Abstract
Expression of ABCA1 is regulated by transcription of the gene and calpain-mediated proteolytic degradation, and inhibition ABCA1 degradation results in increased ABCA1 and HDL biogenesis in vitro. We examined whether this approach could be a potential antiatherogenic treatment. Although probucol inhibits both the activity and degradation of ABCA1, its oxidized products, spiroquinone and diphenoquinone, reduce degradation of ABCA1 without inhibiting its activity or altering transcription of the ABCA1 gene. Accordingly, both compounds enhanced apolipoprotein A-I/ABCA1-dependent generation of HDL in vitro, and increased hepatic ABCA1 and plasma HDL without increasing antioxidant activity in plasma when given to rabbits. Both compounds also decreased vascular lipid deposition in cholesterol-fed rabbits. We therefore conclude that stabilization of ABCA1 against calpain-mediated degradation is a novel and potentially important strategy to increase HDL formation and prevent atherosclerosis. Spiroquinone and diphenoquinone are potential seeds for development of such drugs.
Keywords: ATP binding cassette transporter A1, high density lipoprotein, atherosclerosis, calpain, probucol
HDL plays a central role in the catabolic pathway of cholesterol by transporting it from extrahepatic cells to the liver for conversion to bile acids, and accordingly, is thought to be antiatherogenic. Apolipoprotein-mediated generation of new HDL from cellular lipids is one of the major events in the initial step of this pathway, cellular cholesterol release (1, 2). This reaction was found to be defective in genetic HDL deficiency, Tangier disease (3, 4), and mutations were identified in the gene of ABCA1 as the cause of this disorder (5–7).
Expression of ABCA1 is regulated at the transcriptional level and posttranslationally by calpain-mediated proteolysys. ABCA1 is stabilized against this degradation by helical apolipoproteins (8–10), and destabilized by unsaturated fatty acid (11) or excess unesterified cholesterol (12). Inhibition of calpain increases HDL formation by cultured cells (8), suggesting inhibition of proteolytic degradation of ABCA1 is a potential drug target for increasing HDL. ABCA1 degradation takes place intracellularly and its inhibition results in increased ABCA1 recycling to the cell surface (13). Direct inhibition of internalization of ABCA1 also causes its accumulation in the cell surface (13). HDL formation increases in both cases, indicating that it takes place at the cell surface (13). Inhibition of ABCA1 degradation or internalization would therefore be a potential strategy to increase HDL biogenesis for prevention and/or regression of atherosclerosis.
The hypolipidemic drug, probucol, is known to reduce plasma HDL (14) by inhibiting ABCA1-mediated HDL biogenesis (15) and producing a Tangier disease-like state (16). Interestingly, probucol causes not only inactivation of ABCA1 but also inhibits its degradation (17). We found in preliminary experiments that the crude oxidized products of probucol enhanced HDL formation by cultured cells rather than inhibiting it. Based on these findings, we hypothesized that some of the compounds in this mixture may function as inhibitors of ABCA1 degradation without inhibiting its activity. We therefore investigated the functions of spiroquinone (SQ) and diphenoquinone (DQ), the two potential oxidatized metabolites of probucol (supplementary Material I) (18), in their ability to alter ABCA1 activity and degradation as well as HDL formation in vitro and in vivo, and to alter the development of atherosclerosis in a rabbit model.
MATERIALS AND METHODS
Cell lines and culture conditions
THP-1 cells were maintained in 10% fetal bovine serum-RPMI1640 (Sigma) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Human monocytic cell line cells THP-1 were differentiated to macrophages (THP-1 macrophages) by culturing the cells at a density of 1.0 × 106 cells/ml in the presence of 3.2 × 10−7 M phorbol 12-myristate 13-acetate (Wako) for 24 h (8, 10). Balb/3T3 fibroblasts and HEK293 cells were maintained in 10% fetal bovine serum-DMEM (Sigma). The cells were seeded in culture plates at a density of 3 × 105 cells/ml and cultured for 3 days before use.
Treatment of cells with probucol and its metabolites
Probucol was purchased from Shiono Finesse Co., Ltd., Osaka, Japan. Its oxidant products, SQ and DQ, were synthesized and isolated by the oxidation of probucol (18) and by reduction of 2,6-di-tert-butylphenol (19), respectively. SQ and DQ were chemically stable under the experimental conditions used (details are described in supplementary Material I). Probucol, SQ, or DQ were delivered to cells either after incorporation into acetylated low density lipoprotein (acLDL) as a vehicle (15, 17) or directly as a solution in 2-butanol. Drug-containing acLDL was prepared as described previously (15). Briefly, human LDL was incubated with sonicated lipid microemulsion composed of egg phosphatidylcholine (Avanti), triolein (Wako), and the selected compound in the presence of lipoprotein-free human plasma fraction, reisolated by a dextran sulfate-cellulose column and ultracentrifugation, and acetylated with acetic anhydride. The final preparation contained approximately 0.3 μg of the respective compound per 100 μg protein. THP-1 macrophages were preloaded with probucol or metabolites by incubating with the acLDLs for 24 h. The compounds were alternatively delivered to cells by adding them from stock solutions in 2-butanol to produce a final solvent concentration in the culture medium of 0.5%.
Cellular lipid release by ApoA-I
Apolipoprotein (apo)A-I was isolated from the human HDL fraction as described previously (20). THP-1 macrophages were preloaded with probucol, SQ, or DQ by incubating the compound-containing acLDL and incubated in media containing 0.2% BSA (Sigma) and 10 μg/ml of apoA-I for 24 h. The compounds were also given as a 2-butanol solution as mentioned above by incubating the cells in the presence of the compounds and apoA-I for 24 h. Cholesterol and choline-containing phospholipids released into the media were measured enzymatically (Wako) (21). The cells were dissolved in 0.1N NaOH for protein determination by bicinchoninic acid (BCA) method (Pierce).
Western blotting
After the cells were incubated to load the compounds, they were suspended in 5 mM Tris-HCl buffer (pH 8.5) containing protease inhibitor cocktail (Sigma) and placed on ice for 30 min. The cell suspension was centrifuged at 650 g for 5 min, and the supernatant was centrifuged at 105,000 g for 30 min to precipitate the total membrane fraction. Twenty μg wet-weight liver specimens of the rabbits were treated in the same manner as preparation of the membrane fraction. Protein in these fractions was analyzed by Western blotting using specific polyclonal antibodies against ABCA1 (8), scavenger receptor class B type 1 (SR-B1; Novus Biologicals), and β-actin (Sigma) and visualized by a chemiluminescence method (Amersham Life Science).
Real time quantitative PCR
ABCA1 mRNA was measured by using probes previously reported for human and mouse (8) in a 7300 Real Time PCR System (Applied Biosystems). Cultured cells were lysed in the presence of phenol and guanidine thiocyanate. cDNAs were synthesized by SuperScriptTM First-Strand Synthesis Systems (Invitrogen). For rabbit ABCA1, total RNA was purified from rabbit liver and cDNA was synthesized as described above. A partial sequence of ABCA1 was amplified with synthetic oligonucleotide primers (5′-ACA ATA GTT GTA CGA ATA GCA GGG-3′, 5′-CTC ATC CTG TAG AAA AGA TGT GAG-3′) and cloned into pGEM®-T Easy Vector (Promega). Because the sequence of the partial clone of rabbit ABCA1 analyzed by a capillary sequencer 3100 (ABI) was 97% homologous to human ABCA1, these primers were used for the real-time quantitative PCR. ABCA1 expression was standardized to glyceraldehyde-3-phosphate dehydrogenase and β-actin.
Metabolic analysis of ABCA1
To examine degradation of ABCA1, THP-1 macrophages or Balb/3T3 cells were incubated for 24 h with 9-cis-RA (Sigma) to increase the expression of ABCA1 and treated with SQ or DQ for 30 min in 0.2% BSA-RPMI1640. Cells were washed once with PBS and incubated in 0.2% BSA-RPMI1640 containing 140 μM cycloheximide (Sigma) for the indicated periods, and ABCA1 protein analyzed by Western blotting as described above (8). ABCA1 in the cell surface was analyzed by biotinylation of the surface protein and its precipitation with avidin-beads followed by Western blotting (13). Internalization of ABCA1 was analyzed by biotinylation of surface ABCA1 and cleavage of the biotinylation of ABCA1 that remains in the surface after incubation as described elsewhere (13). To visualize intracellular localization of ABCA1, an expression vector containing ABCA1-green fluorescent protein (GFP) hybrid cDNA was transfected and expressed in HEK293 cells as described previously (22). Expression of ABCA1-GFP protein was confirmed by Western blotting with anti-ABCA1 antibody and with anti-GFP antibody. Intracellular localization of ABCA1-GFP was visually demonstrated as fluorescence images of the cells, placed on a 50-mm round coverslip for mounting in a temperature-controlled chamber at 37°C, and viewed with a LSM510 PASCAL laser scanning confocal microscope (Carl Zeiss). The averaged fluorescent intensity of ABCA1 in the plasma membrane was measured for 60 randomly selected cells using the software of the LSM510 PASCAL microscope.
Animal experiments
Three-month-old male New Zealand White rabbits were fed with LRC-4 diet containing SQ and DQ for 7 days. Plasma lipoproteins were analyzed for HDL and nonHDL fractions separated by ultracentrifugation at densities above and below 1.063 g/ml. The purity of each fraction was verified by agarose electrophoresis, and its cholesterol was measured by the enzymatic method. Expression of ABCA1 in the liver was determined by quantitative PCR for mRNA and by Western blotting for protein as described above. For high-cholesterol experiments, 3-month-old male New Zealand White rabbits were fed with 0.5% cholesterol-containing diet supplemented with SQ or DQ for 8 weeks. Plasma lipoproteins were measured as described above and also analyzed by HPLC as previously described at Skylight Biotech, Tokyo, Japan (23). Aortas were extracted and fixed with 10% neutral buffered formalin solution and lipid deposition in the intima was stained with Oil Red O. The antiatherogenic effect of the drugs was evaluated by measuring the Oil Red O-stained area in the thoracic and abdominal regions. The digitized images were analyzed using Adobe Photoshop and NIH Image to estimate the relative area of lipid deposition.
Antioxidant activity in plasma
To measure antioxidant activity of the compounds in vivo, 3-month-old WHHL rabbits were fed LRC4 diet containing SQ or probucol for 1 month. Five μl of the serum was used for estimation of the antioxidant activity by evaluating its activity to reduce Cu2+ to Cu+ by measuring absorbance at 490 nm of a stable complex of Cu+/bathocuproine (24) based upon the principle developed by MED.DIA, Italy, and modified by the Japan Institute for The Control of Aging, Nikken SEIL Corporation, according to the manufacture's instruction.
CETP mass in plasma
Cholesteryl ester transfer protein (CETP) mass in rabbit plasma was measured by enzyme-linked immuno-sorbent assay as described previously (25) using an assay system provided from Sekisui Medical Co., Ltd. (Tokyo, Japan).
Other methods
Intensity of each electrophoretic band was digitally scanned and semi-quantified by using an EPSON GT-X700 and Adobe Photoshop software. Statistical analysis of the data was performed by one-way ANOVA followed by Scheffé's test. Values represent mean ± SD for at least three independent measurements.
RESULTS
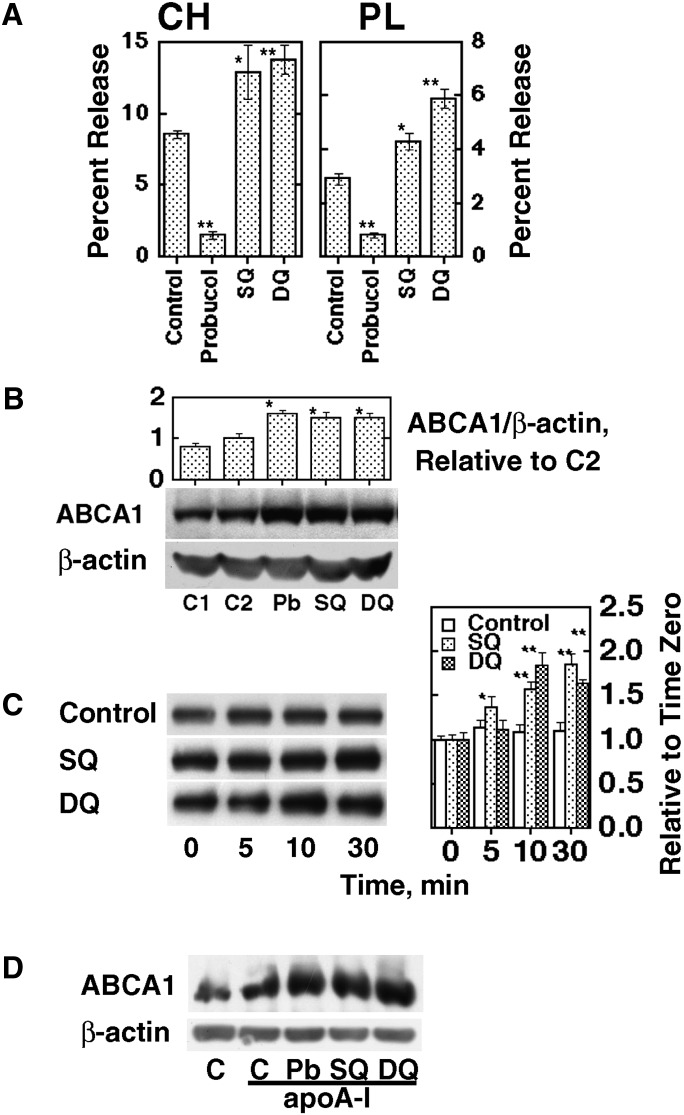
Probucol, SQ, and DQ were incorporated into acLDL and fed to THP-1 macrophages and cellular lipid release by apoA-I was measured. Whereas release of cholesterol and phospholipid was inhibited by probucol (15, 17), SQ and DQ enhanced the lipid release (Fig. 1A). ABCA1 protein was markedly increased by probucol in spite of inhibition of HDL formation, consistent with our previous finding (17) (Fig. 1B). SQ and DQ also markedly increased ABCA1. Other downstream oxidant products of probucol, bisphenol and butylphenol (18), did so but to a lesser extent (data not shown). When the THP-1 macrophages preloaded with acLDL were incubated with SQ and DQ added in a 2-butanol solution, ABCA1 apparently increased in the initial 30 min of the incubation (Fig. 1C). The increase in ABCA1 by SQ and DQ was also apparent in the presence of apoA-I (Fig. 1D), previously shown to inhibit calpain-mediated ABCA1 degradation (13). Similar effects in a dose-dependent manner were seen when SQ and DQ were given to THP-1 macrophages or Balb/3T3 mouse fibroblasts in a 2-butanol solution (supplementary Fig. IA, IB). However, the message of ABCA1 was not influenced by either compound (supplementary Fig. IC), similar to the previous finding with probucol (17). The amount of SQ and DQ in the cells was below the limit of our detection method (1 ng) (supplementary Material I) because of the low concentration of compounds and relatively small number of cells used in the experimental conditions.
Fig. 1.
The effect of probucol, spiroquinone (SQ) or diphenoquinone (DQ) in THP-1 macrophages. A: Cellular lipid release by apoA-I. Cells were incubated with acLDL containing each compound for 24 h at 100 μg/ml protein as 1.9 μM probucol, 2.0 μM SQ, and 2.4 μM DQ and incubated with 10 μg/ml apoA-I for another 24 h. Cholesterol (CH) and phospholipid (PL) in the medium were determined. B: ABCA1 protein in the same condition. Controls (C1 and C2) represent absence and presence of control acLDL. The numbers represent band intensity relative to C2. C: Time-dependent increase of ABCA1 after SQ and DQ were added as a 2-butanol solution (25 nM and 0.05 nM, respectively). The graph represents band intensity relative to time zero. D: Increase of ABCA1 by treatment with SQ and DQ (25 nM and 0.05 nM for 3 h) in the presence of 10 μg/ml apoA-I in the medium. The data represent the mean ± SE for three samples. * P < 0.05, ** P < 0.01 in comparison to control (A), C2 (B), and time zero (C).
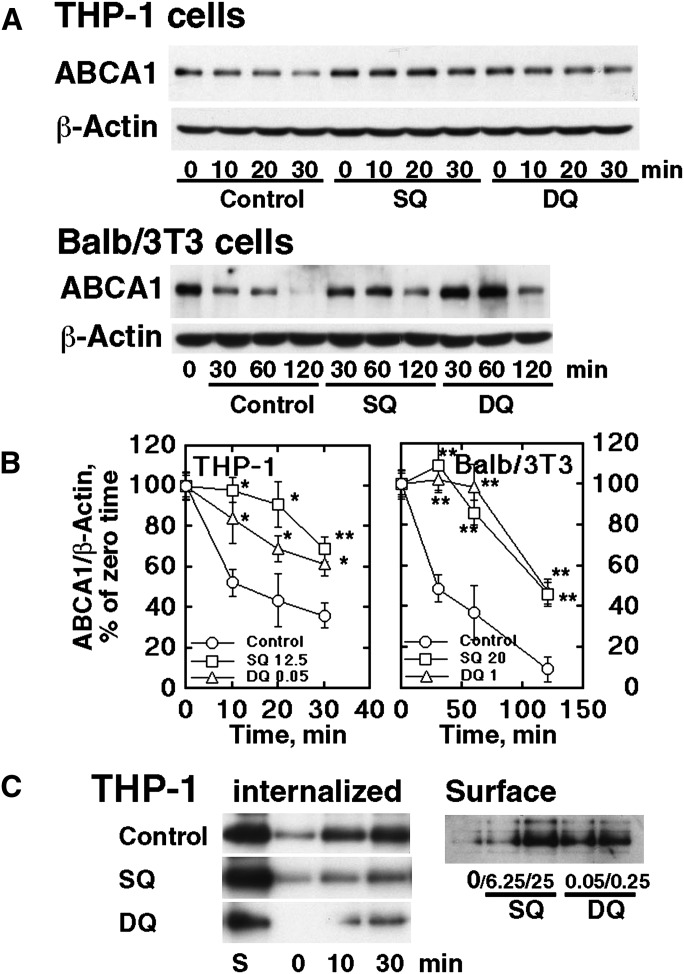
Figure 2, A and B, shows the decay of ABCA1 in the presence of cycloheximide. Both SQ and DQ apparently retarded this process in a very similar manner to the effect observed with probucol (17). Figure 2C demonstrates inhibition of ABCA1 internalization by these compounds. Internalization of surface ABCA1 prelabeled by biotinylation was inhibited by SQ and DQ shown in the left panel. In contrast, ABCA1 in the cell surface was increased by these compounds, shown in the right panel. Inhibition of ABCA1 degradation by these compounds was thus shown to be by inhibiting internalization of ABCA1 (13). The effect of SQ and DQ on intracellular localization of ABCA1 was further examined by using HEK293 cells in which ABCA1-GFP was overexpressed. Figure 3A shows an increase of transfected ABCA1-GFP by SQ and DQ. Fig. 3B shows an increase of fluorescence intensity of ABCA1-GFP as well as images of its intracellular localization. SQ and DQ increased the fluorescence intensity at the cellular surface. In these conditions, SQ and DQ also increased the release of cellular lipid by apoA-I (Fig. 3C).
Fig. 2.
Stabilization of ABCA1 by SQ and DQ. A: Retarded degradation of ABCA1 in the presence of cycloheximide in THP-1 macrophages and Balb/3T3 cells, by SQ (25 nM for THP-1 and 20 nM for Balb/3T3) and DQ (0.05 nM and 1.0 nM). B: Graphic expression of the results typically represented by Fig. 2A after standardization for β-actin. Error bars indicate SE for three measurements. Significant difference from control at each time point is indicated as * P < 0.05 and ** P < 0.01. C: Internalization of ABCA1. Left panel: THP-1 cells were preincubated with SQ (25 nM) and DQ (0.25 nM) for 16 h to equilibrate the cells with the compounds. The surface ABCA1 was then labeled by biotinylatin and the cells were incubated for time indicated. The surface biotinylation was cleaved and the remaining biotinylated ABCA1 was analyzed as the protein internalized. Right panel: Cell surface ABCA1 was analyzed by surface biotinylation after incubation with SQ and DQ (as indicated in nM) for 1 h.
Fig. 3.
Intracellular localization of ABCA1-GFP in HEK293 cells. A: HEK293 cells with stable expression of ABCA1-GFP were cultured with each compound (SQ 50 nM, DQ 0.5 nM) for 12 h. Cellular ABCA1-GFP was analyzed by using anti-ABCA1 antibody. B: Fluorescent image of the living cells was viewed with a laser scanning confocal microscope. The averaged fluorescent intensity in plasma membrane was measured by using the software of the LSM5 Pascal microscope. Sixty cells were analyzed in each group. (low magnitude and high magnitude). C: Release of cholesterol (CH) and phospholipid (PL) by 10 μg/ml apoA-I during the 12-h incubation. The data represent the mean ± SE for three measurements. * P < 0.05, ** P < 0.01 in comparison to each control.
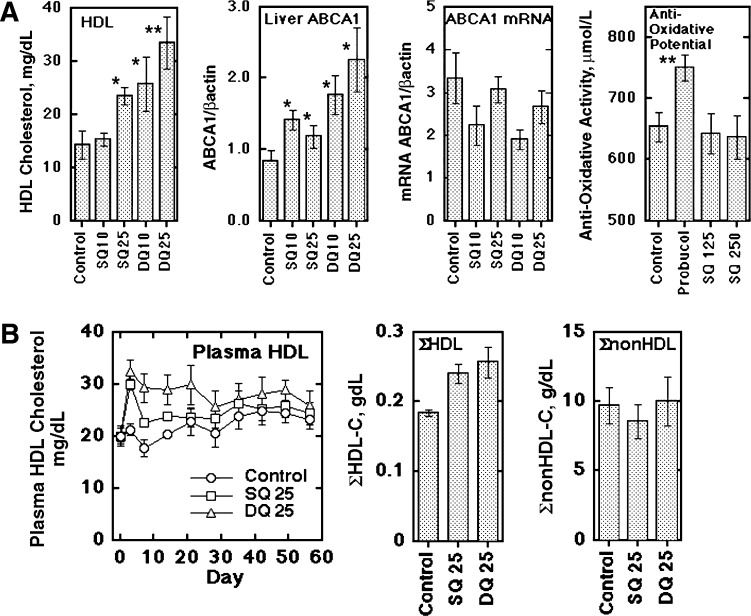
SQ and DQ were given to rabbits to examine their in vivo effects. Figure 4A shows an increase in plasma HDL-cholesterol and of hepatic ABCA1 protein (also in supplementary Fig. II) by SQ and DQ, with no increase in hepatic ABCA1 mRNA. Because probucol has strong antioxidant activity and its antiatherosclerotic effects are assumed to be due to this function, antioxidant activity of SQ, which supposedly has higher antioxidant activity than other probucol oxidation products, has been examined in vivo in comparison to probucol. Although antioxidant activity in plasma was substantially increased in the probucol-treated animals, no significant change was found in plasma antioxidant activity with a higher dose of SQ (Fig. 4A). Figure 4B and supplementary Fig. III show the results in cholesterol-fed rabbits for 8 weeks. Treatment with SQ and DQ did not cause significant change in food intake and body weight (supplementary Fig. IIIA). SQ and DQ increased HDL-cholesterol shown in a time course and in its integrated values for the entire experimental term of 8 weeks (Fig. 4B), as well as in the profile of the HPLC analysis (supplementary Fig. IIIB). SQ and DQ induced a significant increase in HDL-cholesterol, whereas neither compound caused significant change in nonHDL lipoprotein-cholesterol. The HDL-increasing effect seemed somewhat diminished after the 4-week treatment. Plasma CETP markedly increased with cholesterol feeding but did not show a difference among the treatment groups (supplementary Fig. IV). The increase in ABCA1 protein in the liver by SQ or DQ was retained at the end of the experiment, whereas SR-B1 protein showed no change (supplementary Fig. IV). There was no apparent adverse effect in the animals.
Fig. 4.
Effects of SQ and DQ on rabbit plasma lipoproteins. A: The compounds were orally given (mg/kg/day, n = 4) and HDL-cholesterol was measured at day 3. Hepatic ABCA1 protein and mRNA were analyzed at day 7. Plasma antioxidant activity was determined for the animals given 330 mg/kg/day probucol and 125 and 250 mg/kg/day SQ for 1 month. DQ was given in a higher relative dose to SQ than in the in vitro studies because of less solubility in oil, indicating poor absorption. B: Long term effects of SQ and DQ on plasma HDL-cholesterol. SQ or DQ, 25 mg/kg/day, was given to the animals fed with 0.5% cholesterol diet for 8 weeks (n = 8 in each group). Left panel: Plasma HDL-cholesterol. Middle panel: Integrated HDL-cholesterol as sum of HDL-cholesterol for the test period (day 3 and at every week thereafter). Right panel: Integrated nonHDL-cholesterol estimated similarly to HDL. The data represent the mean ± SE. * P < 0.05, ** P < 0.01 in comparison to each control.
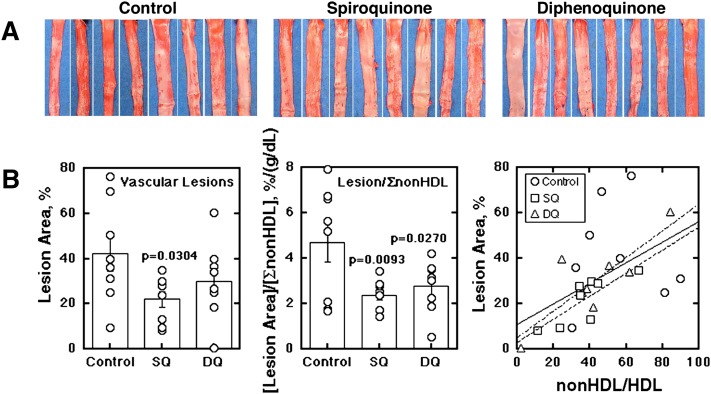
Figure 5 shows the effects of SQ and DQ on the vascular lesions in the cholesterol-fed rabbits characterized as above. Lipid deposition in aortic intima was examined by Oil Red O staining. Relative lipid deposit area was 0.46 ± 0.19 for the controls versus 0.27 ± 0.09 and 0.29 ± 0.13 for the SQ and DQ treatment groups (P = 0.02 and 0.03 against the control), respectively, including the aortic arch regions (supplementary Fig. V). The evaluation for the arch regions, however, may be inaccurate and unreliable as the wall cannot be set flat for photographs, so that further quantitative analysis was performed for the thoracic and abdominal regions of aorta (Fig. 5A). The lesion area was significantly decreased by SQ (Fig. 5B, left). When the lesion area was standardized for the integrated value of nonHDL cholesterol in an individual animal, the reduction in lipid deposition was significant for both SQ and DQ treatment (Fig. 5B, middle). Lipid deposition was a function of (nonHDL-cholesterol)/(HDL-cholesterol) to yield similar parameters in linear regression for each SQ and DQ treatment group and total (Fig. 5B, right), so that the effect of SQ and DQ on the lipid deposition may be attributed to the increase of HDL in association with stabilization of ABCA1.
Fig. 5.
Effects of SQ and DQ on vascular lipid deposit in cholesterol-fed rabbits. After 8 weeks of the experiments described in Fig. 4, lipid deposit in the aortic wall was evaluated by Oil Red O staining. A: Lipid deposit in the thoracic and abdominal aorta. B: Digitalized images were analyzed by an image processing software. Left panel: The lesion relative area (%). Middle panel: The relative lesion area standardized by the integrated nonHDL-cholesterol in each animal. Right panel: Plot of the relative lesion area (%) against the index of (integrated nonHDL-cholesterol)/(integrated HDL-cholesterol). Solid line represents fitting for all the groups (y = 0.46 x + 11.2, r = 0.528); even-broken line for the SQ-fed group (y = 0.51 x + 3.3, r = 0.81); and uneven-broken line for the DQ group (y = 0.59 x + 5.2, r = 0.83).
DISCUSSION
To examine whether inhibition of ABCA1 degradation increases HDL formation and plasma HDL level, we attempted to screen potential candidate chemicals that inhibit degradation of ABCA1, including oxidized products of the ABCA1 inactivator probucol (18). In our preliminary experiments, crude oxidative products of probucol increased cellular HDL formation rather than decreased it. Treatment of cells with SQ and DQ were found to increase ABCA1 protein and apoA-I-mediated HDL formation. Both compounds stabilized ABCA1 against calpain-mediated degradation without changing its transcription. They also increased expression of ABCA1-GFP in HEK293 cells expressing ABCA1-GFP with a nonphysiological promoter. The compounds increased plasma HDL in rabbits by increasing hepatic ABCA1 and suppressed lipid deposition in the arterial wall of cholesterol-fed rabbits. Thus, we conclude that these compounds increase HDL formation through protecting ABCA1 from degradation and thereby reduce atherogenesis in the experimental animals. The effects were apparently independent of antioxidant activity, previously considered to be one of the major antiatherogenic properties of probucol in similar animal models (26, 27), because these compounds did not exhibit significant antioxidant activity in plasma.
We thus demonstrated that pharmacologic inhibition of ABCA1 degradation could increase ABCA1 and plasma HDL and counteract atherogenesis in a model of hypercholesterolemia in vivo. SQ and DQ were shown to cause retardation of ABCA1 degradation seemingly by inhibiting internalization of ABCA1, a prerequisite for calpain-mediated proteolysis (13), rather than by direct inhibition of the calpain reaction. At this stage, we do not have further mechanistic insight into the action of SQ and DQ. The effects might be similar to the effect of cytochalasin D observed in vitro in cultured cells, including an increase of ABCA1 in the cell surface even under conditions where ABCA1 degradation was retarded by the presence of apoA-I (13). Because both SQ and DQ are extremely hydrophobic and likely incorporated into the membrane, these compounds may induce conformational alteration of ABCA1 to stabilize it against internalization for its degradation. However, it is unclear whether SQ and DQ by themselves cause such an effect or their metabolites may secondarily do so. Indeed, such products as bisphenol and butylphenol did show similar activity, but to a lesser extent, as described in the results section. This point should further be examined. It is interesting that probucol inactivates ABCA1 for HDL formation while inhibiting ABCA1 degradation but SQ and DQ only induce the latter effect. There may be a hint in this discrepancy to solve the question on the reaction mechanism of these compounds.
The results demonstrated here showed a novel concept for drug development, enhancement of the function of a specific membrane protein such as transporters or receptors by inhibiting their biological degradation. SQ, DQ, or their related compounds can thus be potential drug candidates to increase HDL formation and prevention/cure of atherosclerosis by inhibiting ABCA1 degradation. Several issues remain to be addressed. The compounds are extremely hydrophobic and need to be improved for oral administration. The apparent tendency to diminish the HDL-raising effect over time may be a problem for long-term administration. Probucol has been used in the market for years, and SQ and DQ may be produced as its metabolites in vivo (18). Further investigation is still required for any unexpected in vivo effects of the compounds, such as their influence on metabolism of membrane proteins in general and the exact mechanism for inhibiting degradation of ABCA1. A wide and thorough survey is needed of their influence on gene expression.
Probucol decreases HDL by inhibiting the activity of ABCA1 (15–17, 28, 29). Despite this HDL-lowering effect, probucol was proposed to have specific antiatherosclerotic properties based on clinical findings of efficient regression of cutaneous and tendinous xanthomas in familial hypercholesterolemia (30). It is also proposed to inhibit atherogenesis in experimental animals because of its ability to inhibit oxidation of LDL (26, 27, 31). We previously discovered that probucol inactivates ABCA1's ability to form HDL while inhibiting calpain-dependent degradation (17), the net result being a severe reduction in HDL. In contrast, we demonstrate here that oxidized products of probucol retain the ability to inhibit ABCA1 degradation but do not inhibit HDL formation by ABACA1. If SQ and/or DQ are produced during the in vivo oxidant metabolism of probucol, these products may induce an increase in active ABCA1 in some tissues. In addition to the effect on ABCA1, probucol has been proposed to induce an increase in activity of CETP (32) or SR-B1 (33) as the causes of the decrease of HDL. However, we found no change in SR-B1 protein by SQ or DQ in rabbit liver (supplementary Fig. IV) or in the mRNAs of apoA-I, LCAT, PLTP, or SR-B1 in the liver of the probucol-fed mice (16). Because the HDL-increasing effects of SQ and DQ were observed in mice as well in our preliminary experiments, the effect of SQ and DQ should not be related to CETP. CETP markedly increased in the rabbit plasma regardless of the drug administration by cholesterol feeding (34) (supplementary Fig. IV) and this effect might somewhat mask the specific increase of plasma HDL by SQ and DQ in this particular model.
Supplementary Material
Acknowledgments
The authors thank Tetsuya Murata, a medical student at Nagoya City University, for his contribution to the initial stage of this project. The authors are also grateful to Takako Sekine, Hisae Takayama, Tomoya Fujisawa, and Takeo Matsukura at Aska Pharmaceutical Co., Ltd. for excellent technical contribution and to Dr. Kuniko Noji, Nagoya City University, for performing the CETP assay.
Footnotes
Abbreviations:
- acLDL
- acetylated LDL
- apoA-I
- apolipoprotein A-I
- CETP
- cholesteryl ester transfer protein
- DQ
- diphenoquinone
- GFP
- green fluorescence protein
- SQ
- spiroquinone
- SR-B1
- scavenger receptor class B type 1
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Science, Sports and Technology of Japan and from Japan Health Science Foundation, and by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Yokoyama S. 2006. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26: 20–27. [DOI] [PubMed] [Google Scholar]
- 2.Hara H., Yokoyama S. 1991. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J. Biol. Chem. 266: 3080–3086. [PubMed] [Google Scholar]
- 3.Francis G. A., Knopp R. H., Oram J. F. 1995. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J. Clin. Invest. 96: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remaley A. T., Schumacher U. K., Stonik J. A., Farsi B. D., Nazih H., Brewer H. B., Jr 1997. Decreased reverse cholesterol transport from Tangier disease fibroblasts. Acceptor specificity and effect of brefeldin on lipid efflux. Arterioscler. Thromb. Vasc. Biol. 17: 1813–1821. [DOI] [PubMed] [Google Scholar]
- 5.Bodzioch M., Orso E., Klucken J., Langmann T., Bottcher A., Diederich W., Drobnik W., Barlage S., Buchler C., Porsch-Ozcurumez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351. [DOI] [PubMed] [Google Scholar]
- 6.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 7.Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denefle P., et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355. [DOI] [PubMed] [Google Scholar]
- 8.Arakawa R., Yokoyama S. 2002. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 277: 22426–22429. [DOI] [PubMed] [Google Scholar]
- 9.Wang N., Chen W., Linsel-Nitschke P., Martinez L. O., Agerholm-Larsen B., Silver D. L., Tall A. R. 2003. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J. Clin. Invest. 111: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arakawa R., Hayashi M., Remaley A. T., Brewer B. H., Jr., Yamauchi Y., Yokoyama S. 2004. Phosphorylation and stabilization of ATP binding cassette transporter A1 by synthetic amphiphilic helical peptides. J. Biol. Chem. 279: 6217–6220. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Oram J. F. 2002. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J. Biol. Chem. 277: 5692–5697. [DOI] [PubMed] [Google Scholar]
- 12.Feng B., Tabas I. 2002. ABCA1-mediated cholesterol efflux is defective in free cholesterol-loaded macrophages. Mechanism involves enhanced ABCA1 degradation in a process requiring full NPC1 activity. J. Biol. Chem. 277: 43271–43280. [DOI] [PubMed] [Google Scholar]
- 13.Lu R., Arakawa R., Ito-Osumi C., Iwamoto N., Yokoyama S. 2008. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler. Thromb. Vasc. Biol. 28: 1820–1824. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama S., Yamamoto A., Kurasawa T. 1988. A little more information about aggravation of probucol-induced HDL-reduction by clofibrate. Atherosclerosis. 70: 179–181. [DOI] [PubMed] [Google Scholar]
- 15.Tsujita M., Yokoyama S. 1996. Selective inhibition of free apolipoprotein-mediated cellular lipid efflux by probucol. Biochemistry. 35: 13011–13020. [DOI] [PubMed] [Google Scholar]
- 16.Tsujita M., Tomimoto S., Okumura-Noji K., Okazaki M., Yokoyama S. 2000. Apolipoprotein-mediated cellular cholesterol/phospholipid efflux and plasma high density lipoprotein level in mice. Biochim. Biophys. Acta. 1485: 199–213. [DOI] [PubMed] [Google Scholar]
- 17.Wu C. A., Tsujita M., Hayashi M., Yokoyama S. 2004. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J. Biol. Chem. 279: 30168–30174. [DOI] [PubMed] [Google Scholar]
- 18.Barnhart R. L., Busch S. J., Jackson R. L. 1989. Concentration-dependent antioxidant activity of probucol in low density lipoproteins in vitro: probucol degradation precedes lipoprotein oxidation. J. Lipid Res. 30: 1703–1710. [PubMed] [Google Scholar]
- 19.Kharasch M. S., Joshi B. S. 1957. Reactions of hindered phenols. II. Base-catalyzed oxidations of hindered phenols. J. Org. Chem. 22: 1439–1443. [Google Scholar]
- 20.Yokoyama S., Tajima S., Yamamoto A. 1982. The process of dissolving apolipoprotein A-I in an aqueous buffer. J. Biochem. 91: 1267–1272. [DOI] [PubMed] [Google Scholar]
- 21.Abe-Dohmae S., Suzuki S., Wada Y., Aburatani H., Vance D. E., Yokoyama S. 2000. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 39: 11092–11099. [DOI] [PubMed] [Google Scholar]
- 22.Abe-Dohmae S., Ikeda Y., Matsuo M., Hayashi M., Okuhira K., Ueda K., Yokoyama S. 2004. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 279: 604–611. [DOI] [PubMed] [Google Scholar]
- 23.Usui S., Hara Y., Hosaki S., Okazaki M. 2002. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 43: 805–814. [PubMed] [Google Scholar]
- 24.Vassalle C., Petrozzi L., Botto N., Andreassi M. G., Zucchelli G. C. 2004. Oxidative stress and its association with coronary artery disease and different atherogenic risk factors. J. Intern. Med. 256: 308–315. [DOI] [PubMed] [Google Scholar]
- 25.Sasai K., Okumura-Noji K., Hibino T., Ikeuchi R., Sakuma N., Fujinami T., Yokoyama S. 1998. Human cholesteryl ester transfer protein measured by enzyme-linked immunosorbent assay with two monoclonal antibodies against rabbit cholesteryl ester transfer protein: plasma cholesteryl ester transfer protein and lipoproteins among Japanese hypercholesterolemic patients. Clin. Chem. 44: 1466–1473. [PubMed] [Google Scholar]
- 26.Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. 1987. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA. 84: 5928–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carew T. E., Schwenke D. C., Steinberg D. 1987. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc. Natl. Acad. Sci. USA. 84: 7725–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favari E., Zanotti I., Zimetti F., Ronda N., Bernini F., Rothblat G. H. 2004. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler. Thromb. Vasc. Biol. 24: 2345–2350. [DOI] [PubMed] [Google Scholar]
- 29.Tomimoto S., Tsujita M., Okazaki M., Usui S., Tada T., Fukutomi T., Ito S., Itoh M, and Y. S 2001. Effect of probucol in lecithin-cholesterol acyltransferase-deficient mice: inhibition of 2 independent cellular cholesterol-releasing pathways in vivo. Arterioscler. Thromb. Vasc. Biol. 21: 394–400. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto A., Matsuzawa Y., Yokoyama S., Funahashi T., Yamamura T., Kishino B. 1986. Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am. J. Cardiol. 57: 29H–35H. [DOI] [PubMed] [Google Scholar]
- 31.Braun A., Zhang S., Miettinen H. E., Ebrahim S., Holm T. M., Vasile E., Post M. J., Yoerger D. M., Picard M. H., Krieger J. L., et al. 2003. Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor SR-BI/apolipoprotein E double knockout mouse. Proc. Natl. Acad. Sci. USA. 100: 7283–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPherson R., Hogue M., Milne R. W., Tall A. R., Marcel Y. L. 1991. Increase in plasma cholesteryl ester transfer protein during probucol treatment. Relation to changes in high density lipoprotein composition. Arterioscler. Thromb. 11: 476–481. [DOI] [PubMed] [Google Scholar]
- 33.Rinninger F., Wang N., Ramakrishnan R., Jiang X. C., Tall A. R. 1999. Probucol enhances selective uptake of HDL-associated cholesteryl esters in vitro by a scavenger receptor B-I-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 19: 1325–1332. [DOI] [PubMed] [Google Scholar]
- 34.Quinet E. M., Agellon L. B., Kroon P. A., Marcel Y. L., Lee Y. C., Whitlock M. E., Tall A. R. 1990. Atherogenic diet increases cholesteryl ester transfer protein messenger RNA levels in rabbit liver. J. Clin. Invest. 85: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.