Abstract
Objectives
Children born to teenage mothers are at risk for more physical and cognitive problems than those born to adult mothers. Our objective was to examine differences in size and intelligence between two cohorts of offspring born to adolescent (n = 357) and adult mothers (n = 668) who attended the same prenatal clinic.
Methods
Two prospective study cohorts were assessed children from gestation through age 6 years. The adult cohort was studied in the mid-1980’s and the teen cohort was evaluated in the mid-1990’s. Both samples were of low socio-economic status. The same study design and measures allowed us to adjust for the covariates of size and IQ.
Results
Offspring of adolescent mothers had a significantly smaller mean head circumference (5 mm) (HC) and higher body mass index (BMI) than offspring of adult mothers. Offspring of adolescent mothers scored significantly lower than the offspring of adult mothers on the Stanford-Binet (SBIS) composite score (4 points), and the quantitative (6.2 points), verbal reasoning (4.8 points), and short-term memory (3.9 points) area scores. Additional predictors of child IQ were maternal IQ, home environment, race, and number of siblings. When child HC was entered into our final regression model for the SBIS, maternal age and HC significantly predicted the composite score, the verbal reasoning, and short-term memory area scores. A 1 cm decrease in HC predicted a 1 point decrease in the SBIS composite score.
Conclusions
Compared to offspring of adult women, children of adolescent mothers have lower mean scores on cognitive measures, smaller head circumference, and higher BMI. These differences were significant after adjusting for differences between the two groups. Adolescent mothers and their children would benefit from interventions such as parenting support, education about nutritional needs, and advice on enriching the environments of their children.
Keywords: children, teenage mothers, cognitive development, growth, IQ, BMI
Children of adolescent mothers may be at greater risk than children of older mothers for poorer growth outcomes [1-5]. In a national longitudinal study of maternal age and neonatal risk status [6], young maternal age predicted lower birthweight and shorter gestational age after controlling for maternal health and sociodemographic status. Less is known about child growth outcomes among older children of adolescent mothers
In addition to growth, children of adolescent mothers may be at risk of cognitive deficits [7-9]. Intellectual, language, and socio-emotional delays have been reported [10] as well as lower academic achievement [11]. These differences are found even after accounting for social and economic disadvantage [8, 9]. Dahinten and colleagues [12] found that children of teen mothers had lower math test scores adjusting for maternal depression, nurturing, and rejecting parenting behaviors. Other studies, however, suggest that the association between maternal age and cognitive status is explained by socio-economic status or family background. Using a national sample, Turley [13] found that lower test scores of children born to younger mothers were due to their family background rather than their mother’s age, while Williams and Decoufle [14] found no association between young maternal age and the rate of mental retardation in 10 year old offspring. Fergusson & Woodward [15] found that controlling for home environment reduced the relation between young maternal age and poorer educational outcomes, but did not eliminate it.
In this report, we compare body size and IQ in 6-year-olds using two cohorts, one of offspring of adult mothers, and the other, children of teenaged mothers. The women attended the same prenatal clinic. The study designs were similar and most of the personnel and instruments were the same between the two studies. The first cohort has 668 offspring of adult-aged women (18 years of age and older). The second cohort has 357 offspring of teenaged women (aged 12 to 18 years of age). Our hypothesis is that children of teenaged mothers will be smaller in head circumference, weight, and height and will have lower IQ’s at age 6 after adjusting for significant covariates.
METHODS
Study Participants
Adult Mother Cohort
The initial study cohort was a consecutive sample of women who attended the prenatal clinic of the Magee-Womens Hospital in Pittsburgh Pennsylvania from 1982 through 1985. The Institutional Review Board of the University of Pittsburgh approved each phase of the study protocol and written informed consent was obtained from each woman. There was no interventional component to this study. Women who were at least 18 years of age were enrolled at their fourth prenatal month clinic visit. Eighty-five percent of the women who were contacted agreed to participate in the study. Two cohorts were selected from the initial sample: 1) All women who drank 3 or more alcoholic drinks per week and a random sample of women who drank less often or not at all were selected for a study of the effects of alcohol use during pregnancy, and 2) All women who used marijuana at the rate of 2 or more joints per month and a random sample of women who used marijuana less often or not at all were chosen for a study of the effects of marijuana exposure during gestation. Women could be in one or in both of the cohorts. The two cohorts combined had a total of 829 women [16]. By delivery, this was reduced to 763 mothers and their live-born singletons newborns. The 66 women who did not participate at this phase of the study included 21 who moved from the Pittsburgh area, 16 who were lost to follow-up, 8 who refused the delivery interview and newborn examination, 2 multiple births, 18 fetal deaths, and 1 adoption. At 6 years, 668 children were examined, which was 88% of the birth cohort. Losses between birth and 6 years occurred due to moves away from Pittsburgh (n = 41), refusals (n = 34), death of a child (n = 4), adoption or foster care (n = 8), and lost to follow-up (n = 8). We considered only those offspring who had complete assessments at birth and 6 years. There were no differences in maternal age, income, substance use, or race between those who were retained and those who were not in the analysis.
Teenage Mother Cohort
Adolescent mothers were interviewed when they came in for their fourth month prenatal visit at the same prenatal clinic at the Magee-Womens Hospital, between 1990 and 1995. The Institutional Review Board of the University of Pittsburgh approved each phase of the study protocol and written informed consent was obtained from all participants. This was a study of substance use during pregnancy among teenagers and the effects of substance exposure on offspring outcomes. The cohort was a sequential sample of all pregnant adolescents. There was no intervention component to this study and no oversampling of substance use. The initial refusal rate was 0.7%. Of the 445 teenagers who were enrolled in the study, 15 subsequently moved out of the area and 1 refused the delivery interview. There were 6 twins, 5 spontaneous abortions, 2 stillborn infants, and 3 liveborn infants died of prematurity. Thus, 413 liveborn singletons were eligible for the six-year follow-up visit.
At the six-year postpartum phase [17], 357 offspring were evaluated. Losses between birth and six years occurred due to moves out of state (n = 9), refusals (n = 10), deaths (n = 6), placement, adoption or foster care (n = 7), and lost to follow-up (n = 24). Almost 7% (n = 23) of the children were not in the custody of their birth mothers. In these cases, information on their environment was provided by their current custodians. We included only offspring who had complete assessments at both time points. There were no differences in age, income, substance use, or race between those who were retained and those who lost to follow-up.
Measures
Child Size
At birth, we measured weight, length, and head circumference. From these we calculated ponderal index (weight in grams × 100 / height in cm3), % low birth weight (LBW: weight <2500 g) and % small-for-gestational age (SGA: birth weight less than the tenth percentile for gestational age) using the standards of Brenner et al. [18]. Measures at 6 years included weight, height, head circumference, body mass index (or BMI weight in kg / height in meters2), and % overweight (BMI > 95th percentile) [19]. In both cohorts, offspring were examined by pediatric nurse clinicians who were trained to reliability and were unaware of the substance exposure status of the children. Twenty-four children born to diabetic mothers and two children with sickle cell anemia were excluded from the analysis.
Child Cognitive Status
Cognitive ability at 6 years was assessed using the Stanford-Binet Intelligence Scale, fourth edition (SBIS) [20]. Outcomes included the composite score and the verbal reasoning, abstract/visual reasoning, quantitative reasoning, and short-term memory area scores. Because the predictors of child IQ differed by race in the adult cohort [21], we analyzed the data with races combined and separately. Twenty-four mentally and physically handicapped children who were unable to complete the SBIS were excluded from the analyses.
In the teen cohort, home environment was measured using the Home Observation for Measurement of the Environment-Short Form (HOME-SF) [22]. In the adult cohort, the Home Screening Questionnaire (HSQ) [23] was used at age six. The HOME-SF was administered in this study at age 10. Both the HSQ and the HOME measure the quality and quantity of support available to the child for cognitive, social, and emotional development. The HSQ correlates well with the HOME [24]. In our analyses of the adult and adolescent cohorts, we elected to use the same instrument whenever possible. This was not possible for the home environment. The HSQ and HOME-SF measures within the adult cohort were standardized and compared using the matched sign test. The difference in the standardized scores was near zero and not statistically different, indicating that the relative distributions of the responses on the HOME and the HSQ did not differ. Therefore, since home environment at the two phases did not change, to maintain comparability, the HOME-SF measured at age 10 was used for the adult cohort.
Maternal cognitive status was measured in both cohorts at the six-year assessment using the vocabulary and block design subtests of the Wechsler Adult Intelligence Scale Revised (WAIS-R) [25]. Maternal intellectual ability is known to be highly correlated with child IQ [26].
Marital status has been found to be related to child cognitive outcome [27]. This measure (married/not married) was assessed in both cohorts. In addition, maternal depression was assessed in both cohorts using the Center for Epidemiologic Studies-Depression scale (CES-D). The CES-D is a 20-item self-report instrument developed for use in general population samples [28]. This scale correlates well with other established measures of depression, such as the Zung (r = .90) and the Beck (r = .81), and has established validity [29].
Since the original aims of both of these studies were to examine prenatal substance exposures on child outcomes, these substances were included as control variables in the current analyses. In both cohorts, prenatal cocaine and other illicit drug use was rare, so these measures were not included. Prenatal tobacco exposure (PTE) was measured as average daily number of cigarettes in the first trimester. Prenatal alcohol exposure (PAE) was measured as average daily number of drinks in the first trimester, and prenatal marijuana exposure (PME) was measured as average daily number of joints in the first trimester.
Data Analysis
Maternal age was defined by the cohort to which the subject belonged: offspring of the adult cohort (0)/offspring of the teen cohort (1). Initially, we tested the effects of each covariate on each outcome by cohort. We examined whether the effect of each covariate on the outcome variable was the same between cohorts by testing whether the slopes of the regression lines were parallel. When the parallelism test failed, a cohort by covariate interaction term was added to the model to represent the different effects of that covariate in the two cohorts. Stepwise multivariate regressions were used next to examine the associations of each of the six-year growth and IQ outcomes with maternal age. The general model tested was,
Y = α1 + β1 (cohort) + β2 (covariate) + β3 (cohort × covariate) + error where Y represented child’s outcome variable. Tolerance, a measure of multicolinearity, was screened to insure that the estimates of the regression slopes were stable. We also screened for outliers and influential cases. These cases were excluded.
Body size outcomes at 6 years included: weight, height, head circumference, body mass index (BMI), and percent overweight. Covariates that were considered in the multivariate analyses were based on the literature. Covariates that differed by cohort were added to the regression model. These included maternal height, prepregnancy maternal weight, race, child’s age, gender, marital status, home environment, number of siblings, PTE, PAE, and PME.
The cognitive outcomes at age 6 included the composite score of the Stanford-Binet Intelligence Scale (SBIS) and the area scores, verbal reasoning, abstract/visual reasoning, quantitative reasoning, and short-term memory. The covariates adjusted in these regression analyses were maternal IQ, race, child gender, home environment, marital status, number of siblings in the household, maternal custody of the child, and PTE, PAE, and PME.
RESULTS
Bivariate Results
At delivery, the average age of the adult mother cohort was 23.0 years (range: 18 - 42); the teen mother cohort was 16.3 years of age (range: 12 - 18). Teen mothers were significantly less likely to be married, and more likely to be African-American and primigravidous than the adult mothers. The adult mothers used more cigarettes, alcohol, and marijuana than did the teen mothers (Table 1). There were no significant differences between the two cohorts on gestational age, birthweight, head circumference, and percent low birthweight or small for gestational age. At birth, the offspring of adult mothers were shorter in length and had a higher ponderal index than offspring of teenage mothers, who were leaner (Table 1).
Table 1.
Characteristics of Mothers and Offspring at Birth in the Adult and Teenaged Cohorts (Unadjusted)
| Mother | Adults N = 653a | Teens N = 355 | P-value* | ||
|---|---|---|---|---|---|
| Mean/% | Range | Mean/% | Range | ||
| Age (years) | 23.0 | (18-42) | 16.3 | (12-18) | <.001 |
| % Married | 31.9 | 0.8 | <.001 | ||
| % African-American | 46.9 | 69.3 | <.001 | ||
| % Primigravidas | 31.0 | 77.0 | <.001 | ||
| Maternal height (inches) | 64.1 | (56-74) | 63.9 | (54-74) | NS |
| Prepregnancy weight (lbs) | 136.3 | (79-312) | 132.6 | (83-250) | NS |
| PTE (cigarettes/day) | 10.8 | (0-70.0) | 5.8 | (0-50.0) | <.001 |
| PAE (drinks/day) | 0.67 | (0-24.7) | 0.38 | (0-8.5) | <.001 |
| PME (joints/day) | 0.46 | (0-9.4) | 0.13 | (0-8.0) | <.001 |
| Offspring | Adults N = 653a | Teens N = 355 | P-value | ||
|---|---|---|---|---|---|
| Mean/% | Range | Mean/% | Range | ||
| % Male | 50.0 | 52.1 | NS | ||
| Gestational age (weeks) | 39.8 | (28-44) | 39.6 | (27-43) | NS |
| Birthweight (grams) | 3205.1 | (1040-4650) | 3158.1 | (996-4863) | NS |
| Birth length (cm) | 49.3 | 38.5-56.0 | 49.8 | (38.5-56.0) | <.01 |
| Head circumference (mm) | 341.1 | 245-380 | 343.1 | (245-389) | NS |
| Ponderal index | 2.7 | 1.13-3.93 | 2.5 | (1.63-3.53) | <.001 |
| % LBW | 10.0 | 8.5 | NS | ||
| %SGA | 10.4 | 9.3 | NS | ||
Children born to diabetic mothers were excluded.
Using t-test for continuous variables, Mann-Whitney for skewed variables, Chi-Square for dichotomous variables.
The unadjusted comparison of maternal and offspring characteristics in the two cohorts at six years is presented in Table 2. There were no differences between the adult and teen mother cohorts in maternal years of education or levels of depressive symptoms. The adult mothers were more likely to be married, and had a higher average IQ than did the teen mothers. There were no significant differences in weight, height, or percent overweight between the offspring of adult mothers and the offspring of teen mothers. However, the offspring of teen mothers had a smaller average head circumference than the offspring of adult mothers and a higher BMI (p = .06), than the offspring of adult mothers.
Table 2.
Characteristics of Mothers and Offspring at 6 years postpartum in the Adult and Teenaged Cohorts (Unadjusted)
| Mother | Adults N = 653a | Teens N = 355 | P-value | ||
|---|---|---|---|---|---|
| Mean/% | Range | Mean/% | Range | ||
| Age (years)b | 30.4 | (24-65) | 24.5 | (19-69) | <0.001 |
| % Married | 35.0 | 20.0 | <0.001 | ||
| Years of School | 12.2 | (7-20) | 12.2 | (7-17) | NS |
| Average monthly income ($)c | 1186 | (0-7500) | 1335 | (0-8000) | <0.050 |
| Depressive symptoms (CES-D) | 37.7 | (20-66) | 37.9 | (20-70) | NS |
| Maternal IQ | 88.4 | (58-122) | 81.4 | (55-130) | <0.001 |
| Offspring | Adults N = 653 | Teens N = 355 | P-value | ||
|---|---|---|---|---|---|
| Mean/% | Range | Mean/% | Range | ||
| Age (years) | 6.5 | (5.5-9.0) | 6.4 | (5.8-9.9) | <0.010 |
| Average number of siblings | 1.5 | (0-6) | 1.2 | (0-5) | <0.001 |
| % not in maternal custody | 4.0 | 8.0 | <0.050 | ||
| Weight (kg) | 23.02 | (14.4-44.1) | 22.99 | (13.1-43.4) | NS |
| Height (cm) | 119.3 | (91.4-142.2) | 118.4 | <.050 | |
| Head Circumference (mm) | 520.5 | (462-565) | 514.7 | (470-556) | <.001 |
| BMI | 16.05 | (11.9-29.1) | 16.33 | (12.7-27.7) | 0.06 |
| % Overweight (BMI > 95th %) | 13.9 | 13.7 | NS | ||
| Stanford Binet Scoresd | |||||
| Composite | 91.6 | (55-131) | 84.6 | (58-125) | <0.001 |
| Verbal Reasoning | 100.5 | (60-136) | 93.8 | (62-127) | <0.001 |
| Quantitative | 93.8 | (49-131) | 84.6 | (56-124) | <0.001 |
| Short-term Memory | 92.6 | (48-150) | 86.9 | (54-130) | <0.001 |
| Abstract/Visual Reasoning | 85.0 | (54-141) | 83.1 | (58-132) | <0.050 |
Children with sickle cell anemia were excluded.
Age includes caregivers.
Adult cohort birth data from mid 1980’s; Teen cohort birth data from mid 1990’s.
Mentally retarded children were excluded.
The unadjusted bivariate comparisons of Stanford Binet composite and area scores for the offspring of the adult and teen mothers are presented in Table 2. Offspring of teen mothers scored significantly lower on the Composite Score and on the verbal reasoning, abstract/visual reasoning, quantitative reasoning, and short-term memory area scales. The average composite score among the offspring of teen mothers was 7 points lower than the score for the offspring of adults. Among the area scores, the largest difference was found with quantitative reasoning (9.2 points) and the smallest difference was found with abstract/visual reasoning (<2 points).
Multivariate Results
Results of the linear regression analyses for growth and IQ measures are presented in Table 3. Head circumference and BMI were the only growth-related outcomes that were significantly different between cohorts at age 6 after controlling for other significant covariates. Head circumference was significantly smaller for the offspring of the teen mother cohort compared to the offspring of the adult mother cohort. The difference in average head circumference between the two cohorts was 5.4millimeters. Other significant predictors of head circumference included child age, gender, maternal height, maternal prepregnancy weight, and number of siblings. The total variance in head circumference explained by the variables was 16%.
Table 3.
Predictors of Size and IQ at Age 6 Using Multiple Regression Analyses
| Size | |||||
|---|---|---|---|---|---|
| Head Circumference R2 = 0.16 | BMI R2 = 0.12 | ||||
| Beta | T | Beta | T | ||
| Cohorta | -5.40 | -5.38# | Cohort | 0.55 | 2.35** |
| Child Age | 4.83 | 5.61# | Child Age | 0.57 | 4.25# |
| Genderb | 7.07 | 7.78# | Maternal Height | -0.07 | -2.42** |
| Maternal Height | 0.43 | 2.51** | Pre-pregnancy Weight | 3.56 | 9.41# |
| Pre-Pregnancy Weight | 12.40 | 5.36# | Number of Siblings | -0.24 | -3.36# |
| Number of Siblings | -1.66 | -3.78# | Home environment | 0.09 | 2.36** |
| Ponderal Index at Birth | 0.64 | 2.36** | |||
| Stanford-Binet | |||||
|---|---|---|---|---|---|
| Composite Score R2 = 0.25 | Verbal R2 = 0.29 | ||||
| Beta | T | Beta | T | ||
| Cohort | -4.09 | -4.61# | Cohort | -4.78 | -6.50# |
| Maternal IQc | 0.32 | 7.84# | Maternal IQ | 0.25 | 7.74# |
| Home Environment | 0.90 | 4.99# | Home Environment | 0.63 | 5.23# |
| Raced | 4.40 | 4.84# | Race | 3.38 | 4.55# |
| Number of Siblings | -1.55 | -4.17# | Number of Siblings | -2.15 | -7.15# |
| Home X Cohort | -0.59 | -1.98* | PME | -1.57 | -1.88* |
| Quantitative R2 = 0.13 | Short-term Memory R2 = 0.16 | ||||
|---|---|---|---|---|---|
| Beta | T | Beta | T | ||
| Cohort | -6.17 | -4.99# | Cohort | -3.90 | -3.81# |
| Maternal IQ | 0.29 | 5.03# | Maternal IQ | 0.38 | 7.50# |
| Home Environment | 0.53 | 2.58** | Home Environment | 0.65 | 3.96# |
| Race | 3.55 | 2.78** | Number of Siblings | -1.16 | -2.71** |
| Number of Siblings | -1.25 | -2.38** | Maternal IQ X Cohort | -0.22 | -2.50** |
Cohort: teen = 1; adult = 0
Gender: male = 1; female = 0
P-value - p < .05;
P-value - p < .01;
P-value p < .001
For ease of interpretation, maternal IQ and home environment were centered around zero
Race: Caucasian = 1; African-American = 0
P-value - p < .05;
P-value - p < .01;
P-value - p < .001
Cohort also significantly predicted BMI. In contrast to the finding at birth, at age 6, offspring of teenagers had higher BMI’s (greater weight per height) than offspring of adults. Other significant predictors of higher BMI were: older child age, shorter maternal height, higher prepregnancy weight, fewer siblings, poorer home environment, and higher ponderal index at birth. The total explained variance was 12%.
The cohort variable predicted child composite scores and most of the area scores of the SBIS, with the exception of the abstract/visual score, after adjusting for other significant covariates (Table 3). There was a 4-point decrease in the average composite score among the offspring of teen mothers compared to the offspring of the adult mothers. In the subscales, the differences by cohort were 6.2, 4.8, and 3.9 points for the quantitative, verbal, and short-term memory scores, respectively. Additional significant predictors of lower child IQ were lower maternal IQ, poorer home environment, African-American race, and more siblings. Twenty-five percent of the variance on the Composite Score was explained by these variables. Because race was a significant predictor of all of the scores, with the exception of short-term memory, regressions were rerun separately by race. For African-Americans, cohort remained a significant predictor of the composite score, and the quantitative and verbal area scores, but not the abstract/visual or short-term memory area scores. For Caucasians, cohort remained a significant predictor of the composite score, quantitative, verbal, and short-term memory scores but not the abstract/visual area score.
There was a significant interaction between home environment and cohort for the composite score. Home environment had a greater influence on the offspring of the adult mothers than on the offspring of the teenage mothers. There was also a significant interaction between maternal IQ and cohort for the short-term memory area score (Figure 1). Children born to teenage mothers scored lower on short-term memory than children of adult mothers and the magnitude of this difference increased with maternal IQ. For example, at a maternal IQ of 86 points, children of teenage mothers scored an average 3.9 points lower on the short-term memory score than children of adult mothers. At a maternal IQ of 96, the short-term memory scores differed by 6.1 points. The interaction of home environment and cohort was also a significant predictor of the abstract/visual score. The effect of home environment was larger on the abstract/visual scores in the offspring of adults than among the offspring of teenagers. The main effect of cohort, however, was not significant.
Figure 1.
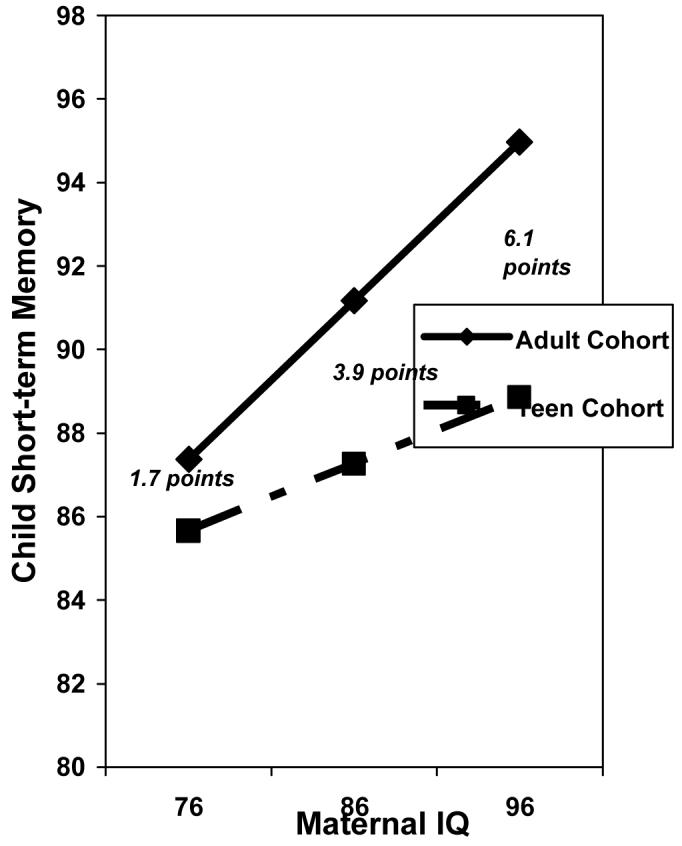
Adjusted Short-term Memory Scores for Offspring in the Adult and Teen Cohorts by Maternal IQ
Head circumference and IQ were significantly related to maternal age. Other research has reported that lower IQ is correlated with smaller head circumference [30-32]. In order to examine this relation, we entered head circumference into our final regression models with the composite score and the area scores as our outcome variables (Table 4). While head circumference significantly predicted the composite, verbal reasoning, and short-term memory scores in the combined cohort analysis, a significant and independent effect of cohort remained. Thus, the difference in head circumferences between cohorts did not explain the association between cohort and child IQ.
Table 4.
Head Circumference and Cohort as Predictors of Cognitive Status
| Composite Score | ||
|---|---|---|
| R2 = 0.25 | Beta | T |
| Cohort | -3.76 | -4.17# |
| Maternal IQ | 0.32 | 7.89# |
| Home Environment | 0.87 | 4.88# |
| Head Circumference | 0.07 | 2.68** |
| Race | 4.36 | 4.76# |
| Number of Siblings | -1.39 | -3.71# |
| Home Environment X Cohort | -0.56 | -1.84* |
| Verbal | ||
|---|---|---|
| R2 = 0.30 | Beta | T |
| Cohort | -4.38 | -5.83# |
| Maternal IQ | 0.26 | 7.75# |
| Home Environment | 0.62 | 5.14# |
| Head Circumference | 0.06 | 2.98** |
| Race | 3.30 | 4.44# |
| Number of Siblings | -2.02 | -6.65# |
| PME | -1.74 | -2.08* |
| Short-term Memory | ||
|---|---|---|
| R2 = 0.14 | Beta | T |
| Cohort | -3.47 | -3.30# |
| Maternal IQ | 0.37 | 7.44# |
| Home Environment | 0.66 | 3.97# |
| Sex | -2.08 | -2.27* |
| Head Circumference | 0.09 | 2.80** |
| Number of Siblings | -0.98 | -2.27* |
| Maternal IQ X Cohort | -0.20 | -2.27* |
P-value p < .05;
P-value p < .01;
P-value p < .001
Ten percent (n = 67) of the adult cohort were 18 years at delivery. The analyses were repeated excluding these cases, and the results reported above remained stable.
DISCUSSION
In this comparison of offspring of adult and teenaged mothers, young maternal age significantly predicted higher BMI and smaller head circumference in six-year-old offspring after adjusting for significant covariates. In addition, offspring of teenagers had lower SBIS composite and quantitative, verbal, and short-term memory area scores compared to offspring of adult mothers.
Although offspring in the two cohorts were not significantly different on most of the birth outcomes, the offspring of adult mothers had a higher ponderal index at birth than the offspring of teenage mothers. This reversed by age six, when the offspring of teenagers had a higher BMI than the offspring of adults. The higher BMI among offspring of teenagers raises public health concern in light of the recent epidemic of childhood obesity in our nation [33] and further research is needed to replicate this finding in other samples. On average, adolescent parents have fewer economical resources and support systems, more stressors [34] and more unhealthy eating behaviors [35, 36]. Teenage mothers who eat less healthy diets are likely to provide a similar diet to their children which might explain their child’s higher BMI at six years of age. Alternatively, it is possible that the increased BMI among offspring of the adolescent mothers can be accounted for by secular changes in body size in the 8-10 years that elapsed between the enrollments of the study cohorts [37].
Children of adolescent mothers had, on average, a smaller head circumference than the offspring of the adult mothers at age 6, although they did not differ on height or weight in the multivariate analyses. Head circumference was not different between the two cohorts at birth, and thus, the difference at 6 years could reflect environmental differences. It is known that the structure of the brain is sensitive to the environment and that environmental variables can affect the brain development of children in humans [38] and laboratory animals [39, 40]. Head circumference correlates with brain volume and is used as an approximate measure of brain growth [41]. Several studies in children have shown that postnatal head growth, as measured directly by MRI or indirectly by head circumference predicted performance on cognitive tests. The larger the brain, the higher the test scores [32, 42-44]. Two additional studies have shown that the brain volume achieved by age 1 predicts intelligence in later childhood [31, 45]. Shaw et al. [38] demonstrated that the thickness of the cerebral cortex is correlated with intelligence. Differences in postnatal brain growth likely reflect the influence of cognitive stimulation and parenting practices during infancy. Cognitive enrichment early in life is associated with improved cognitive performance [46-48]. However, when head circumference and cohort were entered in a final analysis using the combined cohorts in this study, both head circumference and cohort predicted the composite, verbal reasoning, and short-term memory scores. Thus, both of these variables independently predicted cognitive development, and head circumference did not mediate the effect of maternal age on the measures of IQ.
Offspring of the teenage mothers had lower scores on the composite IQ and several of the area scales compared to offspring of adults after controlling for significant covariates such as maternal cognitive status and the home environment. Previous studies have demonstrated that the quality of the home environment is critical during early childhood [26, 38-40]. However, in our study we found that these potential factors have a lesser effect on the children of teenage mothers than the adult mothers. The quality of parenting is also an important factor: Compared with matched samples of older mothers, adolescent mothers were less verbal [49-51], less empathic, less sensitive [52], less warm, had more negative attitudes toward parenting [53], and provided less intellectual stimulation [49], so that by the time their offspring reached school age, they had fallen behind their contemporaries in terms of cognitive abilities.
There were limitations to these analyses. We adjusted for many important factors related to growth and cognition; however, there were additional variables that we could not consider including diet, physical activity levels, paternal IQ and measures of attachment and parenting skills. Each of these is known to be important in predicting growth and cognitive development. In addition, both cohorts were of low socioeconomic status and the results may not represent the association between maternal age and child development among mother-child dyads of higher socioeconomic status. Further, the adult cohort was not a random sample of women who attended the prenatal clinic. The women were selected based on their substance use. This may affect the sample’s generalizability to other populations, although we adjusted for substance use in our analyses to control for these variables. Finally, the two cohorts were sampled from the same site, but they were one decade apart, which also might have led to some differences. Cohort effects for increasing height, weight and BMI have been found in most industrialized countries [e.g., 54 - 57], so it is possible that some of the difference in BMI between the two cohorts may be accounted for by these population trends. On the other hand, IQ scores are also increasing at about 3 points per decade [58] giving credence to our findings of lower IQ scores among the offspring of teenagers who were measured ten years later.
We have found that, at 6 years of age, offspring of adolescent mothers have more cognitive deficits, smaller head circumference and a higher weight to height ratio compared to offspring of adult women. It is important to follow these children of adolescent mothers to determine whether these findings predict additional problems as the children mature. These results suggest that the offspring of adolescent mothers should be identified early and that they may benefit from interventions such as parenting support, education about nutrition needs, and advice on enriching the home environments of the children. Well baby clinics and pediatric offices would be prime locations to target teenage mothers and introduce such interventions.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institute of Drug Abuse (DA03874 PI: N Day; DA09275 PI: M Cornelius) and the National Institute on Alcohol Abuse and Alcoholism (AA06666 PI: N Day; AA08284; PI: M Cornelius). The authors thank the young women and children who made this study possible by contributing their time and sharing their experiences with our interviewers and field staff.
REFERENCES
- 1.Brown H, Fan Y, Gonsoulin W. Obstetric complications in young teenagers. Southern Medical Journal. 1991;84(1):46–48. doi: 10.1097/00007611-199101000-00012. 1991. [DOI] [PubMed] [Google Scholar]
- 2.Fraser A, Brockert J, Ward R. Association of young maternal age with adverse reproductive outcomes. New England Journal of Medicine. 1995;332:1113–1117. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 3.Klein J. Adolescent Pregnancy: Current trends and issues. Pediatrics. 2005;116:281–286. doi: 10.1542/peds.2005-0999. [DOI] [PubMed] [Google Scholar]
- 4.Sommer K, Whitman T, Borkowski J, Gondoli D, Burke J, Maxwell S, Weed K. Prenatal maternal predictors of cognitive and emotional delays in children of adolescent mothers. Adolescence. 2000;35:87–112. [PubMed] [Google Scholar]
- 5.Kongnyuy E, Nana P, Fomulu N, Wiysonge S, Kouam L, Doh A. Adverse perinatal outcomes of adolescent pregnancies in Cameroon. Maternal Child Health Journal. 2008;12:149–154. doi: 10.1007/s10995-007-0235-y. [DOI] [PubMed] [Google Scholar]
- 6.Ketterlinus R, Henderson S, Lamb M. Maternal age, sociodemographics, prenatal health and behavior: Influences on neonatal risk status. Journal of Adolescent Health Care. 1990;11:423–331. doi: 10.1016/0197-0070(90)90090-o. [DOI] [PubMed] [Google Scholar]
- 7.Byrd R, Weitzman M. Predictors of early grade retention among children in the United States. Pediatrics. 1994;93(3):481–487. [PubMed] [Google Scholar]
- 8.Coley R, Chase-Lansdale P. Adolescent pregnancy and parenthood: Recent evidence and future directions. American Psychologist. 1998;53(2):152–166. doi: 10.1037//0003-066x.53.2.152. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran J. Consequences of adolescent pregnancy/parenting: A review of the literature. Social Work Health Care. 1998;27:49–67. doi: 10.1300/J010v27n02_03. [DOI] [PubMed] [Google Scholar]
- 10.Miller C, Miceli P, Whitman T, Borkowski J. Cognitive readiness to parent and intellectual-emotional development in children of adolescent mothers. Developmental Psychology. 1996;32:533–541. [Google Scholar]
- 11.Moore K, Morrison D, Greene A. Effects on the children born to adolescent mothers. In: Maynard RA, editor. Kids having kids: Economic costs and social consequences of teen pregnancy. Urban Institute Press; Washington, DC: 1997. pp. 145–180. [Google Scholar]
- 12.Dahinten V, Shapka J, Wilms D. Adolescent Children of Adolescent Mothers: The Impact of Family Functioning on Trajectories of Development. Journal of Youth and Adolescence. 2007;36(2):195–212. [Google Scholar]
- 13.Turley R. Are children of young mothers disadvantaged because of their mother’s age or family background? Child Development. 2003;74:465–474. doi: 10.1111/1467-8624.7402010. [DOI] [PubMed] [Google Scholar]
- 14.Williams L, Decoufle P. Is maternal age a risk factor for mental retardation among children? American Journal of Epidemiology. 1999;149(9):814–823. doi: 10.1093/oxfordjournals.aje.a009897. [DOI] [PubMed] [Google Scholar]
- 15.Fergusson D, Woodward L. Maternal age and educational and psychosocial outcomes in early adulthood. Paediatric Perinatology Epidemiology. 1991;43(3):479–489. [PubMed] [Google Scholar]
- 16.Day N, Richardson G, Geva D, Robles N. Alcohol, marijuana, and tobacco: Effects of prenatal exposure on offspring growth and morphology at age six. Alcoholism: Clinical and Experimental Research. 1994;18:786–794. doi: 10.1111/j.1530-0277.1994.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 17.Cornelius M, Goldschmidt L, Day N, Larkby C. Prenatal substance use among pregnant teenagers: A six-year follow-up of effects on offspring growth. Neurotoxicology and Teratology. 2002;24:703–710. doi: 10.1016/s0892-0362(02)00271-4. [DOI] [PubMed] [Google Scholar]
- 18.Brenner W, Edelman D, Hendricks C. A standard of fetal growth for the United States of America. American Journal of Obstetrics and Gynecology. 1976;126:555–564. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2000. http://www.cdc.gov/nchs/about/nchs_accessibility.htm] [Google Scholar]
- 20.Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. Fourth Edition Riverside Publ Co.; Chicago, Il: 1986. [Google Scholar]
- 21.Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcoholism: Clinical and Experimental Research. 2006;30(6):1051–1059. doi: 10.1111/j.1530-0277.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker P, Mott F. National Longitudinal Study of Youth Child Handbook. Ohio State University; Center for Human Resource Research: 1989. [Google Scholar]
- 23.Frankenburg W, Coons C. The Home Screening Questionnaire: Its validity in assessing home environment. Journal of Pediatrics. 1986;108:624–626. doi: 10.1016/s0022-3476(86)80853-8. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell B, Bradley R. Home Observation for Measurement of the Environment. University of Arkansas at Little Rock; Little Rock: 1984. [Google Scholar]
- 25.Wechsler D. Wechsler Intelligence Scale for Children. Third Edition Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 26.Tong S, Baghurst P, Vimpani G, McMichael A. Socioeconomic position, maternal IQ, home environment, and cognitive development. Journal of Pediatrics. 2007;151:284–288. doi: 10.1016/j.jpeds.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor D, Batty G, Morton S, Deary I, Macintyre S, Ronalds G, Leon D. Early life predictors of childhood intelligence: Evidence from the Aberdeen children of the 1950’s study. Journal of Epidemiology and Community Health. 2005;59:566–663. doi: 10.1136/jech.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 29.Myers J, Weissman M. Use of a self-report symptom scale to detect depression in a community sample. American Journal of Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 30.Fisch R, Bilek M, Horobin J, Chang P. Children with superior intelligence at 7 years of age. American Journal of Disease in Children. 1976;130:481–487. doi: 10.1001/archpedi.1976.02120060027006. [DOI] [PubMed] [Google Scholar]
- 31.Gale C, O’Callaghan J, Bredow M, Martyn C. The influence of head growth in fetal life, infancy, and childhood on intelligence at ages 4 and 8 years. Pediatrics. 2007;118(4):1486–1492. doi: 10.1542/peds.2005-2629. [DOI] [PubMed] [Google Scholar]
- 32.Gale C, O’Callaghan F, Godfrey K, Law C. Critical periods of brain growth and cognitive function in children. Brain. 2004;127:321–329. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. International Journal of Pediatric Obesity. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 34.Brooks-Gunn J, Chase-Lansdale P. The children of adolescent mothers: Physical, academic and psychological outcomes. Developmental Review. 1986;6:224–251. [Google Scholar]
- 35.Farthing M. Current eating patterns of adolescents in the United States. Nutrition Today. 1991;26:35–39. [Google Scholar]
- 36.Gordon-Larsen P, Adair L, Nelson M, Popkin B. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. American Journal of Clinical Nutrition. 2004;80:569–575. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- 37.Flegal K, Troiano R. Changes in the distribution of body mass index of adults and children in the US population. International Journal of Obesity. 2000;24:807–818. doi: 10.1038/sj.ijo.0801232. [DOI] [PubMed] [Google Scholar]
- 38.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(30):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 39.Bennett E, Diamond M, Krech D, Rosenzweig M. Chemical and anatomical plasticity brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 40.Markham J, Greenough W. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1(4):351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wickett J, Vernon P, Lee D. Relationships between factors of intelligence and brain volume. Person Individ Differ. 2000;29:1095–1122. [Google Scholar]
- 42.Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. New Engl J Med. 1991;325:231–7. doi: 10.1056/NEJM199107253250403. [DOI] [PubMed] [Google Scholar]
- 43.Ounsted M, Moar V, Scott A. Head circumference and developmental ability at the age of 7 years. Acta Paediatr Scand. 1988;77:374–9. doi: 10.1111/j.1651-2227.1988.tb10663.x. [DOI] [PubMed] [Google Scholar]
- 44.Reiss A, Abrams M, Singer H, Ross J, Denckla M. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 45.Fisch R, Bilek M, Horrobin J, Chang P. Children with superior intelligence at 7 years of age. Am J Dis Child. 1976;130:481–7. doi: 10.1001/archpedi.1976.02120060027006. [DOI] [PubMed] [Google Scholar]
- 46.Anderson L, Fullilove M, Scrimshaw S, Fielding J, Normand J, Carande-Kulis V. The effectiveness of early childhood development programs. A systematic review. American Journal of Prevention Medicine. 2003;24(3):32–46. doi: 10.1016/s0749-3797(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 47.Eickmann S, Lima A, Guerra M, Lima M, Lira P, Huttly S, Ashworth A. Improved cognitive and motor development in a community-based intervention of psychosocial stimulation in northeast Brazil. Developmental Medicine and Child Neurology. 2003;45(8):536–41. doi: 10.1017/s0012162203000987. [DOI] [PubMed] [Google Scholar]
- 48.Hill J, Brooks-Gunn J, Waldfogel J. Sustained effects of high participation in an early intervention for low-birth-weight premature infants. Developmental Psychology. 2003;39:730–744. doi: 10.1037/0012-1649.39.4.730. [DOI] [PubMed] [Google Scholar]
- 49.Furstenberg F, Levine J, Brooks-Gunn J. The children of teenage mothers: Patterns of early childbearing in two generations. Family Planning Perspectives. 1990;22:54–61. [PubMed] [Google Scholar]
- 50.Hechtman L. Teenage mothers and their children: Risks and problems: A review. Canadian Journal of Psychiatry. 1989;34:569–574. doi: 10.1177/070674378903400615. [DOI] [PubMed] [Google Scholar]
- 51.Osofsky J, Culp A, Ware L. Intervention challenges with adolescent mothers and their infants. Psychiatry. 1988;51:236–241. doi: 10.1080/00332747.1988.11024397. [DOI] [PubMed] [Google Scholar]
- 52.Becker P. Sensitivity to infant development and behavior: A comparison of adolescent and adult single mothers. Research in Nursing and Health. 1987;10:119–127. doi: 10.1002/nur.4770100303. [DOI] [PubMed] [Google Scholar]
- 53.Newberger E. The helping hand strikes again: The unintended consequences of child abuse reporting. Journal of Clinical Child Psychology. 1983;12:307–311. [Google Scholar]
- 54.Cole TJ. Secular trends in growth. Proceedings of the Nutrition Society. 2000;59(2):317–324. doi: 10.1017/s0029665100000355. [DOI] [PubMed] [Google Scholar]
- 55.Dolman J, Olds TS. Secular changes in fatness and fat distribution in Australian children matched for body size. International Journal of Pediatric Obesity. 2006;1(2):109–13. doi: 10.1080/17477160600684260. [DOI] [PubMed] [Google Scholar]
- 56.Hauspie R, Vercauteren M, Susanne C. Secular changes in growth and maturation: An update. Acta Paediatrica Supplement. 1997;423:20–7. doi: 10.1111/j.1651-2227.1997.tb18364.x. [DOI] [PubMed] [Google Scholar]
- 57.Ogden C, Carroll D, Curtin L, McDowell M, Tabak C, Flegal K. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 58.Lynn R, Pagliari C. The intelligence of American children is still rising. Journal of Biosocial Science. 1994;26:65–67. doi: 10.1017/s0021932000021064. [DOI] [PubMed] [Google Scholar]


