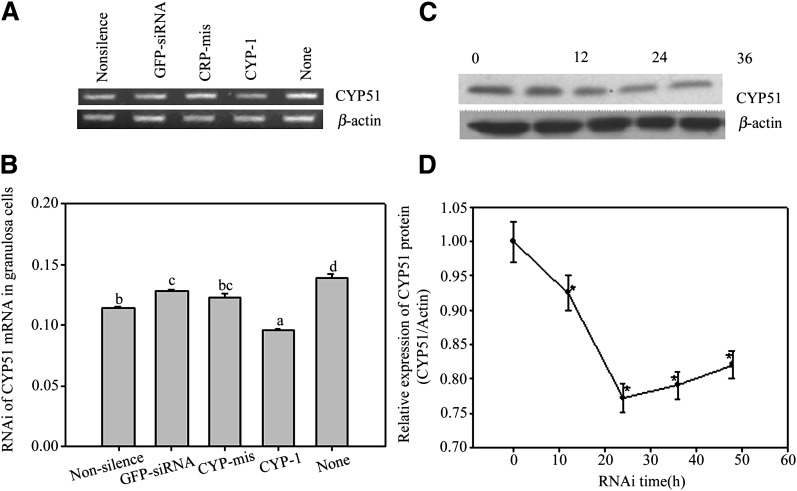
In Fig. 1A–C, the expression of positively transfected EGFP was significantly reduced by EGFP-siRNAs. Fig. 1D shows that 6-FAM labeled CYP51-siRNAs were successfully transfected into follicular granulosa cells. CYP-1 siRNAs decreased CYP51 mRNA levels in follicular granulose cells remarkably compared with controls containing buffer (None), EGFP-siRNA, Non-silence siRNA, and CYP-mis (Fig. 2A, B). The consequence of reduced CYP51 mRNA expression resulted in decreased CYP51 protein levels time dependently in follicular granulosa cells (as shown in Fig. 2C, D) in which CYP-1 inhibition effects showed up at 12 h post-siRNA incubation, and the obvious effects were observed from 24 h to 48 h post-RNAi. Therefore, our study suggested that RNAi reliably knocked down exogenous and endogenous gene expression in follicular granulosa cells.
Fig. 1.
RNAi assay in follicular granulosa cells in vitro. Confocal images show the inhibition of EGFP expression in granulosa cells 24 h post transfection. A: Granulosa cells transfected with EGFP plasmid alone; B: EGFP and EGFP-siRNA cotransfection; C: EGFP and Non-silence-siRNA cotransfection; D: Granulosa cells transfected with 6-FAM labeled 125 nM CYP-1.
Fig. 2.
RNAi assay in follicular granulosa cells in vitro. A, B show the effects of CYP-1, CYP-mis, EGFP-siRNA (Ambion), Non-silence siRNA (plant cell specific Ambion) and None (without siRNAs) on CYP51 mRNA expression in in vitro cultured follicular granulosa cells. Semi-quantitative RT-PCR (A) and real-time PCR (B) show that only CYP-1 moderately decreased CYP51 mRNA levels in granulosa cells (P < 0.05) 24 h post RNAi. The bars that have no same lower case letters showed significant differences, n = 3 (P < 0.05). C, D show time-dependent RNAi on CYP51 protein production in granulosa cells. The CYP51 protein levels in granulosa cells decreased steadily along with RNAi prolonged from 0 h to 20 h post transfection and bit by bit recovered afterward. This figure represents one of the similar results in three independent experiments. The groups with asterisk (*) show significant differences compared with 0 h group, n = 3 (P < 0.05).
Gonadotrophins induced CYP51 expression in in vitro cultured granulose cells
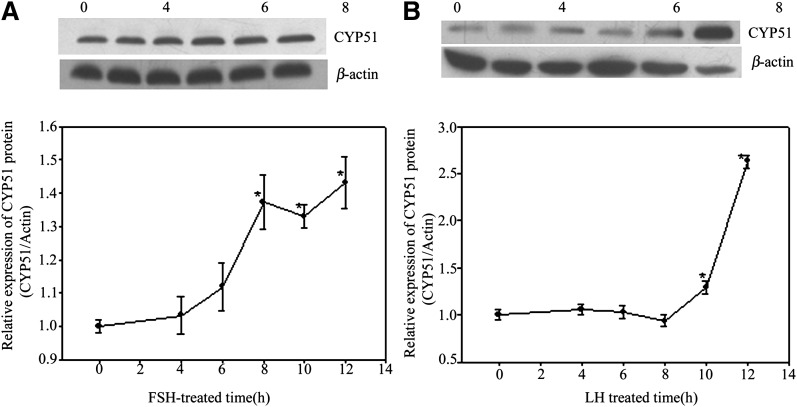
In this study, both FSH and LH upregulated CYP51 expression time dependently in follicular granulosa cells, but the peak expression of CYP51 induced by LH (Fig. 3B) (about 12 h after induction) lagged behind FSH (about 8 h after induction) for nearly 4 h (Fig. 3A). The results implied that in vitro culture of FEOs for 12 h was a reasonable model for later RNAi assays.
Fig. 3.
Gonadotrophins induced CYP51 expression in in vitro cultured granulose cells. Both FSH and LH upregulated CYP51 expression time-dependently in follicular granulosa cells, but the peak expression of CYP51 induced by LH (B) (about 12 h after induction) lagged behind FSH (about 8 h after induction) for nearly 4 h (A). The groups with an asterisk (*) show significant difference compared with 0 h, n = 3 (P < 0.05).
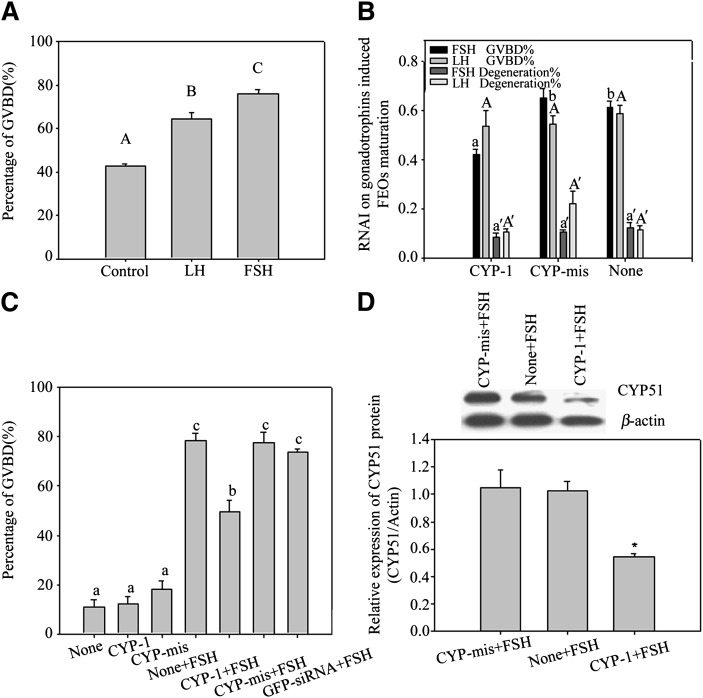
RNAi inhibited FSH-induced FEOs and CEOs meiotic resumption
FEOs in vitro maturation data are shown in Fig. 4A. The results showed that more than 70% of FEOs resumed meiosis after FSH induction for 12 h, but the impact of LH on FEOs maturation was weaker (over 60%) than FSH. Gonadotrophins were respectively added into FEOs culture media to investigate the influences of RNAi on each gonadotrophin-stimulated oocytes maturation (Fig. 4B). During the 12 h culture period, siRNAs moderately decreased the GVBD rate induced by FSH (43% in CYP-1 vs. 66% in control, P < 0.05). On the other hand, siRNAs had little influence on LH-induced FEOs meiosis as oocytes undergoing GVBD showed (58% in CYP-1 vs. 63% in control, P > 0.05) (Fig. 4B).
Fig. 4.
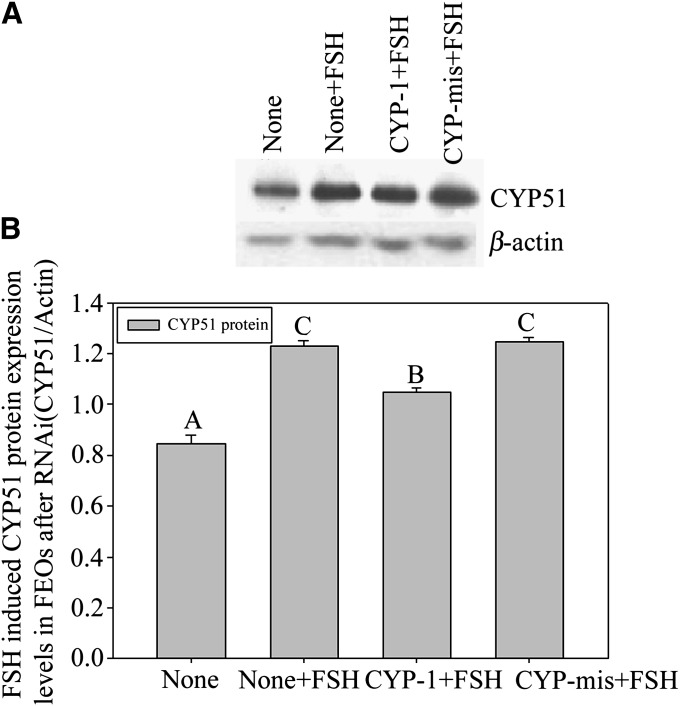
A, B: FEOs in vitro maturation after gonadotrophin induction with or without RNAi treatment. In A, both FSH (more than 70%) and LH (more than 60%) remarkably stimulated FEO maturation in 12 h (P < 0.05), but the LH effect was weaker than FSH. Bars with different capital letters show significant differences (P < 0.05). In B, during the 12 h culture period, siRNAs moderately decreased the GVBD rate induced by FSH (43% in CYP-1 vs. 66% in control, P < 0.05), in which about 23% of FEO meiotic maturation was inhibited from maturation. Instead, LH induced FEO maturation was unaffected (P > 0.05) (58% in CYP-1 vs. 63% in control, P > 0.05). The rate of oocyte degeneration was also presented in B and oocyte degeneration was about 20% in 12 h culture. Different letters on the same colored bars show significant difference (P < 0.05), i.e., letter a only compare with b, a’ only compare with b’, A only compare with B, A’ only compare with B’. C, D: The effects of RNAi on FSH-induced CEO in vitro maturation. In C, the influence of RNAi on CEO maturation. Twelve h after siRNAs transfection, CEOs were cultured in media with or without 10 U/l FSH and further cultured for 12 h. The oocyte maturation results showed that CYP-1 significantly prevented FSH stimulated CEOs meiotic resumption, as the GVBD ratio data proved (P < 0.05). Different lowercase letters on the bars show significant differences, n = 3 (P < 0.05). In D, Western blot of FSH-induced CYP51 expression in CEOs after RNAi. Twelve h after transfection, CEOs were transferred into media containing 10 U/l FSH and further cultured for 8 h. The results show observably decreased CYP51 protein levels in CEOs. This figure represents one of the similar results in three independent experiments. The groups with an asterisk (*) show significant difference compared with None + FSH group, n = 3 (P < 0.05).
Based on the above research, we further studied the relationship between FSH and CYP51 in mice oocytes meiosis in vitro by using the CEOs model. After transfected with siRNAs into CEOs for 12 h plus 12 h culture with FSH, the GVBD ratio of CEOs was decreased significantly by CYP-1 (Fig. 4C) (P < 0.05). Previous reports showed that mice CEOs started to resume meiosis in 8 h if they were treated with FSH existing media (36). Therefore, we detected the protein expression of CYP51 at 8 h after FSH treatment and found that siRNAs decreased CYP51 protein levels within the 8 h culture period (Fig. 4D). The results indicated that CYP51 in cumulus cells participated in FSH-induced oocyte meiotic resumption.
We noticed that the CYP51 expression under gonadotrophin induction was moderately silenced in the culture models. This may be because the activity of existing CYP51 protein in cytoplasm was enhanced to compensate for low level protein production or because the previously administered eCG ex vivo had elevated CYP51 expression level. Another reason may be siRNAs absorption ratio by follicular somatic cells was insufficient. Actually, we had introduced the same CYP51-siRNAs into mice liver through the tail vein, which resulted in as much as 70% of CYP51 mRNAs being knocked down and over 40% of CYP51 protein depletion (37). Although we introduced EGFP/EGFP-siRNAs as the reporter in this study, it was still difficult to judge the CYP51 siRNAs absorption efficiency (lipofectamine mediated and FEOs antrum direct injection) in different experimental models.
RNAi silenced CYP51 gene expression in in vitro cultured FEOs with FSH induction
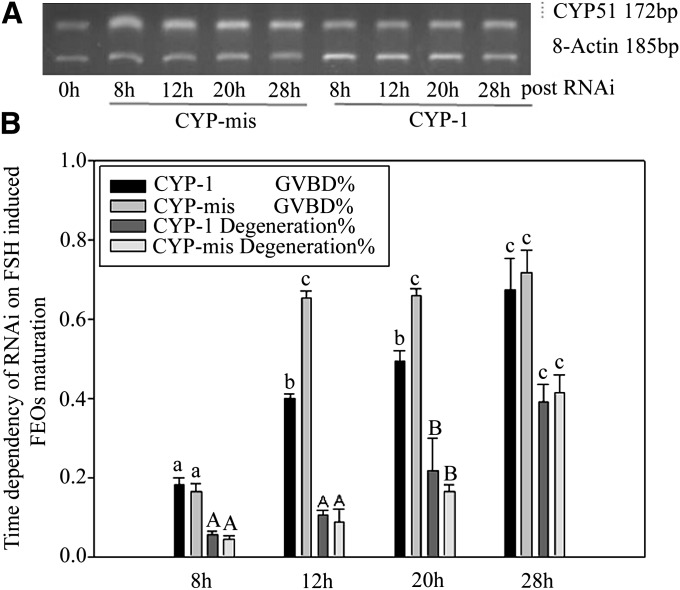
We noticed that silenced CYP51 gene did not interfere with LH-stimulated FEOs meiotic resumption process. Western blot results showed that FSH-stimulated CYP51 protein levels in FEOs was reduced compared with controls 12 h after RNAi (Fig. 5A, B). The data indicated that the downstream signal pathway of LH-induced mice FEOs maturation was different from that of FSH. FSH induced oocytes maturation by promoting CYP51 expression, therefore, MAS signals the pathway to initiate oocyte meiosis, whereas LH uses other signals than MAS. The RNAi effectiveness on silencing gene expression at the mRNA level was checked through RT-PCR after intra antrum injection of siRNAs into FEOs and cultured up to 28 h. The results proved that CYP-1 decreased CYP51 mRNA in FEOs from 8 h to 28 h post-injection compared with CYP-mis (Fig. 6A). Oocytes GVBD ratio data showed that RNAi significantly inhibited FEOs meiosis from 8 h through 20 h (P < 0.05). From 12 h on, although RNAi worked fine in prohibiting FEOs from maturation, the oocyte degeneration ratio was significantly elevated (>20%, P < 0.05) (Fig. 6B). Therefore, 12 h point was a suitable time for evaluating RNAi efficiency in our study.
Fig. 5.
CYP51 protein expression level changes in FEOs in response to FSH induction during 12 h in vitro culture period after RNAi. A: The results showed that FSH stimulated CYP51 protein levels in FEOs were reduced compared with controls 12 h after RNAi. B: Density scanned results related to A. The bars that have no same capital letters show significant differences, n = 3 (P < 0.05). This figure represents one of the similar results in three independent experiments.
Fig. 6.
A: Time dependency of CYP51 mRNA levels after RNAi in follicles cultured with 10 U/l FSH. FEOs were in vitro cultured for up to 28 h after siRNAs injection in FEOs. The result showed that CYP51 mRNA expression was block at a comparatively lower level from 8 to 12 h. The results indicated that RNAi model affected FEOs maturation from 8 to 12 h culture period. This figure represents one of the similar results in three independent experiments. B: Oocytes GVBD ratio data showed that RNAi significantly inhibited FEOs meiosis from 8 h through 20 h (P < 0.05). But from 12 h on, although RNAi worked fine in prohibiting FEOs from maturation, the oocytes degeneration ratio was significantly elevated (more than 20%, P < 0.05). Different lowercase letters on the first two bars among each time point or different capital letters on the last two bars among each time point show significant difference (P < 0.05), i.e., letter a only compare with b and c; A only compare with B and C.
DISCUSSION
In this study, in vitro cultured mouse follicular granulosa cells, CEOs, and FEOs models were established to study the relationship between CYP51 expression and gonadotrophin-induced oocytes meiosis. The results showed that both FSH and LH upregulated CYP51 expression time-dependent in FEO maturation, but the peak expression of CYP51 induced by LH (about 12 h after induction) lagged behind FSH (about 8 h after induction) for nearly 4 h. CYP51-siRNAs were proven to be capable of decreasing, though moderately, CYP51 mRNA and protein levels time dependently in granulosa cells. During 12 h culture of FEOs, CYP51-siRNAs decreased the GVBD rate induced by FSH, but hardly had any influence on LH-induced FEO meiosis. Western blot results also indicated that RNAi moderately reduced CYP51 protein levels in FEOs compared with controls. Therefore, LH-induced FEO in vitro maturation was weaker than FSH, and its action on FEO maturation could not be suppressed by CYP51-siRNAs, demonstrating that CYP51 may not participate in LH-induced FEO meiosis, whereas FSH may facilitate CYP51 to transfer its signals through MAS to induce oocyte meiosis. Based on this conclusion, further evidence of CYP51 expression in response to FSH induction was studied with the in vitro cultured CEOs model. Twelve h after siRNAs transfection, CEOs were cultured for an additional 12 h in media containing FSH. The results showed that the GVBD ratio and CYP51 expression of CEOs was decreased significantly by CYP51-siRNAs, which implied that CYP51 in cumulus cells participated in FSH-induced oocyte meiotic resumption. Our results were confirmed by the studies of Baltsen and Tsafriri (27, 28, 38).
Recent studies demonstrate that FSH and LH go through different signal pathway in oocytes maturation in vitro (18). Physiologically, LH acts via LH receptor (LHR), a member of the large superfamily of G protein-coupled receptors, to cause cAMP accumulation in follicle theca and granulosa cells (39–41). Actually, upregulated cAMP is thought to mediate most of the changes that occur before ovulation because LHR is coupled primarily to stimulatory guanine nucleotide regulatory protein and activates adenylyl cyclase (40). Instead, the research in our laboratory found that FSH induced CYP51 mRNA and protein expression in cumulus cells may via a cAMP dependent protein kinase subunit II (PKAII)/cAMP response element-binding protein (CREB)/Mitogen-activated protein kinase (MAPK)-dependent pathway (42). EGF-like factor, which mimics the LH initiated oocyte maturation, is activated by G protein-coupled receptor signal (43). Similarly, FSH may also exert EGF-like factor to influence mice CEO meiosis by a gap junction-dependent process (44), which implies that LH and FSH may either directly overlap or utilize redundant pathways in oocyte maturation.
The signal differences between two gonadotrophins can be explained in the following aspects. First, FSH receptor and LHR express distinguishably in the follicular somatic cells during follicular growth and oocyte maturation and ovulation takes place after LH surge in vivo (45). In healthy follicles, FSH specific binding sites were detected in the granulose cell layer of all antrum follicles, whereas LH were detected in the theca cell layer and in the granulosa cell layer of large antrum follicles [rat (46), sheep (47), sow (48, 49), and human (49)]. In HX-arrested mice CEOs, only FSH, but not LH, has activity to induce resumption of meiosis because cumulus cells were short of LH receptor (1, 50, 51). In vitro cultured FEOs can maximally mimic the situation in vivo because oocytes are enclosed in a comparatively closed environment from culture media and they are sensitive to both FSH and LH induction. Second, FSH and LH induce oocyte maturation in different patterns. LH can only induce maturation of oocytes of large FEOs in eCG-primed rats, whereas FSH can induce GVBD in oocytes of large and small FEOs in nonstimulated rats (45). FSH induces 180–400 µm FEOs maturation of nonstimulated mice, whereas hCG exhibits a similar but weaker effect on 260–400 µm FEOs (25). Third, the difference between FSH and LH exists among animals. For instance, in rat, LH/hCG stimulates meiotic resumption but does not increase ovarian CYP51 expression at a time that is relevant for the induction of meiosis (52), and the inhibitors of CYP51 do not prevent LH-stimulated rat FEO meiosis (28). In mice, hCG-stimulated MAS surge shows up after oocyte maturation in vivo (38). Combined with our data, the regulation of CYP51 by gonadotrophin may be the difference in the signal pathway of FSH and LH-induced oocyte maturation.
Our study showed that LH induced CYP51 expression in follicular cells but might not contribute to oocytes maturation; instead, a delayed expression may be related to the cases that MAS improves oocyte cytoplasmic maturation [mouse (53, 54), calf (20), human (55)] and protects oocytes from precocious chromatins segregation [mouse (56); and human (55, 57)]. Also, CYP51-mediated MAS production is involved in primary follicle formation in fetal ovary and in sperm meiosis in testis (8, 13, 58).
In summary, our data showed that CYP51 siRNAs reduced CYP51 expression in granulosa cells, CEOs, and FEOs somatic cells in vitro, which moderately inhibited FSH-induced, rather than LH induced, FEOs and FSH-induced CEO meiotic maturation. The results revealed that FSH and LH went through different signal pathways in oocyte maturation in vitro. FSH, partially at least, employed CYP51 as the intermediating regulator, via MAS, to initiate oocytes meiosis.
Acknowledgments
We thank Dr. Byskov for generously providing rabbit anti-CYP51 antibody.
Footnotes
Abbreviations:
- CEO
- cumulus cells-enclosed oocyte
- CYP51
- lanosterol 14-α-demethylase
- CYP-mis
- CYP51-mismatched siRNA
- eCG
- equine chorionic gonadotrophin
- EGFP
- enhance green florescence protein plasmid
- FEO
- follicle-enclosed oocyte
- FSH
- follicle-stimulating hormone
- GVBD
- germinal vesicle breakdown
- hCG
- human chorionic gonadotrophin
- HX
- hypoxanthine
- LH
- luteinizing hormone
- MAS
- meiosis activating sterol
- RNAi
- RNA interference
- siRNA
- small interference RNA
- TBST
- TBS containing 0.1% Tween-20
This research was supported by the National Natural Science Foundation: (No. 30701021) and the China Postdoctoral Science Foundation (No. 20070410099).
REFERENCES
- 1.Lindner H. R., Tsafriri A., Lieberman M. E., Zor. Bauminger U., Barnea A. 1974. Gonadotrophin action on cultured Graafian follicles: induction of maturation division of the mammalian oocyte and differentiation of the luteal cell. Recent Prog. Horm. Res. 30: 79–138. [DOI] [PubMed] [Google Scholar]
- 2.Schoevers E. J., Kidson A., Verheijden J. H. 2003. Effect of follicle-stimulating hormone on nuclear and cytoplasmic maturation of sow oocytes in vitro. Theriogenology. 59: 2017–2028. [DOI] [PubMed] [Google Scholar]
- 3.Grøndahl C., Ottesen J. L., Lessl M., Faarup P., Murray A., Grønvald F. C., Hegele-Hartung C., Ahnfelt-Rønne I. 1998. Meiosis-activating sterol promotes resumption of meiosis in mouse oocytes cultured in vitro in contrast to related oxysterols. Biol. Reprod. 58: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 4.Byskov A. G., Andersen C. Y., Leonardsen L. 2002. Role of meiosis activating sterols, MAS, in induced oocyte maturation. Mol. Cell. Endocrinol. 187: 189–196. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y., Shimada M., Okazaki T., Maeda T. 2003. Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol. Reprod. 68: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita Y., Nishibori M., Terada T., Isobe N., Shimada M. 2005. Gonadotrophin-induced delta14-reductase and delta7-reductase gene expression in cumulus cells during meiotic resumption of porcine oocytes. Endocrinology. 146: 186–194. [DOI] [PubMed] [Google Scholar]
- 7.Byskov A. G. 1974. Does the rete ovarii act as a trigger for the onset of meiosis? Nature. 252: 396–397. [DOI] [PubMed] [Google Scholar]
- 8.Byskov A. G. 1976. Induction of meiosis in fetal mouse testis in vitro. Dev. Biol. 52: 193–200. [DOI] [PubMed] [Google Scholar]
- 9.Byskov A. G. 1978. Regulation of initiation of meiosis in fetal gonads. Int. J. Androl. 1: 29–38. [Google Scholar]
- 10.Byskov A. G., Baltsen M., Andersen C. Y. 1998. Meiosis-activating sterols: background, discovery and possible use. J. Mol. Med. 76: 818–823. [DOI] [PubMed] [Google Scholar]
- 11.Xia G., Byskov A. G., Andersen C. Y. 1994. Cumulus cells secrete a meiosis-inducing substance by stimulating with forskolin and dibutyril cyclic adenosine monophosphate. Mol. Reprod. Dev. 39: 17–24. [DOI] [PubMed] [Google Scholar]
- 12.Byskov A. G., Andersen C. Y., Nordholm L., Thogersen H., Xia G., Wassmann O., Andersen J. V., Guddal E., Roed T. 1995. Chemical structure of sterols that activate oocyte meiosis. Nature. 374: 559–562. [DOI] [PubMed] [Google Scholar]
- 13.Rozman D., Cotman M., Frangez R. 2002. Lanosterol 14alpha-demethylase and MAS sterols in mammalian gametogenesis. Mol. Cell. Endocrinol. 187: 179–187. [DOI] [PubMed] [Google Scholar]
- 14.Debeljak N., Fink M., Rozman D. 2003. Many facets of mammalian lanosterol 14 alpha-demethylase from the evolutionarily conserved cytochrome P450 family CYP51. Arch. Biochem. Biophys. 409: 159–171. [DOI] [PubMed] [Google Scholar]
- 15.Hegele-Hartung C., Kuhnke J., Lessel M. 1999. Nuclear and cytoplasmic maturation of mouse oocytes after treatment with synthetic meiosis-activating sterol in vitro. Biol. Reprod. 61: 1362–1372. [DOI] [PubMed] [Google Scholar]
- 16.Hegele-Hartung C., Grützner M., Lessl M. 2001. Activation of meiotic maturation in rat oocytes after treatment with follicular fluid meiosis-activating sterol in vitro and ex vivo. Biol. Reprod. 64: 418–424. [DOI] [PubMed] [Google Scholar]
- 17.Downs S. M., Ruan B., Schroepfer G. J., Jr 2001. Meiosis-activating sterol and the maturation of isolated mouse oocytes. Biol. Reprod. 64: 80–89. [DOI] [PubMed] [Google Scholar]
- 18.Andersen C. Y., Baltsen M., Byskov A. G. 1999. Gonadotrophin-induced resumption of oocyte meiosis and meiosis-activating sterols. Curr. Top. Dev. Biol. 41: 163–185. [DOI] [PubMed] [Google Scholar]
- 19.Cavilla J. L., Kennedy C. R., Baltsen M. 2001. The effects of meiosis activating sterol on in-vitro maturation and fertilization of human oocytes from stimulated and unstimulated ovaries. Hum. Reprod. 16: 547–555. [DOI] [PubMed] [Google Scholar]
- 20.Donnay I., Faerge I., Grøndahl C., Verhaeghe B., Sayoud H., Ponderato N., Galli C., Lazzari G. 2004. Effect of prematuration, meiosis activating sterol and enriched maturation medium on the nuclear maturation and competence to development of calf oocytes. Theriogenology. 62: 1093–1107. [DOI] [PubMed] [Google Scholar]
- 21.Faerge I., Terry B., Kalous J. 2001. Resumption of meiosis induced by meiosis-activating sterol has a different signal transduction pathway than spontaneous resumption of meiosis in denuded mouse oocytes cultured in vitro. Biol. Reprod. 65: 1751–1758. [DOI] [PubMed] [Google Scholar]
- 22.Marin Bivens C. L., Lindenthal B., O'Brien M. J. 2004. Meiosis-activating sterol promotes the metaphase I to metaphase II transition and preimplantation developmental competence of mouse oocytes maturing in vitro. Biol. Reprod. 70: 1458–1464. [DOI] [PubMed] [Google Scholar]
- 23.Leonardsen L., Strömstedt M., Jacobsen D. 2000. Effect of inhibition of sterol delta 14-reductase on accumulation of meiosis-activating sterol and meiotic resumption in cumulus-enclosed mouse oocytes in vitro. J. Reprod. Fertil. 118: 171–179. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z., Xia G. L., Andersen C. Y. 2000. Effects of amphotericin B and ketoconazole on mouse oocyte maturation: implications on the role of meiosis-activating sterol. Mol. Cell. Endocrinol. 164: 191–196. [DOI] [PubMed] [Google Scholar]
- 25.Xie H., Xia G., Byskov A. G., Andersen C. Y., Bo S., Tao Y. 2004. Roles of gonadotrophins and meiosis-activating sterols in meiotic resumption of cultured follicle-enclosed mouse oocytes. Mol. Cell. Endocrinol. 218: 155–163. [DOI] [PubMed] [Google Scholar]
- 26.Jin S., Zhang M., Lei L., Wang C., Fu M., Ning G., Xia G. 2006. Meiosis activating sterol (MAS) regulate FSH-induced meiotic resumption of cumulus cell-enclosed porcine oocytes via PKC pathway. Mol. Cell. Endocrinol. 249: 64–70. [DOI] [PubMed] [Google Scholar]
- 27.Tsafriri A., Popliker M., Nahum R. 1998. Effects of ketoconazole on ovulatory changes in the rat: implications on the role of a meiosis-activating sterol. Mol. Hum. Reprod. 4: 483–489. [DOI] [PubMed] [Google Scholar]
- 28.Tsafriri A., Cao X., Vaknin K. M., Popliker M. 2002. Is meiosis activating sterol (MAS) an obligatory mediator of meiotic resumption in mammals. Mol. Cell. Endocrinol. 187: 197–204. [DOI] [PubMed] [Google Scholar]
- 29.Byskov A. G., Andersen C. Y., Hossaini A. 1997. Cumulus cells of oocyte-cumulus complexes secrete a meiosis-activating substance when stimulated with FSH. Mol. Reprod. Dev. 46: 296–305. [DOI] [PubMed] [Google Scholar]
- 30.Burton P. M., Swinney D. C., Heller R. 1995. Azalanstat (RS-21607), a lanosterol 14 alpha-demethylase inhibitor with cholesterol-lowering activity. Biochem. Pharmacol. 50: 529–544. [DOI] [PubMed] [Google Scholar]
- 31.Tompkins S. M., Lo C.Y., Tumpey T. M., Epstein S. L. 2004. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. USA. 101: 8682–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svoboda P., Stein P., Schultz R. M. 2001. RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem. Biophys. Res. Commun. 287: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 33.Svoboda P., Stein P., Filipowicz W., Schultz R. M. 2004. Lack of homologous sequence-specific DNA methylation in response to stable dsRNA expression in mouse oocytes. Nucleic Acids Res. 32: 3601–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein P., Svoboda P., Schultz R. M. 2003. Transgenic RNAi in mouse oocytes: a simple and fast approach to study gene function. Dev. Biol. 256: 187–193. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds A., Leake D., Boese Q. 2004. Rational siRNA design for RNA interference. Nat. Biotechnol. 22: 326–330. [DOI] [PubMed] [Google Scholar]
- 36.Su Y. Q., Rubinstein S., Luria A., Lax Y., Breitbart H. 2001. Involvement of MEK-mitogen-activated protein kinase pathway in follicle-stimulating hormone-induced but not spontaneous meiotic resumption of mouse oocytes. Biol. Reprod. 65: 358–365. [DOI] [PubMed] [Google Scholar]
- 37.Xu B., Wang C., Yang J., Mao G., Zhang C., Liu D., Tai P., Zhou B., Xia G., Zhang M. 2008. Silencing of mouse hepatic lanosterol 14-a demethylase down-regulated plasma low-density lipoprotein cholesterol levels by short-term treatment of siRNA. Biol. Pharm. Bull. 31: 1182–1191. [DOI] [PubMed] [Google Scholar]
- 38.Baltsen M. 2001. Gonadotrophin-induced accumulation of 4,4-dimethylsterols in mouse ovaries and its temporal relation to meiosis. Biol. Reprod. 65: 1743–1750. [DOI] [PubMed] [Google Scholar]
- 39.Richards J. S. 2001. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol. Endocrinol. 15: 209–218. [DOI] [PubMed] [Google Scholar]
- 40.Conti M. 2002. Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol. Reprod. 67: 1653–1661. [DOI] [PubMed] [Google Scholar]
- 41.Ascoli M., Fanelli F., Segaloff D. L. 2002. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 23: 141–174. [DOI] [PubMed] [Google Scholar]
- 42.Ning G., Ouyang H., Wang S., Chen X., Xu B., Yang J., Zhang H., Zhang M., Xia G. 2008. CREB up-regulated CYP51 expression involved in FSH-induced mouse oocyte maturation. Mol. Endocrinol. 22: 1682–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J. Y., Su Y. Q., Ariga M., Law E., Jin S. L., Conti M. 2004. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 303: 682–684. [DOI] [PubMed] [Google Scholar]
- 44.Downs S. M., Chen J. 2008. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol. Reprod. Dev. 75: 105–114. [DOI] [PubMed] [Google Scholar]
- 45.Dekel N., Galiani D., Beers W. H. 1988. Induction of maturation in follicle-enclosed oocytes: the response to gonadotrophins at different stages of follicular development. Biol. Reprod. 38: 517–521. [DOI] [PubMed] [Google Scholar]
- 46.Kishi H., Greenwald G. S. 1999. Autoradiographic analysis of follicle-stimulating hormone and human chorionic gonadotrophin receptors in the ovary of immature rats treated with equine chorionic gonadotrophin. Biol. Reprod. 61: 1171–1176. [DOI] [PubMed] [Google Scholar]
- 47.Abdennebi L., Monget P., Pisselet C., Remy J. J., Salesse R., Monniaux D. 1999. Comparative expression of luteinizing hormone and follicle-stimulating hormone receptors in ovarian follicles from high and low prolific sheep breeds. Biol. Reprod. 60: 845–854. [DOI] [PubMed] [Google Scholar]
- 48.Nakano R., Sasaki K., Shima K., Kitayama S. 1983. Follicle-stimulating hormone and luteinizing hormone receptors on porcine granulosa cells during follicular maturation: an autoradiographic study. Exp. Clin. Endocrinol. 81: 17–23. [DOI] [PubMed] [Google Scholar]
- 49.Shima K., Kitayama S., Nakano R. 1987. Gonadotrophin binding sites in human ovarian follicles and corpora lutea during the menstrual cycle. Obstet. Gynecol. 69: 800–806. [PubMed] [Google Scholar]
- 50.Tsafriri A., Lieberman M. E., Koch Y., Bauminger S., Chobsieng P., Zor U., Lindner H. R. 1976. Capacity of immunologically purified FSH to stimulate cyclic AMP accumulation and steroidogenesis in Graafian follicles and to induce ovum maturation and ovulation in the rat. Endocrinology. 98: 655–661. [DOI] [PubMed] [Google Scholar]
- 51.Eppig J. J., Downs S. M. 1987. The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotrophin-induced oocyte maturation. Dev. Biol. 119: 313–321. [DOI] [PubMed] [Google Scholar]
- 52.Vaknin K. M., Lazar S., Popliker M. 2001. Role of meiosis-activating sterols in rat oocyte maturation: effects of specific inhibitors and changes in the expression of lanosterol 14alpha-demethylase during the preovulatory period. Biol. Reprod. 64: 299–309. [DOI] [PubMed] [Google Scholar]
- 53.Marin Bivens C. L., Grøndahl C., Murray A. 2004. A synthetic analogue of meiosis-activating sterol (FF-MAS) is a potent agonist promoting meiotic maturation and preimplantation development of mouse oocytes maturing in vitro. Hum. Reprod. 19: 2340–2344. [DOI] [PubMed] [Google Scholar]
- 54.Griffin A. M., Grondahl C., Fleming S. D. 2004. Action of hypoxanthine and meiosis-activating sterol on oocyte maturation in the mouse is strain specific. Reprod. Biomed. Online. 8: 673–681. [DOI] [PubMed] [Google Scholar]
- 55.Bergh C., Loft A., Lundin K., Ziebe S., Nilsson L., Wikland M., Gröndahl C., Arce J. C. 2004. Chromosomal abnormality rate in human pre-embryos derived from in vitro fertilization cycles cultured in the presence of follicular-fluid meiosis activating sterol (FF-MAS). Hum. Reprod. 19: 2109–2117. [DOI] [PubMed] [Google Scholar]
- 56.Cukurcam S., Hegele-Hartung C., Eichenlaub-Ritter U. 2003. Meiosis-activating sterol protects oocytes from precocious chromosome segregation. Hum. Reprod. 18: 1908–1917. [DOI] [PubMed] [Google Scholar]
- 57.Goud P. T., Goud A. P., Qian C. 1998. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum. Reprod. 13: 1638–1644. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Xu B., Xie H., Zhou B., Ouyang H., Ning G., Li G., Zhang M., Xia G. 2009. Lanosterol metabolic product(s) is involved in primordial folliculogenesis and establishment of primordial folliclepool in mouse fetal ovary. Mol. Reprod. Dev. 76: 514–521. [DOI] [PubMed] [Google Scholar]