Abstract
Our objective was to study the metabolic precursors of surfactant disaturated-phosphatidylcholine (DSPC) in preterm infants with respiratory distress syndrome (RDS) on mechanical ventilation. We performed 46 DSPC kinetic studies in 23 preterms on fat-free parenteral nutrition and mechanical ventilation (birth weight = 1167 ± 451 g, gestational age = 28.5 ± 2.0 weeks). Eight infants received a simultaneous intravenous infusion of U13C-glucose and [16,16,16]2H-palmitate, eight infants received U13C-glucose and 2H2O, and seven received U13C-palmitate and 2H2O. Surfactant DSPC kinetics were calculated from the isotopic enrichments of DSPC-palmitate from sequential tracheal aspirates and its metabolic precursors in plasma or urine. DSPC fractional synthesis rate (FSR) was 17 ± 11, 21 ± 16, and 15 ± 6%/day from glucose, palmitate, and body water, respectively (P = 0.36). DSPC-FSR from U13C-glucose and 2H2O were significantly correlated and yielded similar estimates (difference of –0.1 ± 3%) (P = 0.91). The difference in the 15 infants receiving palmitate versus 2H2O or palmitate versus glucose was +6.0 ± 12%/day (P = 0.21). There was a significant correlation between DSPC-FSRs from plasma glucose and plasma FFA. The contribution of glucose versus palmitate to DSPC-FSR was 49 ± 20% versus 51 ± 20%, respectively. Plasma glucose and FFA showed similar contributions to DSPC-FSR in infants with RDS and fat-free parenteral nutrition. FSRs from 2H2O or glucose were highly correlated.
Keywords: pulmonary surfactant, isotopes, isotope labeling, lecithins, disaturated, metabolic pathways
In mammals, pulmonary surfactant reduces lung surface tension, maintains lung fluid balance, provides lung stability, and reduces the risk of infection. Preterm infants have immature lungs and are prone to develop respiratory distress syndrome (RDS), a disease caused by lack of surfactant production and secretion into the alveoli. The alveolar type II cell is the primary site of synthesis, storage, and secretion of pulmonary surfactant that consists of a mixture of lipids and proteins that are highly enriched with dipalmitoyl-phosphatidylcholine (DPPC) and other phospholipids with saturated fatty acids in the one and two position of the glycerol moiety (1–3). While much work has focused on the pathways of pulmonary phospholipid synthesis in animals, little is known about the provision of its synthetic precursors in animals and even more so in humans.
From a biochemical point of view, the FA moiety of surfactant phospholipids may be derived from the circulating FFA or from FAs denovo synthesized from glucose via acetate and lactate precursors. De novo FA synthesis or lipogenesis has been shown to occur in the lung or in the liver (4, 5). The interplay among these various sources of pulmonary FAs is poorly understood, but it may be influenced by the concentrations of available substrates, dietary and hormonal factors, and altered physiologic states (6–8). The relative contribution of each of the potential sources of FA (glucose, acetate, lactate, and FFA) for incorporation into the lung surfactant disaturated-phosphatidylcholine (DSPC) has been studied in animals by radioactive and, more recently, stable isotopes tracers (4, 9–13).
In humans, we used stable isotope tracer methodology to quantify surfactant DSPC kinetics synthesized from plasma glucose, free palmitate, and body water (14–16), tracers that have not been used simultaneously in previous human studies.
From a clinical point of view, the assessment of the contribution of metabolic substrates on surfactant DSPC synthesis is important because it could influence the nutritional management in the first hours and days of life. Intravenous (i.v.) glucose is commonly given as nutritional support in preterm infants during the first hours of life, and i.v. lipids are often withheld for hours or sometimes for days (17). The effect of early nutritional management in preterm infants on surfactant synthesis is unknown.
The effect of undernutrition on respiratory outcome was studied in animals as long as three decades ago. Brief fasting in rats for up to 72 h induced a decrease in DPPC content of lung lavage fluid (18) associated with increases in minimal surface tension (19, 20) and decreased stability of small liquid bubbles released from lung fragments (21). Because not all these early reports consistently showed alteration on pressure-volume curves, it was suggested that in some instances there was a sufficient surfactant reserve to maintain alveolar stability even if the animals were undernourished (19). Hyperventilation further worsens lung compliance (22). Later it was also observed that prenatal starvation decreased surfactant phospholipids pool and altered lung structure and function in animal models (23, 24), whereas overnutrition or increased FA availability to the lungs could be associated with increased surfactant lipid synthesis (25). No studies have been conducted in animal models of neonatal RDS.
In humans, insufficient nutrition is detrimental to the fetus and to the preterm neonate; newborns who are small for gestational age are at increased risk for adverse respiratory outcomes both in the neonatal period and later in infancy (26, 27). No data are available on the effect of dietary manipulation on surfactant production for either adults or infants.
In this study, we combined three stable isotope methods (14–16) to quantify the rate of surfactant DSPC synthesis from body water, plasma glucose, and plasma FFA palmitate. We have applied this methodology in preterm infants with RDS who required exogenous surfactant at birth and prolonged mechanical ventilation, were receiving fat-free parenteral nutrition, and were receiving no or negligible amounts of enteral feeds.
METHODS
Study patients
Surfactant kinetics were studied in 23 preterm infants whose clinical characteristics are reported in Table 1. The study was approved by the Institutional Ethic Committee, and informed consent was obtained from both parents.
TABLE 1.
Mean ± SD clinical characteristics of the three study groups
| GLUC versus PA N = 8 | GLUC versus H2O N = 8 | PA versus H2O N = 7 | P | |
|---|---|---|---|---|
| Birth weight (g) | 1155 ± 207a | 1412 ± 657b | 898 ± 185a | 0.08 |
| Gestational age (weeks) | 28.5 ± 1.2a | 30.0 ± 2.2b | 26.9 ± 1.1a | <0.01 |
| Prenatal steroids (%) | 63 | 87 | 86 | 0.10 |
| Study age (hours) | 20 ± 13 | 30 ± 23 | 27 ± 35 | 0.72 |
| Surfactant dose>1 (%) | 25a | 37a | 71b | 0.19 |
| Total surfactant dose (mg/kg) | 200 ± 100 | 150 ± 75 | 200 ± 58 | 0.38 |
| Study oxygenation index | 3.3 ± 1.8 | 3.5 ± 1.7 | 3.2 ± 0.9 | 0.90 |
| Conventional ventilation (days) | 5.7 ± 4.6 | 15 ± 14 | 12 ± 9 | 0.22 |
| Intake (kcal/kg) | 31 ± 2 | 31 ± 5 | 30 ± 7 | 0.84 |
| Survival (%) | 100 | 100 | 100 | 0.99 |
| BPD at 36 weeks (%) | 14a | 37a | 71b | 0.10 |
significantly different than a.
All patients were admitted to the Neonatal Intensive Care Unit of the Department of Pediatrics, University of Padova, Italy. Inclusion criteria were: 1) gestational age between 25 and 30 weeks; 2) respiratory failure requiring endotracheal intubation for an estimated length of time of at least 48 h; 3) arterial and venous lines placed for clinical monitoring; and 4) written informed consent. Exclusion criteria were congenital infections and chromosomal abnormalities. Exogenous surfactant (Curosurf, Chiesi Farmaceutici) was administered endotracheally at a dose of 100 mg/kg if the mean airway pressure exceeded 7.5 cm of water or if the inspiratory oxygen fraction was higher than 0.40. Infants received a second dose if the same criteria were met after the first dose. We recorded clinical data and physiological parameters (respiratory rate, heart rate, hemoglobin saturation) hourly during the study. Ventilator parameters and arterial blood gas analysis were recorded before the start of the study and subsequently every 6 h. The oxygenation index was calculated as [(MAP × FiO2)/PaO2] × 100, where MAP is the mean airway pressure, FiO2 the fraction of inspired oxygen, and PaO2 the arterial blood partial oxygen tension at each of these time points.
Study design
Patients were divided into 3 groups: 1) glucose versus palmitate: newborns received a 24 h constant i.v. infusion of U13C-glucose and i.v. albumin bound 2H3-palmitate (15, 28); 2) glucose versus water: patients received a 24 h i.v. infusion of U13C-glucose and constant primed infusion of 2H2O; 3) palmitate versus water: patients received a 24 h i.v. infusion of albumin bound U13C-palmitate and a 48 h bolus administration of 2H2O. U13C-glucose (CIL) was infused at 0.17 mg/kg/min by the same high precision, calibrated syringe pump. U13C-palmitate and 2H3-palmitate (Martek Columbia) were bound to human albumin and infused i.v. at a constant rate of 1.0 ± 0.26 μmol/kg/h by a high precision, calibrated syringe pump (M22, Harvard Apparatus). 2H2O was given as an i.v. bolus of 2 ml/kg at the study start and then 0.125% of fluid intake as 2H2O every 12 h over the next 48 h to maintain body water deuterium enrichment at plateau (16). All infants were on total parenteral nutrition without lipids during the first 24 h of the study.
The isotope tracers were started in all patients within 48 h from birth and were terminated before 96 h of life. The start of the study (t = 0) was defined by the start of the tracer administration. In those patients who received exogenous surfactant, the infusion was started at the time of the first surfactant administration. We used a central venous line (umbilical) for tracer infusion, a peripheral line or the second lumen of the central venous line for 2H2O administration, and an arterial line for blood sampling. Chemical and isotopic purity of all stable isotope tracers were confirmed by gas chromatography and gas chromatography-mass spectrometry.
Blood (0.6 ml) was drawn at time 0, 5.30, 6, 12, 18, and 24 h from the start of the study to determine the isotopic enrichment of plasma FFA palmitate and plasma glucose. The blood was placed in tubes containing EDTA, immediately centrifuged at 1300 g, and plasma was stored at −20°C until analysis.
One milliliter of urine was collected before the start of the study and then every 6 h for 48 h and stored at −20°C until analysis.
Tracheal aspirates were obtained before the start of the infusion and at 3 and 6 h and every 6 h thereafter until 72 h. Afterwards, the samples were collected every 12 h until the extubation or until study day 10. Tracheal aspirates were performed as follows: 0.5 ml normal saline was injected into the endotracheal tube, and after 20 s, suctioning was performed with the tip of the suction catheter beyond the tip of the endotracheal tube. All tracheal aspirates were brought to a final volume of 2 ml. The sample was then gently vortexed and centrifuged at 500 g for 10 min. The supernatant was stored at −20°C until analysis.
Analytical procedure
Plasma lipids were processed as previously described (28, 29). Plasma was delipidated with chloroform and methanol according to Folch (30), lipid classes separated by thin layer chromatography, and their FAs derivatized as methyl esters. The separation and identification of FFA methyl esters from the plasma lipid classes were performed by capillary gas chromatography (28, 29). Isotopic enrichments of plasma FFAs and of the albumin-bound U13C-palmitate and 2H3-palmitate tracers in the plasma were determined by gas-chromatograph coupled with a quadrupole mass spectrometer (Voyager, Thermoquest, Rodano, Milano, Italy) operating in negative ionization mode. Selective ion monitoring was carried out at m/z 255, 256, 257, and 271 for U13C-palmitate and at m/z 255, 256, 257, and 258 for 2H3-palmitate. Enrichments were expressed as mol% excess (MPE). Each sample was measured in duplicate (31).
For plasma glucose, the water fraction of delipidated plasma (30) was passed over anion- and cation-exchange resin. The eluate containing the glucose was derivatized to an aldonitril pentaacetate derivative and enrichment measured by gas-chromatography-combustion interface-isotope ratio mass spectrometry (GC-IRMS) (FinniganTM DELTAplus XL, Thermo Electron Corporation). Enrichments were expressed as Δ ‰. Enrichments were corrected for isotopic dilution contributed by the derivative group (14).
For urine water, urine was filtered with a 0.22 μm filter and diluted 1:500 with reference water. Deuterium in the urine was analyzed by GC-IRMS in triplicate and calibrated against Vienna-Standard Mean Ocean Water (32). Reference water was interspersed every six injections to ensure that memory effect was insignificant (32). Enrichments were expressed as Δ ‰.
Tracheal aspirates’ phospholipids were extracted according to Bligh and Dyer (33) and surfactant DSPC was isolated by thin layer chromatography (34) after oxidation with osmium tetroxide (31). A half spot of DSPC was derivatized (35) and the FA methyl esters were extracted with hexane and stored at −20°C. The amount of DSPC in tracheal aspirates was measured from the FA methyl esters by capillary gas chromatography (31). Tracheal aspirates containing visible blood were not analyzed. Isotopic enrichments were measured by GC-IRMS (14, 16). Each sample was analyzed in duplicate. Enrichments of DSPC-palmitate derived from U13C-glucose and 2H2O were measured in Δ ‰. Enrichments of DSPC-palmitate derived from U13C-palmitate and 2H3-palmitate were expressed in MPE by means of calibration curves ranged from 0 to 1% MPE. MPE by GC-IRMS represents the increase in isotopic enrichment above baseline after tracer(s) administration of 13C and 2H and complete combustion and pyrolisis, respectively. Enrichments were corrected for isotopic dilution contributed by the derivative group.
Calculations
Surfactant kinetics were calculated as previously published (14–16) using the precursor-product approach and assuming isotopic steady-state condition. Secretion time (ST) was defined as the time lag between the start of the tracers infusion and the appearance of the respective labeled FA in surfactant DSPC-palmitate. Fractional synthesis rate (FSR) was defined as the percentage of the total surfactant DSPC pool synthesized from the respective plasma palmitate, glucose, and 2H2O per day. It was calculated from the linear portion of the enrichment rise over time relative to the plateau enrichment of the respective precursors. For 2H2O the plateau enrichment was corrected for the maximum number (N = 22) of deuterium that could be incorporated in the DSPC-palmitate (36). Total FSR is the sum of the two DSPC-FSR derived either from plasma palmitate or from glucose and 2H2O. Half-life (HL) was derived from the final, decreasing, mono-exponential portion of the enrichment versus time curve. Peak time (PT) was the time of maximum enrichment of surfactant DSPC-palmitate from the start of the infusion.
Data analysis
Data are presented as individual values and group mean ± SD. We compared groups of patients receiving different tracers and also compared the results of different tracers being simultaneously administered to the same patients. Thus, comparisons were made by unpaired and paired t-tests between two groups for independent and related samples, respectively, and by ANOVA among the 3 groups. The level of significance accepted was P < 0.05. Correlations were assessed by Pearson correlation.
RESULTS
We performed 46 metabolic studies in 23 preterm infants. Eight infants received simultaneously U13C-glucose and 2H2O, 8 infants received 2H3-palmitate and U13C-glucose, and 7 received U13C-palmitate and 2H2O. The clinical characteristics of the study groups are shown in Table 1. All but one infant had mild or severe RDS and required exogenous surfactant. Among the treated infants, 45% required a second dose of exogenous surfactant after 24 ± 12 h and all showed good clinical response to exogenous surfactant. All infants were on total parenteral nutrition with 7 g/kg/day of carbohydrate and 2 g/kg/day of aminoacids for the first 24 h of the study. Energy intake in the 3 groups is reported in Table 1. i.v. lipids and minimal enteral feeding were started after 96 h of life according with the nutritional policy of the neonatal unit when the study was performed.
Plasma FFA and plasma glucose concentrations were stable during the study period. The mean plasma glucose level was 2.7 ± 0.9 mmol/L and mean plasma FFA levels were 7.0 ± 4.5 mg/dl. In all infants, the tracers were at steady state in plasma from time 6 to 24 h for glucose and palmitate, and from 6 to 48 h for 2H2O since the slope of the linear regression of the precursors’ (i.e., palmitate, glucose, and water) enrichment values versus time was not significantly different from zero. Mean plasma U13C-glucose enrichment was 670 ± 198 Δ ‰ (2.0 ± 0.6 MPE), mean plasma U13C-palmitate MPE was 1.7 ± 0.4, mean plasma 2H3-palmitate MPE was 0.40 ± 0.15, and mean deuterium enrichment in the urine was 18506 ± 6326 Δ ‰ (0.29 ± 0.02 MPE).
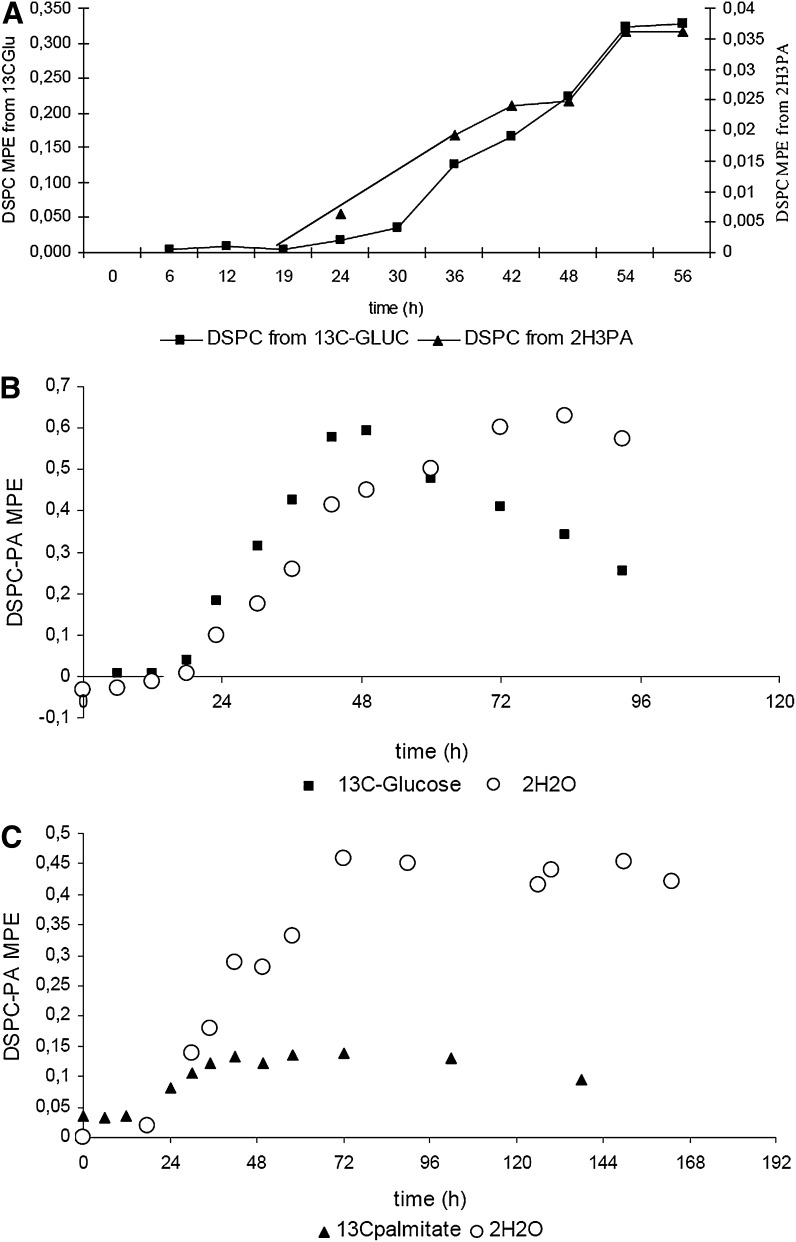
A significant incorporation of the i.v. labeled palmitate and of palmitate deriving from de novo lipogenesis was measurable in the surfactant DSPC-palmitate from all patients. Figure 1 shows the time curves of the 13C and 2H enrichments of DSPC-palmitate deriving from U13C-glucose and from 2H3-palmitate infusion (panel A), the time curves of the 13C and 2H enrichments of DSPC-palmitate deriving from U13C-glucose and from 2H2O (panel B), and the time curves of 13C and 2H enrichments of DSPC-palmitate deriving from 13C-palmitate and 2H2O (panel C).
Fig. 1.
Plots belong to one representative patient. Panel A shows the time curve of the 13C and 2H enrichments of DSPC-palmitate deriving from 13C-glucose (black squares) and from 2H3-palmitate (black triangles) infusion. Panel B shows the time curves of the 13C and 2H enrichments of DSPC-palmitate deriving from 13C-glucose (black squares) and from 2H2O (white circles). Panel C shows the time curves of 13C and 2H enrichments of DSPC-palmitate deriving from 13C-palmitate (black triangles) and 2H2O (white circles). All measurements were performed with GC-IRMS.
Kinetic data (mean ± SD) are shown in Table 2. Comparisons were made with unpaired t-tests. While DSPC-FSR and ST were not different among the three groups, the slow body water turnover delayed significantly the PT in infants receiving 2H2O compared with infants receiving stable isotope glucose and palmitate. Moreover, since many infants were extubated before complete decay in enrichment, surfactant DSPC-palmitate half-life could be reliably calculated in only 10/16 infants from U13C-glucose, 8/15 from palmitate, and 5/15 from 2H2O. Therefore, for the purpose of comparing tracers, only the DSPC-FSR was used for the paired statistical analysis. The mean oxygenation index during the study was significantly and negatively correlated with either surfactant DSPC-FSR derived from de novo lipogenesis (23 infants, R = 0.57, P = 0.007) and from plasma FFA palmitate (15 infants, R = 0.61, P = 0.016). This negative correlation was stronger when the oxygenation index was correlated with the total DSPC-FSR (sum of FSR from lipogenesis and that from FFA-palmitate) (15 infants, R = 0.72, P = 0.002). No significant correlation was found with gestational age and body weight.
TABLE 2.
Mean ± SD kinetic data of the three study groups
| 13C-Glucose N = 16 | 13C and 2H3 Palmitate N = 15 | 2H2O N = 15 | P | |
|---|---|---|---|---|
| FSR (%/day) | 17 ± 11 | 21 ± 16 | 15 ± 6 | 0.36 |
| Secretion time (hours) | 20 ± 6 | 17 ± 12 | 24 ± 14 | 0.32 |
| Peak time (hours) | 58 ± 21 | 68 ± 21 | 98 ± 34 | <0.01 |
| Half-life* (hours) | 78 ± 59 | 170 ± 128 | 125 ± 56 | 0.12 |
* Half-life could be calculated in only 10 infants receiving IV 13C-Glucose, in eight infants receiving stable isotope palmitate, and in five infants receiving 2H2O, because of early extubation.
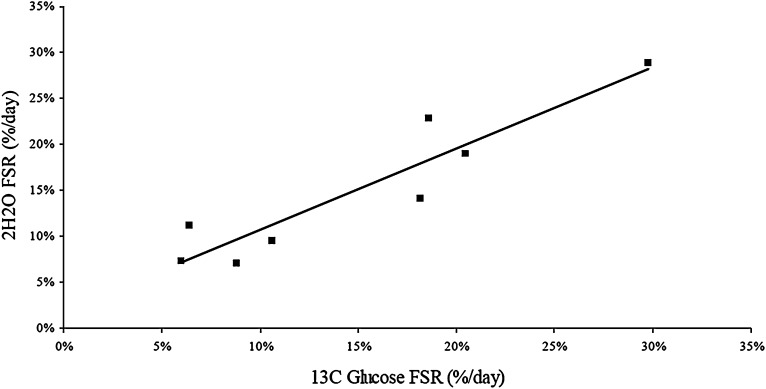
Paired analysis of the 8 infants who received simultaneous U13C-glucose and 2H2O gave a mean DSPC-FSR difference of –0.1 ± 3% (P = 0.91). FSR from U13C-glucose and 2H2O were highly correlated (R2 = 0.93, P = 0.001) (Fig. 2).
Fig. 2.
Correlation between FSR of 13C-glucose and 2H2 O. The two FSRs were signifi cantly correlated ( R2 = 0.93, P = 0.001).
Fifteen infants received palmitate and 2H2O or U 13C-glucose simultaneously. Total DSPC-FSR was 43 ± 30%/day. DSPC-FSR from de novo lipogenesis significantly correlated with the DSPC-FSR from plasma palmitate (R = 0.55, P = 0.04). Paired data analysis of FSR showed a mean difference of +4.5 ± 11.8%/day (palmitate vs. glucose) and + 4.6 ± 16.3%/day (palmitate vs. 2H2O) (P = 0.21 by paired t-test). The percentage of DSPC-FSR from plasma glucose was 49 ± 20% and that from plasma FFA-palmitate was 51 ± 20%.
DISCUSSION
A better understanding of the metabolic precursors for surfactant synthesis may improve the nutritional management of the patients with respiratory failure with primary or secondary surfactant deficiency. In this report, we used the simultaneous administration of stable isotope labeled palmitate, glucose, and water to assess which metabolic precursor is preferentially used for surfactant DSPC synthesis in preterm infants with RDS on parenteral nutrition and low energy intake.
We have recently described a new method to measure pulmonary surfactant DSPC-palmitate synthesis by deuterium incorporation by using low and safe doses of 2H2O (16). As we reported previously, the method is less invasive and less expensive than the other stable isotope methods and it greatly simplifies the measurement of surfactant kinetics in humans, because 2H2O can be given i.v. as well as orally and deuterium enrichment can be measured in plasma or in urine; however, in that study, we did not compare the DSPC-FSR from body water to that obtained from other lipogenic tracers, such as glucose or acetate (14, 37, 38).
Surfactant DSPC production appears to be a carefully timed event in animal experiments with an increased capacity of de novo FA synthesis and of DSPC content during gestation, followed by a sharp decline of de novo FA synthesis in the immediate postnatal period, probably related to postnatal diet and increased availability of dietary and thus plasma lipids (4, 39). In the present study, we found that the DSPC-palmitate FSR from 13C-glucose and from 2H2O were highly correlated (Fig. 2) and that the mean paired difference was –0.1 ± 3% (P = 0.91 by paired t-test). This observation confirmed previous findings in animals and also our hypothesis that tracing the de novo synthesis of palmitate through either U13C-glucose or 2H2O yields similar results (4).
While the glucose and water trace the contribution of the de novo FA-DSPC synthesis pathway, plasma FFA-palmitate evaluates the contribution of circulating palmitate to DSPC synthesis. Two of the 3 study groups received simultaneously a tracer for the de novo FA DSPC synthesis pathway (glucose or water) and a tracer for plasma FFA. DSPC-palmitate kinetic parameters were calculated and comparison considered only for FSR and ST, because 50% of DSPC-palmitate peak time and half-life values were missing due to early extubation. No significant differences in DSPC-palmitate FSR and ST were found between infants receiving plasma palmitate and infants receiving glucose or water, suggesting that preterms on low caloric intake and during the first days of life are able to use indifferently the available metabolic precursors for surfactant DSPC synthesis (Table 2). This was confirmed also by paired analysis (within-groups), where we found that the lipogenic pathway and the incorporation of plasma palmitate contributed almost equally to surfactant synthesis. This observation differs from that of Spence et al. (40) who found that the plasma palmitate pathway contributed twice as much as the de novo synthetic pathway to surfactant synthesis. The infants in that study, though, were chronologically older (2–3 days, 2 weeks, and 6 weeks), had already received surfactant replacement, were developing chronic lung disease, and were receiving enteral feeds, all of which could have contributed to these differences in results.
Moreover, hypertrygliceridemia and abnormal fat accumulation have been found in the lungs of newborns with bronchopulmunary dysplasia, suggesting an abnormal lipid metabolism in preterm infants developing chronic lung disease. (41, 42). An additional methodological explanation can also be considered; the approach of Spence et al. (40) uses 13C-acetate and mass isotopomer distribution analysis to compute the DSPC-palmitate FSR, whereas in our study, we quantify the DSPC-palmitate FSR derived from body water hydrogen. Thus, the two methods measure different aspects of the de novo palmitate synthesis that may not be theoretically equivalent and may not return identical estimates of DSPC-palmitate FSR.
Although the groups of infants in the different tracer protocols were heterogeneous with respect to birth weight and gestational age and we cannot exclude the possibility that this heterogeneity masked small differences in substrate utilization, all study infants had a similar severity of RDS, which is likely to play a more important role in the kinetics of surfactant metabolism (43, 44). Furthermore, and perhaps more importantly, the majority of our data are obtained comparing different tracers in the same individual and applying paired data statistics. We found a significant inverse correlation between the DSPC-FSR and the oxygenation index, suggesting that, in accordance with previous animal studies, lung disease severity affects DSPC synthesis (45–49). Even when the total DSPC-FSR is enhanced, though, there is no preference for lipogenesis versus palmitate incorporation in our study infants who received low amounts of lipid intake.
Several methodological issues are relevant to our results. Surfactant DSPC-palmitate FSR calculated from 2H2O relies on a deuterium isotopic steady state of at least 60 h, because the body water turnover is a slow process. Plasma glucose and palmitate have a much faster turnover, possibly leading to a more imprecise determination of DSPC-palmitate FSR in those infants who received a second surfactant dose during the study. Secretion time and peak time are mainly affected by the administration of unlabeled exogenous surfactant, which diluted the endogenous enriched DSPC-palmitate to a variable degree and postpones the point of maximum enrichment. DSPC-palmitate half-life has a wide variation and it is artificially prolonged, because the slow body water turnover leads to a long residence time of 2H2O, which causes a delay of the DSPC isotopic decay over time due to ongoing synthesis. On the other hand, the simultaneous infusion of two tracers in 23 infants allowed us to make comparisons between the two tracers’ fractional incorporation rates within the same subjects, which clearly strengthened the power of the statistical analysis.
From a nutritional point of view, study infants received fat-free parenteral nutrition with 5.2 ± 1.5 mg/kg/min of glucose, 2 g/kg/day of amino acids, and energy intake of 28–40 kcal/kg/day. Although this energy intake reflects what is often prescribed to preterm infants in the first days of life (17), it provides an energy intake well below energy expenditure. We do not know if the relative contributions of de novo lipogenesis and circulating FAs to the DSPC-FSR could be increased by higher energy intake or by adding fat emulsion to the parenteral nutrition. Higher glucose intake could increase the de novo lipogenesis and decrease the contribution of fat by shutting down lipolysis and reducing plasma FFA. Alternatively, increasing plasma triglycerides and FFA either from increased lipolysis of body stores or by administering i.v. lipid emulsions could increase the availability of FFA precursors. Studies in preterm infants addressing both these issues are in progress.
In vitro data suggested that the alveolar type II cells are capable of active de novo FA synthesis, which is inhibited when exogenous palmitate is available (4, 9). In vivo 3H-acetate and 14C-palmitate have been used by many researchers to investigate the incorporation of FAs from plasma and de novo synthesis into surfactant (10, 45, 50). The major limitation of these studies is that the FA contribution to surfactant phosphatidylcholine incorporation was based only on product enrichment without taking into account the precursor enrichment. Therefore, it is difficult to compare these data directly with the values obtained in our study. More recently, Martini et al. (11, 15, 51, 52) used a simultaneous infusion of uniformly labeled 13C acetate and palmitate in adult pigs to quantify surfactant DSPC kinetics during low-dose glucose infusion. They showed that the phosphatidylcholine-bound palmitate incorporation rate from plasma palmitate was 10 times higher than that derived from de novo synthesis. During high rate glucose infusion, the contribution of de novo synthesis of palmitate to phosphatidylcholine synthesis significantly increased and the contribution from plasma preformed palmitate significantly decreased (12). It is conceivable that these large and fasted animals had significant body fat stores and a high rate of lipolysis, and therefore a large amount of plasma FFA were available for DSPC synthesis. Clearly this does not reflect the metabolic condition of the preterm infants in this study.
The FSR values from plasma palmitate and glucose found in our study were higher than those found by us and others in preterm infants with RDS; these values are closer to the values found in term infants with no lung disease (14, 15, 51, 53–56). However, the purpose of this study was to compare two metabolic tracers in the same infants and, thus, patient selection criteria, degree and stage of respiratory failure, and treatment modality could account for the difference in measurements between this and our previous studies.
In summary, this study provides new data on the contribution of the de novo synthesis and preformed pathways to the DSPC-palmitate synthesis in preterm infants on low energy intake. Further studies on the quality and amount of energy intake are needed to assess the role of nutrition on surfactant metabolism in preterm newborns.
Acknowledgments
The authors are extremely grateful to the nurses of the Neonatal Intensive Care of Padova, who helped us in collecting tracheal samples. Without their help, we would not have accomplished this study.
Footnotes
Abbreviations:
- DPPC
- dipalmitoyl-phosphatidylcholine
- DSPC
- disaturated-phosphatidylcholine
- FSR
- fractional synthesis rate
- GC-IRMS
- gas-chromatography-combustion interface-isotope ratio mass spectrometry
- i.v.
- intravenous
- MPE
- mol% excess
- RDS
- respiratory distress syndrome
REFERENCES
- 1.Jobe A. H. 1993. Pulmonary surfactant therapy. N. Engl. J. Med. 328: 861–868. [DOI] [PubMed] [Google Scholar]
- 2.Batenburg J. J. 1992. Surfactant phospholipids: synthesis and storage. Am. J. Physiol. 262: L367–L385. [DOI] [PubMed] [Google Scholar]
- 3.Rooney S. A. 2001. Regulation of surfactant secretion. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129: 233–243. [DOI] [PubMed] [Google Scholar]
- 4.Maniscalco W. M., Finkelstein J. N., Parkhurst A. B. 1982. De novo fatty acid synthesis in developing rat lung. Biochim. Biophys. Acta. 711: 49–58. [DOI] [PubMed] [Google Scholar]
- 5.Wang M. C., Meng H. C. 1974. Synthesis of phospholipids and phospholipid fatty acids by isolated perfused rat lung. Lipids. 9: 63–67. [DOI] [PubMed] [Google Scholar]
- 6.Buechler K. F., Rhoades R. A. 1980. Fatty acid synthesis in the perfused rat lung. Biochim. Biophys. Acta. 619: 186–195. [DOI] [PubMed] [Google Scholar]
- 7.Bassett D. J., Fisher A. B., Rabinowitz J. L. 1974. Effect of hypoxia on incorporation of glucose carbons into lipids by isolated rat lung. Am. J. Physiol. 227: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 8.Rhoades R. A., Shaw M. E., Eskew M. L. 1975. Influence of altered O2 tension on substrate metabolism in perfused rat lung. Am. J. Physiol. 229: 1476–1479. [DOI] [PubMed] [Google Scholar]
- 9.Maniscalco W. M., Stremmel W., Heeney-Campbell M. 1990. Uptake of palmitic acid by rabbit alveolar type II cells. Am. J. Physiol. 259: L206–L212. [DOI] [PubMed] [Google Scholar]
- 10.Jobe A. 1979. An in vivo comparison of acetate and palmitate as precursors of surfactant phosphatidylcholine. Biochim. Biophys. Acta. 572: 404–412. [DOI] [PubMed] [Google Scholar]
- 11.Martini W. Z., Chinkes D. L., Barrow R. E., Murphey E. D., Wolfe R. R. 1999. Lung surfactant kinetics in conscious pigs. Am. J. Physiol. 277: E187–E195. [DOI] [PubMed] [Google Scholar]
- 12.Martini W. Z., Irtun O., Chinkes D. L., Barrow R. E., Wolfe R. R. 2000. Glucose effects on lung surfactant kinetics in conscious pigs. Am. J. Physiol. Endocrinol. Metab. 279: E920–E926. [DOI] [PubMed] [Google Scholar]
- 13.Martini W. Z., Irtun O., Chinkes D. L., Barrow R. E., Wolfe R. R. 2001. Surfactant phosphatidylcholine in thermally injured pigs. Crit. Care Med. 29: 1417–1422. [DOI] [PubMed] [Google Scholar]
- 14.Bunt J. E., Zimmermann L. J., Wattimena J. L., van Beek R. H., Sauer P. J., Carnielli V. P. 1998. Endogenous surfactant turnover in preterm infants measured with stable isotopes. Am. J. Respir. Crit. Care Med. 157: 810–814. [DOI] [PubMed] [Google Scholar]
- 15.Cogo P. E., Carnielli V. P., Bunt J. E., Badon T., Giordano G., Zacchello F., Sauer P. J., Zimmermann L. J. 1999. Endogenous surfactant metabolism in critically ill infants measured with stable isotope labeled fatty acids. Pediatr. Res. 45: 242–246. [DOI] [PubMed] [Google Scholar]
- 16.Cogo P. E., Gucciardi A., Traldi U., Hilkert A. W., Verlato G., Carnielli V. 2005. Measurement of pulmonary surfactant disaturated-phosphatidylcholine synthesis in human infants using deuterium incorporation from body water. J. Mass Spectrom. 40: 876–881. [DOI] [PubMed] [Google Scholar]
- 17.Grover A., Khashu M., Mukherjee A., Kairamkonda V. 2008. Iatrogenic malnutrition in neonatal intensive care units: urgent need to modify practice. JPEN J. Parenter. Enteral Nutr. 32: 140–144. [DOI] [PubMed] [Google Scholar]
- 18.Gail D. B., Massaro G. D., Massaro D. 1977. Influence of fasting on the lung. J. Appl. Physiol. 42: 88–92. [DOI] [PubMed] [Google Scholar]
- 19.Faridy E. E. 1970. Effect of food and water deprivation on surface activity of lungs of rats. J. Appl. Physiol. 29: 493–498. [DOI] [PubMed] [Google Scholar]
- 20.Weiss H. S., Jurrus E. 1971. Starvation of compliance and surfactant of the rat lung. Respir. Physiol. 12: 123–129. [DOI] [PubMed] [Google Scholar]
- 21.Garbagni R., Coppo F., Grassini G., Cardellino G. 1968. Effects of lipide loading and fasting on pulmonary surfactant. Respiration. 25: 458–464. [DOI] [PubMed] [Google Scholar]
- 22.Thet L. A., Alvarez H. 1982. Effect of hyperventilation and starvation on rat lung mechanics and surfactant. Am. Rev. Respir. Dis. 126: 286–290. [DOI] [PubMed] [Google Scholar]
- 23.Lechner A. J., Winston D. C., Bauman J. E. 1986. Lung mechanics, cellularity, and surfactant after prenatal starvation in guinea pigs. J. Appl. Physiol. 60: 1610–1614. [DOI] [PubMed] [Google Scholar]
- 24.Harding R., Tester M. L., Moss T. J., Davey M. G., Louey S., Joyce B., Hooper S. B., Maritz G. 2000. Effects of intra-uterine growth restriction on the control of breathing and lung development after birth. Clin. Exp. Pharmacol. Physiol. 27: 114–119. [DOI] [PubMed] [Google Scholar]
- 25.Chander A., Fisher A. B. 1988. Choline-phosphate cytidyltransferase activity and phosphatidylcholine synthesis in rat granular pneumocytes are increased with exogenous fatty acids. Biochim. Biophys. Acta. 958: 343–351. [DOI] [PubMed] [Google Scholar]
- 26.Regev R. H., Lusky A., Dolfin T., Litmanovitz I., Arnon S., Reichman B. 2003. Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J. Pediatr. 143: 186–191. [DOI] [PubMed] [Google Scholar]
- 27.Lum S., Hoo A. F., Dezateux C., Goetz I., Wade A., DeRooy L., Costeloe K., Stocks J. 2001. The association between birthweight, sex, and airway function in infants of nonsmoking mothers. Am. J. Respir. Crit. Care Med. 164: 2078–2084. [DOI] [PubMed] [Google Scholar]
- 28.Cogo P. E., Giordano G., Badon T., Orzali A., Zimmermann I. U., Zacchello F., Sauer P. J., Carnielli V. P. 1997. Simultaneous measurement of the rates of appearance of palmitic and linoleic acid in critically ill infants. Pediatr. Res. 41: 178–182. [DOI] [PubMed] [Google Scholar]
- 29.Carnielli V. P., Pederzini F., Vittorangeli R., Luijendijk I. H., Boomaars W. E., Pedrotti D., Sauer P. J. 1996. Plasma and red blood cell fatty acid of very low birth weight infants fed exclusively with expressed preterm human milk. Pediatr. Res. 39: 671–679. [DOI] [PubMed] [Google Scholar]
- 30.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 31.Torresin M., Zimmermann L. J., Cogo P. E., Cavicchioli P., Badon T., Giordano G., Zacchello F., Sauer P. J., Carnielli V. P. 2000. Exogenous surfactant kinetics in infant respiratory distress syndrome: a novel method with stable isotopes. Am. J. Respir. Crit. Care Med. 161: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 32.Gucciardi A., Cogo P. E., Traldi U., Eaton S., Darch T., Simonato M., Ori C., Carnielli V. P. 2008. Simplified method for microlitre deuterium measurements in water and urine by gas chromatography-high-temperature conversion-isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 22: 2097–2103. [DOI] [PubMed] [Google Scholar]
- 33.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 34.Touchstone J. C., Chen J. C., Beaver K. M. 1979. Improved separation of phospholipids in thin layer chromatography. Lipids. 15: 61–62. [Google Scholar]
- 35.Christie W. 1989. The analysis of fatty acids. Gas chromatography and lipids: a practical guide. The Oil Press, Ayr, Scotland. [Google Scholar]
- 36.Diraison F., Pachiaudi C., Beylot M. 1996. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: determination of the average number of deuterium atoms incorporated. Metabolism. 45: 817–821. [DOI] [PubMed] [Google Scholar]
- 37.Merchak A., Patterson B. W., Yarasheski K. E., Hamvas A. 2000. Use of stable isotope labeling technique and mass isotopomer distribution analysis of [(13)C]palmitate isolated from surfactant disaturated phospholipids to study surfactant in vivo kinetics in a premature infant. J. Mass Spectrom. 35: 734–738. [DOI] [PubMed] [Google Scholar]
- 38.Bohlin K., Patterson B. W., Spence K. L., Merchak A., Zozobrado J. C., Zimmermann L. J., Carnielli V. P., Hamvas A. 2005. Metabolic kinetics of pulmonary surfactant in newborn infants using endogenous stable isotope techniques. J. Lipid Res. 46: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 39.Ridsdale R., Post M. 2004. Surfactant lipid synthesis and lamellar body formation in glycogen-laden type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 287: L743–L751. [DOI] [PubMed] [Google Scholar]
- 40.Spence K. L., Zozobrado J. C., Patterson B. W., Hamvas A. 2005. Substrate utilization and kinetics of surfactant metabolism in evolving bronchopulmonary dysplasia. J. Pediatr. 147: 480–485. [DOI] [PubMed] [Google Scholar]
- 41.Seidner S. R., Jobe A. H., Coalson J. J., Ikegami M. 1998. Abnormal surfactant metabolism and function in preterm ventilated baboons. Am. J. Respir. Crit. Care Med. 158: 1982–1989. [DOI] [PubMed] [Google Scholar]
- 42.Wareham J. A., Ferlauto J. J., Wells D. H., Newell R. W. 1989. Hypertriglyceridemia in infants with bronchopulmonary dysplasia. J. Pediatr. 114: 458–460. [DOI] [PubMed] [Google Scholar]
- 43.Cogo P. E., Zimmermann L. J., Pesavento R., Sacchetto E., Burighel A., Rosso F., Badon T., Verlato G., Carnielli V. P. 2003. Surfactant kinetics in preterm infants on mechanical ventilation who did and did not develop bronchopulmonary dysplasia. Crit. Care Med. 31: 1532–1538. [DOI] [PubMed] [Google Scholar]
- 44.Bohlin K., Merchak A., Spence K., Patterson B. W., Hamvas A. 2003. Endogenous surfactant metabolism in newborn infants with and without respiratory failure. Pediatr. Res. 54: 185–191. [DOI] [PubMed] [Google Scholar]
- 45.Guice K. S., Oldham K. T., Wolfe R. R., Simon R. H. 1987. Lung injury in acute pancreatitis: primary inhibition of pulmonary phospholipid synthesis. Am. J. Surg. 153: 54–61. [DOI] [PubMed] [Google Scholar]
- 46.Oldham K. T., Guice K. S., Stetson P. S., Wolfe R. R. 1989. Bacteremia-induced suppression of alveolar surfactant production. J. Surg. Res. 47: 397–402. [DOI] [PubMed] [Google Scholar]
- 47.Gross I., Ilic I., Wilson C. M., Rooney S. A. 1976. The influence of postnatal nutritional deprivation on the phospholipid content of developing rat lung. Biochim. Biophys. Acta. 441: 412–422. [DOI] [PubMed] [Google Scholar]
- 48.Rooney S. A., Gobran L., Gross I., Wai-lee T. S., Nardone L. L., Motoyama E. K. 1976. Studies on pulmonary surfactant. Effects of cortisol administration to fetal rabbits on lung phospholipid content, composition and biosynthesis. Biochim. Biophys. Acta. 450: 121–130. [DOI] [PubMed] [Google Scholar]
- 49.Rubin J. W., Clowes G. H., Jr., Macnicol M. F., Gavin J. W. 1972. Impaired pulmonary surfactant synthesis in starvation and severe nonthoracic sepsis. Am. J. Surg. 123: 461–467. [DOI] [PubMed] [Google Scholar]
- 50.Jobe A., Ikegami M., Sarton-Miller I. 1980. The in vivo labeling with acetate and palmitate of lung phospholipids from developing and adult rabbits. Biochim. Biophys. Acta. 617: 65–75. [DOI] [PubMed] [Google Scholar]
- 51.Cavicchioli P., Zimmermann L. J., Cogo P. E., Badon T., Giordano G., Torresin M., Zacchello F., Carnielli V. P. 2001. Endogenous surfactant turnover in preterm infants with respiratory distress syndrome studied with stable isotope lipids. Am. J. Respir. Crit. Care Med. 163: 55–60. [DOI] [PubMed] [Google Scholar]
- 52.Hellerstein M. K., Neese R. A. 1992. Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. Am. J. Physiol. 263: E988–E1001. [DOI] [PubMed] [Google Scholar]
- 53.Cogo P. E., Zimmermann L. J., Rosso F., Tormena F., Gamba P., Verlato G., Baritussio A., Carnielli V. P. 2002. Surfactant synthesis and kinetics in infants with congenital diaphragmatic hernia. Am. J. Respir. Crit. Care Med. 166: 154–158. [DOI] [PubMed] [Google Scholar]
- 54.Cogo P. E., Zimmermann L. J., Verlato G., Midrio P., Gucciardi A., Ori C., Carnielli V. P. 2004. A dual stable isotope tracer method for the measurement of surfactant disaturated-phosphatidylcholine net synthesis in infants with congenital diaphragmatic hernia. Pediatr. Res. 56: 184–190. [DOI] [PubMed] [Google Scholar]
- 55.Bunt J. E., Carnielli V. P., Darcos Wattimena J. L., Hop W. C., Sauer P. J., Zimmermann L. J. 2000. The effect in premature infants of prenatal corticosteroids on endogenous surfactant synthesis as measured with stable isotopes. Am. J. Respir. Crit. Care Med. 162: 844–849. [DOI] [PubMed] [Google Scholar]
- 56.Bunt J. E., Carnielli V. P., Janssen D. J., Wattimena J. L., Hop W. C., Sauer P. J., Zimmermann L. J. 2000. Treatment with exogenous surfactant stimulates endogenous surfactant synthesis in premature infants with respiratory distress syndrome. Crit. Care Med. 28: 3383–3388. [DOI] [PubMed] [Google Scholar]