Abstract
We evaluated the role of sterol-regulatory element binding protein (SREBP)-1c/peroxisome proliferator activated receptor-γ (PPARγ) pathway on heart lipotoxicity in patients with metabolic syndrome (MS) and aortic stenosis (AS). Echocardiographic parameters of heart function and structural alterations of LV specimens were studied in patients with (n = 56) and without (n = 61) MS undergoing aortic valve replacement. Tissues were stained with hematoxylin-eosin (H and E) and oil red O for evidence of intramyocyte lipid accumulation. The specimens were also analyzed with PCR, Western blot, and immunohistochemical analysis for SREBP-1c and PPARγ. Ejection fraction (EF) was lower in MS compared with patients without MS (P < 0.001); no difference was found in aortic orifice surface among the groups. H and E and oil red O staining of specimens from MS patients revealed several myocytes with intracellular accumulation of lipid, whereas these alterations were not detected in biopsies from patients without MS. Patients without MS have low levels and weak immunostaining of SREBP-1c and PPARγ in heart specimens. In contrast, strong immunostaining and higher levels of SREBP-1c and PPARγ were seen in biopsies from the MS patients. Moreover, we evidenced a significative correlation between both SREBP-1c and PPARγ and EF and intramyocyte lipid accumulation (P < 0.001). SREBP-1c may contribute to heart dysfunction by promoting lipid accumulation within myocytes in MS patients with AS; SREBP-1c may do it by increasing the levels of PPARγ protein.
Keywords: aortic stenosis, heart function, surgery
Metabolic syndrome (MS) is strongly associated with left ventricular (LV) hypertrophy and cardiac function derangements that lead to heart failure (HF) (1). The structural basis of the progression from well-compensated hypertrophy to HF is still largely unknown in MS patients. Emerging evidence suggests that inherited and acquired cardiomyopathies, such as impaired glucose tolerance and diabetes, are associated with marked intracellular lipid accumulation in the heart (2, 3). In the normal body, most triglyceride is stored in adipocytes; the amount of triglyceride stored in nonadipocyte tissues (liver, and myocardium) is minimal and very tightly regulated. However, several-fold increased cardiomyocyte triglyceride stores are observed in animal models of obesity and diabetes (4).This lipid accumulation may contribute to cardiomyocyte death by nonoxidative and oxidative (5) metabolic pathways and to HF. Even in humans, myocardial lipid content was recently reported to increase with the degree of adiposity and contribute to cardiac dysfunction (6), suggesting that myocardial lipid content may be a biomarker and putative therapeutic target for cardiac disease in patients with MS.
Genes involved in lipid metabolism are nutritionally regulated at the transcriptional level in a coordinated fashion (7). Sterol-regulatory element binding protein (SREBP)-1c is a transcription factor that controls lipogenesis and is induced during overnutrition to facilitate the conversion of glucose to fatty acids and triglycerides for the storage of excess energy (8). Uncontrolled activation of nuclear SREBP-1c in the liver can cause hepatosteatosis and multiple biochemical features of the MS (9). Moreover, it has been proposed that peroxisome proliferator activated receptor-γ (PPARγ) itself is a direct target gene of SREBP-1c, providing a mechanisms by which SREBP-1c and PPARγ could cooperate to enhance lipogenesis (10). Thus, it is conceivable that the SREBP-1c/PPARγ pathway deregulation might be important in the pathogenesis of lipotoxic cardiomyopathy. We hypothesized that an increase in cardiac levels of both SREBP-1c and PPARγ is involved in the adaptations of the heart to myocardial metabolic derangements and that it is potentially an important stimulus of heart adiposity and HF in MS patients. To investigate this possibility, we examined myocyte lipid accumulation and cardiac levels of SREBP-1c and PPARγ protein and mRNA in patients with and without MS who underwent surgical aortic valve replacement for aortic stenosis (AS). This is a classic model of pressure-induced concentric remodelling in humans (11).
METHODS
Patients
We selected 282 consecutive patients who underwent aortic valve replacement for isolated AS (area≤0.715 cm2) (Table 1). The WHO criteria were used to classify patients as being with or without the MS (12): waist circumference (WC) >102 cm for men, >88 cm for women; blood pressure elevation >130/85 mm Hg; low HDL cholesterol <40 mg/dl in men, <50 mg/dl in women; high triglycerides >150 mg/dl; hyperglycemia, fasting glucose >100 mg/dl. The MS is considered present when at least three of the five traits are present. Among the above patients, 56 presented at least three traits of MS (4 ± 0.8) and 61 without MS (MS-traits: 1.1 ± 0.6). The remaining 165 patients were excluded because of the presence of at least one of the following conditions: diabetes, unstable angina, previous myocardial infarction, coronary stenosis >70%; renal, hepatic, rheumatic, cancerous, or other severe diseases were excluded. Insulin sensitivity was estimated from the homeostasis model assessment (HOMA) [(glucose in mmol/L × insulin in µU/ml)/22.5] (13). All patients underwent coronary angiography before valve replacement. On the basis of ejection fraction (EF) determined by echocardiography at the time of admission, MS patients were subdivided into three groups: EF >50% (n = 18); EF 50% to 30% (n = 24); EF <30% (n = 14). The institutional Ethics Committee of the Sassari Hospital approved the study; all patients gave informed consent.
TABLE 1.
Characteristics of the patients undergoing aortic valve replacement surgery according to the presence or absence of metabolic syndrome
| Variables | No MS (n = 61) | MS (n = 56) | P |
|---|---|---|---|
| Age, y | 70.3 ± 7 | 70.6 ± 6 | NS |
| Male gender, n (%) | 44 (72) | 38 (69) | NS |
| Body mass index, kg/m2 | 25.6 ± 5 | 29.3 ± 4 | <0.001 |
| Waist circumference, cm | 81.1 ± 3.2 | 93.2 ± 4.1 | <0.001 |
| Waist circumference women, cm | 80.4 ± 2.8 | 87.7 ± 1.9 | <0.001 |
| Waist circumference men, cm | 82.3 ± 3.9 | 95.8 ± 5.4 | <0.001 |
| Risk factors | |||
| Hypertension, n (%) | 22 (36) | 31 (55) | <0.01 |
| Obesity, n (%) | 10 (16) | 29 (52) | <0.001 |
| Hypercholesterolemia, n (%) | 29 (47) | 26 (46) | NS |
| Smokers, n (%) | 11 (18) | 9 (16) | NS |
| Laboratory | |||
| Glycemia, mg/dl | 89.2 ± 5.4 | 113.4 ± 7.2 | <0.001 |
| Triglycerides, mg/dl | 109.4 ± 47 | 187.6 ± 41 | <0.001 |
| HDL cholesterol, mg/dl | 52.3 ± 9.1 | 39.3 ± 8.9 | <0.01 |
| HDL cholesterol women, mg/dl | 53.9 ± 6.4 | 40.4 ± 9.1 | <0.01 |
| HDL cholesterol men, mg/dl | 51.7 ± 6.9 | 38.8 ± 8.9 | <0.01 |
| Total cholesterol, mg/dl | 187.5 ± 26 | 192.9 ± 24 | NS |
| LDL cholesterol, mg/dl | 105.8 ± 19.4 | 106.4 ± 18.3 | NS |
| Insulin, µU/L | 7.9 ± 3.2 | 17 ± 4.8 | <0.001 |
| HOMA score | 1.7 ± 0.4 | 4.2 ± 0.5 | <0.001 |
| Coronary artery disease < 70% | 30 (49) | 29 (52) | NS |
| Left main coronary stenosis, n (%) | 3 (5) | 5 (9) | NS |
| Stenosis of LAD, n (%) | 12 (19) | 11 (20) | NS |
| Stenosis of LCA, n (%) | 15 (25) | 13 (24) | NS |
| Active therapy | |||
| ACE inhibitors, n (%) | 6 (9) | 5 (9) | NS |
| AT2 receptor antagonists, n (%) | 3 (5) | 2 (4) | NS |
| Diuretics, n (%) | 33 (54) | 29 (52) | NS |
| β-Blockers, n (%) | 44 (72) | 40 (71) | NS |
| Clonidine, n (%) | 12 (19) | 11 (20) | NS |
| Statins, n (%) | 27 (44) | 25 (45) | NS |
| Echocardiographic parameters | |||
| Aortic valve area, cm2 | 0.71 ± 0.05 | 0.72 ± 0.06 | NS |
| Mean aortic valve gradient, mmHg | 57.3 ± 4 | 58.6 ± 3 | NS |
| LV mass index, g/m2 | 118.2 ± 11.9 | 123.7 ± 12.6 | NS |
| LV septum, mm | 16.3 ± 3 | 15.4 ± 2.2 | NS |
| LVEDD, mm | 49.5 ± 10 | 46.7 ± 6 | NS |
| LVESD, mm | 29.8 ± 5 | 31.5 ± 6 | NS |
| Cardiac output, L* min−1 | 4.3 ± 1.8 | 3.9 ± 2.2 | NS |
| Ejection fraction, % | 53.1 ± 9.8 | 41.2 ± 9.9 | <0.001 |
| Myocardial performance index | 0.42 ± 0.09 | 0.51 ± 0.06 | <0.001 |
| scFS, % | 118 ± 14 | 107 ± 12 | <0.001 |
| scMWS, % | 102 ± 16 | 94 ± 18 | <0.05 |
| esWS, dyne/cm2 | 115 ± 24 | 103 ± 21 | <0.005 |
| Heart specimen analysis | |||
| Myocyte, No/mm2 | 249 ± 32 | 259 ± 26 | NS |
| Vacuolated myocytes, % | 0 | 18.4 ± 6.1 | <0.001 |
| Red oil staining positive myocytes, % | 0 | 15.1 ± 2.6 | <0.001 |
| Triacylglycerol, µg/mg | 20.7 ± 8 | 69.1 ± 11 | <0.001 |
| SREBP-1c positive myocytes, % | 1.5 ± 0.9 | 30.1 ± 6.3 | <0.001 |
| PPARγ positive myocytes, % | 0.9 ± 0.3 | 18.7 ± 3.7 | <0.001 |
| PPARα positive myocytes, % | 25.4 ± 12.3 | 26.7 ± 14.1 | NS |
| PPARα protein, arbitrary units | 51.3 ± 18.4 | 53.6 ± 21.3 | NS |
| SCD-1, arbitrary units | 4.3 ± 2.9 | 4.8 ± 3.1 | NS |
| SERCA2a, arbitrary units | 88.4 ± 11. | 60.8 ± 9.6 | <0.001 |
Data are presented as mean ± SD or n (%). MS = metabolic syndrome; HDL = high-density lipoprotein; LDL = low-density lipoprotein; HOMA = homeostasis model assessment; LDL = low-density lipoprotein. LAD = left anterior descending artery; LCA = left circumflex artery; LVEDD = left ventricular end-diastolic diamenter; LVESD = left ventricular end-systolic diameter; scFS = stress-corrected mean velocity of fiber shortening; scMWS = stress- corrected midwall fractional shortening; esWS = end-systolic wall stress; SREBP-1c = sterol regulatory element-binding protein-1c; PPAR-γ = perixome proliferator-activated receptor-γ; PPAR-α= perixome proliferator-activated receptor-α; SCD-1 = stearoyl-CoA desaturase 1; SERCA2a = sarco-endoplasmic reticulum ATPase 2a.
Echocardiography
Each patient underwent an M-mode and Doppler-echocardiographic study (ATL, hdi-5000-Philips, Milan, Italy). Measurements were made according to the recommendations of the American Society of Echocardiography (14). Aortic valve annulus was measured at end-diastole in 2-dimensional parasternal long-axis. Effective aortic valve area was calculated using the continuity equation with the time-velocity integral ratio and indexed for body surface area. The LV cardiac output was calculated as the product of heart rate and stroke volume and was indexed for body surface area. The myocardial performance index (MPI), which measures both systolic and diastolic parameters of ventricular function, was calculated as previously described (15). The echocardiograms were interpreted blindly, without knowledge of MS presence/absence, by the operator.
Tissue sampling
During open-heart surgery, myectomy samples weighing ∼10 to 30 mg were removed from the LV septum. Half of each biopsy was immediately frozen in liquid nitrogen and stored at −80°C, whereas the other half was mildly fixed in 1% paraformaldehyde, embedded in paraffin sectioned to a thickness of 5µm, and mounted on slides.
Measurement of RNA
Each sample was analyzed for the presence of transcripts encoding SREBP-1c, PPARγ, and adipose most-abundant gene transcript 1 (apM1). Levels of SREBP-1c and PPARγ were measured by RT-PCR amplification by using the following sense and antisense primer sequences: SREBP-1c Up 5′ GCGCAAGACAGCAGATTTATTC 3′, SREBP-1c Low: 3′ TAGATGCGGAGAAGCTGCCTA 3′; PPARγ Up 5′TCCAACTCCCTCATGGCAATTG 3′; PPARγ Low 5′ ATGAGACATCCCCACTGCAAG 3′; apM1 Up 5′GCTGGGAGCTGTTCTACTGC-3′, apM1 Low 5′ GTAAAGCGAATGGGCATGTT 3′. Appropriate regions of the hypoxanthine-phosporybosil transferase (HPRT) cDNA were amplified as control. HPRT Up: 5′ CCTGCTGGATTACATTAAAGCACTG 3′, HPRT Low: 5′ CCTCGTGGGGTCCTTTTCACCAGC 3′. (Roche Diagnostics, Italy). Each RT-PCR experiment was repeated at least three times.
Western blot analysis.
SREBP-1c, PPARγ, PPARα, stearoyl-CoA desaturase 1 (SCD-1), and sarco-endoplasmic reticulum ATPase (SERCA2a), proteins were determined by Western blotting analysis as previously described (16). The samples incubated in a buffer containing the specific antibody against SREBP-1c (sc-367; 1:100 dilution; Santa Cruz Biotechnology, Inc.), PPARγ (sc-6284; 1:100 dilution; Santa Cruz Biotechnology, Inc.), anti-PPARα (sc-9000; 1:100 dilution; Santa Cruz Biotechnology, Inc.), anti- SCD-1 (sc-14719; 1:100 dilution; Santa Cruz Biotechnology, Inc.), and anti-SERCA2a (sc-8095; 1:100 dilution; Santa Cruz Biotechnology, Inc.). The densitometric measurements were performed using the gel image system Fluor-S equipped with the analysis software Quantity One (Bio-Rad, Rome, Italy).
Immunohistochemical staining.
Immunohistochemical analyses were performed as previously described (16). Tissues were stained with hematoxylin and eosin (H and E). The sections of heart tissue were incubated with anti-SREBP-1c (sc-367; 1:100 dilution; Santa Cruz Biotechnology, Inc.), monoclonal anti-PPARγ (sc-6284; 1:100 dilution; Santa Cruz Biotechnology, Inc.), monoclonal anti-PPARα (sc-9000 1:100 dilution; Santa Cruz Biotechnology, Inc.), and anti-myoglobin (sc-65982; 1:100 diluition; Santa Cruz Biotechnology, Inc.). Oxidative stress was determined by measuring thiobarbituric acid-reactive substances (TBARS) such as malondialdehyde (MDA) (16) and by the appearance of nitrotyrosine, a biomarker for nitrosative stress. Tyrosine nitration, an index of the nitrosylation of proteins by peroxynitrite and/or reactive oxygen species (ROS), was determined by immunohistochemistry as previously described (17). Sections were incubated overnight with anti-nitrotyrosine rabbit polyclonal antibody (1:500 in PBS, v/v). The sections were then scored for intensity of immunostaining (0 = absent, 1 = faint, 2 = moderate, and 3 = intense) for each antibody and the average value was calculated for each section. A portion of each specimen was also snap-frozen, and sections were stained with oil red O. For MDA, a portion of frozen heart tissue was ground in liquid nitrogen and homogenized. After cooling, 1:2 adduct of MDA and TBA was extracted into 4 ml of n-butanol and the absorbance was measured at 540 nm against 1,1,3,3-tetramethoxypropane used as the standard. Numbers of vacuolated myocytes, oil red O, SREBP-1c, PPARα, and PPARγ-positive cells were obtained from the entire section. Section size and number of myocytes per mm2 were determined and percentages of positive myocytes were calculated.
Triacylglycerol measurements in heart tissues.
A solution (500 µl) of 2 mM NaCl/20 mM EDTA/50 mM sodium phosphate buffer, pH 7.4, was added to myectomy samples removed from the LV septum. Then, 10 µl of homogenate was mixed with 10 µl of tert-butyl alcohol and 5 µl of Triton X-100/methyl alcohol mixture (1:1 v/v) for the extraction of lipids. Triacylglycerol was measured with a Sigma diagnostic kit.
Statistics
Data are presented as mean ± SD. Continuous variables were compared among the groups with one-way ANOVA for normally distributed data and the Kruskal-Wallis test for nonnormally distributed data. When differences were found among the groups, the Bonferroni correction was used to make pairwise comparisons. A p value <0.05 was considered statistically significant. Pearson r was used for bivariate correlation analysis. All calculations were performed by SPSS-12 (SPPS, Inc.).
RESULTS
Characteristics of the study participants are reported in Table 1. MS patients did not differ from controls in terms of age, smoking, total and low-density lipoprotein cholesterol concentration, coronary artery disease, and active therapy. The MS patients had a higher prevalence of hypertension and obesity compared with patients without MS. By selection, significant differences were found for the components of the MS: body mass index (BMI), WC, fasting insulin, HOMA values, glucose, triglyceride, and HDL cholesterol levels. No differences were found in the anthropometric parameters, risks factors, laboratory analysis, and active therapy among the groups of MS patients subdivided on the basis of EF (EF >50%; EF 50% to 30%; EF <30%) (Table 2).
TABLE 2.
Characteristics of the patients with metabolic syndrome undergoing aortic valve replacement surgery according to the ejection fraction values
| Variables | Ejection Fraction >50% (n = 18) | Ejection Fraction 30%-50% (n = 24) | Ejection Fraction <30% (n = 14) |
|---|---|---|---|
| Age, y | 70.6 ± 5.3 | 70.5 ± 5.2 | 70.6 ± 6.2 |
| Laboratory | |||
| Glycemia, mg/dl | 109.1 ± 5.3 | 112.9 ± 7.3 | 118.2 ± 7.1* |
| Triglycerides, mg/dl | 179.1 ± 45 | 185 ± 34.8 | 192 ± 49.7 |
| Total cholesterol, mg/dl | 190.7 ± 15.4 | 191.1 ± 22.1 | 192.2 ± 23.6 |
| HDL cholesterol, mg/dl | 41.5 ± 9.4 | 40.3 ± 8.1 | 40.2 ± 9.9 |
| LDL cholesterol, mg/dl | 104.4 ± 13.3 | 103.1 ± 14.3 | 109.4 ± 28.4 |
| Insulin, µU/L | 15.4 ± 4.4 | 16.3 ± 4.7 | 18.4 ± 5.5 |
| HOMA score | 4.2 ± 0.4 | 4.2 ± 0.5 | 4.4 ± 0.5 |
| Echocardiographic parameters | |||
| Aortic valve area, cm2 | 0.73 ± 0.06 | 0.71 ± 0.05 | 0.71 ± 0.06 |
| Mean aortic valve gradient, mmHg | 59.1 ± 3.1 | 58.6 ± 2.9 | 58.1 ± 3.8 |
| LV mass index, g/m2 | 123.3 ± 8.6 | 123.75 ± 13.6 | 124.2 ± 15.6 |
| LV septum, mm | 15.1 ± 2.2 | 15.4 ± 2.3 | 15.9 ± 2.1 |
| Ejection fraction, % | 52.1 ± 1.1 | 40.7 ± 5.7* | 27.7 ± 1.0*+ |
| LVEDD, mm | 47.7 ± 7.7 | 46.8 ± 6.1 | 45.4 ± 8.5 |
| LVESD, mm | 29.2 ± 7 | 32.1 ± 5 | 33.4 ± 4 |
| Myocardial performance index | 0.46 ± 0.05 | 0.51 ± 0.06* | 0.56 ± 0.06*+ |
| Histological analysis | |||
| Myocyte, No/mm2 | 258.7 ± 23.7 | 258.9 ± 27.7 | 259.5 ± 29.7 |
| Vacuolated myocytes, % | 13.8 ± 4.2 | 17.6 ± 4.3* | 25.4 ± 4.3*† |
| Red oil staining positive myocytes, % | 13.0 ± 1.4 | 15.2 ± 2.5* | 17.2 ± 2.8*† |
| SREBP-1c positive myocytes, % | 25.6 ± 5.1 | 30.4 ± 6.0* | 35.3 ± 6.3*† |
| PPARγ positive myocytes, % | 16.0 ± 3.3 | 18.7 ± 3.3* | 22.1 ± 2.8*† |
Data are presented as mean ± SD or n (%). *P < 0.05 compared with group with ejection fraction >50%. †P < 0.05 compared with ejection fraction between 30%-50%.
Hemodynamic status
All patients showed characteristics of LV hypertrophy (Table 1). LV systolic function (EF, MPI) was impaired in MS patients: MS patients had lower EF and higher MPI (P < 0.01; Table 1). No difference in the aortic orifice surface among the groups was found (Table 1). Moreover, MS patients had EF not correlated with aortic orifice surface (R = 0.123, P = 0.154), whereas a significant correlation between aortic orifice surface and EF (R = 0.555, P < 0.001) in patients without MS was found.
Lipid accumulation in cardiomyocytes
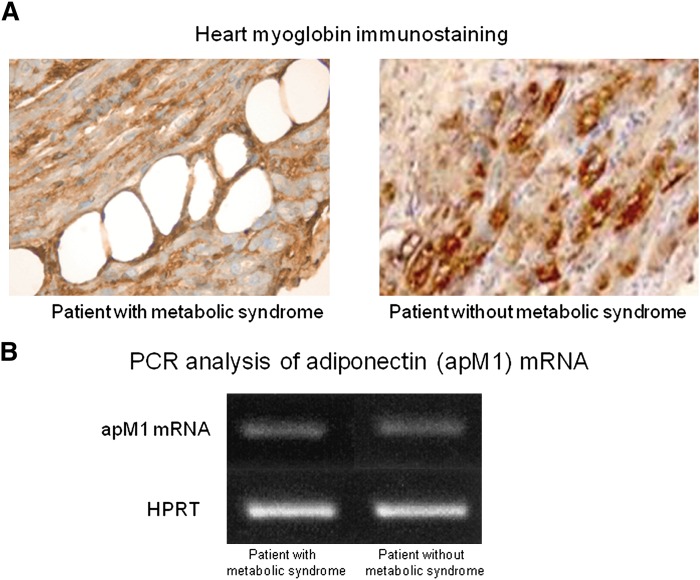
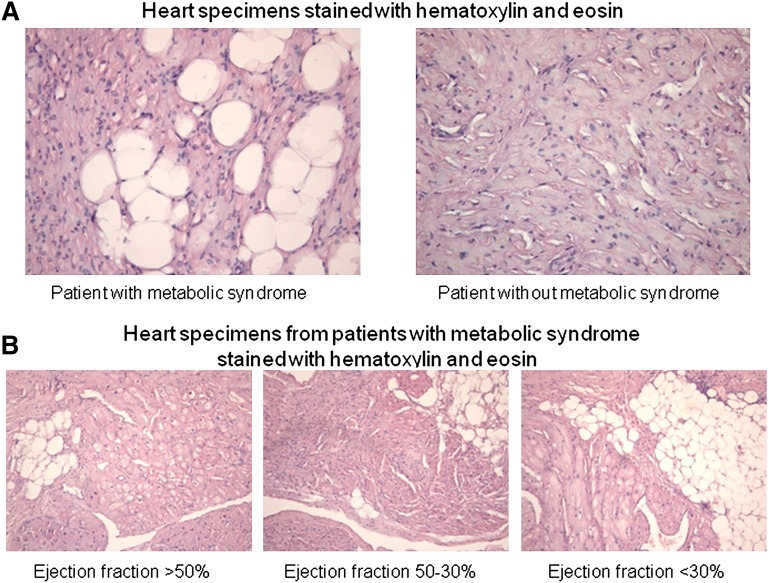
We did not observe adipocytes in ventricular sections. This morphological observation was supported by RT-PCR of an adipocytes-specific gene transcript (apM1) that encodes adiponectin: the apM1 transcript was almost undetectable in ventricular biopsies from all patients (Fig. 1). Moreover, the positive myoglobin staining of vacuolated cells indicates lipid accumulation in cardiomyocytes (figure, supplementary Appendix). However, H and E staining of heart specimens from MS patients revealed vacuolated cells consistent with intracellular accumulation of lipid (Fig. 2). Oil red O staining showed high lipid deposition in myocytes of MS patients (Table 1). We detected vacuolated myocytes and droplets of lipid in 53 of 56 specimens from MS patients, whereas the same myocardial alterations were not detected in biopsies from patients without MS. Moreover, triacylglycerol content was significantly higher in specimens from MS patients than in specimens of patients without MS (Table 1). Moreover, we observed that vacuolated myocytes and myocytes with lipid droplets increased with depressed EF (Fig. 2, Table 2). A positive correlation between WC and percentage of the vacuolated myocytes was observed (R = 0.315, P < 0.01). Multiple linear regression analysis revealed that WC and serum triglycerides were both independent determinants of myocardial lipid content (R = 0.324, P < 0.01; R = 0.312, P < 0.01), whereas age, BMI, and HOMA were unrelated to myocardial triglyceride content.
Fig. 1.
Representative immunohistochemical analysis of myoglobin protein from ventricular biopsy specimens (×600). The specimens from both patients with and without metabolic syndrome show a strong immunostaining for myoglobin protein in hypertrophied myocytes (A). Representative PCR analysis of apM1 mRNA content in heart specimens from patients without metabolic syndrome: apM1 transcripts were almost undetectable in heart specimens of patients with and without metabolic syndrome (B).
Fig. 2.
Vacuolated myocytes in representative ventricular-biopsy specimens hematoxylin and eosin. The specimen from a patient without metabolic syndrome shows hypertrophied myocytes without vacuoles (×600). The specimens from a patient with metabolic syndrome shows a high number of vacuolated myocytes (×600) (A). Specimens from patients with metabolic syndrome show a progressive increase in vacuolated myocytes according to the ejection fraction (×400) (B).
Molecular analysis of ventricular specimens
SREBP1-c/ PPARγ pathways.
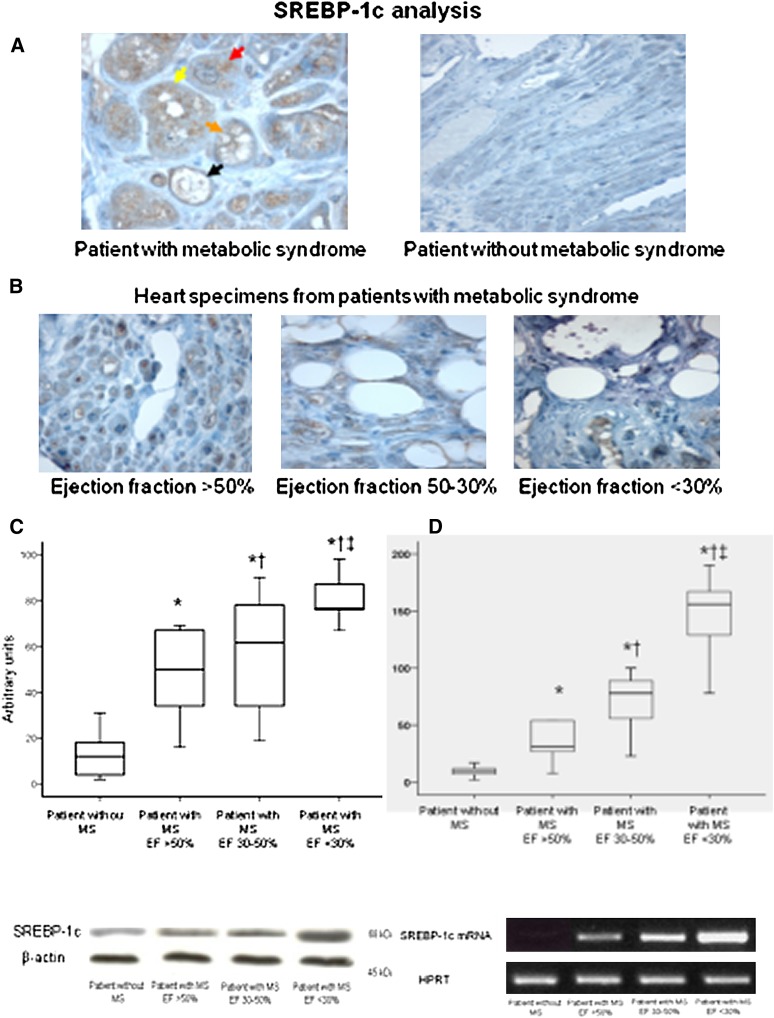
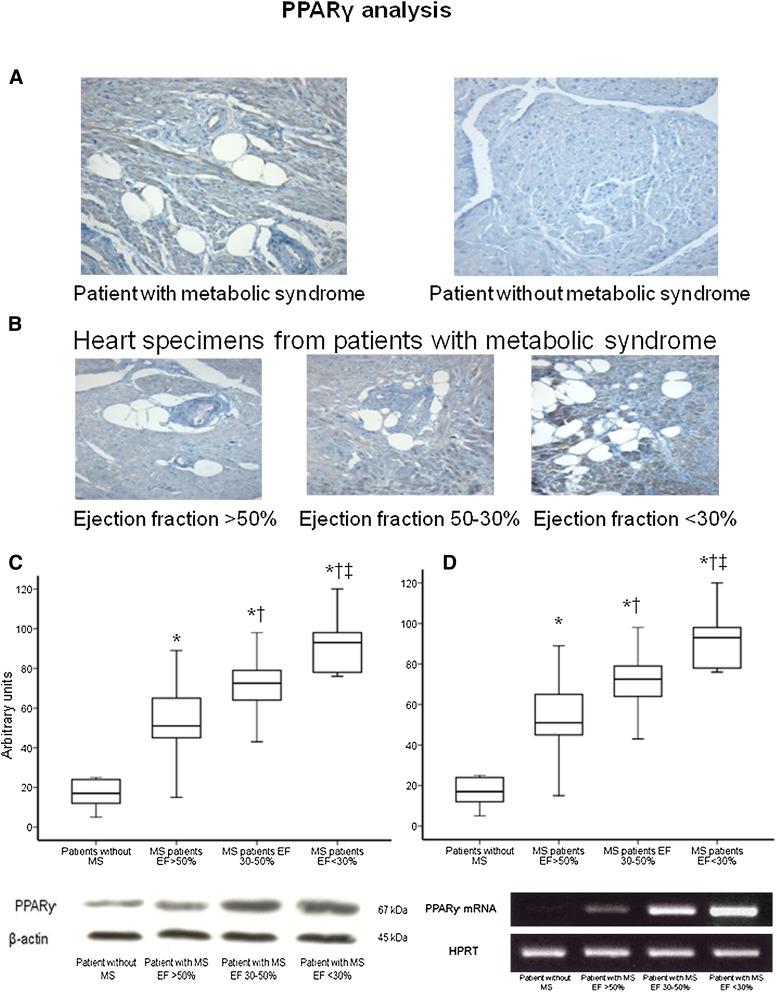
Figures 3 and 4 show the results of the analysis of SREBP-1c/ PPARγ proteins and mRNA levels from ventricular specimens from the groups of patients. In patients without MS, cardiac SREBP-1c/ PPARγ proteins and mRNAs were not or were weakly detected (Figs. 3, 4). In contrast, higher levels of SREBP-1c/ PPARγ proteins and mRNAs were seen in biopsy from the MS patients as well as strong immunostaining for SREBP-1c/ PPARγ (Figs. 3, 4). There was a significant correlation between PPARγ and SREBP-1c protein levels (R = 0.598, P < 0.001). The WC and plasma triglycerides were significantly correlated with SREBP-1c protein levels (R = 0.512, P < 0.001; R = 0.565, P < 0.001). Multiple linear regression analysis revealed that serum triglycerides and WC were both independent determinants of myocardial SREBP-1c expressions (R = 0.323, P < 0.01; R = 0.339, P < 0.01) as well as PPARγ expressions (R = 0.310, P < 0.01; R = 0.329, P < 0.01), whereas age, BMI, and HOMA were unrelated to SREBP-1c/ PPARγ expressions. Moreover, there was a significant negative correlation between SREBP-1c/ PPARγ protein levels and EF (R = −0.618, P < 0.001; R = −0.556, P < 0.001, respectively), in heart specimens from patients with MS (Figs. 3, 4, Table 2). No correlation was observed between SREBP-1c/ PPARγ expressions and EF in patients without MS (R = 0.198, P = 0.261; R = 0.121, P = 0.116, respectively).
Fig. 3.
Representative immunohistochemical analysis of SREBP-1c protein from ventricular biopsy specimens (×600). The specimen from a patient without metabolic syndrome shows a weak immunostaining for SREBP-1c protein in hypertrophied myocytes. The specimen from a patient with metabolic syndrome shows a strong immunostaining in hypertrophied myocytes as well as in vacuolated myocytes in which the staining is localized around the vacuoles. In addition, we observe several stages representative for the different progression from intense staining to completely vacuolated myocyte in the same specimen: in the section analyzed the specimen shows a hypertrophied myocyte with a strong immunostaining without vacuoles (red arrow); yellow arrow evidences a few vacuoles in the myocyte with a strong immunostaining; orange arrow indicates numerous vacuoles in the myocytes; at this stage the vacuoles are surrounded by intense staining in the cytoplasm; finally, at this stage, probably, the vacuoles flow together in a large vacuole that occupies the cytoplasm completely (black arrow) (A). Specimens from patients with metabolic syndrome show a progressive increase in both vacuolated myocytes and immureactivity for SREBP-1c protein according to the ejection fraction (×400) (B). Western blot analysis of SREBP-1c protein contents in heart specimens from patients with metabolic syndrome according to the ejection fraction. ‡ P < 0.05 versus patients with ejection fraction between 30% and 50%. † P < 0.05 versus patients with ejection fraction >50%. * P < 0.05 versus patients without metabolic syndrome. Representative western blot analysis of SREBP-1c protein content in heart specimens from patients with metabolic syndrome according to the ejection fraction. C: Box plot showing SREBP-1 mRNA levels in ventricular biopsies from patients without metabolic syndrome and from patients with metabolic syndrome according to ejection fraction. The mRNA levels were measured with RT-PCR. Representative PCR analysis of SREBP-1c mRNA content in heart specimens from patients without metabolic syndrome and from patients with metabolic syndrome according to the ejection fraction (D).
Fig. 4.
Representative immunohistochemical analysis of PPARγ protein from ventricular biopsy specimens (×400). The specimen from a patient without metabolic syndrome did not show immunostaining for PPARγ protein in hypertrophied myocytes. The specimen from a patient with metabolic syndrome shows a strong immunostaining for PPARγ protein in hypertrophied myocytes as well as in vacuolated myocytes in which the staining is localized around the vacuoles (A). Specimens from patients with metabolic syndrome show a progressive increase in both vacuolated myocytes and immureactivity for PPARγ protein according to the ejection fraction (×400) (B). Western blot analysis of PPARγ protein contents in heart specimens from patients with metabolic syndrome according to the ejection fraction. ‡ P < 0.05 versus patients with ejection fraction between 30% and 50%. † P < 0.05 versus patients with ejection fraction >50%. * P < 0.05 versus patients without metabolic syndrome. Representative western blot analysis of PPARγ protein content in heart specimens from patients with metabolic syndrome according to the ejection fraction (C). Box plot showing PPARγ mRNA levels in ventricular biopsies from patients without metabolic syndrome and from patients with metabolic syndrome according to ejection fraction. The mRNA levels were measured with RT-PCR. Representative PCR analysis of PPARγ mRNA content in heart specimens from patients without metabolic syndrome and from patients with metabolic syndrome according to the ejection fraction (D).
PPARα.
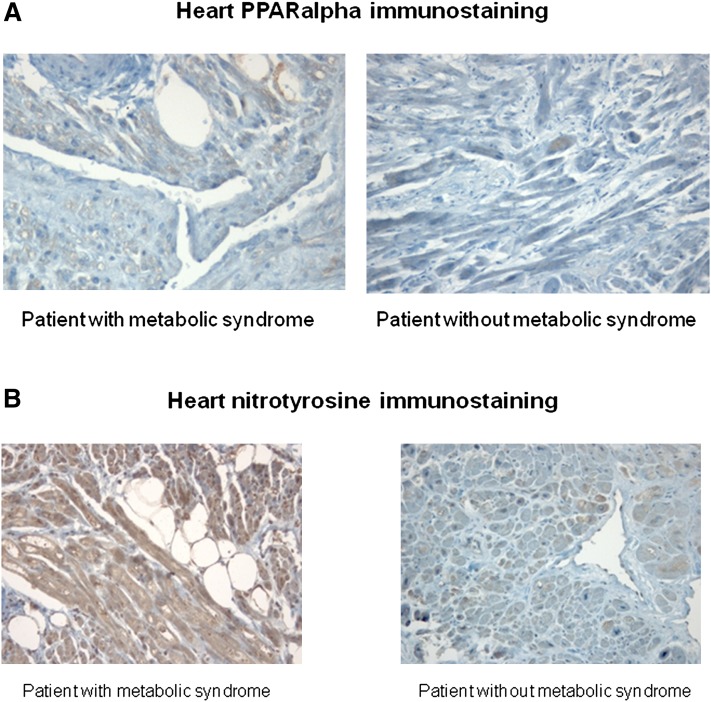
Similar levels and immunostaining of PPARα and SCD-I proteins (Fig. 5, Table 1) were seen in biopsy from patients with and without MS. There were no differences among the groups.
Fig. 5.
Representative immunohistochemical analysis of PPARα protein from ventricular biopsy specimens (×400). The specimens from patients with and without metabolic syndrome show a similar immunostaining for PPARα protein in myocytes (A). Representative immunohistochemical analysis of nitrotyrosine from ventricular biopsy specimens (×200). The specimen from patients with metabolic syndrome shows a strong immunostaining for nitrotyrosine in hypertrophied myocytes (B).
SERCA2a.
The level of SERCA2a protein is 30% lower in heart specimens from patients with MS compared with heart specimens from patients without MS (Table 1). Moreover, a negative correlation between SERCA2 levels and percentage of the vacuolated myocytes was observed (R = −0.319, P < 0.01).
Oxidative stress-related parameters.
Levels of MDA were higher in heart specimens from MS patients compared with specimens from patients without MS (49.6 ± 2.1 nmol/mg vs. 15.8 ± 2.4 nmol/mg, P < 0.001). When immunostaining for the nitrotyrosine antigen was compared, differences were found between tissues from patients with and without MS. Significantly intense nitrotyrosine immunostaining was present in tissue from MS patients compared with tissues from patients without MS (score 3.4 ± 0.43 vs. 1.2 ± 0.31, P < 0.001) (Fig. 5). Moreover, a positive correlation between nitrotyrosine levels and percentage of the vacuolated myocytes was observed (R = 0.408, P < 0.01).
DISCUSSION
The goal of our study was to correlate the myocyte lipid accumulation with the expression of SREBP-1c and PPARγ in patients with MS undergoing aortic valve replacement.
Heart function and myocyte lipid accumulation
We provide evidence that MS is strongly associated with poor functional cardiac outcome in patients with pressure-overloaded heart: MS patients present reduced EF and impaired MPI compared with patients without MS. Moreover, we observed that the levels of SERCA2a protein are 30% lower in heart specimens from patients with MS compared with heart specimens from patients without MS. This is in agreement with human studies showing that SERCA2a concentrations are reduced (18). Indeed, molecular alteration in metabolic cardiomyopathy results in significant contractile and Ca2+ handling abnormalities, in part, mediated by decreased SERCA2a expression. These metabolic alterations evoke a reduced cardiac contractility due to direct changes in heart muscle function independent of vascular disease (19). Abnormal calcium handling with diminished Ca2+ entry into the cytoplasm during systole and delayed lowering of diastolic Ca2+ levels is an important contributor to contractile dysfunction in the presence of the metabolic alterations (19). In MS patients, we evidenced that a strong correlation also exists between the progression of cardiac dysfunction and myocytes lipid accumulation because, with increases of vacuolated myocytes and oil red O staining-positive myocytes, MPI increases and EF decreases. These alterations, importantly, are directly cardiotoxic and cause LV remodeling and HF in patients with AS and MS. In this context, the EF was correlated to the aortic stenosis in no-MS patients, whereas a close correlation exists between cardiac function and myocardial morphology in patients with AS and MS because the cardiac functional impairments were independent from the stenosis severity and were associated with a marked cardiomyocyte lipid accumulation.
Changes of molecular myocardial pathways associated with myocyte lipid accumulation
Although it is unclear how metabolic environments of MS induce cardiac lipid accumulation, emerging evidence suggests that caloric excess is associated with altered gene expression (20). Regulation of gene expression by nutrients in mammals is an important mechanism allowing them to adapt to the nutritional environment (21). Experimental models have demonstrated that the transcription of genes coding for lipogenic and glycolytic enzymes in liver and/or adipose tissue is upregulated by overfeeding.
SREBP-1c.
SREBP-1c is a transcription factor that controls lipogenesis and is induced during overnutrition to facilitate the conversion of glucose to fatty acids and triglycerides for the storage of the excess energy (8). Uncontrolled activation of nuclear SREBP-1c that causes hepatosteatosis and possibly MS (22, 23) may have a role in the intramyocyte lipid accumulation observed in heart specimens from MS. Interestingly, we detected strong immunoreactivity to SREBP-1c in both hypertrophied and vacuolated myocytes, particularly around the cells completely vacuolated in specimens of the human heart affected by MS. These results imply, therefore, that SREBP-1c could play a role in the regulation of intracellular lipid stores in the human heart, as it has been previously observed in liver cells (23). The mechanism by which SREBP-1c exercises its lipogenic function, however, remains unknown and not demonstrated by our study, but several insights have been made that point to a relationship with PPARγ.
PPARγ.
PPARγ seems to be a direct target gene of SREBP-1c (10). The induction of PPARγ by SREBP-1c seems to play a role in the regulation of intracellular lipid stores in human heart, as it has been previously observed in rodents (24, 25). Activation of PPARγ was shown to strongly induce adipogenesis in nonadipogenic cells and cardiomyocytes, particularly when coexpressed with SREBP-1c (7, 25, 26). In line with this, our contention is that myocardial metabolic derangements in MS may be associated with SREBP-1c and PPARγ overexpressions that in turn probably evoke the storage of the lipid excess in the myocytes.
Overall, our mental scheme would be: (I) caloric surplus induced by MS→(II) increased expression of the lipogenic transcription factor SREBP-1c→ (III) increased expression of PPARγ→(IV) increased lipogenesis→(V) ectopic lipid deposition in myocardium→(VI) nonoxidative and/or oxidative cardiomyocyte death→(VII) heart dysfunction. This scheme may be congruent with the known actions of the excess in the caloric environment on the disposition of unused calories, initially as fat in adipocytes (27, 28), but ultimately as ectopic fat in nonadipocytes, such as myocytes (29).
Study limitations.
Some limitations merit emphasis: our study is a cross-sectional design on small sample size and not probative to definitively ascribe a role for this construct; therefore, these results require further confirmation. Also we have not assessed some of the most relevant downstream genes to SREBP-1c such as fatty acid synthetase (FAS). Hovewer, FAS has been shown to be regulatable by SREBP-1c and sensitive to nutritional perturbations in vivo (30) and thus, we may hypothesize that FAS may a role in the heart metabolic alteration of patients with MS.
These findings are potentially important from a fundamental standpoint because they hypothesize a molecular mechanism by which MS may influence the evolution of heart dysfunction. These findings are also potentially important from a practical standpoint because they raise the interesting possibility that modification of the SREBP-1c/PPARγ pathway might provide a novel form of therapy for cardiac dysfunction of patients with MS.
Footnotes
Abbreviations:
- apM1
- adipose most-abundant gene transcript 1
- AS
- aortic stenosis
- BMI
- body mass index
- EF
- ejection fraction
- H and E
- hematoxylin-eosin
- HF
- heart failure
- HOMA
- homeostasis model assessment
- HPRT
- hypoxanthine-phosporybosil transferase
- LV
- left ventricular
- MDA
- malondialdehyde
- MPI
- myocardial performance index
- MS
- metabolic syndrome
- PPAR
- peroxisome proliferator activated receptor
- SERCA
- sarco-endoplasmic reticulum ATPase
- SREBP
- sterol-regulatory element binding protein
- WC
- waist circumference
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of an appendix.
REFERENCES
- 1.Burchfiel C. M., Skelton T. N., Andrew M. E., Garrison R. J., Arnett D. K., Jones D. W., Taylor H. A., Jr 2005. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 112: 819–827. [DOI] [PubMed] [Google Scholar]
- 2.McGavock J. M., Victor R. G., Unger R. H., Szczepaniak L. S. 2006. Adiposity of the heart, revisited. Ann. Intern. Med. 144: 517–524. [DOI] [PubMed] [Google Scholar]
- 3.McGavock J. M., Lingvay I., Zib I., Tillery T., Salas N., Unger R., Levine B. D., Raskin P., Victor R. G., Szczepaniak L. S. 2007. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 116: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. 2000. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. USA. 97: 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S., Adrogue J. V., Golfman L., Uray I., Lemm J., Youker K., Noon G. P., Frazier O. H., Taegtmeyer H. 2004. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 18: 1692–1700. [DOI] [PubMed] [Google Scholar]
- 7.Rosen E. D., Walkey C. J., Puigserver P., Spiegelman B. M. 2000. Transcriptional regulation of adipogenesis. Genes Dev. 14: 1293–1307. [PubMed] [Google Scholar]
- 8.Tontonoz P., Kim J. B., Graves R. A., Spiegelman B. M. 1993. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 13: 4753–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller-Wieland D., Kotzka J. 2002. SREBP-1: gene regulatory key to syndrome X? Ann. N. Y. Acad. Sci. 967: 19–27. [DOI] [PubMed] [Google Scholar]
- 10.Fajas L., Schoonjans K., Gelman L., Kim J. B., Najib J., Martin G. 1999. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol. Cell. Biol. 19: 5495–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opie L. H., Commerford P. J., Gersh B. J., Pfeffer M. A. 2006. Controversies in ventricular remodelling. Lancet. 367: 356–367. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Definition of metabolic syndrome in definition, diagnosis and classification of diabetes and its complications. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. WHO/NCD/NCS/99.2. 1999. Geneve, World Health Organization-Department of Noncommunicable Disease Surveillance. [Google Scholar]
- 13.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 14.Gardin J. M., Adams D. B., Douglas P. S., Feigenbaum H., Forst D. H., Fraser A. G. 2002. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography’s Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J. Am. Soc. Echocardiogr. 15: 275–290. [DOI] [PubMed] [Google Scholar]
- 15.Marfella R., Siniscalchi M., Esposito K., Sellitto A., De Fanis U., Romano C., Portoghese M., Siciliano S., Nappo F., Sasso F. C., et al. 2003. Effects of stress hyperglycemia on acute myocardial infarction: role of inflammatory immune process in functional cardiac outcome. Diabetes Care. 26: 3129–3135. [DOI] [PubMed] [Google Scholar]
- 16.Mihara M., Uchiyama M. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86: 271–278. [DOI] [PubMed] [Google Scholar]
- 17.Marfella R., Esposito K., Nappo F., Siniscalchi M., Sasso F. C., Portoghese M., Di Marino M. P., Baldi A., Cuzzocrea S., Di Filippo C., et al. 2004. Expression of angiogenic factors during acute coronary syndromes in human type 2 diabetes. Diabetes. 53: 2383–2391. [DOI] [PubMed] [Google Scholar]
- 18.Barrans J. D., Allen P. D., Stamatiou D., Dzau V. J., Liew C. C. 2002. Global gene expression profiling of end-stage dilated cardiomyopathy using a human cardiovascular-based cDNA microarray. Am. J. Pathol. 160: 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young M. E., McNulty P., Taegtmeyer H. 2002. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 105: 1861–1870. [DOI] [PubMed] [Google Scholar]
- 20.Mingrone G., Rosa G., Greco A. V., Manco M., Vega N., Hesselink M. K., Castagneto M., Schrauwen P., Vidal H. 2003. Intramyocitic lipid accumulation and SREBP-1c expression are related to insulin resistance and cardiovascular risk in morbid obesity. Atherosclerosis. 170: 155–161. [DOI] [PubMed] [Google Scholar]
- 21.Roche H. M., Phillips C., Gibney M. J. 2005. The metabolic syndrome: the crossroads of diet and genetics. Proc. Nutr. Soc. 64: 371–377. [DOI] [PubMed] [Google Scholar]
- 22.Biddinger S. B., Almind K., Miyazaki M., Kokkotou E., Ntambi J. M., Kahn C. R. 2005. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 54: 1314–1323. [DOI] [PubMed] [Google Scholar]
- 23.Horton J. D., Shimomura I., Ikemoto S., Bashmakov Y., Hammer R. E. 2003. Overexpression of sterol regulatory element-binding protein-1a in mouse adipose tissue produces adipocyte hypertrophy, increased fatty acid secretion, and fatty liver. J. Biol. Chem. 278: 36652–36660. [DOI] [PubMed] [Google Scholar]
- 24.Schadinger S. E., Bucher N. L., Schreiber B. M., Farmer S. R. 2005. PPARgamma regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 288: E1195–E1205. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y. L., Hernandez-Ono A., Siri P., Weisberg S., Conlon D., Graham M. J., Crooke R. M., Huang L. S., Ginsberg H. N. 2006. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J. Biol. Chem. 281: 37603–37615. [DOI] [PubMed] [Google Scholar]
- 26.Son N. H., Park T. S., Yamashita H., Yokoyama M., Huggins L. A., Okajima K., Homma S., Szabolcs M. J., Huang L. S., Goldberg I. J. 2007. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J. Clin. Invest. 117: 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong K. M., Belperio J. B., Keane M. P., Burdick M. D., Strieter R. M. 2007. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator activated receptor-gamma. J. Biol. Chem. 282: 22910–22920. [DOI] [PubMed] [Google Scholar]
- 28.Unger R. H. 2008. Reinventing type 2 diabetes pathogenesis, treatment, and prevention. JAMA. 299: 1185–1187. [DOI] [PubMed] [Google Scholar]
- 29.Marfella R., Barbieri M., Paolisso G. 2008. Insulin therapy and lipid overload in type 2 diabetes. JAMA. 300: 789–790. [DOI] [PubMed] [Google Scholar]
- 30.Yang J., Sambandam N., Han X., Gross R. W., Courtois M., Kovacs A., Febbraio M., Finck B. N., Kelly D. P. 2007. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ. Res. 100: 1208–1217. [DOI] [PubMed] [Google Scholar]