To the Editor:
Anaphylaxis is a severe allergic reaction that has a rapid course and can result in death.1 Similar to other allergic diseases, the incidence of anaphylaxis has increased over the past few decades.2-4 Recent estimates suggest a lifetime prevalence of 0.05% to 2.0%,5 with food allergy being the most common cause.2,6 One approach was to indirectly estimate the incidence of anaphylaxis by analyzing medication-dispensing data for epinephrine.7 Camargo et al8 used this method to demonstrate a north-south gradient of EpiPen (Dey L.P., Napa, Calif) prescriptions, with the highest rates found in northern states. Our study scrutinized the incidence of pediatric anaphylaxis with a specific focus on a north-south comparison. To do this, we analyzed a large national billing database of US pediatric hospitals for all patient encounters billed as anaphylaxis.
Data for this study were obtained from the Pediatric Health Information System, an administrative database that contains administrative and billing data from a group of tertiary care pediatric hospitals in the United States that are affiliated with the Child Health Corporation of America (Shawnee Mission, Kan), a business alliance of children’s hospitals. We included all pediatric hospitals that contributed complete data from emergency department visits, inpatient admissions, and observation unit stays during a 5-year period from January 2003 to December 2007. There were 24 pediatric hospitals in 18 states across the United States that provided complete data during the entire study period. These hospitals were divided geographically at the line of 39°N latitude, extending roughly from Washington, DC, to Sacramento, California (see Fig E1 in this article’s Online Repository at www.jacionline.org). As determined by the US Geological Society (www.usgs.gov), cities with a degree of latitude greater than 39°N were considered northern cities (n = 11), and cities south of 39°N were considered southern cities (n = 13).
FIG E1.
Map of the United States divided into north and south. This represents a map of the United States divided geographically at the line of 39°N latitude. We considered hospitals in cities above this line to be northern hospitals and those below the line to be southern hospitals.
We queried all cases in which the primary billing International Classification of Diseases, ninth revision, diagnosis code was specific for anaphylaxis. The diagnoses of anaphylaxis were classified by the type of anaphylaxis (see Table E1 in this article’s Online Repository at www.jacionline.org). We did not include cases in which an anaphylaxis code was secondary or in which the code was not specific for anaphylaxis. Thus we did not include sting anaphylaxis because the code 989.5 (“toxic effect of venom”) is not limited to or specific for anaphylaxis. We then used the Pediatric Health Information System database to calculate the total number of patient encounters at each of the hospitals during the study period. The number of anaphylaxis cases was divided by the total number of patient encounters to calculate an incidence (cases per 1,000 encounters). Incidences were calculated for each geographic region, each type of patient, and each coded type of anaphylaxis. Rates were compared, and rate ratios (RRs) with 95% CIs were calculated. Statistical significance was achieved with a 2-sided P value of less than .05, and analyses were performed with SPSS version 14 software (SPSS, Inc, Chicago, Ill).
TABLE E1.
International Classification of Diseases, ninth revision, codes evaluated to determine cases of anaphylaxis
| “Food” anaphylaxis |
| 995.60 to 995.69: anaphylactic shock due to food (and specific foods) |
| “Immunization/serum” anaphylaxis |
| 999.4: anaphylactic shock caused by immunization or serum |
| “Medication” anaphylaxis |
| 977.9: anaphylactic shock caused by overdose or wrong substance given or taken |
| 977.9: anaphylactic shock caused by specified drug |
| “Unspecified/other” anaphylaxis |
| 995.0: anaphylactic shock or reaction NOS |
The database was searched for cases having a primary International Classification of Diseases, ninth revision, code specific for anaphylaxis.
We identified 6,457 cases of anaphylaxis among 8,589,583 patient encounters over the 5-year period. This is an incidence of 0.75 cases per 1,000 patient encounters. The median age of the subjects with anaphylaxis was 4.1 years, with 53.8% being male. Across the country, the incidence increased 50% during our study period, from 0.64 cases per 1,000 encounters in 2003 to 0.96 cases per 1,000 encounters in 2007 (P < .001).
The basic demographics of the cases of anaphylaxis are presented in Table I. Analysis of the northern hospital data revealed 3,704 cases of anaphylaxis among 4,199,103 patient encounters (rate of 0.88 cases per 1,000 encounters) compared with 2,753 cases of anaphylaxis among 4,390,480 patient encounters in the southern hospitals (rate of 0.63 cases per 1,000 encounters). Overall, this difference was statistically significant (RR, 1.41; 95% CI, 1.34-1.48; P < .001). Additionally, we compared degree of latitude with rate of anaphylaxis and found a statistically significant positive correlation (r = 0.555, P = .005). As seen in Table I, northern hospitals had statistically higher rates of anaphylaxis for all large subgroups of sex, race, and patient location.
TABLE I.
Basic demographics of cases of anaphylaxis
| Northern United States (11 hospitals) |
Southern United States (13 hospitals) |
||||
|---|---|---|---|---|---|
| Absolute no. | Rate (per 1,000) | Absolute no. | Rate (per 1,000) | P value | |
| Cases of anaphylaxis | 3,704 | 0.88 | 2,753 | 0.63 | <.001 |
| Total patient visits | 4,199,103 | 4,390,480 | |||
| Median age (y) | 4.3 | 4.0 | |||
| Sex | |||||
| Male (cases) | 2,018 | 0.90 | 1,453 | 0.61 | <.001 |
| Total visits | 2,244,487* | 2,372,381* | |||
| Female (cases) | 1,686 | 0.86 | 1,300 | 0.64 | <.001 |
| Total visits | 1,954,393* | 2,018,075* | |||
| Race/ethnicity | |||||
| White/non-Hispanic (cases) | 2,285 | 1.06 | 1,072 | 0.87 | <.001 |
| Total visits | 2,159,145 | 1,230,543 | |||
| Black (cases) | 671 | 0.62 | 924 | 0.56 | .044 |
| Total Visits | 1,082,313 | 1,650,409 | |||
| Hispanic (cases) | 207 | 0.52 | 475 | 0.44 | .045 |
| Total visits | 401,791 | 1,089,432 | |||
| Asian (cases) | 114 | 2.11 | 38 | 0.75 | <.001 |
| Total visits | 53,943 | 50,809 | |||
| Native American (cases) | 7 | 0.77 | 2 | 0.76 | .991 |
| Total visits | 9,113 | 2,627 | |||
| Other (cases) | 249 | 0.87 | 145 | 0.71 | .054 |
| Total visits | 285,845 | 203,651 | |||
| Unknown (cases) | 171 | 0.83 | 97 | 0.60 | .010 |
| Total visits | 206,953 | 163,009 | |||
| Patient location | |||||
| Emergency department (cases) | 2,640 | 0.78 | 1,725 | 0.49 | <.001 |
| Total visits | 3,395,655 | 3,524,166 | |||
| Inpatient (cases) | 1,064 | 1.32 | 1,028 | 1.19 | .012 |
| Total visits | 803,448 | 866,314 | |||
There were 24 visits in the south and 223 visits in the north without a sex listed.
Overall, the most common cause of anaphylaxis was food induced (n = 2,082 [32.2%]). This was followed by anaphylaxis caused by immunization or serum (n = 1,800 [27.9%]), medication (n = 1,627 [25.2%]), and unspecified or “other” causes (n = 946 [14.7%]). The comparison of northern hospitals with southern hospitals according to each type of anaphylaxis is seen in Fig 1. Anaphylaxis cases caused by food, immunization or serum, and “other” were all more common in the northern hospitals. In particular, the incidence of food anaphylaxis was almost double in the north compared with that in the south (0.31 vs 0.17; RR, 1.81; 95% CI, 1.66-1.98; P < .001). There was no statistical difference in the rates of medication-induced anaphylaxis between the north and south (0.19 vs 0.19; RR, 1.02; 95% CI, 0.92-1.12; P = .73).
FIG 1.
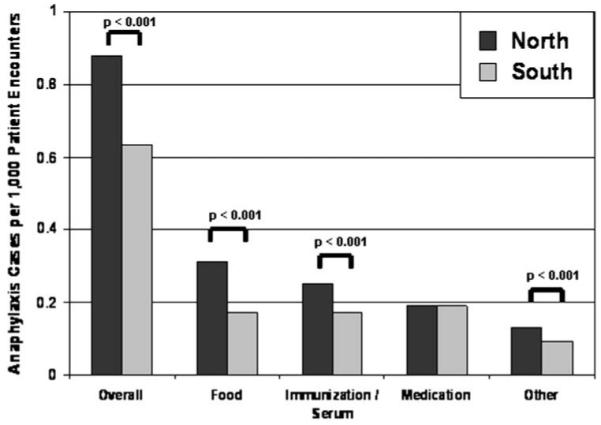
Rates of anaphylaxis by type of anaphylaxis. Each bar represents the identified cases of anaphylaxis per 1,000 patient encounters. P values represent the comparison of rates in the north with rates in the south (χ2 test).
This study is the first in the United States to evaluate the incidence of anaphylaxis on a national scale. Although we were unable to evaluate the true incidence in the general public, we were able to evaluate the rate of anaphylaxis cases per patient encounters at 24 hospitals in 18 states across the United States. We found a similar distribution of anaphylaxis as in previous studies,2,6 with food-induced cases being the most common. The median age of our cases was low (approximately 4 years), with a male predominance (53.8%).
Although evaluated from a different perspective, our study also suggests higher rates of anaphylaxis in northern areas of the United States. Previous studies have used epinephrine distribution data, but instead, our study used primary billing diagnostic codes, thus eliminating prescription-writing bias. It has been suggested that this north-south gradient might be due to differences in vitamin D status. Although some studies have shown an inverse relationship between vitamin D status and risk of atopic illnesses,9 more studies are needed in this area. Additionally, future studies are needed to evaluate for a north-south gradient for other atopic illnesses, such as asthma, allergic rhinitis, and eczema.
Of note, our study is representative of cases evaluated and treated at freestanding pediatric hospitals. As such, these hospitals are often referral centers providing tertiary care for all children in a certain city or state. It is difficult to assess how this might reflect incidence calculations for the general public. This might overestimate numbers if a large number of difficult anaphylaxis cases are referred to these hospitals. In contrast, it might underestimate incidence because anaphylaxis is an acute illness that is often treated immediately at local smaller hospitals. Also, our method of case identification by means of diagnostic billing codes might lead to errors in incidence calculation if anaphylaxis is inaccurately billed. However, both of these limitations occur in the north and south and should not affect the geographic comparison provided in our study.
Acknowledgments
W. J. S. is supported by a National Institutes of Health (NIH) National Research Service Award grant (T32-AI-007512). W. P. is supported by an NIH K-23 grant (AI-054972) and an NIH R-01 grant (AI-073964).
Footnotes
Disclosure of potential conflict of interest: W. J. Sheehan receives a T-32 Training Grant from the National Institutes of Health. W. Phipatanakul receives research support from the National Institutes of Health. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 2.Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–5. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yocum MW, Butterfield JH, Klein JS, Volcheck GW, Schroeder DR, Silverstein MD. Epidemiology of anaphylaxis in Olmsted County: a population-based study. J Allergy Clin Immunol. 1999;104:452–6. doi: 10.1016/s0091-6749(99)70392-1. [DOI] [PubMed] [Google Scholar]
- 4.Simons FE, Sampson HA. Anaphylaxis epidemic: fact or fiction? J Allergy Clin Immunol. 2008;122:1166–8. doi: 10.1016/j.jaci.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman P, Camargo CA, Jr, Bohlke K, Jick H, Miller RL, Sheikh A, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97:596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 6.de Silva IL, Mehr SS, Tey D, Tang ML. Paediatric anaphylaxis: a 5 year retrospective review. Allergy. 2008;63:1071–6. doi: 10.1111/j.1398-9995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 7.Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol. 2002;110:647–51. doi: 10.1067/mai.2002.127860. [DOI] [PubMed] [Google Scholar]
- 8.Camargo CA, Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120:131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]


