Abstract
During screening of genes upregulated by lipopolysaccharide (LPS; endotoxin) treatment of bone marrow-derived mouse macrophages, it was unexpectedly found that cholesterol 25-hydroxylase (Ch25h) was strongly upregulated. Treatment of macrophages with 10 ng/ml of LPS for 2 h resulted in a 35-fold increase in the expression of Ch25h. In contrast, LPS treatment did not increase the expression of Cyp27a1 or Cyp7b1. The increased Ch25h expression was found to be independent of Myeloid differentiation protein 88 signaling but dependent on Toll-like receptor 4 signaling. LPS treatment of macrophages caused a 6- to 7-fold increase in cellular 25-hydroxycholesterol concentration. When macrophages were treated with increasing concentrations of 25-hydroxycholesterol, a dose-dependent release of CCL5 into the culture medium was observed. Intravenous injection of LPS in eight healthy volunteers resulted in an increase in plasma 25-hydroxycholesterol concentration. The possibility is discussed that 25-hydroxycholesterol may have a role in the inflammatory response, in addition to its more established role in the regulation of cholesterol homeostasis.
Keywords: 25-hydroxycholesterol, oxysterols, endotoxin, inflammation, sepsis, macrophages
Cholest-5-ene-3β,25-diol (25-hydroxycholesterol) has long been recognized as a cholesterol autooxidation product, formed during storage of cholesterol or artifactually produced during work-up of tissue samples (1, 2). Enzymatic formation of 25-hydroxycholesterol by rat liver mitochondria was described in 1974 (3), and it was later shown that 25-hydroxycholesterol is formed as a by-product during cholesterol oxidation by the mitochondrial enzyme sterol 27-hydroxylase in liver in pig (4) and mouse (5). In addition to the formation of 25-hydroxycholesterol by sterol 27-hydroxylase (CYP27A1), a specific human microsomal cholesterol 25-hydroxylase has been cloned and characterized (6). This enzyme does not belong to the cytochrome P450 family but is related to the eukaryotic stearoyl-CoA desaturases (6). Low levels (3-5 ng/ml) of 25-hydroxycholesterol are present in human plasma (7), but the relative contribution of CYP27A1 and cholesterol 25-hydroxylase to its formation is not known.
25-Hydroxycholesterol is a potent regulatory oxysterol and may participate in several aspects of lipid metabolism (8). A family of oxysterol binding proteins with high affinity for 25-hydroxycholesterol has been identified (9). Overexpression of oxysterol binding proteins in Chinese hamster ovary cells resulted in significant changes in genes involved in lipid metabolism (10). Side chain oxidized oxysterols, such as 25-hydroxycholesterol, have been implicated in the regulation of cholesterol homeostasis for a long time but only recently was the mechanism clarified. These oxysterols bind to proteins called Insigs, thereby blocking the sterol regulatory element binding protein signaling that regulates cholesterol biosynthesis (11). Furthermore, 25-hydroxycholesterol has been shown to activate the nuclear receptor liver X receptor α (LXRα) (12, 13), involved in the regulation of lipid metabolism. It has been questioned whether oxysterols are natural ligands for LXR (14), but some support for their role as ligands has been obtained from experiments in triple knockout mice deficient in the biosynthetic enzymes for 24-, 25-, and 27-hydroxycholesterol. While cholesterol feeding induced several LXR-responsive genes in wild-type mice, an impaired response was seen in the triple knockout mice (15). Recently, it was found that 25-hydroxycholesterol binds to the Niemann-Pick, Type C1 protein, a protein involved in cholesterol transport from lysosomes to the endoplasmic reticulum (16). Altogether, these studies suggest an important role for 25-hydroxycholesterol in the regulation of lipid metabolism.
Lipopolysaccharide (LPS; endotoxin) is an integral component of the cell wall of Gram-negative bacteria, such as Escherichia coli. LPS interaction with the Toll-like receptor 4 (TLR4), MD-2, and CD14 receptor complex induces a host response to invading pathogens. There are two main signaling pathways activated by TLR4, depending on the recruitment of separate sets of adaptor proteins: the Myeloid differentiation protein 88 (MyD88)-dependent pathway, involving Mal/TIRAP and MyD88, and the MyD88-independent pathway, involving the TRIF and TRAM adaptors (17). The MyD88-dependent pathway leads to activation of the transcription factor NF-κB, whereas TRIF activates the transcription factor IRF3. Together, these pathways result in the induction of pro-inflammatory genes with κB-binding motifs or the interferon-sensitive response element in their promoter regions.
During screening of genes upregulated by LPS treatment of bone marrow-derived mouse macrophages, it was unexpectedly found that cholesterol 25-hydroxylase was strongly upregulated. In this investigation, we have studied the LPS regulation of cholesterol 25-hydroxylase in mouse macrophages. We have also observed increased levels of plasma 25-hydroxycholesterol in healthy human volunteers exposed to endotoxin challenge.
EXPERIMENTAL PROCEDURES
Cell culture
The local Animal Care and Use Committee approved the experimental protocol used in the study, and all mice were bred in-house. Mice were anesthetized with isoflurane and euthanized by cervical dislocation. Bone marrow was isolated from 6 to 8 week old C57BL/6 and MyD88-deficient mice. Macrophages were derived from these marrows by culture in DMEM media supplemented with 10% FBS and 10 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) for 6 days. Bone marrow-derived macrophages were treated with either E. coli 0111 LPS (Sigma-Aldrich), 25-hydroxylcholesterol (Sigma-Aldrich), or control medium at the concentrations and times specified below. The mouse macrophage-like cell line RAW264.7 was cultured in DMEM media supplemented with 10% FBS.
Microarray expression analysis
For microarray analysis of the mRNA expression profile in bone marrow-derived macrophages, cells were treated at day 8 ex vivo for 2 h with 10 ng/ml E. coli 0111 LPS, and their gene expression profiles were compared with that of medium-treated control cells incubated for the same time. Cells from four individual mice of each genotype were used, and each mouse served as its own medium-treated control. In the time-course experiment, cells from two mice were treated with 10 ng/ml LPS or live E. coli (one bacterium per macrophage) for increasing times, and their gene expression profiles were compared with that of the preceding time point in a loop design. Total RNA for microarray analysis was isolated with TRIzol reagent (Invitrogen) and RNeasy columns (Qiagen). RNA integrity was assessed using the 2100 Bioanalyzer (Agilent Technologies). The Operon Mus musculus ver. 1.1 probe set (Qiagen) consisting of >13,000 oligonucleotide probes (70-mers) was printed in the Massachusetts General Hospital (Cambridge, MA) microarray core facility using an Omnigrid 100 (GeneMachines) on CodeLink-activated slides (Amersham). RNA was reverse transcribed and differentially labeled with Cy3 and Cy5 dyes (Amersham) using the Atlas PowerScript fluorescent labeling kit (BD Biosciences). Labeled samples were hybridized overnight using an automated hybridization station (Genomic Solutions). Fluorescent images from the arrays were acquired using a GenePix 4000B microarray scanner and its accompanying software (Axon Instruments). Data were normalized using the BioArray Software Environment (http://base.thep.lu.se/). Higher-level data and statistical analysis was performed using the GeneSpring (Silicon Genetics) software. A detailed analysis of the entire microarray data set has been published elsewhere (18).
Quantitative real-time PCR
Bone marrow-derived macrophages were treated with PBS (vehicle) or 10 ng/ml LPS for 2 h. Total RNA was isolated using Trizol reagent (Invitrogen) and the E.Z.N.A. Total RNA kit (Omega Bio-Tek). RNA integrity and purity were analyzed with an Experion automated electrophoresis system (Bio-Rad) and a ND-1000 spectrophotometer (NanoDrop Technologies). Purified total RNA was reverse transcribed with MMLV reverse transcriptase (Invitrogen), and quantitative real-time PCR was performed using SYBR Green PCR Master Mix and a 7900HT Fast real-time PCR system (Applied Biosystems). Relative mRNA expression was calculated from standard curves, constructed by serial dilution of gel-purified PCR products, and normalized to 18S rRNA expression. Primer pairs used were as follows: cholesterol 25-hydroxylase (Ch25h) forward primer 5′-TGCTACAACGGTTCGGAGC-3′ and reverse primer 5′-AGAAGCCCACGTAAGTGATGAT-3′, Cyp27a1 forward primer 5′- CCAGGCACAGGAGAGTACG-3′ and reverse primer 5′-GGGCAAGTGCAGCACATAG-3′, and Cyp7b1 forward primer 5′- AGCTGCTTACTGATGACGACC-3′ and reverse primer 5′-AGTGAGCCACAGAATGCAAAT-3′.
Oxysterol measurements
Bone marrow-derived macrophages were treated with PBS (vehicle) or 10 ng/ml LPS for 6 or 24 h. Supernatant was collected, and dead cells and cell debris were removed by centrifugation. Adherent macrophages were washed twice with PBS before harvesting by scraping in 200 µl PBS. Supernatants and scraped cells were stored at −80°C until analyzed. Total protein concentration was measured with the BCA protein assay kit (Pierce). For 25-hydroxycholesterol measurements, cells or cell culture medium were extracted according to Folch, Lees, and Sloane Stanley (19) and subsequently analyzed by isotope dilution GC-MS using deuterium-labeled internal standard as described earlier (7).
ELISA
RAW264.7 cells were treated with either increasing concentrations of 25-hydroxycholesterol or fixed concentrations of 25 or 50 ng/ml 25-hydroxycholesterol for 18 h. Cells were also treated with 25 or 50 ng/ml 25-hydroxycholesterol 30 min prior to stimulation with LPS for 18 h. Culture media were collected and stored at −80°C for cytokine analysis. The amount of released Chemokine (C-C motif) ligand 5 (CCL5; RANTES) was determined by ELISA according to the manufacturer’s instructions (RnD Systems).
LPS injection in healthy subjects: study protocol
Healthy male volunteers (n = 8), age 26 ± 3 years, weight 74 ± 9 kg, length 182 ± 5 cm, and body mass index 22 ± 2 kg/m2 (mean ± SD) participated in the study (20). All subjects were metabolically and otherwise healthy, as determined by medical history, physical examination, analyses of cell blood count, and biochemical profile. The study started in the morning at 8 AM after an overnight fast, and lasted for 8 h. At 4 h, all subjects received an intravenous injection of US Standard Reference E. coli endotoxin at 4 ng/kg (Lot EC-6; US Pharmacopeia). Venous blood samples were collected in evacuated tubes containing EDTA, before and 2 and 4 h after endotoxin administration. Informed consent was obtained from all subjects to participate in this study, which was approved by the local ethics committee of Karolinska Institutet at Huddinge University Hospital, Huddinge, Sweden.
The endotoxin model
Healthy volunteers were exposed to an endotoxin challenge. In small doses (4 ng/kg of body weight), endotoxin can safely be given to healthy individuals. It has been extensively used as a standardized human model for the early phase of sepsis (21, 22). Endotoxin initiates the immune response. This response is clinically manifested as the systemic inflammatory response syndrome of septic shock caused by Gram-negative bacteria. It should be recognized that administration of small doses of endotoxin is not a model of septic shock. Yet, endotoxin challenge elicits typical responses seen in sepsis, such as in hemodynamics, pulmonary function, metabolism, as well as in the coagulation/fibrinolytic function and in effects upon inflammatory cells, although not as pronounced. The cytokine response shows a typical pattern with an early rise of the pro-inflammatory mediators tumor necrosis factor-α, followed by interleukin-1 (IL-1), IL-6, and IL-8 similar to what is seen in sepsis (21, 22). From a metabolical point of view, endotoxin challenge elicits pronounced changes in amino acid metabolism after 3 h with decreases of both plasma glutamate and glutamine by 25% (23).
RESULTS
Expression of cholesterol 25-hydroxylase mRNA is increased by LPS treatment
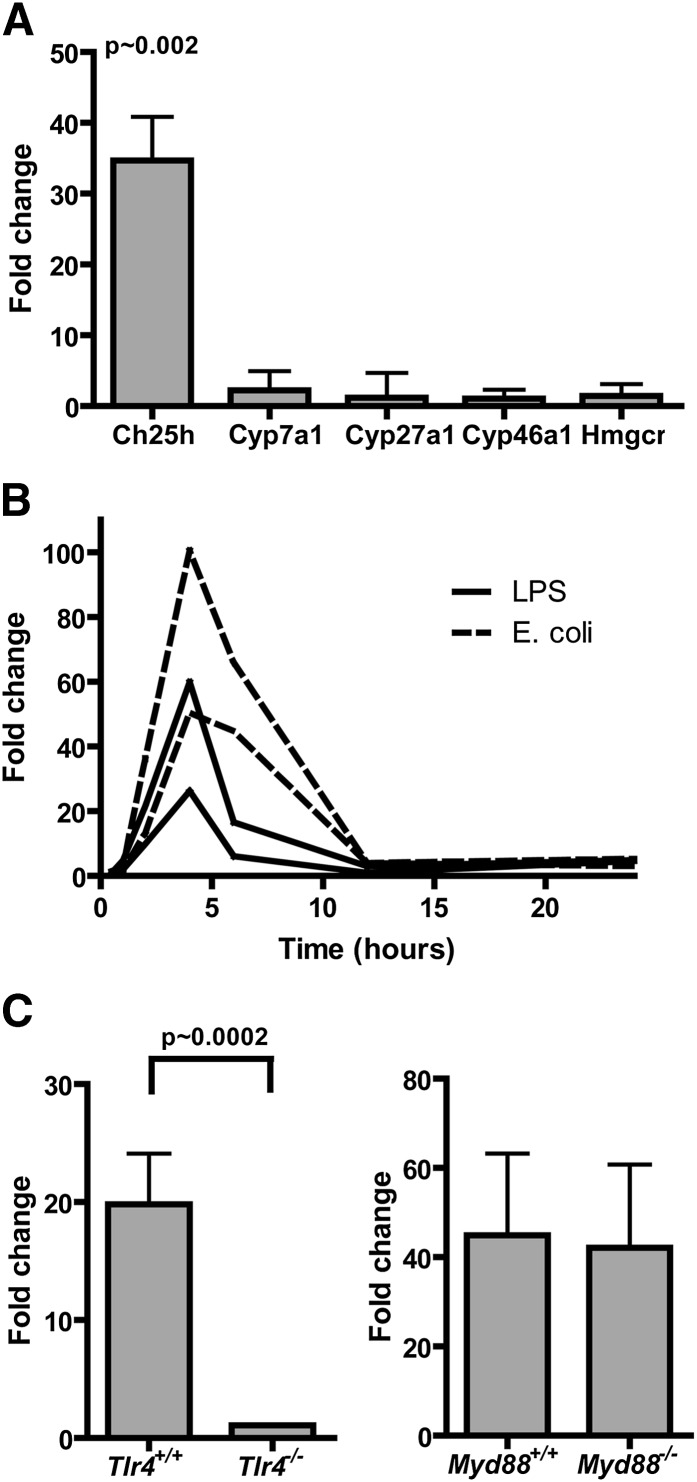
While studying the expression profile induced by LPS and live E. coli in mouse bone marrow-derived macrophages (18), we noted that the expression of several genes involved in cholesterol and oxysterol metabolism were not changed considerably by LPS treatment with the exception of the cholesterol 25-hydroxlyase gene Ch25h (Fig. 1A). Ch25h mRNA expression was increased 35-fold (P = ∼0.002) in macrophages treated with 10 ng/ml repurified LPS for 2 h compared with medium-treated controls. The increased Ch25h mRNA expression was found to be transient with maximum expression reached at about 4 h after addition of LPS, and at 12 h, the expression had returned to a similar level of expression as before the LPS treatment (Fig. 1B). Furthermore, the increased Ch25h expression was found to be independent of MyD88 signaling but dependent on TLR4 signaling, as the LPS-induced Ch25h expression was not impaired in mice with a targeted disruption of the MyD88 gene but severely impaired in TLR4-deficient mice (Fig. 1C). These data imply that LPS-induced Ch25h expression is controlled by the MyD88-independent TLR4/TRIF dependent pathway.
Fig. 1.
The cholesterol 25-hydroxylase gene is induced by LPS. Bone marrow-derived macrophages were differentiated in vitro with GM-CSF for 8 d and then treated with 10 ng/ml repurified E.coli LPS or media only for 2 h (A). Total RNA was harvested, purified, and labeled, and gene expression analyzed by microarrays (Operon) with oligonucleotide reporters representing approximately 13,000 genes. Cells from four individual mice were used and analyzed in duplicate (n = 8 microarrays total). In B, macrophages were treated with repurified E. coli LPS, live E. coli (one bacterium per macrophage), or media only for 0, 0.5, 1, 2, 4, 6, 12, and 24 h as indicated. Each line represents macrophages taken from one individual mouse. In C, macrophages were derived from Tlr4−/−, Myd88−/−, or C57Bl/6 control mouse bone marrow and treated with 10 ng/ml repurified E. coli LPS or media only for 2 h. Cells from four individual mice were used and analyzed in duplicate (n = 8 microarrays total). Fold changes represent expression compared with media controls, i.e., a fold change of 1 indicates no change from media only treated cells. Average data ± SD are presented. Statistics were calculated with a Mann-Whitney test.
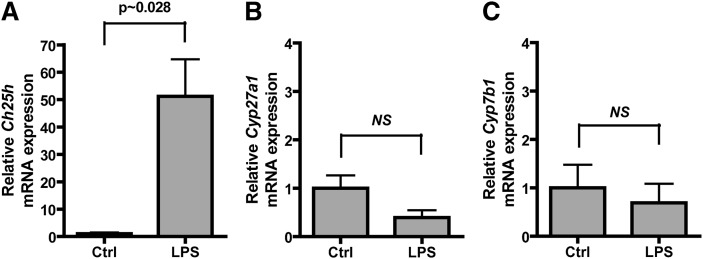
To confirm that the mRNA expression measured using microarrays was correct, we treated mouse bone marrow-derived macrophages with either PBS or 10 ng/ml repurified E. coli LPS for 2 h and analyzed the mRNA expression by quantitative real-time PCR. Ch25h mRNA was found to be increased in LPS-treated macrophages by 51-fold over PBS-treated control macrophages, whereas Cyp27a1 and Cyp7b1 mRNA was not changed compared with control macrophages (Fig. 2A–C). The LPS-induced upregulation of Ch25h mRNA expression presented a possibility that 25-hydroxycholesterol may have a function in inflammation separate from other oxysterols.
Fig. 2.
The cholesterol 25-hydroxylase gene, but not Cyp27a1 or Cyp7b1, was induced by LPS. Bone marrow-derived macrophages were differentiated in vitro with GM-CSF for 6 d and then treated with PBS (vehicle) or 10 ng/ml E. coli LPS for 2 h. Total RNA was isolated, and mRNA expression of Ch25h (A), Cyp27a1 (B), and Cyp7b1 (C) was analyzed by real-time PCR. Expression was normalized to β-actin expression and presented as expression relative to PBS-treated control cells. Average data ± SD are presented. Statistics were calculated with a Mann-Whitney test (n = 4 individual cultures).
25-hydroxycholesterol production and release from mouse macrophages is increased upon LPS treatment
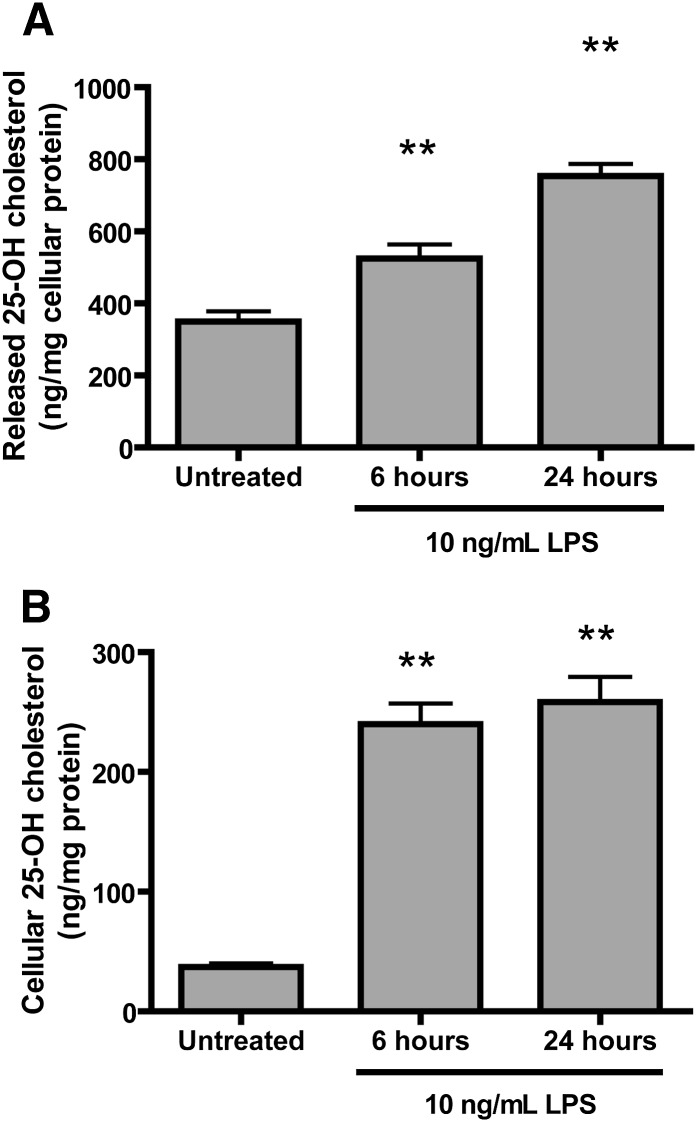
To study if LPS treatment of macrophages also induces the production and release of 25-hydroxycholesterol, we treated mouse bone marrow-derived macrophages with 10 ng/ml LPS for 6 or 24 h. The medium from untreated and LPS-treated cells was saved, and the adherent macrophages were thoroughly washed with PBS before they were scraped. Cellular oxysterols and oxysterols released into culture medium were extracted and analyzed by GC-MS and normalized to total cellular protein. LPS treatment increased macrophage 25-hydroycholesterol by 6- to 7-fold compared with untreated cells (Fig. 3B) and increased 25-hydroxycholesterol released into the culture medium by 1.5-fold at 6 h and by 2.2-fold at 24 h.
Fig. 3.
LPS-treated macrophages produce and release 25-hydroxycholesterol. Bone marrow-derived macrophages were differentiated in vitro with GM-CSF for 6 d and then treated with PBS (vehicle) or 10 ng/ml E. coli LPS for 6 or 24 h. Medium (A) and cells (B) were collected and analyzed for 25-hydroxycholesterol content and normalized to total cellular protein. Average data ± SD are presented. ** P < 0.01 in a one-way ANOVA with Dunnett’s multiple testing correction compared with untreated cells (n = 4 individual cultures).
25-hydroxycholesterol induces release of pro-inflammatory CCL5 cytokine but does not inhibit LPS-induced CCL5 release
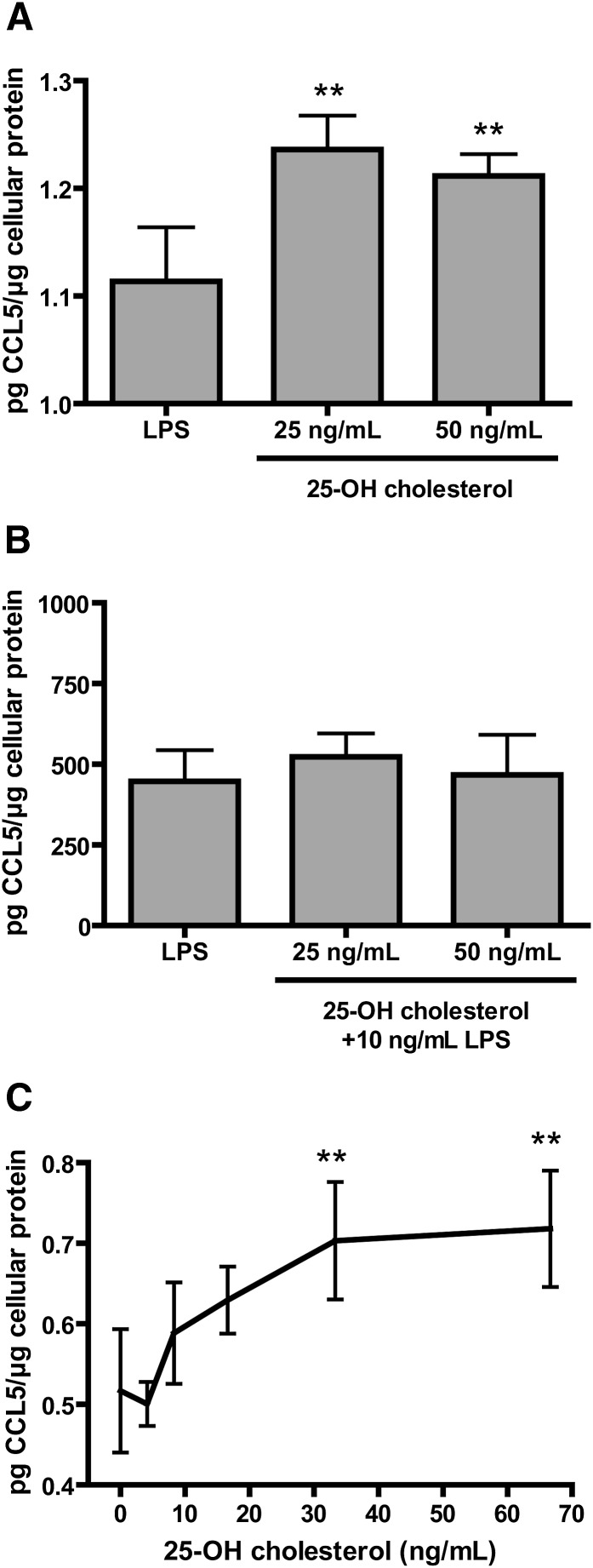
To investigate if 25-hydroxycholesterol may have a function in promoting inflammation, we treated RAW264.7 mouse macrophages with 25 or 50 ng/ml 25-hydroxycholesterol for 18 h and then measured CCL5 released into the medium normalized to total cellular protein. This is the same oxysterol concentrations found to be released from macrophages upon LPS treatment (Fig. 3A). Administration of CCL5 has previously been shown to increase sepsis-induced lethality in a cecal ligation and puncture model of septic peritonitis in mice, whereas CCL5 neutralization improved survival (24). The 25-hydroxycholesterol treatment induced a small but significant increase in CCL5 released into the medium compared with control macrophages. Next, to study if 25-hydroxycholesterol might have a role in limiting inflammation, we reasoned that pretreatment of macrophages with 25-hydroxycholesterol might impair LPS-induced release of CCL5, thus limiting inflammation. In Fig. 4B, pretreatment of RAW264.7 macrophages with 25 or 50 ng/ml 25-hydroxycholesterol for 30 min, prior to LPS treatment for 18 h, did not alter the amount of CCL5 released into the medium. RAW264.7 macrophages treated with increasing concentrations of 25-hydroxycholesterol responded by producing CCL5 in a dose-dependent manner, confirming that the small but significant induction of CCL5 release was dependent on 25-hydroxycholesterol.
Fig. 4.
25-Hydroxycholesterol treatment of macrophages induced CCL5 expression. Mouse macrophage-like cells were treated under various conditions and the CCL5 released into the culture media was measured by ELISA. A: The mouse macrophage-like cell line RAW264.7 (in DMEM with 10% FCS) was treated with 25-hydroxycholesterol for 18 h. B: RAW264.7 cells (in DMEM with 10% FCS) were pretreated with 25-hydroxycholesterol for 30 min before treatment with 10 ng/ml LPS for 18 h. C: RAW264.7 cells (in DMEM with 2% FCS) were treated with varying concentrations of 25-hydroxycholesterol for 18 h. Average data ± SD are presented. ** P < 0.01 in a one-way ANOVA with Dunnett’s multiple testing correction compared with untreated cells (n = 4 individual cultures). Culture medium contained <1 ng/ml 25-hydroxycholesterol.
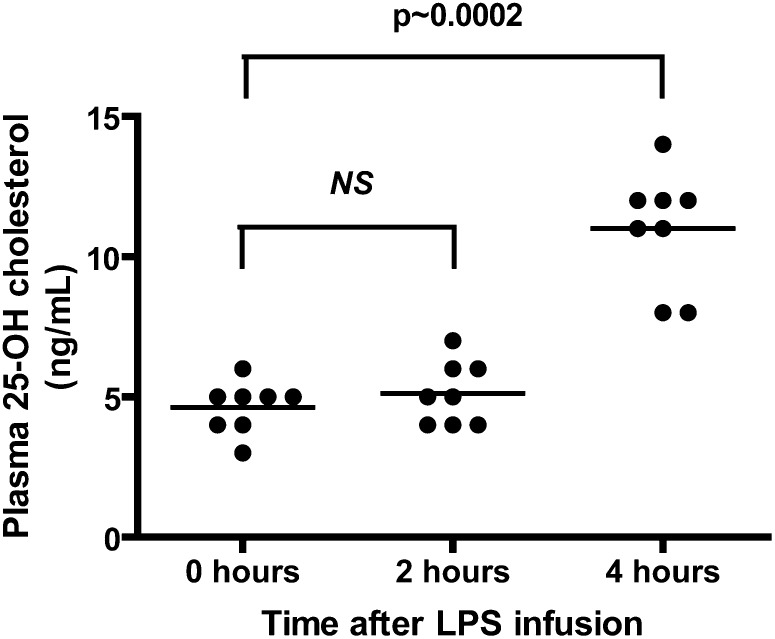
25-hydroxycholesterol increases in the plasma of healthy volunteers after LPS challenge
To test if 25-hydroxycholesterol levels are increased during human endotoxaemia and sepsis, we administered 4 ng/kg of US Standard Reference E. coli LPS by intravenous injection to eight healthy volunteers and measured plasma oxysterols in sampled blood over time. Blood plasma levels of 25-hydroxycholesterol were significantly increased over baseline levels 4 h after endotoxin challenge (Fig. 5). These results indicate that 25-hydroxycholesterol may play a role in human endotoxaemia and sepsis.
Fig. 5.
LPS infusion in healthy volunteers raises plasma 25-hydroxycholesterol levels after 4 h. Mann-Whitney test compared with time 0 (n = 8 healthy volunteers).
DISCUSSION
Very little is known about the regulation of cholesterol 25-hydroxylase. In an animal model where the gene responsible for the metabolism of 25-hydroxycholesterol, Cyp7b1, was disrupted, high circulatory levels of 25-hydroxycholesterol and 27-hydroxycholesterol were observed (25). These animals, lacking oxysterol 7α-hydroxylase, appeared to have a normal overall cholesterol metabolism, and no alterations in inflammatory parameters were reported. Triple knockout mice deficient in cholesterol 24-hydroxylase, cholesterol 25-hydroxylase, and sterol 27-hydroxylase have been generated and used in studies on LXR signaling. The gene disruptions resulted in reduced endogenous levels of 24-, 25-, and 27-hydroxycholesterol to below levels of detection. However, phenotypic data on these mice were not reported (15). It has been reported previously that 25-hydroxy vitamin D3 and 1,25-dihydroxy vitamin D3 can upregulate cholesterol 25-hydroxylase in human primary prostate stromal cells (26). In this work, we show that LPS induces cholesterol 25-hydroxylase mRNA expression, which is accompanied by increased 25-hydroxycholesterol release from mouse macrophages.
It has been shown previously that 25-hydroxycholesterol stimulates the release of pro-inflammatory cytokines in several cellular systems. Treatment of human monocytes or THP-1 cells with 25-hydroxycholesterol stimulated the release of IL-8 (27, 28). Enhanced pro-inflammatory cytokine secretion was also observed in U937 cells after 25-hydroxycholesterol challenge (29, 30). This oxysterol also caused a dose-dependent increase in IL-1β secretion from human monocyte-derived macrophages and in addition stimulated IL-1β secretion induced by LPS (28). 25-Hydroxycholesterol treatment resulted in an enhanced production of both IL-1β and IL-8 in Caco-2 cells (31) and was also shown to be a potent inducer of inflammation in retinal pigment epithelial cells (32). In macrophages, the pro-inflammatory cytokine CCL5 has been shown to have a critical role for cell survival (33); consequently, we studied the effect of 25-hydroxycholesterol on the release of CCL5 from mouse macrophages. Although a dose-dependent release of CCL5 was observed, the amount released was so low compared with LPS-induced CCL5 release that it can be questioned if it has any biological meaning. On the other hand, it may be that tissue macrophages have a higher production of cytokines compared with isolated cells. Liu, Hulten, and Wiklund (27) showed that tissue macrophages produced 7 times more IL-8 than monocytes and 100 times more than monocyte-derived macrophages.
It has been reported previously that LPS treatment markedly decreased the activity and mRNA levels of CYP27 in livers from Syrian hamsters and mice (34). In this study, no such decrease was observed in the mouse macrophages after 2 h (Fig. 2). Furthermore, LPS treatment of healthy volunteers did not alter plasma levels of 27-hydroxycholesterol during the time window studied (data not shown).
Many in vitro cell culture studies have been conducted with 25-hydroxycholesterol, and a wide array of biological functions for 25-hydroxycholesterol have been suggested (8). In addition to activation of LXR and regulation of lipid metabolism (12, 13), 25-hydroxycholesterol has been attributed both pro- and anti-inflammatory functions (35, 36), as well as a role in the induction of programmed cell death (37–39). Interestingly, transcriptional profiling in macrophages has revealed that inflammatory signaling induced by LPS via TLR4 can regulate expression of LXR-dependent lipid metabolism genes, whereas LXR activation in turn negatively regulates expression of inflammatory genes (40, 41). Thus, LXR activation results in inhibition of macrophage gene expression of inflammatory genes, such as iNOS, COX-2, IL-6, IL-1β, and G-CSF (30). In addition, LXR agonists show anti-inflammatory activity on both acute and chronic inflammation in murine in vivo models (30). Furthermore, the crosstalk between LXRs and TLRs has been found to be mediated by the TRIF-dependent activation of IRF-3 (42). Taken together, these reports and our findings raises the possibility that 25-hydroxycholesterol may have a role in the crosstalk between inflammatory signaling and lipid metabolism.
The potential role of 25-hydroxycholesterol in the crosstalk between inflammatory signaling and lipid metabolism may have consequences for atherosclerosis, a disease of the arteries driven by inflammation and lipid accumulation. Increased production of 25-hydroxycholesterol by macrophages, the prototypic cells of the atherosclerotic lesion, during inflammation could lead to LXR activation, which has been shown to increase cholesterol efflux and reverse cholesterol transport from lipid loaded macrophages.
In conclusion, we show here that 25-hydroxycholesterol, shown to act as an efficient LXR ligand in vitro, is markedly induced by LPS in mouse macrophages. Interestingly, LPS challenge in humans also increased plasma 25-hydroxycholesterol levels.
Footnotes
Abbreviations:
- CCL5
- Chemokine (C-C motif) ligand 5
- Ch25h
- cholesterol 25-hydroxylase
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- IL
- interleukin
- LXR
- liver X receptor
- LPS
- lipopolysaccharide
- MyD88
- Myeloid differentiation protein 88
- TLR4
- Toll-like receptor 4
This work was supported by grants from Torsten och Ragnar Söderbergs Stiftelser, Karolinska Institutet Research Foundations, The Swedish Heart Lung Foundation, The Swedish Research Council, The Albert Påhlsson Foundation, The Crafoord Foundation, The Magnus Bergvall Foundation, The Lars Hierta Foundation, The Malmö University Hospital Foundation, The Swedish Society of Medicine, The Thelma Zoega Foundation, and The Knut and Alice Wallenberg Foundation.
REFERENCES
- 1.Fieser L. F., Huang W-Y., Bhattacharyya B. K. 1957. Cholesterol and companions. X. The diol fraction. J. Org. Chem. 22: 1380–1384. [Google Scholar]
- 2.van Lier J. E., Smith L. L. 1967. Sterol metabolism. I. 26-Hydroxycholesterol in the human aorta. Biochemistry. 6: 3269–3278. [DOI] [PubMed] [Google Scholar]
- 3.Björkhem I., Gustafsson J. 1974. Mitochondrial ω-hydroxylation of cholesterol side chain. J. Biol. Chem. 249: 2528–2535. [PubMed] [Google Scholar]
- 4.Lund E., Björkhem I., Furster C., Wikvall K. 1993. 24-, 25- and 27-hydroxylation of cholesterol by a purified preparation of 27-hydroxylase from pig liver. Biochim. Biophys. Acta. 1166: 177–182. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Pandak W. M., Erickson S. K., Ma Y., Yin L., Hylemon P., Ren S. 2007. Biosynthesis of the regulatory oxysterol, 5-cholesten-3beta,25-diol 3-sulfate, in hepatocytes. J. Lipid Res. 48: 2587–2596. [DOI] [PubMed] [Google Scholar]
- 6.Lund E. G., Kerr T. A., Sakai J., Li W-P., Russell D. W. 1998. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 273: 34316–34327. [DOI] [PubMed] [Google Scholar]
- 7.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225: 73–80. [DOI] [PubMed] [Google Scholar]
- 8.Russell D. W. 2000. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta. 1529: 126–135. [DOI] [PubMed] [Google Scholar]
- 9.Lehto M., Olkkonen V. M. 2003. The OSBP-related proteins: a novel protein family involved in vesicle transport, cellular lipid metabolism, and cell signalling. Biochim. Biophys. Acta. 1631: 1–11. [DOI] [PubMed] [Google Scholar]
- 10.Lagace T. A., Byers D. M., Cook H. W., Ridgway N. D. 1997. Altered regulation of cholesterol and cholesteryl ester synthesis in Chinese-hamster ovary cells overexpressing the oxysterol-binding protein is dependent on the pleckstrin homology domain. Biochem. J. 326: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radhakrishnan A., Ikeda Y., Kwon H. J., Brown M. S., Goldstein J. L. 2007. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA. 104: 6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janowski B. A., Willy P. J., Devi T. R., Falck J. R., Mangelsdorf D. J. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature. 383: 728–731. [DOI] [PubMed] [Google Scholar]
- 13.Forman B. M., Ruan B., Chen J., Schroepfer G. J., Jr., Evans R. M. 1997. The orphan nuclear receptor LXRα is positively and negatively regulated by distinct products of mevalonate metabolism. Proc. Natl. Acad. Sci. USA. 94: 10588–10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björkhem I. 2002. Do oxysterols control cholesterol homeostasis? J. Clin. Invest. 110: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W., Chen G., Head D. L., Mangelsdorf D. J., Russell D. W. 2007. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 5: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Infante R. E., Abi-Mosleh L., Radhakrishnan A., Dale J. D., Brown M. S., Goldstein J. L. 2008. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 283: 1052–1063. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill L. A., Bowie A. G. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7: 353–364. [DOI] [PubMed] [Google Scholar]
- 18.Bjorkbacka H., Fitzgerald K. A., Huet F., Li X., Gregory J. A., Lee M. A., Ordija C. M., Dowley N. E., Golenbock D. T., Freeman M. W. 2004. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol. Genomics. 19: 319–330. [DOI] [PubMed] [Google Scholar]
- 19.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 20.Fredriksson K., Fläring U., Guillet C., Wernerman J., Rooyackers O. 2009. Muscle mitochondrial activity increases rapidly after an endotoxin challenge in human volunteers. Acta Anaesthesiol. Scand. 53: 299–304. [DOI] [PubMed] [Google Scholar]
- 21.Martich G. D., Boujoukos A. J., Suffredini A. F. 1993. Response of Man to Endotoxin. Immunobiology. 187: 403–416. [DOI] [PubMed] [Google Scholar]
- 22.Lin E., Lowry S. 1998. The human response to endotoxin. Sepsis. 2: 255–262. [Google Scholar]
- 23.Vesali R. F., Klaude M., Rooyackers O., Wernerman J. 2005. Amino acid metabolism in leg muscle after an endotoxin injection in healthy volunteers. Am. J. Physiol. Endocrinol. Metab. 288: E360–E364. [DOI] [PubMed] [Google Scholar]
- 24.Ness T. L., Carpenter K. J., Ewing J. L., Gerard C. J., Hogaboam C. M., Kunkel S. L. 2004. CCR1 and CC chemokine ligand 5 interactions exacerbate innate immune responses during sepsis. J. Immunol. 173: 6938–6948. [DOI] [PubMed] [Google Scholar]
- 25.Li-Hawkins J., Lund E. G., Turley S. D., Russell D. W. 2000. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J. Biol. Chem. 275: 16536–16542. [DOI] [PubMed] [Google Scholar]
- 26.Wang J. H., Tuohimaa P. 2006. Regulation of cholesterol 25-hydroxylase expression by vitamin D3 metabolites in human prostate stromal cells. Biochem. Biophys. Res. Commun. 345: 720–725. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Hulten L. M., Wiklund O. 1997. Macrophages isolated from human atherosclerotic plaques produce IL-8, and oxysterols may have a regulatory function for IL-8 production. Arterioscler. Thromb. Vasc. Biol. 17: 317–323. [DOI] [PubMed] [Google Scholar]
- 28.Lemaire-Ewing S., Berthier A., Royer M. C., Logette E., Corcos L., Bouchot A., Monier S., Prunet C., Raveneau M., Rebe C., et al. 2009. 7beta-Hydroxycholesterol and 25-hydroxycholesterol-induced interleukin-8 secretion involves a calcium-dependent activation of c-fos via the ERK1/2 signaling pathway in THP-1 cells: oxysterols-induced IL-8 secretion is calcium-dependent. Cell Biol. Toxicol. 25: 127–139. [DOI] [PubMed] [Google Scholar]
- 29.Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Berthier A., Bessede G., Corcos L., Gambert P., Neel D., Lizard G. 2005. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 21: 97–114. [DOI] [PubMed] [Google Scholar]
- 30.Prunet C., Montange T., Vejux A., Laubriet A., Rohmer J. F., Riedinger J. M., Athias A., Lemaire-Ewing S., Neel D., Petit J. M., et al. 2006. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry A. 69: 359–373. [DOI] [PubMed] [Google Scholar]
- 31.Bai B., Yamamoto K., Sato H., Sugiura H., Tanaka T. 2005. Combined effect of 25-hydroxycholesterol and IL-1beta on IL-8 production in human colon carcinoma cell line (Caco-2). Inflammation. 29: 141–146. [DOI] [PubMed] [Google Scholar]
- 32.Joffre C., Leclere L., Buteau B., Martine L., Cabaret S., Malvitte L., Acar N., Lizard G., Bron A., Creuzot-Garcher C., et al. 2007. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr. Eye Res. 32: 271–280. [DOI] [PubMed] [Google Scholar]
- 33.Tyner J. W., Uchida O., Kajiwara N., Kim E. Y., Patel A. C., O’Sullivan M. P., Walter M. J., Schwendener R. A., Cook D. N., Danoff T. M., et al. 2005. CCL5–CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat. Med. 11: 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Memon R. A., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. 2001. In vivo and in vitro regulation of sterol 27-hydroxylase in the liver during the acute phase response. Potential role of hepatocyte nuclear factor-1. J. Biol. Chem. 276: 30118–30126. [DOI] [PubMed] [Google Scholar]
- 35.Rosklint T., Ohlsson B. G., Wiklund O., Noren K., Hulten L. M. 2002. Oxysterols induce interleukin-1beta production in human macrophages. Eur. J. Clin. Invest. 32: 35–42. [DOI] [PubMed] [Google Scholar]
- 36.Englund M. C., Karlsson A. L., Wiklund O., Bondjers G., Ohlsson B. G. 2001. 25-Hydroxycholesterol induces lipopolysaccharide-tolerance and decreases a lipopolysaccharide-induced TNF-alpha secretion in macrophages. Atherosclerosis. 158: 61–71. [DOI] [PubMed] [Google Scholar]
- 37.Rusinol A. E., Thewke D., Liu J., Freeman N., Panini S. R., Sinensky M. S. 2004. AKT/protein kinase B regulation of BCL family members during oxysterol-induced apoptosis. J. Biol. Chem. 279: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 38.Ayala-Torres S., Moller P. C., Johnson B. H., Thompson E. B. 1997. Characteristics of 25-hydroxycholesterol-induced apoptosis in the human leukemic cell line CEM. Exp. Cell Res. 235: 35–47. [DOI] [PubMed] [Google Scholar]
- 39.Christ M., Luu B., Mejia J. E., Moosbrugger I., Bischoff P. 1993. Apoptosis induced by oxysterols in murine lymphoma cells and in normal thymocytes. Immunology. 78: 455–460. [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P. 2003. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9: 213–219. [DOI] [PubMed] [Google Scholar]
- 41.Zelcer N., Tontonoz P. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castrillo A., Joseph S. B., Vaidya S. A., Haberland M., Fogelman A. M., Cheng G., Tontonoz P. 2003. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell. 12: 805–816. [DOI] [PubMed] [Google Scholar]